Abstract
In this study, we investigated the sedative and hypnotic effects of the aqueous extract of Aloe vera on rats. In order to evaluate the overall hypnotic effects of the Aloe vera extract, open field and loss of righting reflex tests were primarily used. The sedative and hypnotic effects of the extract were then confirmed by detection of remarkable raise in the total sleeping time through analysis of electroencephalographic (EEG) recordings of animals. Analysis of the EEG recordings showed that there is concomitant change in Rapid Eye Movement (REM) and None Rapid Eye Movement (NREM) sleep in parallel with the prolonged total sleeping time. Results of the current research show that the extract has sedative-hypnotic effects on both functional and electrical activities of the brain.
Key Words: Aloe vera, Insomnia, Sedative-Hypnotic effects, Electroencephalography, Electromyography
Introduction
Insomnia is a sleep disorder characterized by inability of efficiently falling into and staying asleep to restore normal states of energy and wakefulness (1, 2). Reportedly one-third of the general adult population (2) particularly females (3) experience insomnia at some point in their lives. Although the prevalence of insomnia estimates very largely based on the diagnostic methods (4), there is no doubt on the enormous economic impact of sleep disorders (5, 6). Insomnia may be associated with obesity (7), increased risk for metabolic syndrome (8), coronary artery disease (9, 10), depression (11), and anxiety (12, 13), as well as being a cause of concentration and memory problems (14, 15).
Given primary insomnia seems to be the most common diagnosis (2), intensive pharmacological treatment is inevitable in many patients. Although efficient therapeutics like benzodiazepines are available for insomnia, clinical applications are limited because of concerns about their potential abuse, dependence, and adverse effects (16). Behavioral therapies also have empirical evidence for relieving insomnia, however they have remained generally unemployed because of the time-intensive nature and need for expert trainees for effective application (17). Regarding the limitations and unfeasibility of existing therapies of insomnia, alternative and traditional medicine can be interesting as new treatment of insomnia.
Old materia medicas offer a variety of remedies for sleeping ailments, namely Crocus sativus (safron), Egyptian lotus, Solanum nigrum and Aloe vera (A. vera) (18). Aloe vera L. (Aloe barbadensis Miller) named as Sabr-e-zard (19), a succulent plant belonging to the Liliaceae family is amongst well known Iranian traditional medicine(20).
A. vera is one of the most widely used herbal medicines well known because of its local anti-inflammatory and healing properties. Besides being one of the most popular herbal medicines worldwide (21), it is also increasingly used in food industries (22). The clear gel isolated from the plant leaves, which has a variety of nutrients and bioactive molecules, is widely used in skin care, cosmetics and as “nutraceuticals” (23). Systemic consumption has also been empirically confirmed to improve a variety of health elements including immune system (24), angiogenesis (25), and gastrointestinal integrity (26).
A. vera therapeutic efficacies are relied on various bioactive compounds. Amylase and salicylates for instance render the extract as an anti-inflammatory and antibacterial agent (27). Sedative and hypnotic effects of A. vera have been reported in several Iranian and international old pharmacopoeias (18, 20, 28). A. vera extract contains certain biochemical components such as flavonoids (29) and amino acids (30) which have been previously documented to affect sleep quality.
Regarding many surveys implying beneficial effects of A. vera in CNS diseases (31, 32, 33), the present work aims to find experimental support for the reported hypnotic effects of A. vera in traditional medicine. In order to do that, the effect of aqueous extract of A. vera leaves on locomotion and pentobarbital induced sleeping of rats was investigated. More details about influence of A. vera on the sleep architecture were obtained through investigation of Electroencephalogram (EEG) and Electromyogram (EMG) recordings of the animal.
Results and Discussions
Behavioral examination of sedative-hypnotic properties of A. vera aqueous extract
Investigation of pentobarbital-induced loss of righting reflex
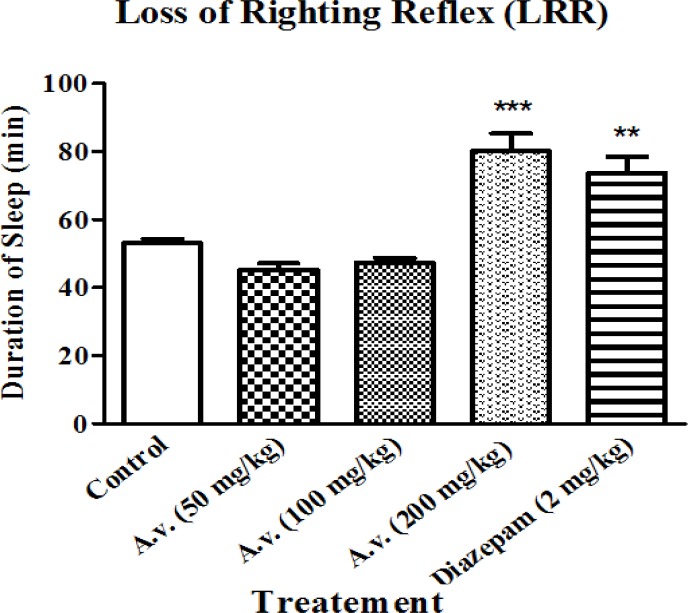
As the present work essentially aims to investigate hypnosis in response to A. vera administration, changes in loss of righting reflex was considered to estimate hypnotic effects of the extract. Administration of the extract did not influence onset of pentobarbital induced sleeping (data are not shown here) but prolonged the representative loss of righting reflex as the main characteristic of hypnotic agents. As it is presented in Figure 1, administration of the extract (200 mg/kg) led to prolonged loss of righting reflex compared to the control group of animals. Prolongation of loss of righting reflex in the animals was statistically equal to that of the animals which had received diazepam (2 mg/kg) as the positive control.
Figure 1.
Aloe vera (A. vera) aqueous extract prolonged pentobarbital induced loss of righting reflex. Rats received pentobarbital (40 mg/kg, i.p.) 30 min following Aloe vera extract (50, 100, 200 mg/kg, i.p.) or diazepam (2 mg/kg, i.p.). A. vera hypnotic effects were evaluated based on increasing the sleeping time in test animals. Data are represented as mean ± SD (n=6). *** p < 0.001, ** p < 0.01 compared to control group
Investigation of locomotion activity
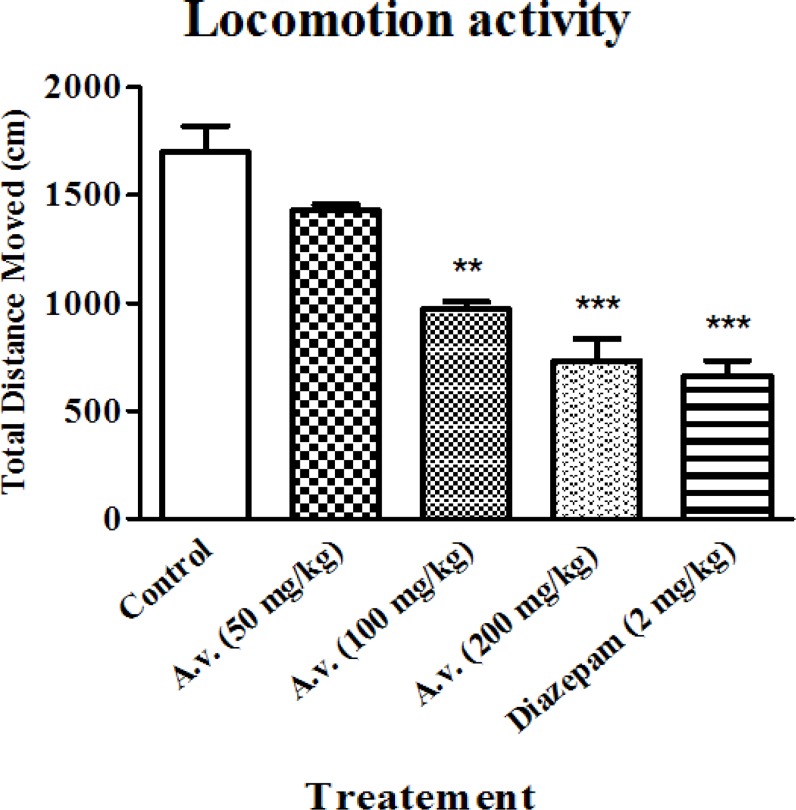
While hypnotic properties of the extract were primarily determined in loss of righting reflex examination, locomotion activity alteration was also considered as an index for the sedative effect. The open field results in conjunction with data obtained from loss of righting reflex test, confirmed sedative-hypnotic effects of the extract. Figure 2 shows significantly repressed locomotion activity of the animals after administration of the extract (100 and 200 mg/kg) probably as a result of its sedative effect. The results show that administration of the extract at dose of 200 mg/kg is as efficient as diazepam (2 mg/kg) in suppressing the locomotor activity of the animals.
Figure 2.
Aloe vera (A. vera) aqueous extract repressed animals' locomotor activity. 30 min after Aloe vera extract (50, 100, 200 mg/kg, i.p.) or diazepam (2 mg/kg, i.p.), animals were subjected to open field test and animals’ total distance moved were compared as an indicative for locomotor activity. Data are represented as mean ± SD (n=6). *** p < 0.001, ** p < 0.01 compared to control group
Investigation of sleep parameters
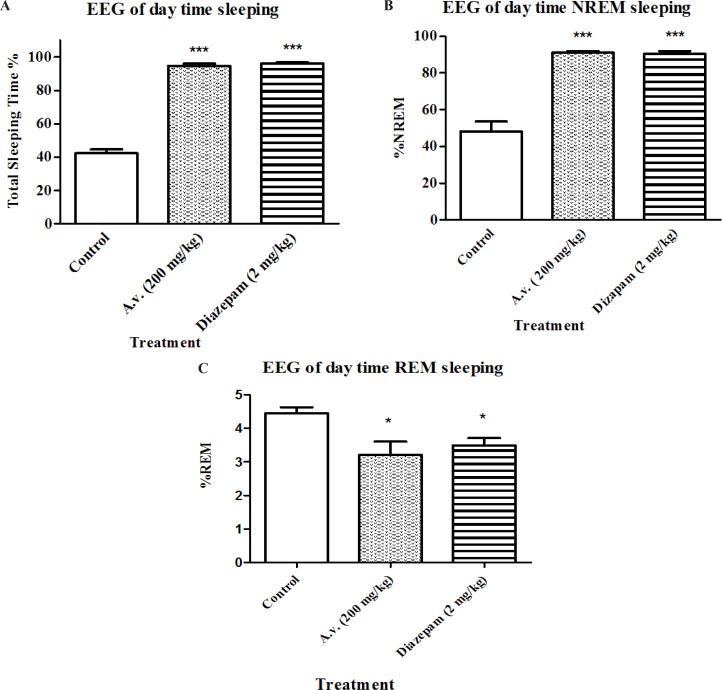
In order to be able to define significant sedative-hypnotic effects for the extract, results of loss of righting reflex test, as a widely used screening test for hypnotic compounds, needs to be confirmed by complementary methods. Therefore EEG recording was performed to determine duration of awaking, NREM and REM sleep states. Figures 3 A, 3 B and 3 C represent changes in sleep pattern of the animals which received the A. vera extract (200 mg/kg) in four hours during daytime. Animals treated with A. vera had following sleep parameters: total sleep time [F(2,15)=358, p < 0.05], percent of REM sleep [F(2,15)=15.5, p < 0.05] and percent of NREM sleep [F(2,15)=14, p < 0.05]. Results of Bonferroni’s post-test of EEG recording show that A.vera as well as diazepam increased the sleeping time (p < 0.001) and NREM sleep duration (p < 0.001) and decreased REM sleep (p < 0.001) compared to the control group.
Figure 3.
Impact of Aloe vera (A. vera) extract administration on electroencephalographic architecture of sleep. Day-time EEG and EMG recordings were conducted in freely moving rats following 30 min post Aloe vera or diazepam administration for 4 hours. Accordingly, Aloe vera aqueous extract (200 mg/kg, i.p.) prolonged total sleeping time (A) as well as NREM sleep (B) while reduced sleeping time spent in REM (C). Data are represented as mean ± SD (n=6). * p < 0.05, ***p < 0.001 compared to control group
Active pharmacological ingredients of A. vera , concentrated in the gel and rind of the plant leaves have been evidently shown to exert analgesic, anti-inflammatory, antioxidant and anticancer effects (38). Amongst identified therapeutic indications for A. vera in folk medicine, treatment of insomnia has not been addressed yet by any scientific experiment. The present work evaluates sedative and hypnotic effects of A. vera through performing open field as well as pentobarbital induced-sleeping prolongation, as screening tests, on rats. Whereas open field test is not specific for sedation–related behaviors, suppressed locomotion activity in conjunction with hypnosis determined by prolongation of loss of righting reflex in animals, may highlight sedative properties of the A. vera extract. Increase of total sleeping time observed in investigations of EEG recordings of the same animals during the tests, could provide rational proof for the suppressed locomotion reported in our behavioral examinations.
To our knowledge, the only relevant survey by now has been a multicentre clinical open study on a topical moisturizer preparation containing A. vera extract for which 100% sleep improvement have been reported in subjects bearing atopic dermatitis (39). According to the study design however, this could be simply accounted for the relief from itching discomfort probably resulting from histamine release suppression by A. vera (26, 38) .
The essential characteristic of sleep is full reversal with efficient external stimuli. Therefore, virtual hypnotic impact of bioactive compounds could be accurately evaluated by EEG recording in normal, rather than drug induced sleeping animals. The EEG records of our day-time sleeping studies illustrate substantial electrophysiological effects of A. vera on sleep length that seemingly were efficient enough to produce the behavioral responses we observed in open field and loss of righting reflex test.
Besides prolongation of total sleeping time, A. vera administration led to a significant shift toward more NREM sleep. Such alterations in REM and NREM sleep parameters may provide useful data about outcomes of certain therapeutics. REM and NREM sleep are two distinct stages which are different not only in cerebral electrophysiological status but also in simultaneous resting muscular tonus. That is whilst thought-like mental activity takes place during NREM sleep. REM sleep is mostly associated with hallucinations concurrent with muscular paralysis. Several investigations on memory performance have also revealed that REM sleep contributes to consolidation of procedural memory (40) while NREM improves declarative memory (41).
The hypnotic activity of herbal medicines has been frequently attributed to different phytochemicals compounds such as flavonoids and saponines (29, 42). In the case of protein rich plants however, presence of certain amino acids may be of prominent importance (43, 44).
Versatile non-amino acid neurotransmitters such as acetylcholine and catecholamine are involved in governing normal sleep quality. That is centrally acting anticholinergic, dopaminergic, noradrenergic, and serotonergic agents cause a decrease in duration and density of REM sleep (45, 46). Recent evidence has elucidated significant changes in cerebral neurotransmitters in mice treated with A. vera extract of which diminished levels of nore-epinephrine and serotonin are conspicuous (47). Regardless of probable impact on sleep parameters, we postulated such alterations might not apply to our set of experiments in which no long-term dosing protocols were included.
A. vera may be expected to elevate acetylcholine levels based on some reports implying its choline-esterase inhibition property (48). It has been shown that REM sleep duration is decreased by central cholinergic system augmentation and parasympathetic tone is dominant in NREM sleep (49, 50). The observed changes in REM and NREM sleep can be partly explained by presence of compounds with anti-choline-esterase activity in A. vera. The implication of any of the mentioned neurotransmitters however, needs further elucidation as our experiments did not include any contributing examination.
Conclusion
Several investigations have provided experimental evidences for CNS-ailments that have been traditionally claimed to be improved by A. vera administration of which convulsion (51), cerebral ischemia (25) and multiple sclerosis (33) seemingly are the foremost ones. Present work provides positive evidences which support sedative and hypnotic effects of A. vera extract obtained by corresponding traditional method described in folk medicine. While investigating the corresponding properties of cold-dried extracts seem extremely intriguing for further works, our results also remained a question whether A. vera exerts ameliorating effects in the context of insomnia in human.
Acknowledgments
This work is a part of a Ph.D. research thesis (No.157) financed by School of Traditional Medicine, Shahid Beheshti University of Medical Sciences. Authors acknowledge the school for their support in performing the present research.
References
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep medicine reviews. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Roth T. Insomnia: definition, prevalence, etiology, and consequences. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2007;3(5 Suppl):S7. [PMC free article] [PubMed] [Google Scholar]
- 3.Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population: influence of previous complaints of insomnia. Archives of Internal Medicine. 1992;152(8):1634. [PubMed] [Google Scholar]
- 4.Hermes ED, Rosenheck RA. Prevalence, Pharmacotherapy, and Clinical Correlates of Diagnosed Insomnia Among Veterans Health Administration Service Users Nationally. Sleep Medicine. 2014 doi: 10.1016/j.sleep.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Sarsour K, Kalsekar A, Swindle R, Foley K, Walsh JK. The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep. 2011;34(4):443. doi: 10.1093/sleep/34.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoller MK. Economic effects of insomnia. Clinical Therapeutics: The International Peer-Reviewed. Journal of Drug Therapy. 1994 [PubMed] [Google Scholar]
- 7.Wittels E. Obesity and hormonal factors in sleep and sleep apnea. The Medical clinics of North America. 1985;69(6):1265–80. doi: 10.1016/s0025-7125(16)30986-5. [DOI] [PubMed] [Google Scholar]
- 8.Troxel WM, Buysse DJ, Matthews KA, Kip KE, Strollo PJ, Hall M, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33(12):1633. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the Risk of Acute Myocardial Infarction A Population Study. Circulation. 2011;124(19):2073–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia With Objective Short Sleep Duration Is Associated With Type 2 Diabetes A population-based study. Diabetes Care. 2009;32(11):1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao K-W, Yu S, Cheng S-P, Chen I-J. Relationships between personal, depression and social network factors and sleep quality in community-dwelling older adults. Journal of Nursing Research. 2008;16(2):131–9. doi: 10.1097/01.jnr.0000387298.37419.ff. [DOI] [PubMed] [Google Scholar]
- 12.Sagaspe P, Sanchez-Ortuno M, Charles A, Taillard J, Valtat C, Bioulac B, et al. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain and cognition. 2006;60(1):76–87. doi: 10.1016/j.bandc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Franzen PL, Buysse DJ, Rabinovitz M, Pollock BG, Lotrich FE. Poor sleep quality predicts onset of either major depression or subsyndromal depression with irritability during interferon-alpha treatment. Psychiatry research. 2010;177(1):240–5. doi: 10.1016/j.psychres.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagoda Z, Klodziński S, Maslowski J. Problems of sleep and dreams in inmates of the Auschwitz-Birkenau concentration camp. Przeglad lekarski. 1976;34(1):28–66. [PubMed] [Google Scholar]
- 15.Most EI, Scheltens P, Van Someren EJ. Prevention of depression and sleep disturbances in elderly with memory-problems by activation of the biological clock with light-a randomized clinical trial. Trials. 2010;11(1):19. doi: 10.1186/1745-6215-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health NIo. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28(9):1049. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 17.Krystal AD. The changing perspective on chronic insomnia management. The Journal of clinical psychiatry. 2004;65:20. [PubMed] [Google Scholar]
- 18.Sina I. Al-Qanun fi al-tibb. Rome: Typgraphia Mediciea. 1993;1593:539–43. [Google Scholar]
- 19.Ghahraman A, Okhovvat A. Matching the old medicinal plant names with scientific terminology. Tehran: Tehran University Publication; 2004. [Google Scholar]
- 20.Hussain M, Kareem HN. Makhzan-ul-advia. Urdu translation from Persian by Hakeem Noor Kareem. Kanpur: Newal Kishore Press; 1888. [Google Scholar]
- 21.Zhang AL, Story DF, Lin V, Vitetta L, Xue CC. A population survey on the use of 24 common medicinal herbs in Australia. Pharmacoepidemiology and drug safety. 2008;17(10):1006–13. doi: 10.1002/pds.1610. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Z, Zhou H, Tian R, Zhang X, Pan Y. Effects and mechanism of aloe vera extracts on control of botrytis in postharvest apples. Transactions of the Chinese Society of Agricultural Engineering. 2014;30(4):255–63. [Google Scholar]
- 23.Cragg GM, Newman DJ. Natural product drug discovery in the next millennium. Pharmaceutical Biology. 2001;39(s1):8–17. doi: 10.1076/phbi.39.s1.8.0009. [DOI] [PubMed] [Google Scholar]
- 24.Lissoni P, Giani L, Zerbini S, Trabattoni P, Rovelli F. Biotherapy with the pineal immunomodulating hormone melatonin versus melatonin plus aloe vera in untreatable advanced solid neoplasms. Natural immunity. 1998;16(1):27–33. doi: 10.1159/000069427. [DOI] [PubMed] [Google Scholar]
- 25.Choi S, Kim K-W, Choi J-S, Han S-T, Park Y-I, Lee S-K. Angiogenic activity of β-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian gerbil. Planta medica. 2002;68(04):330–5. doi: 10.1055/s-2002-26750. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Agunu A, Diana M. The effect of Aloe vera A. Berger (Liliaceae) on gastric acid secretion and acute gastric mucosal injury in rats. Journal of ethnopharmacology. 2004;93(1):33–7. doi: 10.1016/j.jep.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Joseph B, Raj SJ. Pharmacognostic and phytochemical properties of Aloe veralinn–an overview. Int J Pharm Sci Rev Res. 2010;4(2):106–10. [Google Scholar]
- 28.Shah MH. The general principles of Avicenna's Canon of Medicine: Naveed Clinic. 1966. [Google Scholar]
- 29.San AMM, Thongpraditchote S, Sithisarn P, Gritsanapan W. Total Phenolics and Total Flavonoids Contents and Hypnotic Effect in Mice of Ziziphus mauritiana Lam Seed Extract. Evidence-Based Complementary and Alternative Medicine. 2013:2013. doi: 10.1155/2013/835854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannai M, Kawai N. New therapeutic strategy for amino acid medicine: glycine improves the quality of sleep. Journal of pharmacological sciences. 2011;118(2):145–8. doi: 10.1254/jphs.11r04fm. [DOI] [PubMed] [Google Scholar]
- 31.Parihar M, Chaudhary M, Shetty R, Hemnani T. Susceptibility of hippocampus and cerebral cortex to oxidative damage in streptozotocin treated mice: prevention by extracts of Withania somnifera and Aloe vera. Journal of clinical neuroscience. 2004;11(4):397–402. doi: 10.1016/j.jocn.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Madhusudhan N, Basha PM, Rai P, Ahmed F, Prasad GR. Effect of maternal fluoride exposure on developing CNS of rats: Protective role of Aloe vera, Curcuma longa and Ocimum sanctum. 2010. [PubMed] [Google Scholar]
- 33.Mirshafiey A, Aghily B, Namaki S, Razavi A, Ghazavi A, Ekhtiari P, et al. Therapeutic approach by Aloe vera in experimental model of multiple sclerosis. Immunopharmacology and immunotoxicology. 2010;32(3):410–5. doi: 10.3109/08923970903440184. [DOI] [PubMed] [Google Scholar]
- 34.Evans WC. Trease and Evans' pharmacognosy: Elsevier Health Sciences. 2009. [Google Scholar]
- 35.Achermann P. EEG analysis applied to sleep. Epileptologie. 2009;26:28–33. [Google Scholar]
- 36.Campbell IG. EEG recording and analysis for sleep research. Current Protocols in Neuroscience. 2009;10 doi: 10.1002/0471142301.ns1002s49. 2. 1-2. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30(6):547–58. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- 38.Joseph B, Raj SJ. Pharmacognostic and phytochemical properties of Aloe vera Linnan overview. International Journal of Pharmaceutical Sciences Review & Research. 2010;4(2) [Google Scholar]
- 39.Pires M, Sitart J, Cestari S, Rodrigues R, Tkcaz R. National multicenter clinical open study for the evaluation of efficacy, safety and tolerability of a moisturizer composed by alpha bisabolol, aloe vera in the treatment of mild atopic dermatitis. REVISTA BRASILEIRA DE MEDICINA. 2006;63(8):378. [Google Scholar]
- 40.Fogel SM, Smith CT, Cote KA. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behavioural brain research. 2007;180(1):48–61. doi: 10.1016/j.bbr.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 41.Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiology of learning and memory. 2006;86(2):241–7. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Cho S, Yoon M, Kim D, Kim J-S, Yang H, Lee C-H, et al. Effect of the licorice flavonoid isoliquiritigenin on the sleep architecture and profile in mice. Food Science and Biotechnology. 2012;21(4):1221–5. [Google Scholar]
- 43.Bakalian MJ, Fernstrom JD. Effects of L-tryptophan and other amino acids on electroencephalographic sleep in the rat. Brain research. 1990;528(2):300–7. doi: 10.1016/0006-8993(90)91671-3. [DOI] [PubMed] [Google Scholar]
- 44.Moja EA, Mendelson WB, Stoff DM, Gillin JC, Wyatt RJ. Reduction of REM sleep by a tryptophan-free amino acid diet. Life sciences. 1979;24(16):1467–70. doi: 10.1016/0024-3205(79)90029-8. [DOI] [PubMed] [Google Scholar]
- 45.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189(4196):55–8. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 46.Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Neurophysiology and Neurochemistry of Sleep and Wakefulness: Springer; 1972. pp. 166–307. [DOI] [PubMed] [Google Scholar]
- 47.Sultana N, Najam R. Alterations in neurobehavioral and brain neurotransmitters by Aloe vera (L.) burm Fand vitamine E. International Journal of Research in Ayurveda & Pharmacy. 2012;3(5) [Google Scholar]
- 48.Kaithwas G, Dubey K, Bhatia D, Sharma AD, Pillai K. Reversal of Sodium nitrite indusced impairment of spontaneous alteration by Aloe vera gel: Involvement of cholinergic system. 2007. [Google Scholar]
- 49.Arankowsky-Sandoval G, Aguilar-Roblero R. Cholinergic reduction of REM sleep duration is reverted by auditory stimulation. Brain research. 1986;375(2):377–80. doi: 10.1016/0006-8993(86)90762-6. [DOI] [PubMed] [Google Scholar]
- 50.Markov D, Goldman M, Doghramji K. Normal Sleep and Circadian Rhythms: Neurobiological Mechanisms Underlying Sleep and Wakefulness. Sleep Medicine Clinics. 2012;7(3):417–26. [Google Scholar]
- 51.R Rathor N, Arora T, Manocha S, Patil AN, Mediratta PK, Sharma KK. Anticonvulsant activity of Aloe vera leaf extract in acute and chronic models of epilepsy in mice. Journal of Pharmacy and Pharmacology. 2013 doi: 10.1111/jphp.12181. [DOI] [PubMed] [Google Scholar]