Abstract
Critical events of HIV-1 pathogenesis occur in lymphoid tissues where HIV-1 is typically accompanied by infections with other pathogens (HIV co-pathogens). Co-pathogens greatly affect the clinical course of the disease and the transmission of HIV. The apicomplexan parasite Toxoplasma gondii is a common HIV co-pathogen associated with AIDS development. Here, we examined the interaction of T. gondii and HIV in coinfected human lymphoid tissue ex vivo. Both pathogens readily replicate in ex vivo infected blocks of human tonsillar tissue. Surprisingly, we found that live T. gondii preferentially inhibits R5 HIV-1 replication in coinfected tissues. This effect is reproduced by treatment of the tissue blocks with recombinant C-18, a T. gondii -encoded cyclophilin that binds to CCR5. These ex vivo findings raise the possibility that, in addition to being a co-factor in HIV disease, T. gondii may influence the outcome of viral infection by preferentially suppressing R5 variants.
Keywords: HIV, Toxoplasma gondii, Cyclophylin, ex-vivo, human lymphoid tissue
1. Introduction
HIV infection is associated with coinfection by other microbes that typically worsen the clinical course of HIV disease. However, several microbial agents have been shown to suppress HIV-1 replication either in vivo or in vitro [1-8]. Indeed, even known HIV-1 copathogens such as HHV-6 [9] upregulate growth of HIV-1 of one tropism (X4), but suppress HIV-1 variants of another tropism (R5) [3]. This indicates that, in vivo, the net effect of non-HIV microbes on HIV-disease progression is the result of complex processes that may include differential induction of chemokines and their receptors that are used as co-receptors for host cell entry by R5 and X4viruses [10].
Our group has investigated HIV interactions with co-pathogens in the context of human lymphoid tissues ex vivo [3, 4, 6, 11, 12]. This explant model represents both the in vivo microenvironment and cellular/cytokine interactions more faithfully than isolated cell cultures. Here, in this system we examine the effect of the apicomplexan parasite Toxoplasma gondii on HIV replication. This parasite is one of the original opportunistic pathogens detected in the early AIDS epidemic [13, 14] and, if not properly managed by chemotherapy, triggers life-threatening neurological consequences in immunocompromised HIV+ individuals [15]. With the advent of HAART, T. gondii incidence as an opportunistic infection has declined dramatically but it remains a frequent AIDS co-pathogen in developing countries with ineffective HIV control [16, 17].
In previous studies of T. gondii –HIV-1 interaction, this parasite has been shown to stimulate viral replication in human monocytes [18] and to trigger proviral gene expression in vivo in an HIV transgenic mouse model [19]. These results were attributed to the potent stimulatory effects of the parasite on NF-κB activation and on the synthesis of macrophage proinflammatory cytokines. More recently, a T. gondii gene product: Cyclophilin-18 (C-18), previously characterized as a CCR5 ligand mimic [20], was shown to inhibit infection of T lymphocytes and monocytes by R5 but not X4 HIV-1 isolates [21]. The structural elements mediating this interaction were identified in both the parasite protein and the CCR5 co-receptor [21, 22]. These observations raised the question of whether live replicating T. gondii parasites might negatively affect HIV-1 replication in vivo (possibly through their production of C-18). We demonstrate that live T. gondii selectively inhibits R5 HIV-1 replication in coinfected tissues and that recombinant C-18 partially reproduces this effect.
2. Materials and Methods
2.1. HIV-1 and T .gondii strains
R5SF162 and X4LAI.04 virus stocks were obtained from the Viral Quality Assurance Laboratory (Rush Medical School, Chicago, IL). JR-CSF was provided by D. Kabat (Oregon Health and Science University, Portland, OR).
T. gondii culture and purification
RH88 T. gondii tachyzoites (ATCC, Rockville, MD) were maintained in the Hs27 cell line cultured in DMEM supplemented with 2mM glutamine, 100U/ml penicillin, 0.1mg/ml streptomycin and 5% FCS (Hyclone, Logan, UT). Parasites were collected from infected monolayers, washed 3 times in complete tonsil medium (RPMI 1640 supplemented with 1mM sodium pyruvate, non essential amino-acids, 2.5μg/ml fungizone, 50μg/ml gentamicin and 15% Fetal Calf Serum), and counted.
2.2. Tissues and infection
Human tonsils obtained from routine tonsillectomy were dissected into 2-3 mm blocks and set up in culture as previously described [3, 23]. Briefly, 3 wells containing 9 blocks each were prepared for each experimental condition. Culture medium was sampled and changed every 3 days for up to 12 days after infections.
Tonsilar tissues from multiple donors were infected with R5SF162 or X4LAI.04 HIV-1 by applying 0.3 to 1ng of HIV-1 p24gag on the top of each tissue blocks. 3 to 104 extemporaneously purified T. gondii tachyzoites were added per block, 2 to 3 hours before HIV-1 inoculation. Recombinant C-18 (rC-18) was added at 10μg/ml, a concentration found to effectively inhibit R5 HIV-1 replication [24], two hours prior HIV-1 inoculation and was replenished at the same concentration with each medium change. In several experiments recombinant human IL-1α (rIL-1α, Peprotech, Rocky Hill, NJ) was added at 3ng/ml and replenished at each medium change.
2.2. Measurement of HIV-1 and T. gondii replication
Productive HIV infection was assessed by measuring p24gag in culture medium with the Alliance p24 kit, (PerkinElmer, Waltham, MA). T. gondii replication was monitored by a sandwich ELISA. Briefly, Maxisorp plates (Nunc, Rochester, NY) were coated for two hours with 100μl of a 1μg/ml solution of the monoclonal anti-p30 antibody (T8075-30, US Biological. MA) in PBS and blocked with PBS 0.05% Tween 1% BSA pH 7.4. 100μl of either culture medium or standard made of purified tachyzoites were added into each well for 2h at 37°c. After 6 washes, 100μl/well of a 0.5μg/ml solution of mouse anti-P30 biotinylated antibodies (clone M26303) were added for 1 h at 37°C. After 6 washes, 100μl of a 0.1μg/ml Streptavidin-Horseradish Peroxidase (Amersham Biosciences, Piscataway, NJ) solution were added for 30 min at 37°C. After 6 washes, 200μl of TMB substrate (KPL, Gaithersburg, MD) were added for 30 minutes, the reactions were stopped with 50μl of 3N H2SO4 per well. The number of tachyzoites was determined by interpolation of the standard curve of purified tachyzoites (millions/ml). T. gondii replication was also monitored by flow cytometry and immuno-histochemistry (see below).
2.3. Flow cytometric analysis
Flow cytometry was performed on cells isolated from control and infected tissue blocks. For phenotyping, cells were stained with antibodies labeled as follows: CD3-Cy7-PE, CD4-Cy5.5-PerCp, CD8-APC (Caltag-Invitrogen, Carlsbad, CA), CCR5- APC-Cy7, CXCR4-Cy5-PE (BD-Pharmingen, San Diego, CA). To identify productively infected cells, stained cells were fixed and permeabilized with Fix&Perm (Caltag-Invitrogen) and stained with anti-p24gag PE-labeled antibodies (Beckman Coulter, Miami, FL) and anti-T. gondii monoclonal mouse antibody (Fitzgerald Industries, Concord, MA) conjugated in house to AlexaFluor-488 (Molecular Probes-Invitrogen). Data were acquired using DIVA- 3.0 on a BD LSRII. Data were analyzed with FlowJo (Tree Star, Ashland, OR). Counting beads (Caltag-Invitrogen) were added to estimate absolute cell counts and results were normalized by tissue weight. For immuno-histochemestry, cell suspensions from T. gondii infected tissues were fixed by 4% paraformaldehyde for 20 min, permeabilized by 0.25%Triton X100 for 5 min and stained with a 1/40 dilution of AlexaFluor-488 labeled anti- T. gondii monoclonal antibody for 30 min at RT. After washing, the cells were mounted and fixed on a microscope slide.
2.4. Cytokine & Chemokine measurements
The levels of 17 cytokines/chemokines MIP-1α, MIP-1β, RANTES, IFN-γ, IL-2, IL-4, TNF-α, SDF-1β, IL-1α, IL-1β, IP-10, IL-16, GM-CSF, MIG, IL-7, IL-12 and IL-15 were measured in singly infected and co-infected tissues using a multiplexed bead array as described in [4]. Data were collected and analyzed by Bioplex Manager-3.0 (BioRad Laboratories, Hercules, CA) using a 5-parameter fitting algorithm.
2.5. Expression of data and statistical analyses
Viral replication is expressed as the concentration of HIV-1 p24gag in culture medium accumulated during 12 days in culture (or as indicated). As reported earlier [4], there was a substantial donor-to-donor variability in HIV replication. T. gondii replication in tissues is expressed as the number of tachyzoites released per ml of culture medium. Cytokine secretion is expressed as fold increase (ratio between production in infected and control tissues). Statistical significance was assessed by paired Student's t-test for experiments with a sample size higher than 30, for lower sample sizes, non parametric test such as the Willcoxon signed rank test or the Mann-Whitney paired test were used when data did not pass the normality test. P values <0.05 were considered statistically significant. When non-parametric tests were used to assess statistical significance, the median and interquartile range (IQR) are given.
3. Results
3.1. T. gondii replicates in human lymphoid tissue ex vivo
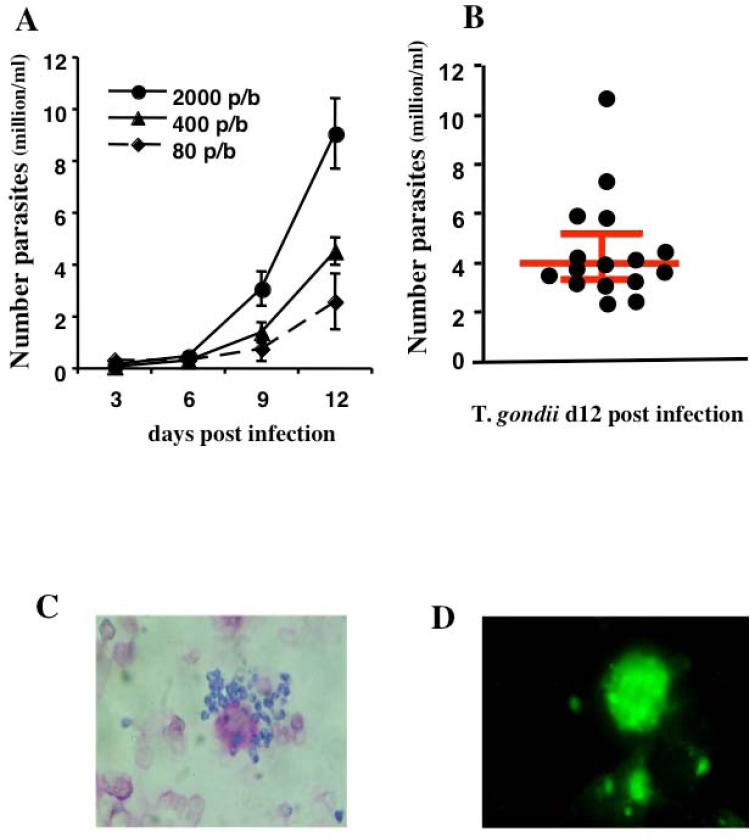
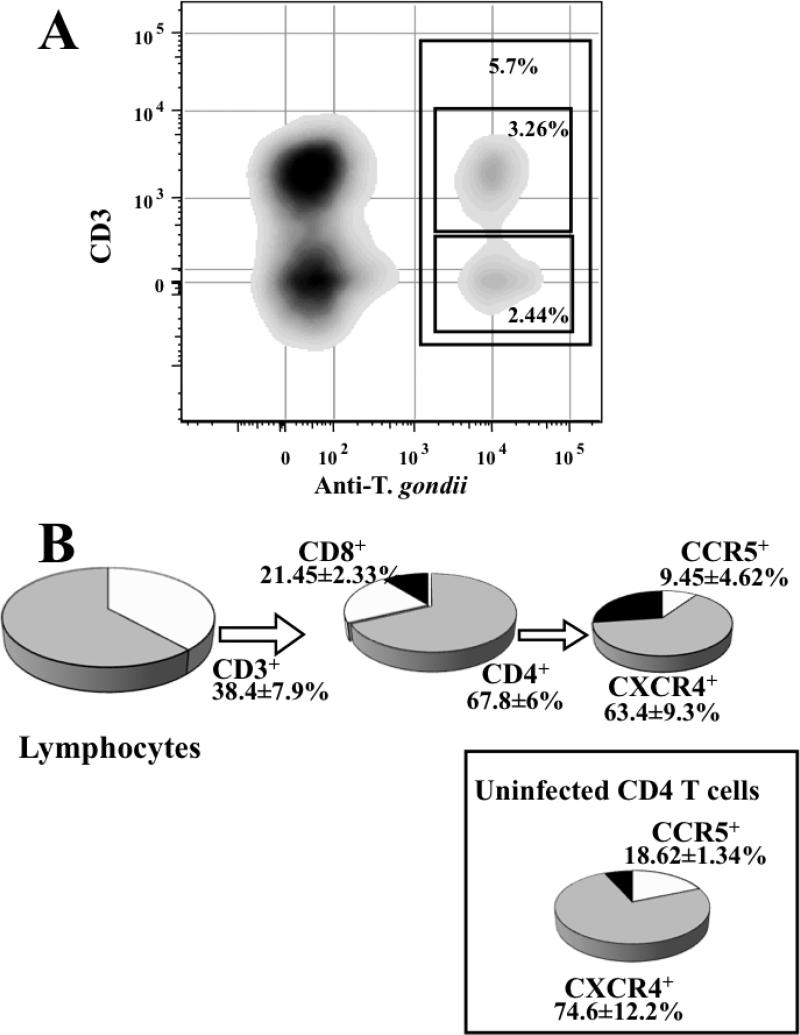
To monitor T. gondii replication in blocks of human tonsils we used an ELISA that quantifies a major parasite surface protein, p30. This assay demonstrated that on average, after 12 days post inoculation, tissue blocks inoculated with 400 tachyzoites released 4.5 ± 0.5 × 106 (3.92× 106, IQR[3.26 × 106, 5.15 × 106], n=16) parasites into the culture medium (Fig. 1A & B). T. gondii replication was confirmed by microscopic analysis of infected tissue blocks on day 12 post-inoculation. Parasites were readily detected in tissue blocks stained with Giemsa (Fig. 1C) or with fluorescent anti- Toxoplasma antibodies (Fig. 1D). We detected and quantified cells productively infected with T. gondii by flow cytometry. Cells mechanically isolated from inoculated tissue were stained with antibodies against for CD3, CD4, CD8, CXCR4, and CCR5, permeabilized and stained for T. gondii p30. As shown in Figure 2A, inoculation with 400 tachyzoites resulted in lymphocyte infection. On average, of 5.97 ± 1.52% (4.717, IQR[7.9, 10.40]) of lymphocytes were infected by T. gondii (n=6). Among those, p30 was expressed in T cells (CD3+) as well as in cells that did not express CD3, which, in human tonsils, comprise mainly B cells. Among p30+ lymphocytes, 38.37 ± 7.89% (42.18, IQR[18.15, 54.77]) were CD3+ of which 21.45 ± 2.33% (19.83, IQR[16.39,28.13]) were CD8+ and 67.87 ± 6.05% were CD4+ (71.63, IQR[52.23, 79.75]). Within the p30+CD4+ population, 9.45 ± 4.62% (6.97, IQR[1.064, 20.30]) were CCR5+ and 63.45 ± 9.32% (62.84, IQR[41.63, 86.68]) were CXCR4+ (n=6) while in these tissues, among non infected CD4+ T cells, 18.62 ± 11.34% (6.90, IQR[0, 48.97]) were CCR5+ and 74.63 ± 12.23% (85.57, IQR[38.33, 100]) cells were CXCR4+ cells (Fig. 2B). Therefore, the ratios of the CD4+CCR5+ to CD4+CXCR4+ cell numbers among the infected and uninfected cells in infected tissues blocks were not significantly different (p= 0.185, n=6).
FIGURE 1. Replication of T. gondii in human lymphoid tissue.
Tonsil blocks were infected with purified tachyzoites grown in the human fibroblastic cell line Hs27. Blocks were inoculated with various amounts of T. gondii ranging from 80 to 2000 tachyzoites per block. T. gondii replication was monitored by measuring concentration of p30 in culture medium of infected tissue blocks by ELISA (A). Panel (B) shows T. gondii replication in 16 different donors with the median and interquartile range at 12 days post infection. The replication of T gondii was confirmed by staining cells isolated from infected tissue blocks with Giemsa (C) or by staining with fluorescent anti-Toxoplasma antibodies (D).
FIGURE 2. Infection of human lymphoid tissue by T. gondii.and phenotype of infected cells.
Tonsil blocks were inoculated with 400 purified tachyzoites. Cells mechanically isolated 12 days post infection were stained with antibodies against the surface markers CD3, CD4, CD8, CXCR4, CCR5, permeabilized and stained with fluorescent monoclonal antibodies against p30. (A) The bi-exponential contour –plot, gated on lymphocytes, represents the cell staining with anti-CD3 PE-Cy7 and anti-T. gondii p30-Alexa 488 in a typical experiment. The gate represents the fraction of T. gondii infected cells in lymphocytes and their repartition within CD3+ and CD3- cells. (B) The pie charts represent the relative proportion of CD4 ( ) and CD8 (
) and CD8 ( ) T cells among infected cells as well as the relative proportion of CXCR4- (
) T cells among infected cells as well as the relative proportion of CXCR4- ( ) and CCR5- (
) and CCR5- ( ) expressing cells among CD4 infected cells, the proportion of CXCR4- and CCR5-expressing cells in non infected (p30-) CD4 T cells in infected tissues is given in the box. The values represent the mean and SEM of 6 experiments.
) expressing cells among CD4 infected cells, the proportion of CXCR4- and CCR5-expressing cells in non infected (p30-) CD4 T cells in infected tissues is given in the box. The values represent the mean and SEM of 6 experiments.
We evaluated the cytopathicity of T. gondii infection in tonsilar tissue. Tissue blocks were inoculated with various amounts of tachyzoites and, after 12 days in culture, tissue lymphocytes were enumerated. At high multiplicities of infection, as measured by propidium iodide staining, T. gondii induced the death of 76.08 ± 17.39% and 71.36 ± 14.21% of lymphocytes in tissues inoculated with 104 and 2.103 tachyzoites per block respectively (n=3) while inoculation with 400 tachyzoites, resulted in the death of 10.57 ± 7.58% (8.32, IQR [−11.04, 34.02]) of lymphocytes, which was not statistically significant (P=0.074, n=9). When analyzed at the subpopulation level, this depletion translated into the loss of 25.09 ± 10.38% (18.97, IQR[4.775, 52.80]) of CD3+ cells (p=0.0391), 29.28 ± 7.99% (18.38, IQR[8.705, 53.03]) of CD4+ T cells (p=0.0039), and 20.61 ± 18.18% (31.4, IQR[4.305, 59.15]) of CD8+ T cells (p=0.1250). Among CD3+CD4+ T cells, 32.96 ± 7.76% (33.95, IQR[14.0, 55.89]) of CCR5+CD4+ T cells (p=0.0078) and 30.47 ± 6.87% (18.95, IQR[13.21, 50.63]) of CXCR4+CD4+ T cells (p=0.0039) were depleted in infected tissue. Thus, although T. gondii infection results in an overall T cell depletion, this depletion does not discriminate between CCR5+ and CXCR4+ CD4+ T cells.
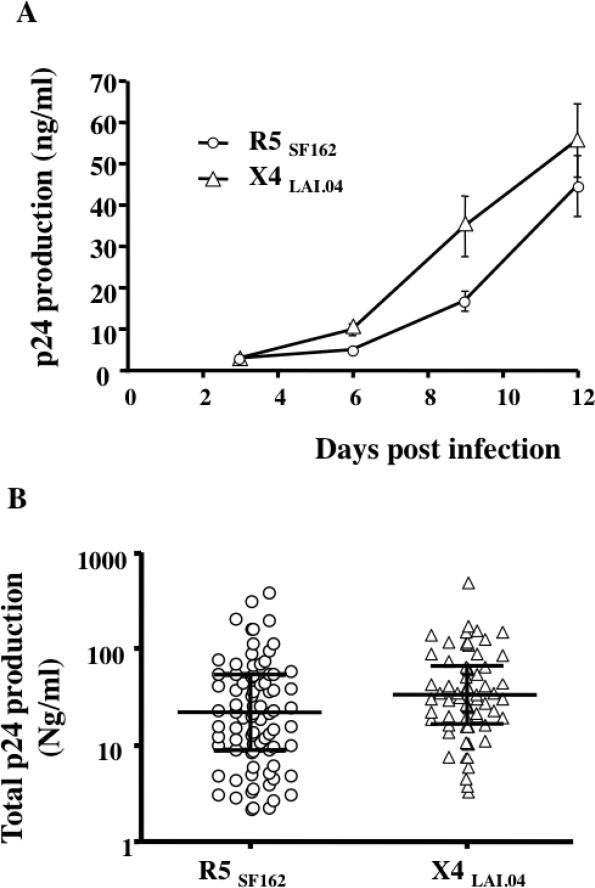
Tonsils from 81 and 66 donors were infected with R5SF162 or X4LAI.04 respectively. As reported earlier [4], viral replication became evident between day 6 and day 9 post- HIV-1 infection and increased during the course of the experiment. A typical viral replication kinetic is presented in Figure 3A. However, the absolute level of viral replication varied from donor to donor and the total p24gag production in medium bathing 27 tissue blocks over 12 days of culture varied between 2.18 and 386.92 ng/ml for R5SF162 (21.86, IQR[8.965, 54.75], n=81) and 3.31 and 508.36 ng/ml for X4LAI.04 (31.67, IQR[16.82, 66.70], n=66) (Fig. 3B). To evaluate the effect of T. gondii on HIV replication, we selected tissues in which HIV replication was higher than 5ng/ml of p24gag, which constituted 82.7% and 95.5% of R5SF162 and X4LAI.04 infected tissues respectively. On average these tissues produced 53.08 ± 8.52 ng/ml of R5SF162 (26.21, IQR[12.96, 56.41], n=67) and 57.98 ± 9.17 ng/ml of X4LAI.04 (34.84, IQR[19.61, 66.73], n=63) p24gag protein respectively over the 12-day infection period.
FIGURE 3. Ex-vivo replication of R5SF162 and X4LAI.04 in human lymphoid tissue.
Blocks of human lymphoid tissues were inoculated with 0.3 to 1ng of p24gag/block of R5SF162 or X4LAI.04. A typical kinetic of viral replication is presented in panel A. The absolute levels of viral replication and the cumulative release of p24 gag into the 3 ml of the culture medium varied from donor to donor. The panel B represents the levels of p24gag produced during infection of tissue blocks by R5SF162 (n=81) and X4LAI.04 (n=66) over the 12 days of culture. The median and inter-quartile ranges are drawn on each data set.
3.2 T. gondii and HIV-1 replication in coinfected tissues
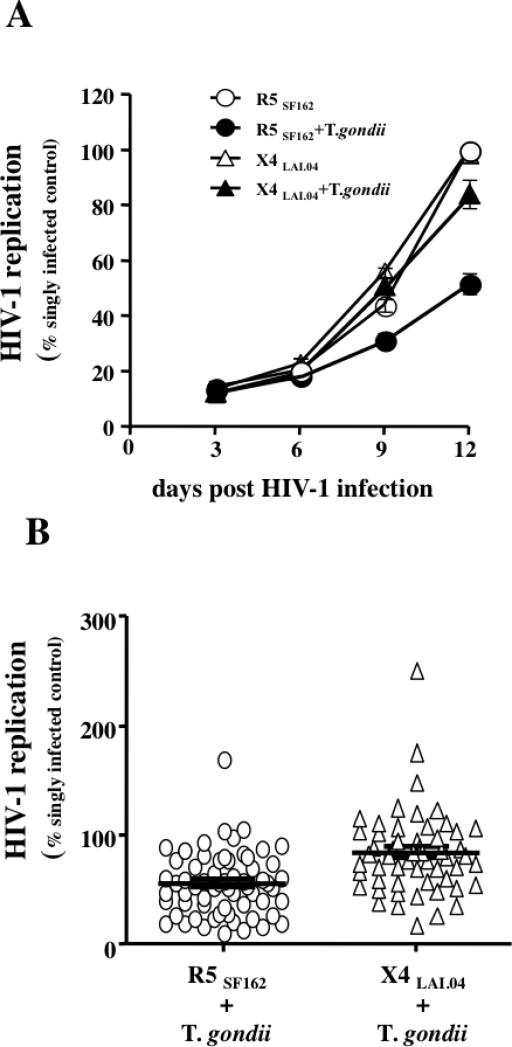
To investigate whether T. gondii and HIV-1 affect each other's replication in coinfected tissues ex vivo, we inoculated matched tissue blocks with a combination of these two microbes or with each of them individually. T. gondii replication was mildly but significantly suppressed by HIV-1 infection: In R5SF162– and X4LAI.04–coinfected tissues, T. gondii replication respectively reached 81.3±6% (91.4, IQR [73.90, 99.4], n=16, p=0.008) and 63.7±6.6% (62.89, IQR[48.7, 71.9], n=15, p<0.0001) of its replication in tissues infected with T. gondii alone. The replication of X4LAI.04 in T. gondii coinfected tissues was not significantly different from its replication in singly HIV-infected tissues and reached 84±5% (81.72, IQR[58.62, 104.3]) of the latter (n=55; p=0.0939). In contrast, R5SF162 replication in T. gondii -coinfected tissues was suppressed to the level of 55.8±3.6% (53.89, IQR[3.97,77.9]) (n=63, p<0.0001) of its replication in donor-matched control tissues singly infected (Fig. 4 A&B). T. gondii inhibited the replication of R5JRC-SF HIV-1 to the level of 49.4±9.13% (54.11, IQR[23.38, 70.70], n=7, p=0.0026) of its replication in tissue infected only with HIV-1.
FIGURE 4. Effect of T. gondii on the replication of HIV-1 in co-infected tissues.
Blocks of human lymphoid tissues inoculated with 400 tachyzoites were co-infected with R5SF162 and X4LAI.04 HIV-1 or left singly infected with HIV-1 as a control. HIV-1 replication was monitored by measuring the concentration of p24 gag in the culture medium bathing infected tissue blocks. (A) The graph represents a typical kinetic of replication of HIV-1 R5SF162 (○) and X4LAI.04 (△) in singly infected tissues blocks or in tissue blocks co-infected with T. gondii and R5SF162 (●) or T. gondii and X4LAI.04 (▲). (B) Effects of T. gondii infection on total HIV-1 replication in co-infected tissues expressed as percentage of the cumulative HIV-1 replication in matched singly infected tissues. Each dot represents one experiment (n=63 for R5SF162, n=55 for X4LAI.04). The line represents the median and inter-quartile range of each distribution.
In HIV-1 and T. gondii coinfected tissues, there was no correlation between the level of T. gondii replication and the level of inhibition of either X4 or R5 HIV-1 replication (r2=0.07, p=0.39 and r2=0.026, p=0.059 respectively). Thus, the lower inhibition of X4in T. gondii co-infected tissues is not due to a decreased T. gondii replication compared to tissues co-infected with R5 HIV-1.
3.3 Analysis of cytokine and chemokine production
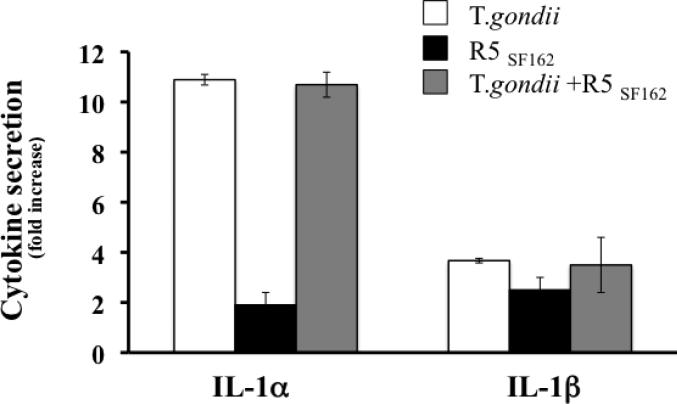
Since several microbes were shown to affect HIV-1 by changing the cytokine/chemokine secretion spectra, we analyzed cytokine/chemokine production in T. gondii infected tissue blocks. Using a multiplexed bead array assay we measured the concentration of MIP-1α, MIP-1β, RANTES, IFN-γ, IL-2, IL-4, TNF-α, SDF-1β, IL-1α, IL-1β, IP-10, IL-16, GM-CSF, MIG, IL-7, IL-12 and IL-15 in culture medium from singly T. gondii - or R5 HIV-1-infected and R5 HIV-1/T.gondii-coinfected tissues at day 3, 6, 9 and 12 post infection. TNF-α, IL-12 and Il-15 were not detected using our assay (limit of detection 12 pg/ml), the remaining 14 cytokines/chemokines were detected at every time point (data not shown). As previously reported [23], none of the measured cytokines was upregulated in R5 HIV-1-infected tissues. In contrast, both in T. gondii singly infected tissues and in tissues coinfected with HIV-1 and T. gondii, at day 12 post infection, IL-1 was the only cytokine significantly increased in infected tissues. IL-1α was up-regulated 10.05±1.6 fold (n=10) (9.342, IQR[6.48-12.74], p=0.002) while Il-1β was upregulated 3.58±0.48 fold (3.15, IQR[2.632-4.031], p= 0.002) (Fig 5).
FIGURE 5. Production of IL-1α in human tonsil blocks infected with T. gondii and HIV-1.
Blocks of human lymphoid tissues from 10 donors were left untreated, inoculated with T. gondii tachyzoites □, infected by R5SF162 ■, or infected by both T. gondii and HIV ■. The production of IL-1 α and IL-1β were measured in the medium bathing the blocks, collected at each medium change. The bar graph represents the mean and the SEM of the total IL-1 produced during the 12 days of culture expressed as a fold increase over the total IL-1 produced in control matched non-infected tissues.
3.4 Effect of IL-1 on HIV-1 replication
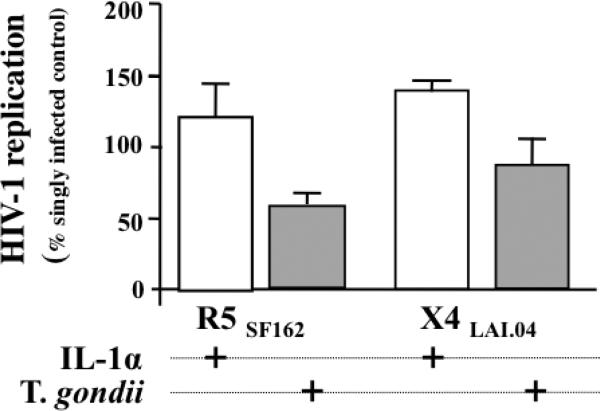
We investigated whether IL-1α up-regulation by T. gondii plays a role in T. gondii - mediated inhibition of R5SF162 replication, by adding IL-1α to medium bathing R5SF162 HIV-1-infected tissues. Since the biological effects of IL-1α and Il-1β are similar on immune cells except on eosinophils [25], we added the former to the cultures. In T. gondii infected tissues, at day 12 post infection when the anti-HIV effect is maximal, the average Il-1α concentration is 254±50 pg/ml. To mimic the 10 times IL-1α concentration increase triggered by T. gondii infection inside the tissue blocks, we added IL-1α at 3ng/ml. In contrast to T. gondii infection, rIL-1α stimulated replication of both R5SF162 and X4LAI.04 to the levels of 122.4±23.4% (126.5 IQR[71.71, 171.1], n=5, p=0.218) and 141.2±6.9% (141.2, IQR[134.4, 148.1], n=2, p=0.5) relative to singly HIV-1-infected tissues, respectively. In the same tissues, T. gondii infection inhibited R5SF162 replication to 56.25±8.45 % (57.85, IQR[42.08, 69.062], p=0.0312) of and X4LAI.04 86.03±18.8% (p=0.5) relative to singly HIV-1-infected tissues (Fig 6).
FIGURE 6. Effect of IL-1α and T. gondii on HIV-1 replication in human tonsil blocks.
Blocks of human lymphoid tissues from two donors were inoculated with 400 T. gondii tachyzoites per block and treated with IL-1α, or kept untreated. Subsequently, these blocks were further infected with R5SF162 orX4LAI.04 HIV-1. The treatment of the tissues blocks is indicated by a “+” under each column. The columns represent the mean and the SEM (n=5 for R5SF162 and n=2 forX4LAI.04) of HIV-1 replication in treated tissues expressed as percent of HIV-1 replication in untreated control tissues.
3.5 Recombinant C-18 inhibits R5 HIV-1 replication in lymphoid tissue
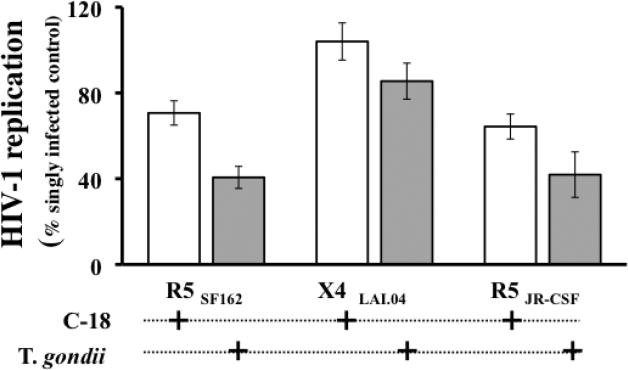
Since T. gondii encodes a CCR5-binding cyclophilin C-18 [20], we tested the effect of recombinant C18 (rC-18) on R5SF162 and X4LAV.04 HIV replication in human lymphoid tissue. rC-18 was added two hours prior to HIV-1 infection and replenished with every medium change until the end of the culture at day 12 post infection. We used rC-18 at 10 μg/ml, a concentration previously shown to inhibit HIV-1 replication [24]. At this concentration, rC-18 inhibited R5 replication to the level of 70.66±5.68% relative to R5SF162 infected donor-matched tissues, while T. gondii inhibited the same tissues to the level of 40.61±5.14% of R5SF162-infected control (n=13, p=0.0001 and p<0.0001 respectively) (Fig. 7). Similarly, rC-18 inhibited replication of R5JRC-SF to the level of 65.65±6.94% relative to R5JRC-SF -infected donor-matched control tissue blocks, while in the same tissues T. gondii inhibited R5JRC-SF to the level of 41.89±10.62% of R5JRC-SF - infected controls (n=5, p=0.0078 and 0.0054 respectively).
FIGURE 7. Comparison of the effects of C-18 and T. gondii on HIV-1 replication in human tonsil blocks.
Blocks of human lymphoid tissues from several donors were inoculated with 400 T. gondii tachyzoites per block or treated with recombinant C-18 in the concentration of 10 μg/ml or kept without further treatment. Subsequently, these blocks were infected with R5SF162 , X4LAI.04, or R5JR-CSF HIV-1. The treatment of the tissues blocks is indicated by “+” under each column. The columns represent the mean and the SEM of HIV-1 total replication in treated tissues expressed as percent of HIV-1 replication in matched untreated tissues from 13 donors for R5SF162 , 10 donors for X4LAI.04, and 5 donors for R5JR-CSF
Neither the presence of rC-18 nor the infection by T. gondii significantly affected the replication of X4LAI.04. In presence of rC18, X4LAI.04 replication reached 104±8.65% of the replication of X4LAI.04 in matched untreated tissues (n=10, p=0.6275), while in T,gondii infected tissues, X4LAI.04 replication reached 85.51±8.4 % of X4LAV.04 replication in matched untreated tissues (n=10, p=0.1183).
4. Discussion
Progression of HIV-1 disease is typically accompanied by infections with other pathogens that greatly affect the transmission of HIV and the clinical course of the disease. Clinical and experimental evidence demonstrate that copathogens locally interact with HIV-1 in coinfected tissues. In particular, the level of replication, as well as the evolution of HIV-1, may be influenced by copathogen-triggered modulation of cell activation, expression of receptors/coreceptors and chemokines release. This creates a complex system of interactions that integrates negative and positive signals for HIV-1 replication. In vivo, these frequently result in the acceleration of HIV disease progression. Nevertheless, these signals need to be dissected to develop new approaches to suppress HIV replication either by simulating the copathogens’ negative signals or by suppressing positive signals which upregulate HIV [12].
T. gondii is a common HIV copathogen that worsens the clinical course of HIV disease [15] However, its genome also encodes a cyclophilin (C-18) that binds to CCR5 and is able to suppress R5 HIV-1 replication in PBMC [24]. In the present work we found that live T. gondii infection significantly suppresses replication of R5 but not X4 HIV-1. Such pattern of interactions was shown earlier for, HHV-6, HHV-7, vaccinia virus (VV) and GBV-C [4, 6, 8]. Coinfection with GBV-C suppresses both R5 and X4 HIV-1 [26]. R5 HIV-1 suppression by HHV-6 and GBV-C involves the release of a CC chemokine that binds to CCR5 [3, 26]. However, we found that, in human lymphoid tissue infected ex vivo with T. gondii, none of these chemokines was upregulated. Out of 17 cytokines/chemokines tested, only IL-1α was upregulated. Although this cytokine is known to stimulate HIV-1 infection [27] rather than to suppress it, we tested its effect in our model. We added IL-1α in the amounts that mimick intra-tissue levels similar to those generated by T. gondii infection. In agreement with earlier reports [27, 28], IL-1α upregulated HIV-1 replication in human tonsils, ruling out its contribution to the R5 suppression in T.gondii/HIV-1-coinfected tissues ex vivo.
Another pathogen that, upon coinfection, suppresses replication of R5 rather than X4 HIV-1 variants is HHV-7 which downregulates CD4, the HIV-1 receptor, on the surface of both infected and uninfected T cells [4]. In this case, the differential effect on R5and X4 HIV-1 was due to the former's higher dependency on the number of CD4 molecules on T cell surface. In T. gondii infected tissue blocks there was no loss of CD4 expression on T cells, suggesting that the down-regulation of CD4 is not a mechanism by which T. gondii suppresses HIV-1 in coinfected tissues.
An additional pathogen suppressing predominantly R5 HIV-1 is vaccinia virus. This virus depletes significant numbers of CD4 T cells and down-regulates the HIV-1 coreceptor CCR5 essentially decreasing the number of CD4+CCR5+ T cells [6]. T. gondii does not seem to affect R5 HIV-1 replication in such a way, since flow cytometry did not reveal any decrease in CCR5+CD4+ T cells in T. gondii -infected tissues compared to matched uninfected control.
In their complex interaction with HIV-1, some pathogens combine several of the mechanisms mentioned above. For example, GBV-C induces the release of an inhibitory chemokines while it also independently decreases the surface expression of HIV receptors [29, 30].
T. gondii encodes a protein, cyclophylin C-18 [20] that directly binds to the HIV-1 coreceptor CCR5 N-terminus and suppresses R5 infection in stimulated PBMC [24]. It also inhibits cell-cell fusion without induction of CCR5 downmodulation. To test whether this protein may mediate T. gondii's suppression of R5 in human lymphoid tissue ex vivo, we compared, in our system, the effect of C-18 on R5 HIV-replication with that of replicating T. gondii. Similar to live T. gondii tachyzoites, recombinant C-18 significantly suppressed replication of R5, but not of X4 HIV-1. The strong cross-immuno-reactivity of anti-C18 antibodies with various human cyclophillins present in tissue cultures prevented us from measuring C-18 with ELISA in T. gondii-infected lymphoid tissues. However, the fact that C-18 is secreted by infected cells in vitro [20] and that this cyclophilin is a suppressor of R5 HIV-1 in human tissues ex vivo as well as the exclusion of the alternative mechanisms discussed above, suggest that C-18 is a probable mediator of T. gondii -triggered R5 HIV-1 inhibition. Nevertheless, other as yet unknown mechanisms may contribute to the T. gondii triggered suppression of R5 HIV-1. Whatever these mechanisms are, the ability of T. gondii and T. gondii-encoded cyclophilin C-18 to suppress HIV-1 replication is important for our understanding of the complex mechanisms of co-pathogenesis. In vivo other factors, probably of systemic nature, not simulated in isolated tissue blocks, may overcome this negative effect of T. gondii on HIV-1 replication making T. gondii a dangerous pathogen in immunocompromised patients. Nevertheless, based on our results, it is now important to find what is the contribution of the phenomenon reported here to the general T. gondii/HIV physiopathology. In particular, T. gondii's effects on HIV-1 infection at different stages of HIV-1 disease and on the dominance of viruses of different coreceptor tropism should be now specifically addressed in a properly chosen cohort. If the mechanisms described above occur in vivo, T. gondii may suppress HIV-1 at the early stages of disease when R5dominates but not at the later stages when virus acquires ability to use CXCR4, which is associated with accelerated progression to AIDS. Moreover, suppression of R5 HIV-1 may contribute to the switch to X4 HIV-1 in the course of HIV-1 disease, and might alter the risk of HIV-1 infection between T. gondii-infected and control individuals, as this parasite preferentially suppresses R5 HIV-1 variants, which transmit disease.
Acknowledgements
We thank Dr. M. Santi and the entire staff of the Department of Pathology of Children's National Medical Center for their generous assistance in obtaining human tonsillar tissues.
We Thank Dr. D. Kabat of the Department of Biochemistry and Molecular Biology and Proteomics Shared Resource, Oregon Health and Science University, Portland, OR, for providing JR-CSF.
This research was supported, in part, by the Intramural Research Program of the NIH, National Institute of Child Health and Human Development and by the Intramural AIDS Targeted Anti-viral Program (IATAP) of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casoli C, Vicenzi E, Cimarelli A, Magnani G, Ciancianaini P, Cattaneo E, Dall'Aglio P, Poli G, Bertazzoni U. HTLV-II down-regulates HIV-1 replication in IL-2-stimulated primary PBMC of coinfected individuals through expression of MIP-1alpha. Blood. 2000;95:2760–2769. [PubMed] [Google Scholar]
- 2.Goyal S, Kannangai R, Abraham AM, Ebenezer DL, Sridharan G. Lack of increased frequency of human immunodeficiency virus infection in individuals with dengue-like illness in South India. Indian J Med Microbiol. 2007;25:300–301. [PubMed] [Google Scholar]
- 3.Grivel JC, Ito Y, Faga G, Santoro F, Shaheen F, Malnati MS, Fitzgerald W, Lusso P, Margolis L. Suppression of CCR5-but not CXCR4-tropic HIV-1 in lymphoid tissue by human herpesvirus 6. Nat Med. 2001;7:1232–1235. doi: 10.1038/nm1101-1232. [DOI] [PubMed] [Google Scholar]
- 4.Lisco A, Grivel JC, Biancotto A, Vanpouille C, Origgi F, Malnati MS, Schols D, Lusso P, Margolis LB. Viral interactions in human lymphoid tissue: Human herpesvirus 7 suppresses the replication of CCR5-tropic human immunodeficiency virus type 1 via CD4 modulation. J Virol. 2007;81:708–717. doi: 10.1128/JVI.01367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, Griffin DE. Suppression of human immunodeficiency virus replication during acute measles. J Infect Dis. 2002;185:1035–1042. doi: 10.1086/340027. [DOI] [PubMed] [Google Scholar]
- 6.Vanpouille C, Biancotto A, Lisco A, Brichacek B. Interactions between human immunodeficiency virus type 1 and vaccinia virus in human lymphoid tissue ex vivo. J Virol. 2007;81:12458–12464. doi: 10.1128/JVI.00326-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watt G, Kantipong P, de Souza M, Chanbancherd P, Jongsakul K, Ruangweerayud R, Loomis-Price LD, Polonis V, Myint KS, Birx DL, Brown AE, Krishna S. HIV-1 suppression during acute scrub-typhus infection. Lancet. 2000;356:475–479. doi: 10.1016/S0140-6736(00)02557-5. [DOI] [PubMed] [Google Scholar]
- 8.Xiang J, Wunschmann S, Diekema DJ, Klinzman D, Patrick KD, George SL, Stapleton JT. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med. 2001;345:707–714. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- 9.Lusso P, Gallo RC. Human herpesvirus 6 in AIDS. Immunol Today. 1995;16:67–71. doi: 10.1016/0167-5699(95)80090-5. [DOI] [PubMed] [Google Scholar]
- 10.E. Berger A, P. Murphy M, M. Farber J. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism and disease. Ann. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 11.Grivel JC, Garcia M, Moss W, Margolis L. measles virus inhibits HIV-1 replication in human lymphoid tisue ex vivo. J. Inf. Dis. 2005 doi: 10.1086/430743. in press. [DOI] [PubMed] [Google Scholar]
- 12.Margolis L. Cytokines--strategic weapons in germ warfare? Nat Biotechnol. 2003;21:15–16. doi: 10.1038/nbt0103-15. [DOI] [PubMed] [Google Scholar]
- 13.Hauser WE, Luft BJ, Conley FK, Remington JS. Central-nervous-system toxoplasmosis in homosexual and heterosexual adults. N Engl J Med. 1982;307:498–499. doi: 10.1056/nejm198208193070814. [DOI] [PubMed] [Google Scholar]
- 14.Luft BJ, Conley F, Remington JS, Laverdiere M, Wagner KF, Levine JF, Craven PC, Strandberg DA, File TM, Rice N, Meunier-Carpentier F. Outbreak of central-nervous-system toxoplasmosis in western Europe and North America. Lancet. 1983;1:781–784. doi: 10.1016/s0140-6736(83)91847-0. [DOI] [PubMed] [Google Scholar]
- 15.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 16.Jones JL, Hanson DL, Dworkin MS, Kaplan JE, Ward JW. Trends in AIDS-related opportunistic infections among men who have sex with men and among injecting drug users, 1991-1996. J Infect Dis. 1998;178:114–120. doi: 10.1086/515593. [DOI] [PubMed] [Google Scholar]
- 17.Palella FJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 18.Bala S, Englund G, Kovacs J, Wahl L, Martin M, Sher A, Gazzinelli RT. Toxoplasma gondii soluble products induce cytokine secretion by macrophages and potentiate in vitro replication of a monotropic strain of HIV. J Eukaryot Microbiol. 1994;41:7S. [PubMed] [Google Scholar]
- 19.Gazzinelli RT, Ropert C, Campos MA. Role of the Toll/interleukin-1 receptor signaling pathway in host resistance and pathogenesis during infection with protozoan parasites. Immunol Rev. 2004;201:9–25. doi: 10.1111/j.0105-2896.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 20.Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, Reis e Sousa C, Fairlamb A, Ribeiro JM, Sher A. Molecular mimicry of a CCR5binding-domain in the microbial activation of dendritic cells. Nat Immunol. 2003;4:485–490. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- 21.Golding H, Khurana S, Yarovinsky F, King LR, Abdoulaeva G, Antonsson L, Owman C, Platt EJ, Kabat D, Andersen JF, Sher A. CCR5N-terminal region plays a critical role in HIV-1 inhibition by Toxoplasma gondii-derived cyclophilin-18. J Biol Chem. 2005;280:29570–29577. doi: 10.1074/jbc.M500236200. [DOI] [PubMed] [Google Scholar]
- 22.Yarovinsky F, Andersen JF, King LR, Caspar P, Aliberti J, Golding H, Sher A. Structural determinants of the anti-HIV activity of a CCR5antagonist derived from Toxoplasma gondii. J Biol Chem. 2004;279:53635–53642. doi: 10.1074/jbc.M410550200. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Grivel JC, Chen S, Kiselyeva Y, Reichelderfer P, Margolis L. CXCR4-tropic HIV-1 suppresses replication of CCR5-tropic HIV-1 in human lymphoid tissue by selective induction of CC-chemokines. J Infect Dis. 2004;189:506–514. doi: 10.1086/381153. [DOI] [PubMed] [Google Scholar]
- 24.Golding H, Aliberti J, King LR, Manischewitz J, Andersen J, Valenzuela J, Landau NR, Sher A. Inhibition of HIV-1 infection by a CCR-5 binding cyclophilin from Toxoplasma gondii. Blood. 2003 doi: 10.1182/blood-2003-04-1096. [DOI] [PubMed] [Google Scholar]
- 25.Whitcomb EA, Dinarello CA, Pincus SH. Differential effects of interleukin-1 alpha and interleukin-1 beta on human peripheral blood eosinophils. Blood. 1989;73:1904–1908. [PubMed] [Google Scholar]
- 26.Xiang J, George SL, Wunschmann S, Chang Q, Klinzman D, Stapleton JT. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1. Lancet. 2004;363:2040–2046. doi: 10.1016/S0140-6736(04)16453-2. [DOI] [PubMed] [Google Scholar]
- 27.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Briesen H, von Mallinckrodt C, Esser R, Muller S, Becker K, Rubsamen-Waigmann H, Andreesen R. Effect of cytokines and lipopolysaccharides on HIV infection of human macrophages. Res Virol. 1991;142:197–204. doi: 10.1016/0923-2516(91)90057-a. [DOI] [PubMed] [Google Scholar]
- 29.Bisson GP, Strom BL, Gross R, Weissman D, Klinzman D, Hwang WT, Kostman JR, Metzger D, Stapleton JT, Frank I. Effect of GB virus C viremia on HIV acquisition and HIV set-point. AIDS. 2005;19:1910–1912. doi: 10.1097/01.aids.0000188427.25657.e7. [DOI] [PubMed] [Google Scholar]
- 30.Stapleton JT, Xiang J, Williams CF. HIV and GB virus C coinfection. Lancet Infect Dis. 2006;6:187–188. doi: 10.1016/S1473-3099(06)70419-4. author reply 188-189. [DOI] [PubMed] [Google Scholar]