Abstract
Smith-Magenis syndrome (SMS) is a rare genetic neurodevelopmental disorder characterised by behavioural disturbances, intellectual disability and early onset obesity. The physical features of this syndrome are well characterised; however, behavioural features, such as sleep disturbance, are less well understood and difficult to manage. Sleep issues in SMS are likely due to a combination of disturbed melatonin cycle, facial anatomy and obesity-related ventilatory problems. Sleep disorders can be very distressing to patients and their families, as exemplified by our patient's experience, and can worsen behavioural issues as well as general health. This case demonstrates the successful use of non-invasive ventilation in treating underlying obesity hypoventilation syndrome and obstructive sleep apnoea. As a consequence of addressing abnormalities in sleep patterns, some behavioural problems improved.
Background
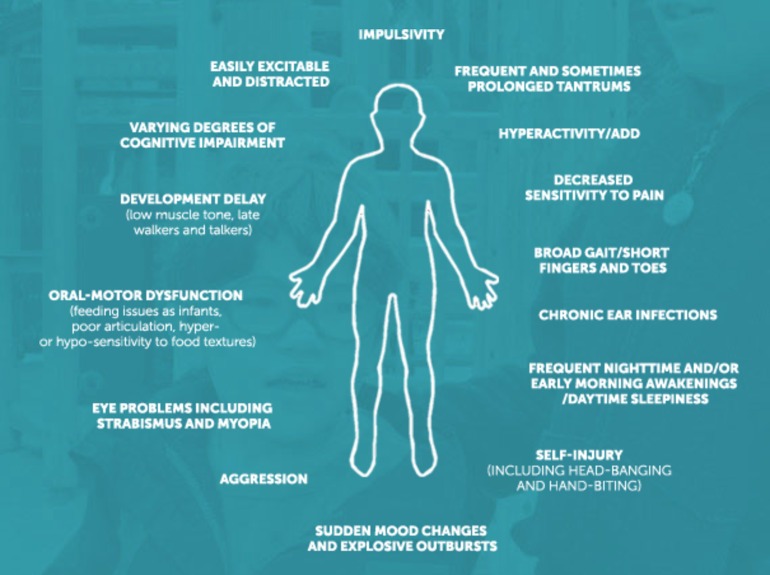
Smith-Magenis syndrome (SMS) is a rare neurodevelopmental disorder caused by a microdeletion of chromosome 17p11.2, which occurs de novo in virtually all cases.1–3 The estimated prevalence is between 1 in 15 000 and 1 in 25 000 live births.2 It was first described in 1982, since then the clinical phenotype has been well described and clear associations have been made with sleep disturbances, intellectual disability and early onset obesity. In addition, patients display a wide range of behavioural abnormalities, including self-injury, aggression, tantrums and attention deficits.4 5 Figure 1 summarises common features of the syndrome.
Figure 1.
Key behavioural features of Smith-Magenis syndrome. Reproduced with permission from the Smith-Magenis Research Foundation.
Sleep disorder in SMS can be very distressing to patients and their families (see ‘patient's experience’), and may also be responsible for some behavioural problems.4 5 Many studies have found that patients with SMS have disturbed circadian rhythms in melatonin and have hypothesised that this is the likely cause of sleep disturbance.6 However, melatonin replacement therapy is often ineffective as treatment. These patients also have a combination of anatomical features which may lead to disordered sleep: obesity is common and can be severe, and SMS facies are characterised by flattening of the middle of the face and the bridge of the nose (figure 2).
Figure 2.
An adult woman and young girl with typical facial features of Smith-Magenis syndrome. Photo kindly provided by the Smith-Magenis Foundation UK.
Case presentation
A 23-year-old man with known SMS attended respiratory clinic due to his deteriorating breathlessness. He had the typical facial appearance associated with SMS. In addition, the family reported a history of excessive eating, behavioural problems and learning difficulties.
Pharmacological treatment included melatonin. However, his family reported that this treatment was not effective in normalising his sleep pattern.
His mother described a lifelong history of noisy breathing with snoring from infancy. The patient had been investigated extensively and been found to have narrow nasal airway passages, which led to surgical repair of a deviated septum during childhood. Unfortunately, this did not improve his symptoms.
There was also a history of excessive daytime sleepiness with an Epworth score of 23. He slept upright in a chair, reporting a long sleep latency of about 1 hour and woke up intermittently with choking episodes. Consequently, he took frequent naps during the day. After extensive investigations, he was diagnosed with obesity hypoventilation syndrome (OHS) and obstructive sleep apnoea (OSA).
Periods of worse sleep associated with exacerbations of his behavioural issues. Main problems reported by his parents included ‘temper tantrums’ where he would become very aggressive, displaying challenging and attention seeking behaviours on a daily basis. They also reported a lack of concentration and difficulty finishing tasks; both of which were often associated with frustration and distress.
In his social history, he lived with his family and was dependent on his mother for his care needs. There were no other family members with relevant medical conditions.
On examination, he was clinically obese with a body mass index of 41. Auscultation of his chest revealed generally good air entry but quiet breath sounds throughout the lung fields with no added sounds. The heart sounds were quiet with no murmurs. Blood pressure was 132/84 mm Hg. His jugular venous pressure was not noticeably elevated, but there was bilateral pitting oedema up to his thighs. His throat appeared crowded with a Mallampati score of 4.
Investigations
Preintervention
An inpatient sleep study (polysomnography) was performed for a duration of just over 6 hours. Although the study was compromised by a few loss of signal for oxygen saturation, it was nevertheless deemed valid for analysis. The study reported 24 obstructive events per hour (/hour) with no central events. Apnoeas (average duration 18 s) occurred at 24/hour and hypopnoea (average 27 s) occurred at 10/hour, giving an apnoea–hypopnea index (AHI) of 34/hour, which is suggestive of severe OSA. The respiratory disturbance index (RDI) was 71/hour. There was no evidence of Cheyne-Stokes breathing. There were 55/hour desaturations <90%, although this was limited by some signal loss. The average heart rate was 92 bpm. Total leg movement occurred at 16/hour with the periodic leg movement index of 2.3/hour. The snore index was 492/hour. In summary, these findings suggest severe OSA.
Spirometry showed a restrictive pattern with forced expiratory volume (FEV1) of 53% predicted, forced vital capacity (FVC) of 58% predicted and FEV1/FVC ratio of 77%. The peak flow was 61% predicted.
ECG showed sinus rhythm with signs of right-axis deviation and right ventricular strain. This was consistent with cardiopulmonary MRI findings, which demonstrated significant right ventricular hypertrophy. All chamber sizes were normal and biventricular function was preserved.
Echocardiography was difficult, given his obesity. Right ventricular outflow tract velocity time integral was 13 cm and tricuspid regurgitation velocity was 3.3 m/s. Right ventricular systolic velocity was 10 cm/s. Pulmonary vascular resistance was calculated at 2.7 Wood units. Findings were consistent with elevation in pulmonary artery pressure. Left ventricle was not dilated and had good systolic function.
Early morning arterial blood gas (ABG) on room air showed: pH 7.32, pCO2 9.0 kPa, pO2 7.1 kPa, HCO3 28.0 mmol/L.
Postintervention
Early morning ABG on:
Continuous positive airway pressure (CPAP) with pressure of 11 cm H2O: pH 7.33, pCO2 8.6 kPa, pO2 6.7 kPa, HCO3 27.8 mmol/L.
Non-invasive ventilation (NIV) 20/10 cm H2O on room air: pH 7.34, pCO2 8.1 kPa, pO2 8.1 kPa, HCO3 27.5 mmol/L.
NIV 24/10 cm H2O on room air: pH 7.35, pCO2 7.7 kPa, pO2 7.7 kPa, HCO3 26.9 mmol/L.
Overnight oxygen monitoring showed mean oxygen saturations of 92.3% with a median of 92.9%. The patient spent 37 min below 90% oxygen saturations during the 5 hour 45 min tracing, with some dipping at the 4% level at the rate of 17/hour. Trace was limited by artefact and the oxygen saturations probe being off the patient's finger for much of the night.
Treatment
Initial treatment included weight management. In addition to treating his OSA, he was initially given a trial of CPAP which improved baseline oxygen saturations and led to some improvement in his symptoms. However, early morning ABG results showed raised carbon dioxide levels.
As a result of this, he was switched to nocturnal NIV which was increased to 24 cm H2O inspiratory positive airway pressure and 10 cm H2O expiratory positive airway pressure, which the patient tolerated well. This improved the patients ABGs: pH increased from 7.33 to 7.35 and SpO2 increased from 6.7 to 7.7 kPa.
Supplemental oxygen was not given as the overnight oximetry on NIV was inconclusive due to significant artefact. After discussion with the patient's family, a decision was reached not to attempt a further overnight oximetry, given his significant symptomatic improvement.
Outcome and follow-up
After the initial trial of CPAP, the patient and his family noticed reduced snoring and nocturnal choking, they also reported an improvement in the patient's daytime sleepiness.
Significant improvement was noted after switching to nocturnal NIV, the nasal mask was tolerated well; the patient used it each night with no oxygen entrained. The patient reported much better sleep quality, control of snoring and was much less sleepy during the day. He also reported improvement of his nocturia and peripheral oedema. Repeat overnight oximetry showed an improvement in mean oxygen saturations and less reported dips.
The family reported that in addition to a better sleeping pattern, they had also noticed a significant improvement in his overall quality of life. He appeared to be less agitated and distressed throughout the day, and there were less frequent temper tantrum episodes. They felt that the reduction in daytime drowsiness seemed to improve his concentration and attention span which had generally improved his overall behaviour.
With assistance from a weight management programme, he was able to lose 6 kg in 1 year.
Discussion
Sleep disorders are commonly reported in patients with SMS, the majority of research evidence attributes this to an inverted diurnal secretion of melatonin.7 8 Several pharmacological treatments have been trialled, including melatonin replacement, β-agonists and growth hormone replacement. However, the consensus is that if these do not improve sleep disturbance, then the underlying cause is genetically determined and therefore resistant to interventions.8
However, in some patients with a combination of otolaryngological abnormalities due to characteristic facial features, obesity and general hypotonia, there may be a treatable component. Investigations into respiratory patterns, including full sleep studies or overnight oxygen oximetry, are rarely discussed but are important to assess if there is a treatable component to sleep disturbance. A case report in 2010 supported this; Leoni et al7 described a paediatric patient with SMS who presented with repeated oxygen desaturations during rapid eye movement (REM) sleep but with no apnoeas or hypopneas. They suggest that this could be associated with a subclinical restrictive respiratory impairment or an impairment of central respiratory control during REM sleep and advise recording of respiratory parameters in all patients with SMS who complain of sleep disturbance.7
More recently, Takenouchi et al8 described a patient with SMS who had significant sleep disturbance (frequent nocturnal awakenings and early awakenings), daytime somnolence and snoring since infancy which interfered with daytime activities. The team carried out an overnight polysomnography which showed severe sleep apnoea (AHI 97.5/hour).8 CPAP was started which significantly improved his daytime somnolence, snoring and nocturnal wakening, leading to better communication skills and improved concentration throughout the day.8
In conclusion, disordered sleep in SMS is likely multifactorial. Obesity-related ventilatory disorders should be considered, assessed and managed. NIV may dramatically improve sleep and help with behavioural issues. Further case series are needed to support the screening of sleep-disordered breathing in SMS.
Patient's perspective.
[He] had breathing problems since birth […] especially at night and would cough, choke, vomit, waking up at least 4–6 times a night. We all suffered constant sleep deprivation as he would get up and disturb his siblings. I would find him asleep on the floor and the stairs. He would fall asleep throughout the day on his school desk, going up the stairs, behind the bathroom door. His temper tantrums were frequent even as a toddler; he would bang his head on the floor or the wall, rip up his books screaming. As he got older the tantrum became more violent. As he approached his 20s his health issues escalated. He was flooding the bed three times every night. His snoring and choking worsened, he simply could not sleep […] he was exhausted, falling asleep through the day, snoring very loud, with pitting oedema up to his knees.
From the first night [he] had BiPAP his health improved. I could put him to bed knowing that within 2 min he would be asleep and be warm and dry. His daytime napping has improved and he can be more engaged during the day. The BiPAP has improved his health and life immeasurably. He feels a lot healthier and is [...] losing weight. I feel strongly that [BiPAP] should be offered to people with Smith-Magenis. I have always thought [his] breathing problems were anatomical with his very small nasal passages his flattened bridge of nose.
Learning points.
Obesity-related ventilatoy disorders should be considered and investigated in patients with Smith-Magenis syndrome if they report daytime sleepiness and breathlessness.
Treating obstructive sleep apnoea/obesity hypoventilation syndrome with nocturnal NIV can improve breathing during sleep and in turn can reduce daytime sleepiness.
Improving sleep may also help behavioural problems, concentration and general daytime performance.
Acknowledgments
The authors would like to thank the patient and his mother for their permission to write this case report and their touching contribution to ‘patient's perspective’. The authors would also like to thank the SMS Foundation UK (www.smith-magenis.co.uk) and the SMS Research Foundation (www.smsresearchfoundation.org) for their support and kind permission to reproduce the figures in this report.
Footnotes
Contributors: VC and SZ contributed equally and are therefore joint first authors, listed in alphabetical order. Significant input and guidance was received from RA, who was the patient's responsible consultant.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Morse R, Rojahn J, Smith A. Effects of behavior problems, family functioning, and family coping on parent stress in families with a child with Smith-Magenis syndrome. J Dev Phys Disabil 2014;26:391–401. 10.1007/s10882-014-9367-3 [DOI] [Google Scholar]
- 2.Elsea SH, Girirajan S. Smith-Magenis syndrome. Eur J Hum Genet 2008;16:412–21. 10.1038/sj.ejhg.5202009 [DOI] [PubMed] [Google Scholar]
- 3.Niederhofer H. Efficacy of risperidone treatment in Smith-Magenis syndrome (del 17 p11.2). Psychiatr Danub 2007;19:189–92. [PubMed] [Google Scholar]
- 4.Greenberg F, Guzzetta V, De Oca-Luna RM et al. . Molecular analysis of the Smith-Magenis syndrome; a possible contiguous gene syndrome associated with del(17)(p11.2). Am J Hum Genet 1991;49:1207–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg F, Lewis RA, Potocki L et al. . Multi-disciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2). Am J Med Genet 1996;62:247–54. [DOI] [PubMed] [Google Scholar]
- 6.Potocki L, Glaze D, Tan DX et al. . Circadian rhythm abnormalities of melatonin in Smith-Magenis syndrome. J Med genet 2000;37:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leoni C, Cesarini L, Dittoni S et al. . Hypoventilation in REM sleep in a case of 17p11.2 deletion (Smith-Magenis syndrome). Am J Med Genet A 2010;152A:708–12. 10.1002/ajmg.a.32700 [DOI] [PubMed] [Google Scholar]
- 8.Takenouchi T, Saito H, Oishi N et al. . Daytime somnolence in an adult with Smith-Magenis syndrome. Am J Med Genet A 2013;161A:1803–5. 10.1002/ajmg.a.35936 [DOI] [PubMed] [Google Scholar]