Abstract
Following orthopaedic surgery, a 56-year-old woman developed heparin-induced thrombocytopenia (HIT), complicated by extensive proximal deep vein thrombosis. The patient did not respond to multiple conventional therapies; however, a prompt treatment response occurred after starting rivaroxaban at standard dosing. This case represents the first documentation of efficacy for rivaroxaban in the setting of treatment refractory HIT and strengthens the limited existing evidence for this agent in HIT.
Background
Heparin-induced thrombocytopenia/thrombosis (HIT) is a prothrombotic phenomenon that occurs when IgG antibodies to heparin-platelet-factor-4 (PF4) complexes bind through the platelet FcγIIa receptor.1 Platelet activation ensues, resulting in venous or arterial thrombosis in up to 50% of cases.2 Rivaroxaban, an oral direct Xa inhibitor, does not cause such platelet activation in the presence of heparin-PF4 antibodies, nor does it provoke PF4 release from platelets in plasma.3 Accordingly, rivaroxaban may be an effective anticoagulant for treating patients with HIT and thrombosis, though safety and efficacy data are lacking. Several case reports have documented successful use of rivaroxaban as upfront management of HIT, with satisfactory effect.4–7 Most recently, Linkins et al8 have published a small prospective cohort study in 12 patients with confirmed HIT, which demonstrates the effectiveness and safety of rivaroxaban in this condition. In this case report, we describe a patient with severe HIT, refractory to conventional treatment with therapeutic dose fondaparinux and supratherapeutic levels of danaparoid that responded promptly to rivaroxaban.
Case presentation
A 56-year-old previously well woman presented to the emergency department with extensive unilateral lower limb swelling and pain, 10 days following an elective total knee replacement for osteoarthritis. Prophylactic enoxaparin (40 mg daily) was administered postoperatively for 5 days, but it was discontinued at discharge. There was no re-exposure to heparin or enoxaparin prior to re-presentation.
Investigations
Doppler ultrasonography revealed a proximal deep vein thrombosis in the left femoral and external iliac veins. A full blood examination revealed that the patient's platelet count had fallen from a baseline of 287×109/L to a nadir of 23×109/L. Examination of the blood film revealed a mild neutrophilia and marked thrombocytopenia with no further diagnostic features. HIT was suspected and subsequently confirmed when ELISA testing returned strongly positive (141%, kit positive cut-off >10.5%).
Treatment
Therapeutic fondaparinux (7.5 mg/day) was started on day 3, but proved ineffective and was ceased on day 8 as the D-dimer remained elevated at 5.09 mg/L (normal range <0.2 mg/L) and the platelet count remained 48×109/L. A therapeutic danaparoid infusion was started on day 9. However, a supratherapeutic anti-Xa level (∼1.3 U/mL; recommended level 0.5–0.8 U/mL) was required to suppress plasma D-dimer and stabilise the platelet count (figure 1). Further investigations including CT scanning of the chest, abdomen and pelvis failed to reveal an occult malignancy, although an asymptomatic pulmonary embolus was detected at this time. HIT-associated disseminated intravascular coagulation was also excluded based on serially normal fibrinogen levels. In an attempt to suppress HIT antibody production, prednisolone (1 mg/kg) was added at day 13; however, this too proved ineffectual. On day 19, given clinical stability and with consideration of an urgent family matter, the patient's treatment was switched to oral rivaroxaban (15 mg twice daily); within 5 days the platelet count doubled to 94×109/L and was 176×109/L after 1 week of treatment. There was an accompanying precipitous decline in the D-dimer as well as marked symptomatic improvement.
Figure 1.
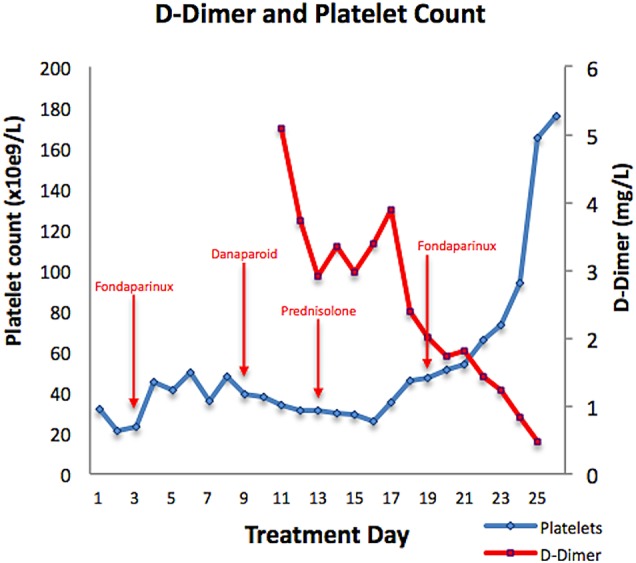
Platelet count and D-dimer charted against time and across treatment modalities.
Outcome and follow-up
The rivaroxaban dose was adjusted to 20 mg daily after 3 weeks and ultimately ceased after 6 months of therapy. The treatment was well tolerated with no adverse effects and the patient remains clinically well, with no recurrence of thromboembolism.
Discussion
Our case is unique among existing literature, objectively demonstrating rapid normalisation of D-dimer, platelet levels and clinical improvement in a patient with treatment-resistant HIT. Given the rarity of HIT itself, there are no consensus definition or management guidelines for treatment refractory disease, and such cases remain a challenging clinical scenario. Fondaparinux and danaparoid are indirect factor Xa inhibitors and suitable non-heparin anticoagulants for use in HIT.2 Additional alternative therapeutic options include parenteral direct thrombin inhibitors such as bivalirudin or argatroban (lepirudin no longer being available in Australia).2 Both agents require continuous intravenous infusions and frequent monitoring and dose adjustment is mandatory; argatroban also affects the prothrombin time, thereby complicating the transition to warfarin, and bivalirudin has minimal evidence in HIT and is infrequently used in our institution. There are no randomised prospective trials comparing the various non-heparin anticoagulants in HIT and, in many instances, practice is significantly dependent on physician and institutional experience with the available agents.
Rivaroxaban is an attractive alternative treatment for HIT, having demonstrated no evidence of platelet activation in the presence of HIT antibodies assessed via serotonin release assay, platelet aggregometry or flow cytometric analyses.3 Additionally, there is no evidence that rivaroxaban provokes release of PF4 from either quiescent or activated platelets.3 Furthermore, rivaroxaban's anti-Xa activity was unchanged following incubation with purified PF4, suggesting that no interaction between these compounds occurs.3
While other case reports and a small cohort study suggest that rivaroxaban appears to be effective as upfront therapy in HIT, its efficacy in the treatment resistant setting bolsters existing evidence and supports the theoretical underpinnings for this agent. Although this case contributes to the evidence for rivaroxaban in HIT, validation in a larger cohort study is required.
Patient's perspective.
In late February 2015, I had a full knee reconstruction done. Five days after the operation I returned home and on the fifth day at home I realised something had gone awfully wrong. My leg swelled up from top to toe, it went blue-black in colour like an elephant foot and I was in terrible pain. The pain was so severe, and walking even a short distance was excruciating and slow.
When in hospital I was diagnosed with HIT; every day I wondered if I was going to survive. Was I going to lose my leg? Were my doctors going to get the right mix with my medication to cure my problem? I was very scared as my platelets were very unstable, rising one day and then plummeting down the next. My condition was not responding to the usual medication for treating HIT. Some days I would be so tired and lethargic, I could not stay awake and I also experienced strange jolting movements and pain.
Medical staff informed me that they were going to try a new medication, one that had never been used before for an HIT case like mine, but they explained to me that using this drug could have a good outcome. Even though I was apprehensive, I really had nothing to lose. I felt a trial of the drug was worthwhile and I gave full consent due to the fact multiple other medications had failed.
The new drug was effective and my body responded quite quickly, changes in tests were noticed straight away and it was not long after the changes that I could be discharged. This was such a relief after spending a whole month in hospital. I felt free and that I had my life back; after going home I continued to improve and continued to receive lots of follow-up appointments to monitor and receive feedback on my progress. I am well on the road to recovery but am aware that this condition can return. I use compression stockings and exercise regularly.
In reflecting on my time as an in-patient, I am very grateful for the attention paid to me by medical staff and their caring manner. I do feel that I may have benefited from a port or other means of regularly performing blood tests as this could have saved me a lot of excessive pain and anguish, but this is only a minor symptom. I would like to thank the medical staff for always remembering there is a patient in the bed, and this will never be forgotten. I benefited greatly from all the positive feedback and reassurance that they would get me better and that there was a solution out there to help me. They always kept me thinking ahead and informed on what they were going to try next.
Learning points.
As a direct Xa inhibitor, rivaroxaban has a sound theoretical basis for efficacy in HIT, but published data are limited to case reports and a small cohort study of upfront treatment in uncomplicated cases.
This case demonstrates the effectiveness of rivaroxaban in a case of HIT that was refractory to multiple conventional therapies.
While this report demonstrates the promising utility of rivaroxaban in HIT, larger studies are needed to definitively establish safety and efficacy.
Footnotes
Contributors: JMLC is a primary treating registrar and the author of the paper and involved in background research and formulation. GG involved in supervising treating consultant; article formulation, editing and oversight. SC supervised treating consultant, gave input into investigational strategy, and participated in article editing and oversight. NC is a consulting physician and thrombosis expert and involved in article formulation and editing.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Amiral J, Bridey F, Dreyfus M et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. J Thromb Haemost 1992;68:95–6. [PubMed] [Google Scholar]
- 2.Linkins LA, Dans AL, Moores LK et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e495S–530S. 10.1378/chest.11-2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walenga JM, Prechel M, Jeske WP et al. Rivaroxaban—an oral, direct Factor Xa inhibitor—has potential for management of patients with heparin-induced thrombocytopenia. Br J Haematol 2008;143:92–9. 10.1111/j.1365-2141.2008.07300.x [DOI] [PubMed] [Google Scholar]
- 4.Ng HJ, Than H, Teo EC. First experiences with the use of rivaroxaban in the treatment of heparin-induced thrombocytopenia. Thromb Res 2015;135: 205–7. 10.1016/j.thromres.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 5.Sartori M, Favaretto E, Cini M et al. Rivaroxaban in the treatment of heparin-induced thrombocytopenia. J Thromb Thrombolysis 2015;40:392–4. 10.1007/s11239-015-1208-4 [DOI] [PubMed] [Google Scholar]
- 6.Hantson P, Lambert C, Hermans C. Rivaroxaban for arterial thrombosis related to heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis 2015;26:205–6. 10.1097/MBC.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 7.Abouchkara L, Khabbaz Z, Abouassi S et al. Rivaroxaban for treatment of heparin-induced thrombocytopenia after cardiac surgery: A case report. J Thoracic Cardiovasc Surg 2015;150:e19–20. 10.1016/j.jtcvs.2015.04.054 [DOI] [PubMed] [Google Scholar]
- 8.Linkins LA, Warkentin TE, Pai M et al. Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. J Thromb Haemost 2016;14:1206–10. 10.1111/jth.13330 [DOI] [PubMed] [Google Scholar]


