Abstract
Salvia genus is one of the largest genera of the Lamiaceae family. Its species have been used for a wide variety of disorders in the local traditional medicine systems. Therefore, the genus has been the subject of several phytochemical and biological studies. The aim of the study was to identify the major antioxidant compound(s) from the methanol extract of Salvia verticillata using activity-guided fractionation. The crude extract showed strong antioxidant activities in DPPH and β-carotene/linoleic acid tests. The ethyl acetate fraction also exhibited a potent free radical scavenging activity compared to the other fractions. Further fractionation and purification of the ethyl acetate fraction using chromatography methods yielded a compound with high antioxidant capacity. The isolated active compound was determined as chrysoeriol. It showed a dose-dependent free radical scavenging activity with an IC50 (DPPH scavenging) value of 93.32 (80.23 – 108.57) mM.
Key Words: Antioxidant activity, Chrysoeriol, Labiatae, Salvia verticillata, TLC-DPPH bioautography analysis
Introduction
Recently, the role of reactive oxygen species (ROS) in biological systems and pathological conditions has been in the focus of research interests. ROS are the major free radicals (FRs) in human body that are considered to be the prim causes of oxidative damage (1). They can attack cellular biomolecules such as lipids, proteins and DNA, leading to various chronic disorders e.g. neurological disorders, cancer, diabetes, inflammation, etc. (2). FRs can also induce oxidation of lipids in fat-based foods, affecting the quality and acceptability of the products (3).
The main task of the antioxidants is defense of organisms and/or food and pharmaceutical products from the harmful effects of FRs. Generally, antioxidants are able to scavenge FRs, and therefore to inhibit or retard the oxidation process (4). Synthetic antioxidants such as BHT, BHA, and TBHQ are commonly used in the food industry to delay the oxidation degradation and to improve the shelf life (5). However, the safety of the synthetic antioxidants has been questioned and some restrictions have been placed on their application (6). For the reason, there is a growing interest in identifying the effective and safe natural antioxidants and several natural sources are being examined (2, 7). Various plant-derived natural compounds such as polyphenols and terpenoids have been found to act as antioxidants and free radical scavengers (8, 9).
The plants of the Lamiaceae family are regarded as an important source for natural antioxidants. For instance, sage (Salvia officinalis) and rosemary (Rosmarinus officinalis) are well known for their potent antioxidant properties (10). Salvia L. is one of the largest genera of the Lamiaceae family with over 1000 species worldwide. Some members of the genus are used as culinary herb, spice, tea and in perfume and cosmetic industries. Also, some Salvia species have been used in folk medicine because of their diverse biological activities. Phytochemical investigations have shown that Salvia taxa are mainly rich in terpenoids and phenolic compounds (11-13).
Iran is an important country for Salvia species in the world. The flora of Iran includes 58 species of the genus (14). However, the majority of Salvia species growing wild in Iran have not been evaluated from phytochemical and pharmacological point of view. In the research for new antioxidant sources, we have studied the antioxidant capacity of some Salvia species from Iran and have found that Salvia verticillata has high antioxidant activities (15). Other researchers have also shown the prominent antioxidant activities of the species (16). Phytochemical analysis have revealed the presence of volatile constituents (17-22), ditrpenoids (23, 24), triterpenoids (23, 25), phenolic acids (25, 26) and flavonoids (25, 27) in different parts of Salvia verticillata. In the present work, an activity-guided isolation was carried out to identify the major antioxidant compound(s) from aerial parts of Salvia verticillata.
Experimental
General
1H and 13C NMR spectra were recorded in DMSO-d6 using TMS as internal standard on a Varian 400 spectrometer at 400 MHz and 100 MHz, respectively. EI-MS spectra were obtained on a Finigan-Mat spectrometer (70 ev). UV-Vis spectra were recorded on a Shimadzu Multispect-1501 UV-Vis spectrophotometer in methanol.
All chemicals used in this study were purchased from Sigma-Aldrich Chemical Co. (France) or Merck Company (Germany).
Plant material
Salvia verticillata L. was collected from Tehran province during the flowering period in summer 2012 and authenticated by M. Kamalinejad at the Herbarium of the Department of Pharmacognosy, School of Pharmacy, Shahid Beheshti University of Medical Sciences where the voucher specimens (No. 1072) have been preserved. The plant was dried at ambient temperature with active ventilation.
Extraction, fractionation and isolation
The air-dried aerial parts of the plant (250 g) were extracted thrice using 80% methanol by maceration method. The supernatant was filtered and concentrated in vacuo to give the crude extract (27.8 g).
The crude extract (25 g) was suspended in water and then partitioned successively in different solvents, namely n-hexane (HX), chloroform (CF), ethyl acetate (EA), and water (W). The fractions were filtered and concentrated by rotary evaporator.
Since the EA fraction showed the highest DPPH (1,1'-diphenyl-2-picrylhydrazyl) radical scavenging activity among the other fractions, it was further fractionated using a silica gel 60 column (70 – 230 mesh) by a chloroform/ethyl acetate step-gradient elution. The collected fractions (F1 – F11) were evaluated by DPPH test. Fraction F8, which elicited radical scavenging activity, was submitted to repeated preparative layer chromatography on coated plates with silica gel 60F254 (230 – 400 mesh) using CHCl3/EtOAC/HCOOH (45:45:10, v/v/v) as the best developing system. The bands that showed DPPH scavenging activity were scraped off, eluted by ethyl acetate, and monitored by TLC on precoated plates with silica gel 60F254. Further purification and recrystallisation resulted in the active compound. The pure compound was identified by 1H, 13C NMR, MS spectra and by comparison with the literature data.
Antioxidant assays
DPPH radical scavenging activity
The free radical scavenging activities of the crude extract, fractions and the pure compound were evaluated by measuring their ability to scavenging DPPH radicals.
The preliminary test was performed with a rapid TLC scavenging technique (TLC-bioautography analysis) according to a modified method described by Nickavar et al., 2014 (28). An aliquot of each sample (5 μL, MeOH) was directly deposited onto the TLC plate (silica gel 60F254 TLC plates) and developed in CHCl3/EtOAC/HCOOH (45:45:10, v/v/v). The developed plate was allowed to air-dry and followed by spraying with a DPPH solution (0.2%, MeOH). 30 min later, active compounds appeared as yellow spots against a purple background. Gallic acid was used as the positive control.
The spectrophotometric assay was carried out according to the method explained by Nickavar and Esbati, 2012 with some modifications (29). An aliquot of each sample (200 μL, MeOH) was mixed with 2 mL of a 0.1 mM DPPH solution in MeOH. After 30 min, the absorbance of each sample was recorded at 517 nm. Different concentrations were prepared for each sample and analyzed in triplicate. The percentage of scavenged DPPH was calculated according to the following equation (Equation 1):
| Equation 1 |
Where AC is the absorbance of the control (200 μL of MeOH with 2 mL DPPH solution), AB is the absorbance of the blank (200 μL sample with 2 mL MeOH) and AS is the absorbance of the sample.
β-Carotene/linoleic acid bleaching assay
Antioxidant activities of the crude extract, fractions and the pure compound were evaluated using β-carotene/linoleic acid bleaching model system as described by Nickavar and Esbati, 2012 with some modifications (29). 2 mL of β-carotene (200 μg/mL, CHCl3) was added to a flask containing linoleic acid (45 mg) and tween-40 (400 mg). Chloroform was evaporated under a stream of nitrogen. 100 mL of distilled water saturated with oxygen was added and shaken vigorously. 0.5 mL of different concentrations of each sample in methanol was transferred into different test tubes containing 4.5 mL of the above mixture. As soon as the emulsion was added to each tube, the zero time absorbance was measured at 470 nm using a spectrophotometer. The samples were then subjected to thermal autoxidation by keeping them in a constant temperature water bath at 50 °C for 2 h. Subsequently absorbance values were recorded after incubation. The antioxidant activity was calculated according to the following equation (Equation 2):
| Equation 2 |
Statistical analysis
The IC50 values were calculated from logarithmic regression curves (I% against sample concentration) with normalized data and presented with their respective 95% confidence limits. The assays were performed in triplicate. All the statistical analysis was accomplished using the computer software GraphPad prism 3.02 for windows (GraphPad Software, San Diego, CA, USA).
Results and Discussion
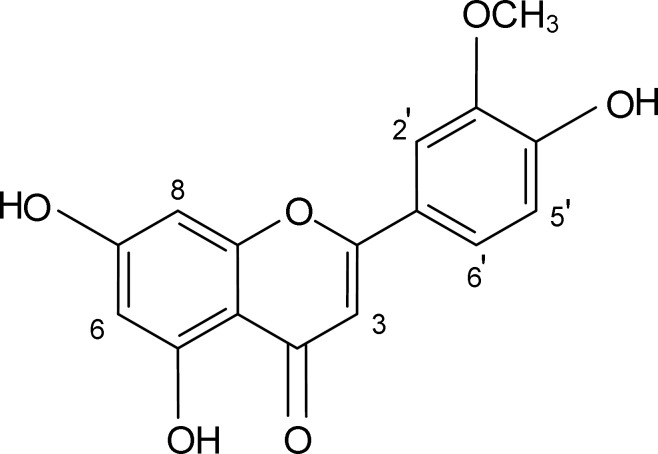
The conventional antioxidant tests were first carried out to determine the antioxidant capacity of the crude extract of Salvia verticillata. The crude extract exhibited remarkable scavenging capacity toward DPPH radical and strong inhibitory effect on bleaching of β-carotene in a concentration-dependent manner with IC50 values of 134.10 (114.80 – 156.70) mcg/mL and 194.00 (170.40 – 220.90) mcg/mL, respectively (Table 1). In order to separate the active components presented in the crude extract, solvent fractionation was performed with n-hexane, chloroform, ethyl acetate and water, successively. As shown in Table 1, the DPPH radical was significantly scavenged by the ethyl acetate fraction with an IC50 value of 23.89 (20.61 – 27.68) mcg/mL. The fraction was monitored by the TLC-DPPH method and at least two bands were observed to possess high scavenging activity. Therefore, it was fractionated for further purification by chromatographic techniques. Finally, a pure active compound (Figure 1) was isolated. The compound was obtained as a yellow amorphous powder with Rf = 0.72 on TLC plate (silica gel 60F254) with CHCl3/EtOAC/HCOOH (45:45:10, v/v/v) solvent system. The spectral characteristics of the compound were as follows:
Table 1.
Antioxidant activities and IC50 values of the crude extract and fractions of Salvia verticillata and its active compound chrysoeriol
| Sample | IC 50 (DPPH scavenging) | IC 50 (β-Carotene bleaching) |
|---|---|---|
| Crude extract | 134.10 (114.80 – 156.70) [mcg/mL] | 194.00 (170.40 – 220.90) [mcg/mL] |
| HX fraction | NAa | - |
| CF fraction | NAa | - |
| EA fraction | 23.89 (20.61 – 27.68) [mcg/mL] | - |
| H2O fraction | 46.48 (41.65 – 51.87) [mcg/mL] | - |
| Rutin | 45.99 (38.74 – 54.66) mM | NAa |
| Vitamin C | 135.70 (112.42 – 163.81) mM | NAa |
| Gallic acid | 75.00 (67.83 – 82.88) mM | 1091.58 (952.27 – 1252.06) mM |
| Chrysoeriol | 93.32 (80.23 – 108.57) mM | 561.18 (518.22 – 607.81) mM |
NA: not active
Figure 1.
Chemical structure of chrysoeriol
1H NMR (400 MHz, in DMSO-d6), δ: 3.88 (s, 3H, OCH3-3'), 6.12 (br. s, 1H, H-6), 6.40 (br. s, 1H, H-8), 6.54 (s, 1H, H-3), 6.83 (d, J = 8.1Hz, 1H, H-5'), 7.32 (br. s, 1H, H-2'), 7.36 (d, J = 8.1 Hz, 1H, H-6').
13C NMR (100 MHz, in DMSO-d6), δ: 54.2 (OCH3), 92.7 (C-8), 97.6 (C-6), 101.6 (C-3), 102.3 (C-10), 110.1 (C-2'), 115.2 (C-5'), 120.2 (C-6'), 129.8 (C-1'), 145.6 (C-3'), 148.4 (C-4'), 153.8 (C-2), 155.1 (C-9), 160.9 (C-5), 167.1 (C-7), 170.5 (C-4).
EI-MS (70 ev), m/z (I%): 300 (12%), 286 (82%), 153 (27%), 151 (22%).
UV-Vis: λmax (in CH3OH) = 267 (sh.), 356 nm.
Detailed analysis of the spectral data showed that the compound was chrysoeriol and all of data matched with those reported in the literature (2, 30). It scavenged DPPH radicals in a dose dependent manner and the IC50 valve was 93.32 (80.23 – 108.57) mM.
Phenolic compounds, particularly flavonoids, of the genus Salvia have received much attention due to their relevant biological properties (31). Chrysoeriol has already been isolated from some Salvia species such as Salvia candidissima and Salvia palaestina (32, 33). However, to the best of our knowledge, this is the first report on the isolation of chrysoeriol from Salvia verticillata and its antioxidant activity.
Acknowledgements
We would like to thank Shahid Beheshti University of Medical Sciences for financial support of this research project as a part of J. Rezaee’s Pharm.D. thesis (Pharm.D. graduate of the School of Pharmacy, Shahid Beheshti University of Medical Sciences).
References
- 1.Suganthy N, Kesika P, Pandian SK, Devi KP. Mangrove plant extracts: Radical scavenging activity and the battle against food-borne pathogens. Forsch Komplementmed. 2009;16:41–48. doi: 10.1159/000209852. [DOI] [PubMed] [Google Scholar]
- 2.Gu L, Wu T, Wang Z. TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT - Food Sci Technol. 2009;42:131–136. [Google Scholar]
- 3.Roby MHH, Sarhan MA, Selim KAH, Khalel KI. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crop Prod. 2013;43:827–831. [Google Scholar]
- 4.Koşar M, Göger F, Başer KHC. In-vitro antioxidant properties and phenolic composition of Salvia virgata Jacq. from Turkey J Agric Food Chem. 2008;56:2369–2374. doi: 10.1021/jf073516b. [DOI] [PubMed] [Google Scholar]
- 5.Moyo M, Ndhlala AR, Finnie JF, Van Staden J. Phenolic composition, antioxidant and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chem. 2010;123:69–76. [Google Scholar]
- 6.Yeşilyurt V, Halfon B, Öztürk M, Topçu G. Antioxidant potential and phenolic constituents of Salvia cedronella. Food Chem. 2008;108:31–39. [Google Scholar]
- 7.Głód BK, Wantusiak PM, Piszcz P, Lewczuk E, Zarzycki PK. Application of micro-TLC to the total antioxidant potential (TAP) measurement. Food Chem. 2014;173:749–754. doi: 10.1016/j.foodchem.2014.10.058. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Park KH, Lee MH, Kim HT, Seo WD, Kim JY, Baek IY, Jang DS, Ha TJ. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013;136:843–852. doi: 10.1016/j.foodchem.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 9.Cretu GC, Morlock GE. Analysis of anthocyanins in powdered berry extracts by planar chromatography linked with bioassay and mass spectrometry. Food Chem. 2014;146:104–112. doi: 10.1016/j.foodchem.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Yeap Foo L. Antioxidant activities of polyphenols from sage (Salvia officinalis) Food Chem. 2001;75:197–202. [Google Scholar]
- 11.Wu YB, Ni ZY, Shi QW, Dong M, Kiyota H, Gu YC, Cong B. Constituents from Salvia species and their biological activities. Chem Rev. 2012;112:5967–6026. doi: 10.1021/cr200058f. [DOI] [PubMed] [Google Scholar]
- 12.Kamatou GPP, Makunga NP, Ramogola WPN, Viljoen AM. South African Salvia species: A review of biological activities and phytochemistry. J Ethnopharmacol. 2008;119:664–672. doi: 10.1016/j.jep.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Li Q, Zhang C, Zhang N, Cui Z, Huang L, Xiao P. An ethnopharmacological investigation of medicinal Salvia plants (Lamiaceae) in China. Acta Pharmaceut Sin B. 2013;3:273–280. [Google Scholar]
- 14.Mozaffarian V. A Dictionary of Iranian Plant Names. Farhang Mo'aser, Tehran. 1996:477–480. [Google Scholar]
- 15.Nickavar B, Kamalinejad M, Izadpanah H. In-vitro free radical scavenging activity of five Salvia species. Pak J Pharm Sci. 2007;20:291–294. [PubMed] [Google Scholar]
- 16.Tosun M, Ercisli S, Sengul M, Ozer H, Polat T, Ozturk E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol Res. 2009;42:175–181. [PubMed] [Google Scholar]
- 17.Chalchat JC, Gorunovic MS, Petrovic SD, Maksimovic ZA. Chemical compositions of two wild species of the genus Salvia L. from Yugoslavia: Salvia aethiopis and Salvia verticillata. J Essent Oil Res. 2001;13:416–418. [Google Scholar]
- 18.Krstic L, Malencic D, Anackov G. Structural investigations of trichomes and essential oil composition of Salvia verticillata. Bot Helv. 2006;116:159–168. [Google Scholar]
- 19.Pitarokili D, Tzakou O, Loukis A. Essential oil composition of Salvia verticillata, S. verbenaca, S. glutinosa and S. candidissima growing wild in Greece. Flav Frag J. 2006;21:670–673. [Google Scholar]
- 20.Rajabi Z, Ebrahimi M, Farajpour M, Mirza M, Ramshini H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Ind Crop Prod. (2014);61:233–239. [Google Scholar]
- 21.Rzepa J, Wojtal Ł, Staszek D, Grygierczyk G, Labe K, Hajnos M, Kowalska T, Waksmundzka-Hajnos M. Fingerprint of selected Salvia species by HS-GC-MS analysis of their volatile fraction. J Chromatogr Sci. 2009;47:575–580. doi: 10.1093/chromsci/47.7.575. [DOI] [PubMed] [Google Scholar]
- 22.Sefidkon F, Khajavi MS. Chemical composition of the essential oils of two Salvia species from Iran: Salvia verticillata L. and Salvia santolinifolia Boiss. Flav Frag J. 1999;14:77–78. [Google Scholar]
- 23.Janicsák G, Zupkó I, Nikolova MT, Forgo P, Vasas A, Máthé I, Blunden G, Hohmann J. Bioactivity-guided study of antiproliferative activities of Salvia extracts. Nat Prod Commun. 2011;6:575–579. [PubMed] [Google Scholar]
- 24.Nagy G, Günther G, Máthé I, Blunden G, Yang MH, Crabb TA. Diterpenoids from Salvia glutinosa, S. austriaca, S. tomentosa and S. verticillata roots. Phytochem. 1999;52:1105–1109. [Google Scholar]
- 25.Demirezer OL, Gurbuz P, Kuruuzum-Uz A, Guvenalp Z, Kazaz C, Donmez AA. Chemical constituents of two sages with free radical scavenging activity. Nat Prod Commun. 2012;7:187–190. [PubMed] [Google Scholar]
- 26.Yumrutas O, Sokmen A, Ozturk N. Determination of in-vitro antioxidant activities and phenolic compounds of different extracts of Salvia verticillata ssp. verticillata and spp. amasiaca from Turke'ys flora. J App Pharm Sci. 2011;1:43–46. [Google Scholar]
- 27.Ulubelen A, Topçu G. Flavonoids and terpenoids from Salvia verticillata and Salvia pinnata. J Nat Prod. 1984;47:1068. [Google Scholar]
- 28.Nickavar B, Adeli A, Nickavar A. TLC-bioautography and GC-MS analyses for detection and identification of antioxidant constituents of Trachyspermum copticum essential oil. Iran J Pharm Res. 2014;13:127–133. [PMC free article] [PubMed] [Google Scholar]
- 29.Nickavar B, Esbati N. Evaluation of the antioxidant capacity and phenolic content of three Thymus species. J Acupunct Meridian Stud. 2012;5:119–125. doi: 10.1016/j.jams.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Awaad AS, Maitland DJ, Soliman GA. Hepatoprotective activity of Schouwia thebica webb. Bioorg Med Chem Lett. 2006;16:4624–4628. doi: 10.1016/j.bmcl.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Yeap Foo L. Polyphenolics of Salvia - A review. Phytochem. 2002;59:117–140. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 32.Topcu G, Tan N, Ulubelen A, Sun D, Watson WH. Terpenoids and flavonoids from the aerial parts of Salvia candidissima. Phytochem. 1995;40:501–504. [Google Scholar]
- 33.Miski M, Ulubelen A, Johansson C, Mabry TJ. Antibacterial activity studies of flavonoids from Salvia palaestina. J Nat Prod. 1983;46:874–875. doi: 10.1021/np50030a007. [DOI] [PubMed] [Google Scholar]