Abstract
A woman aged 42 years with a 1-month history of rapidly expanding bilateral breast masses presented with severe leucocytosis, anaemia, blurry vision, headaches and shortness of breath. Evaluation revealed chronic myeloid leukaemia in lymphoid blast crisis with extramedullary leukaemia involving her breasts.
Background
Chronic myeloid leukaemia (CML) was considered historically to be a fatal disease. It was the first malignancy identified to have a consistent chromosomal marker. The Philadelphia Chromosome, which involves a reciprocal translocation between chromosomes 9 and 22, was later discovered to result in the BCR-ABL1 oncoprotein. This oncoprotein has tyrosine kinase activity that causes a massive clonal expansion of multipotent stem cells leading to significant leucocytosis. The advent of tyrosine kinase inhibitors (TKIs) transformed CML from a fatal disease to a more prevalent chronic illness.1
CML is described as a triphasic disease consisting of a chronic, an accelerated and a blastic phase. In the developed world, CML is often diagnosed after leucocytosis is noted on a routine haemogram or when a patient presents with non-specific symptoms such as fatigue, weight loss, abdominal pain and early satiety. Without treatment, chronic phase (CP) CML typically progresses to the blastic phase within 3–5 years.2 Once in the blast phase, the prognosis is very poor. TKIs are highly effective in inducing a remission and decreasing the incidence of blast crisis.
Here, we present the case of a woman with precursor B-cell Philadelphia chromosome-positive ALL with leptomeningeal involvement and bilateral breast lymphoblastic infiltrates arising out of a previously undiagnosed CML. This case highlights the unusual extramedullary involvement of the breasts as well as the impact of socioeconomic factors that led to diagnosing her in the blastic phase, as is rarely seen in the developed world since the advent of TKIs.
Case presentation
A Guatemalan woman aged 42 years with no significant medical history presented with worsening back pain, blurry vision, decreased hearing, drenching night sweats, hot flashes, shortness of breath and pleuritic chest pain. She was evaluated at an urgent care clinic 1-month prior when she noticed a tender right breast mass during breastfeeding. She received antibiotic therapy for a presumed breastfeeding-associated mastitis. The right breast mass continued to grow, and she developed a new left breast mass, but she received no follow-up care until she presented to our hospital. She was an undocumented and uninsured patient with very limited medical literacy, which, she said, prevented her from seeking follow-up care.
On admission to the hospital, she was afebrile with a blood pressure of 150/82, a heart rate of 123 bpm and an oxygen saturation of 98% on 2 L via nasal cannula. Her physical examination was significant for a large, firm, slightly mobile and non-tender mass in the upper quadrant of the right breast and a non-mobile mass that was fixed to the chest wall involving her entire left breast. Her cardiac, respiratory and neurologic exams were unremarkable.
Investigations
Peripheral blood showed a white cell count count of 327.28/μL (67% blasts, 15% neutrophils, 5% bands, 5% metamyelocytes, 5% myelocytes and 3% lymphocytes), a haemoglobin of 7.4 g/dL and a platelet count of 313 000/μL. Peripheral blood smear showed a left shift with numerous blast cells (figure 1).
Figure 1.
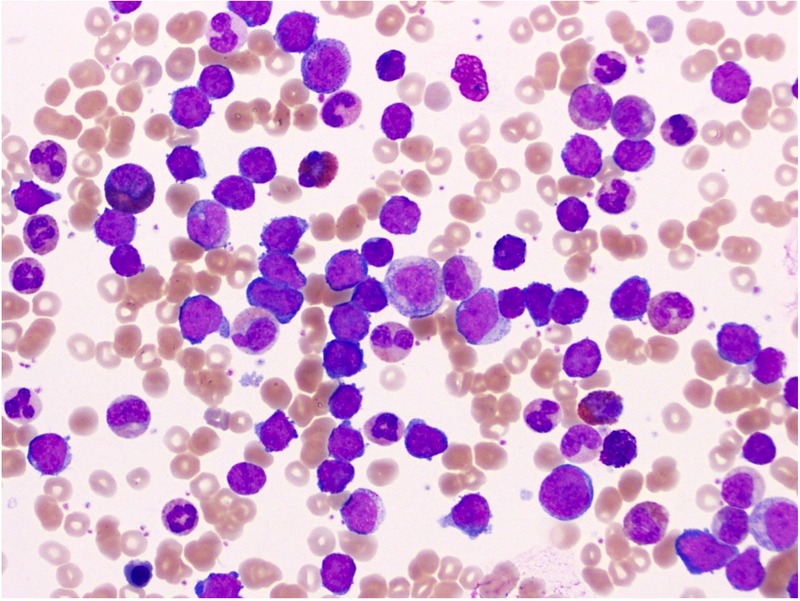
Peripheral blood smear showing a left shift with blast cells.
Peripheral blood immunohistochemistry had a marked increase in CD34, CD19 and TdT positive blasts, and flow cytometry showed 11% B lymphoblasts. TdT+ lymphoid blasts coexpressed B-cell marker CD19, CD34 (partial), CD38, HLA-DR, CD13 (partial) and CD7 (dim/partial), but were negative for CD20, CD10 and surface light chains. Bone marrow biopsy had 100% cellularity with 50–60% of the cell population being CD34, TdT and CD19 positive blasts (figure 2). The overall findings were consistent with an acute B-cell lymphoblastic transformation of an underlying undiagnosed CML. Cytogenetic studies showed normal karyotype 46, XX with translocation (9, 22) (q34;q11.2), which was confirmed by fluorescence in situ hybridization.
Figure 2.
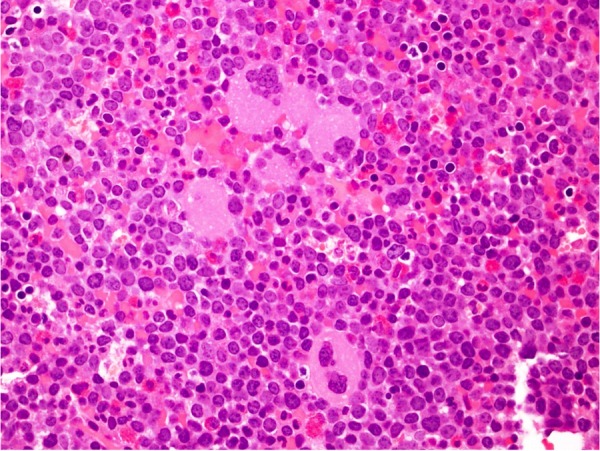
Bone marrow with myeloid and megakaryocytic hyperplasia.
Since she was reporting a headache and blurry vision in the setting of CML blast crisis, she underwent a lumbar puncture. The cerebrospinal fluid (CSF) showed marked lymphocytosis with numerous blasts (figure 3). A brain MRI showed enhancing areas along the left tentorial leaflets and within the retroclival space (figure 4). It also showed diffuse dural thickening and a possible irregular prominence and enhancement within the intraorbital optic nerves, left greater than right, which can be seen in leukemic infiltration of the dura.
Figure 3.
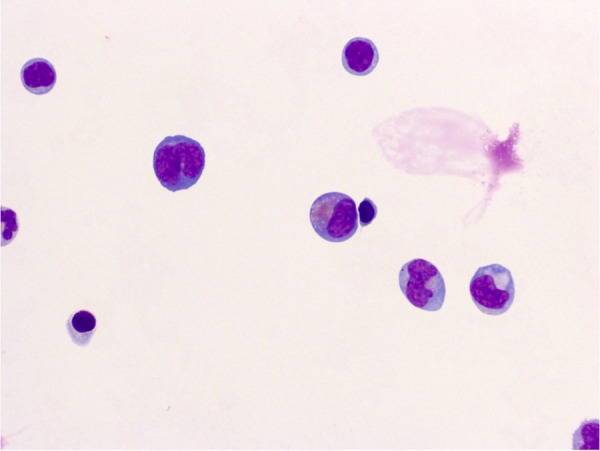
CSF showing marked lymphocytosis including larger atypical blast forms.
Figure 4.
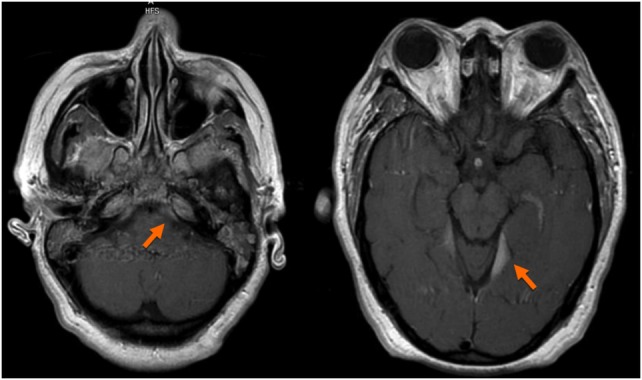
Brain MRI showing areas of enhancement along the left tentorial leaflets and within the retroclival space as well as diffuse dural thickening, likely due to leukemic infiltration of the dura.
Ultrasound of the right breast showed an ill-defined, predominantly hypoechoic lesion with irregular margins, measuring 5.5×5.4×3.8 cm (figure 5). CT angiography of the chest was negative for pulmonary embolus, but showed bilateral pleural effusions, prominent axillary and mediastinal lymphadenopathy and hepatosplenomegaly. Bilateral breast fine-needle aspirations showed atypical immature lymphocytes, consistent with extramedullary involvement of her blast crisis of CML (figure 6).
Figure 5.
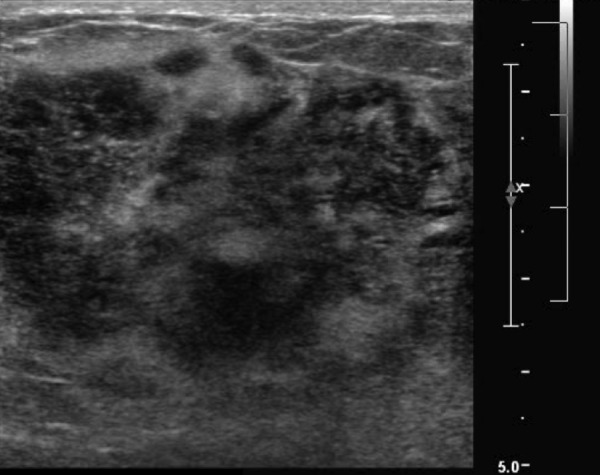
Right breast ultrasound showing an ill-defined, predominantly hypoechoic lesion with irregular margins.
Figure 6.
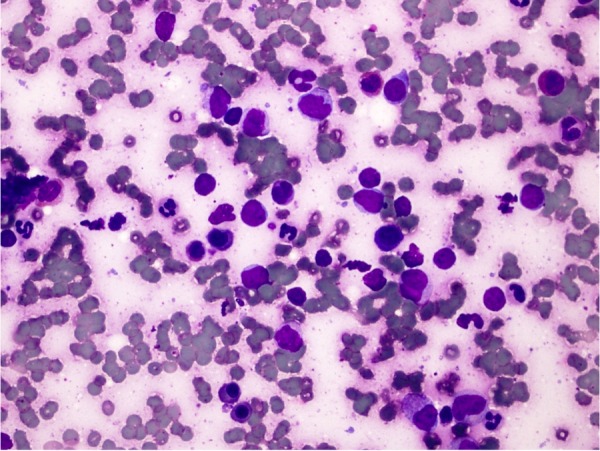
Breast FNA with atypical immature lymphocytes and background erythroid and myeloid left shift. FNA, fine-needle aspiration.
Differential diagnosis
The differential diagnosis for this patient presenting with leucocytosis and lymphoid blasts >20% in the peripheral blood includes CML in lymphoid blast crisis and acute lymphoblastic leukaemia (ALL). Patients with CML classically have an increase in the granulocytic cell lineages (eosinophils, neutrophils and basophils) and a myeloid bulge, which refers to an increase in the number of immature myeloid precursors in the peripheral blood.
Our patient's labs were notable for an increase in metamyelocytes and myelocytes in equal ratio. This implies a process of increased myeloid proliferation with maturation arrest, which is a hallmark of CML.
Our patient also harboured the 210 kD (p210) transcribed protein from the t(9, 22) chromosomal abnormality, which is strongly associated with CML, and not the 190 kD (p190) transcribed protein, which is more often associated with Ph+-ALL. Many experts consider differentiating between the p210 and the p190 protein to be a useful tool in differentiating CML in lymphoid blast crisis from Ph+ALL.3
Our patient's classic complete blood count (CBC) along with the p210 molecular abnormality leave little doubt that she had a previously undiagnosed CML and was now presenting in lymphoid blast crisis.
Treatment
The patient was started empirically on cytoreductive therapy with hydroxyurea and TKI therapy with imatinib, while awaiting the results of her laboratory studies. Owing to the extreme leucocytosis and neurological symptoms of blurry vision and headaches, she underwent 3 days of leukopheresis. Her white cell count decreased to 94/μL. When her flow cytometry and molecular studies confirmed Philadelphia positive CML in lymphoid blast crisis, she was transitioned from imatinib to dasatinib and began treatment with hyper-CVAD that consisted of hyperfractioned cyclophosphamide, vincristine, adriamycin and dexamethasone, followed by methotrexate and cytarabine. Owing to symptomatic leukemic meningitis, she received intrathecal methotrexate and cytarabine.
She tolerated the treatment well, though her hospital course was complicated by a Clostridium difficile infection.
Outcome and follow-up
After one cycle of chemotherapy, her white cell count normalised to 5.16/μL without peripheral blasts. Her breast masses clinically resolved, and a follow-up ultrasound showed no residual masses. A repeat lumbar puncture prior to cycle 2 did not identify any abnormal cells in the CSF. A repeat brain MRI did not have any areas of enhancement and showed resolution of the previously noted dural thickening. A follow-up chest X-ray showed resolution of the previously visualised bilateral pleural effusions.
After completing three cycles of hyper-CVAD, she achieved a haematological remission. Unfortunately, the duration of her haematological remission was brief, and she did not achieve a cytogenetic or molecular remission. She has since relapsed with recurrent blast crisis and is undergoing salvage therapy. The goal is for her to undergo an allogenic bone marrow transplant.
Discussion
The genetic hallmark of CML, which was the first genetic abnormality associated with cancer, is the oncogene BCR-ABL from the resultant translocation of the ABL gene (from chromosome 9) onto the BCR gene (on chromosome 22). Although the function of BCR is largely unknown, ABL is a tyrosine kinase that phosphorylates various proteins that participate in the cell division and adhesion. This protein, in its normal structure and function, is tightly regulated by its regulatory domain (SH3 domain); nonetheless, this domain is not expressed in this fusion protein and it acts as a constitutively ‘on’ tyrosine kinase—propagating increased cell division.4 5 The working molecular hypothesis behind the pathogenesis of CML is some genomic hit (the only known risk factor being ionising radiation) and can lead to acquisition of the BCR-ABL translocation resulting in CP CML with an expanding granulocyte compartment. The continued, uncontrolled activity of BCR-ABL is thought to cause increased oxidative stress leading to DNA damage, impaired DNA repair and additional mutations causing progression to the blast phase. Some common mutations seen in the molecular profiling of patients in the blast phase include trisomy 8, 19, p53, p16 and the clonal evolution of BCR-ABL or point mutations of the BCR-ABL tyrosine kinase domain itself conferring resistance to TKIs.6–8
The pathophysiology behind CML transition to blast crisis secondary to unregulated BCR-ABL activity emphasises the need to reduce the BCR-ABL-positive cell pool as early and as significantly as possible. This has been possible with the advent of TKIs. Prior to the TKIs, conventional chemotherapy was ineffective with progression to blast crisis for most patients within 5 years. However, with the use of TKIs, the median survival in CP is estimated at 25–30 years and progression to advanced phase CML or BC has been reduced to 1.5% per year during the first 5 years.9
CML is unique among haematologic malignancies in that the malignant cell is a pluripotent stem cell, and progression of disease can manifest as either a myeloid blast phase or a lymphoid blast phase. Patients with a myeloid blast crisis are treated like de novo AML with cytarabine and idarubicin, known commonly as 7+3, in combination with a TKI. Patients with a lymphoid blast crisis are treated with a multidrug regimen including vincristine and steroids. The goal of therapy is to achieve a second CP and proceed eventually to an allogenic stem cell transplant, which offers the best chance of long-term survival.
There are no large randomised trials comparing induction regimens since the advent of TKI therapy, since the blast phase has become a rare event in developed countries. Although vincristine, dexamethasone and a TKI alone have been shown to be highly effective in treating lymphoid blast crisis, the study only included 13 patients with untreated CML and showed the best results in older patients.10 Another small study including 11 patients with de novo lymphoid blast phase showed good outcomes when hyper-CVAD is given in combination with imatinib. Since our patient was young with high-risk features including extramedullary disease, she was treated with the more aggressive hyper-CVAD induction in combination with dasatinib. We substituted dasatinib for imatinib because of theoretically improved central nervous system (CNS) penetration in our patient with known leptomeningeal involvement.11
Although extramedullary acute leukaemia is not unheard of, particularly in the context of leukaemia cutis and myeloid sarcoma in AML, CML lymphoid blast crisis is very rare to involve the breast. Typical metastatic cancers to the breast include various lymphomas, lung cancer, ovarian cancer and rhabdomyosarcoma with the common clinical presentation as an outer upper quadrant mobile mass, much like the right breast mass noted in our patient.12 On the other hand, typical ALL relapse sites include the bone marrow, CNS and occasionally the ovaries. In a PubMed literature review of ‘ALL’ and ‘extramedullary involvement of the breast’ (not differentiating from CML blast crisis or not), there were only 14 case reports with another 3 focusing on radiological findings and only 5 of the 14 reports are in adult patients (one T cell, 2 pre-B cell and 2 unavailable immunophenotypes), highlighting the rarity of this manifestation of ALL, let alone CML in ALL transformation.13–16
Learning points.
TKIs are an essential component of therapy regardless of the phase of CML to prevent further DNA damage and uncontrolled cellular division.
Although extramedullary haematopoiesis is not rare in CML blast crisis, the breast is a very uncommon site.
Extramedullary blastic infiltrates are responsive to standard of care combination chemotherapy and TKIs.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Yong ASM, Barrett JA. Chronic myelogenous leukemia. In: Rodgers GP, Young N, eds. The Bethesda handbook of clinical hematology. Philadelphia, PA: Lippincott Williams & Wilkins, 2013:170–1. [Google Scholar]
- 2.Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer 2007;7:441–53. 10.1038/nrc2147 [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Talpaz HM, Dhingra K et al. Significant of P210 Versus P190 molecular abnormalities in adults with Philadelphia chromosome-positive acute leukemia. Blood 1991;78:2411–18. [PubMed] [Google Scholar]
- 4.Alimena G, De Cuia MR, Diverio D et al. The karyotype of blastic crisis. Cancer Genet Cytogenet 1987;26:39–50. 10.1016/0165-4608(87)90131-2 [DOI] [PubMed] [Google Scholar]
- 5.Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol 2002;107:76 doi:46636 [DOI] [PubMed] [Google Scholar]
- 6.Fabarius A, Leitner A, Hochhaus A et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood 2011;118:6760–8. 10.1182/blood-2011-08-373902 [DOI] [PubMed] [Google Scholar]
- 7.Koptyra M, Falinski R, Nowicki MO et al. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood 2006;108:319–27. 10.1182/blood-2005-07-2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hehlmann R. How i treat CML blast crisis. Blood 2012;120:737–47. 10.1182/blood-2012-03-380147 [DOI] [PubMed] [Google Scholar]
- 9.Druker BJ, Guilhot F, O'Brien SG et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006;355:2408–17. 10.1056/NEJMoa062867 [DOI] [PubMed] [Google Scholar]
- 10.Rea D, Legros L, Raffoux E et al. High-dose imatinib mesylate combined with vincristine and dexamethasone as induction therapy in patients with resistant Philadelphia-positive acute lymphoblastic leukemia and lymphoid blast crisis of chronic myeloid leukemia. Leukemia 2006;20:400–3. 10.1038/sj.leu.2404115 [DOI] [PubMed] [Google Scholar]
- 11.Porkka K, Koskenvesa P, Lundán T et al. Dasatinib crosses the blood–brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 2008;112:1005–12. 10.1182/blood-2008-02-140665 [DOI] [PubMed] [Google Scholar]
- 12.Basara I, Orguc SJ. Giant breast involvement in acute lymphoblastic leukemia: MRI findings. J Breast Cancer 2012;15:258–60. 10.4048/jbc.2012.15.2.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gogia A, Mehta P, Pramanik R et al. Isolated breast relapse mimicking breast cancer in elderly patient with acute lymphoblastic leukemia. Turk J Hematol 2014;31:203–4. 10.4274/tjh.2013.0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karbasian-Esfahani M, Wiernik PH, Yeddu M et al. Leukemic infiltration of the breast in acute lymphocytic leukemia (ALL). Hematology 2008;13:101–6. 10.1179/102453308X315933 [DOI] [PubMed] [Google Scholar]
- 15.Chim CS, Shek TW, Liang R. Isolated relapse of acute lymphoblastic leukemia in the breast masquerading as gynecomastia. Am J Med 2000;108:677–9. 10.1016/S0002-9343(99)00397-6 [DOI] [PubMed] [Google Scholar]
- 16.Luzurier A, Van Der Gucht A, Blanc E et al. Breast infiltration by relapsed acute lymphoblastic leukaemia on FDG PET/CT. Eur J Nucl Med Mol Imaging 2015;42:811–12. 10.1007/s00259-014-2977-2 [DOI] [PubMed] [Google Scholar]


