Abstract
Checkpoint blockade therapy has been proven to be highly active across many cancer types but emerging evidence indicates that the therapeutic benefit is limited to a subset of patients in each cancer entity. The presence of CD8+ T cells within the tumor microenvironment or the invasive margin of the tumor, as well as the up-regulation of PD-L1, have emerged to be the most predictive biomarkers for clinical benefit in response to checkpoint inhibition. Although the up-regulation of immune inhibitory mechanisms is one mechanism of immune escape, commonly used by T-cell-inflamed tumors, exclusion of an anti-tumor specific T-cell infiltrate displays another even more potent mechanism of immune escape. This review will contrast the mechanisms of immunogenic, T-cell-inflamed, and the novel concept of non-immunogenic, non-T-cell-inflamed, adaptive immune escape.
Keywords: checkpoint blockade, immune evasion, immunotherapy, oncogenes
Introduction
Cancer immunotherapy has revolutionized the treatment and survival prospects of cancer patients. In particular the approval of ipilimumab, an anti-CTLA-4 antibody, as well as nivolumab and pembrolizumab, antibodies against programmed death-1 (PD-1), has paved the way for their use (alone or in combinations) as immunotherapeutic agents (1–3).
The characteristics of immunotherapeutic interventions are that clinical benefit is mostly associated with a durable response, a key feature that leads to on-average long survival rates in the patient groups that experience benefit (2). The latest clinical data indicate that PD-1 inhibition is therapeutically active in up to 30% of patients with a variety of cancers, including melanoma, lung cancer, head and neck cancer, renal cell cancer etc. (3). Furthermore, combination of anti-CTLA-4 therapy with PD-1 blockage increased the fraction of responding patients with malignant melanoma to 57%, indicating that combination therapy can be beneficial in increasing the proportion of responding patients (4). Nevertheless, understanding the molecular mechanisms that result in sensitivity or insensitivity towards immunotherapy will be the undertaking of the upcoming years.
One of the most useful features discriminating immunotherapy-sensitive versus immunotherapy-insensitive cancer patients is the presence or absence of tumor-specific T cells. In a recent study, Tumeh et al. have provided evidence that the presence of CD8+ T cells within the tumor or the invasive margin of the tumor is highly correlated with response towards PD-1 inhibition (5). The observation that the presence or absence of a T-cell infiltrate is a strong, if not the most potent, indicator for a productive anti-tumor immune response has long been proposed was elegantly introduced in the immune score concept Galon et al. (6–8).. Since then many clinical observations have subsequently linked the presence of CD8+ T cells to a type I interferon (IFN-α/β) signature (9, 10). The sum of these observations has led to the discrimination of cancer patients into T-cell-inflamed (positive for a CD8+ T-cell infiltrate as well as a type I interferon signature) and non-T-cell-inflamed patients (lacking both features) (9, 11).
This review will discuss and contrast two mechanisms of tumor immune escape leading either to sensitivity or to insensitivity towards immunotherapy mediated through modulation of the adaptive immune response against the tumor.
Mounting a spontaneous and productive anti-tumor immune response
Recognition of the tumor by the immune system is the most proximal event required to occur in order to mount a productive anti-tumor immune response. Over the past years, much effort has been undertaken in order to understand the so-called innate immune sensing. This mechanism requires the recruitment of antigen-presenting cells (APC) into the tumor microenvironment (TME). It has been shown that the recruitment of a subset of dendritic cells (DC), driven by the transcription factor Batf3, is crucial for the mounting of an anti-tumor immune response (12–14). Subsequently, work using mouse models that feature transplantable tumor cells has proven that those DC are mediating a type I interferon response, required for mounting a potent anti-tumor immune response (13, 14).
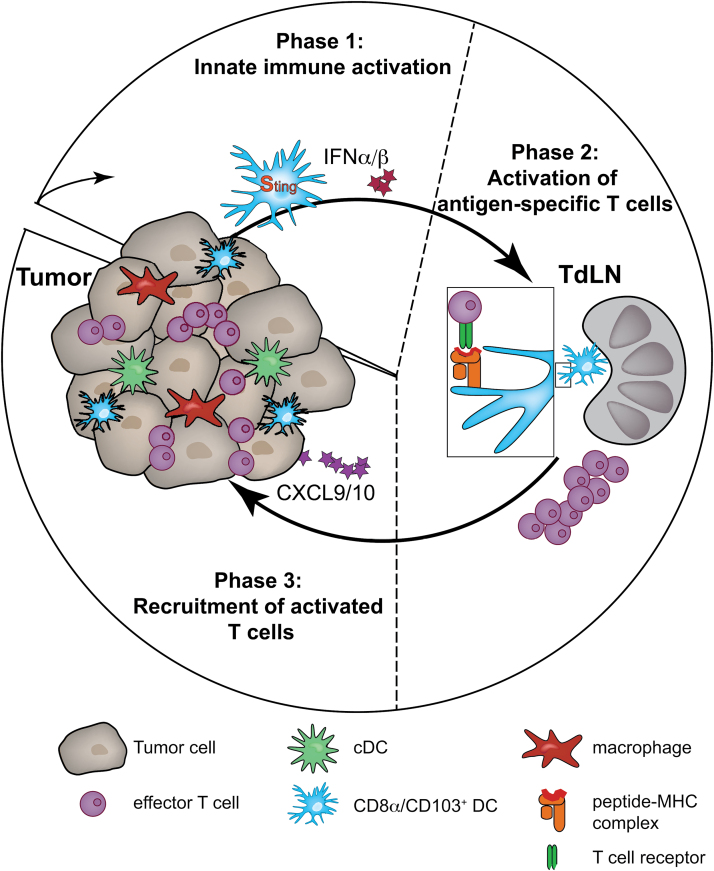
More recent work has further characterized the subset of DC and has provided strong evidence that the CD103+/CD8α+ subset of Batf3-driven DC is responsible for the type I interferon signature and the translocation into the tumor-draining lymph node (TdLN, Fig. 1), whereas non-migratory ‘conventional’ DC (cDC) only minimally affect this response (15). Pre-clinical and clinical studies have proven the requirement of the induction of type I interferon for the activation of a potent anti-tumor immune response. Seminal work has provided strong evidence that activation of DC under sterile, non-pathogen-driven tumor conditions is predominantly mediated through sensing of cytosolic DNA through the cGAS–Sting pathway and that pharmacological activation of this pathway enhances the type I interferon signature and the anti-tumor immune response (16, 17).
Fig. 1.
Induction of a potent and productive anti-tumor immune response. In Phase 1 (innate immune activation) APC, in particular CD8α+CD103+ DC, migrate into the tumor and produce IFN-α/β downstream of Sting activation. In Phase 2 (activation of antigen-specific T cells) in the TdLN, migratory CD8α+CD103+ DC deliver tumor-derived antigens to the TdLN and through cross-presentation activate tumor-specific CD8+ T cells. In Phase 3 (recruitment of activated T cells) subsequent to activation of effector T cells, those cells migrate into the tumor microenvironment via CXCL9/CXCL10 chemokine gradients.
Taking these studies together it can be concluded that the tumor needs to be infiltrated by CD103+ DC that will, upon recognition of the tumor (via DNA–cGAS–Sting), migrate into the TdLN before activating T cells in an antigen-specific manner (Fig. 1, Phase 1).
A second, rate-limiting factor is the presence of antigens capable of being recognized by specific T cells. Recent studies have provided evidence that tumors with strong de novo-derived antigens, so-called neo-antigens, respond better to checkpoint blockade (18, 19). Lack of immunogenic antigens would presumably result in an ineffective priming response in the TdLN (Fig. 1, Phase 2). Although the presence of antigens could indeed be a rate-limiting factor, preliminary data from our own laboratory have indicated that the absence of a local anti-tumor immune response does not exclude the presence of strong antigens. Analysis of The Cancer Genome Atlas (TCGA) metastatic melanoma dataset provided evidence for comparable expression of differentiation antigens, cancer-germline antigens, as well as mutated self-proteins generating peptides presented by HLA-A*0201 (data presented at the ASCO meeting 2015, Chicago) (20). Thus, it can be concluded that an additional mechanism explains sensitivity versus insensitivity towards immunotherapy.
In order for effector T cells, activated in the TdLN by Batf3-driven DC, to traffic into the TME, the appropriate chemokines need to be expressed locally. One candidate chemokine receptor, known to be expressed on activated effector T cells, is CXCR3 and recent work has suggested a non-redundant role of this receptor in the migration of anti-tumor CD8+ T cells into the tumor mass (21). Interestingly CXCL9 and CXCL10, which are the chemokine ligands of CXCR3, have been strongly correlated with the T-cell-inflamed phenotype, whereas the non-T-cell-inflamed subset lacks these chemokines (9, 22, 23). Furthermore, as in vitro activation of the Sting pathway in DC also triggers CXCL9 and CXCL10 (16), it is plausible to consider that the minimal defect in non-T-cell-inflamed tumors might be attributed to poor recruitment and/or activation of Batf3-lineage DC into the TME, a concept that is being pursued further (Fig. 1, Phase 3).
Escape in the context of a T-cell-inflamed TME
The existence of the T-cell-inflamed TME by itself provides evidence for escape mechanisms that allow the co-existence of an anti-tumor immune response and the tumor itself. The presence of CD8+ T cells has in multiple studies been associated with the up-regulation of immune inhibitory mechanisms mediating immune suppression, whereas elimination of immunogenic tumor cells, leaving only non-immunogenic tumor cells, displays a similarly potent form of immune escape (11, 24, 25).
Escape through immune suppression
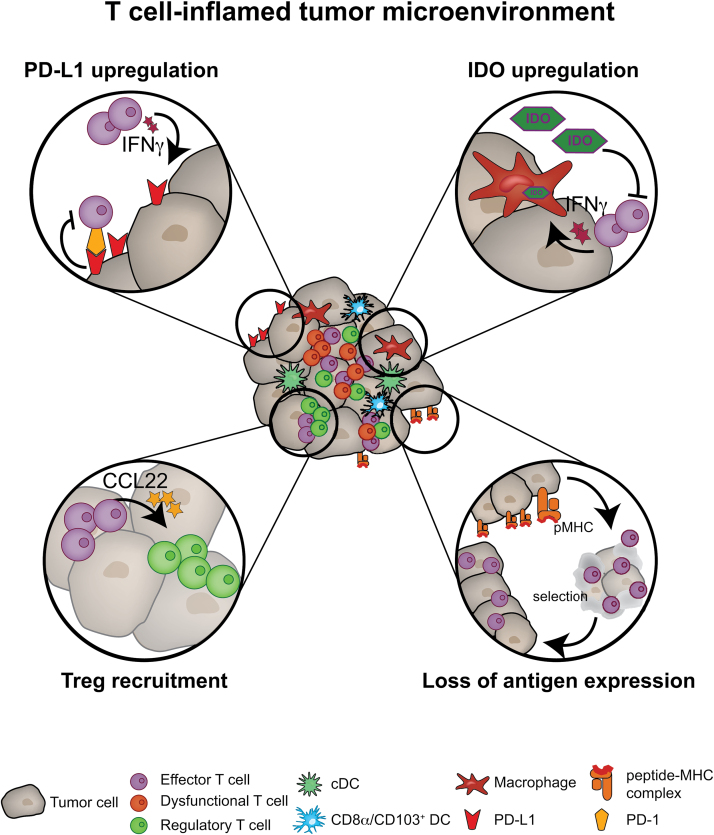
Gene expression profiling indicated the presence of transcripts encoding indoleamine-2,3-dioxygenase (IDO) in these tumors, a molecule that had previously been demonstrated to contribute to peripheral tolerance (26) (Fig. 2). Interrogation for additional candidates revealed that these tumors also expressed PD-L1 and FoxP3 transcripts (11, 27). The recruitment of FoxP3+ Tregs is directly linked with the presence of activated effector T cells that produce CCL22, the dominant chemokine to recruit the predominantly CCR4+ Tregs (11) (Fig. 2). CCL22 can also be produced by other components of the TME, including M2-like macrophages (28).
Fig. 2.
Immune escape in a T-cell-inflamed tumor microenvironment. The figure shows details of the four dominant immune escape mechanisms in the T-cell-inflamed tumor microenvironment: PD-L1 up-regulation and subsequent inhibition of T cells through PD-L1 engagement with PD-1; IDO up-regulation; recruitment of regulatory T cells (Tregs) through CCL22 (derived from effector T cells); and selection of tumor cells with reduced antigenic immunogenicity.
The presence of Tregs can impact the TME and in particular the CD8+ effector T-cell function through three mechanisms: (i) soaking up IL-2 (29); (ii) CTLA-4 (a co-inhibitor) competing with CD28 (a co-stimulator) for binding to CD80 or CD86; and (iii) CD39/CD73-mediated generation of adenosine (30–33). Those mechanisms act directly on effector T cells by impairing their functionality leading to a reduction of the anti-tumor destruction. Additionally, it is a conceivable concept that through interaction between Tregs and DC the latter will be imprinted to acquire a tolerogenic phenotype, which in turn might impact the stimulation of effector T cells (34–36).
Pre-clinical and clinical studies have indicated that, besides the recruitment of Tregs, the secretion of IFN-γ by tumor-specific CD8+ T cells results in the up-regulation of PD-L1 and most likely also PD-L2 on tumor cells (11, 37) (Fig. 2). Both PD-L1 and PD-L2 are ligands for PD-1 (38). This receptor is commonly expressed on activated T cells, as well as chronically exhausted T cells during chronic viral infection (39). The engagement of the receptor with its ligands induced an inhibitory signal towards the T cells, inhibiting their effector functions (40). This mechanism of self-inhibition of the immune system is being targeted using antibodies that block PD-1 or PD-L1 and displays currently the most effective form of checkpoint blockade.
Altogether, these immune inhibitory pathways inhibit T-cell function and result in the development of a dysfunctional T-cell phenotype (Fig. 2). Several groups have provided strong indications that T cells found within the TME are no longer capable of lysing target cells, producing IL-2 or proliferating (41–43). The dysregulation of T-cell function can be seen as an additional, T-cell-intrinsic immune inhibitory mechanism, which prevents an effective anti-tumor immune response. These immune evasion mechanisms are part of the natural host response implying that they are most likely independent of the tumor context and thereby display targets for therapeutic interventions with a low risk of resistance.
Because of the presence of a variety of immune regulatory factors in the same TME, it can be assumed that targeting multiple pathways simultaneously will result in synergistic therapeutic effects. There are at least three rational combinations: (i) an IDO inhibitor combined with either anti-CTLA-4 or anti-PD-1 mAbs; (ii) anti-LAG-3 plus anti-PD-1; and (iii) an anti-4-1BB mAb in various combinations. Already the combination of anti-CTLA-4 plus anti-PD-1 has revealed a higher response rate than either agent alone in metastatic melanoma, albeit with a higher rate of adverse events (4, 44). By using pre-clinical mouse models, combinations of CTLA-4, PD-L1 and/or IDO have indicated that the therapeutic effect is associated with re-activation of CD8+ T cells directly within the TME (43). Similar results could have been obtained using combinations of Lag-3 and PD-1, as well as CTLA-4 and 4-1BB-agonist (45, 46). Although several of those combinations are already generating exciting response rates in clinical trials, more pre-clinical mouse models will be needed to dissect the molecular mechanism of each of the combination in order to tailor their application to the specific tumor phenotype of the patient.
The major biologic correlate that is restored with blockade or activation of these pathways is the ability of tumor-infiltrating CD8+ T cells to produce IL-2 and to proliferate when analyzed ex vivo, linking the mechanisms of T cell-intrinsic dysfunction to the expression of extrinsic immune inhibitory mechanisms. Consistently, the clinical response with anti-PD-1 mAbs in metastatic melanoma was found to occur predominantly in patients with pre-existing CD8+ T cell infiltrates, located predominantly in regions of PD-L1 up-regulation (3, 5, 37). Thus, it is conceivable that the clinical response with active immunotherapies is also mediated through restored function of pre-existing tumor-infiltrating lymphocytes (TIL).
Escape through reduced immunogenicity
Besides up-regulation of immune inhibitory mechanisms, the tumor can also evade the anti-tumor immune response by down-regulation of its immunogenicity. Seminal work by Robert Schreiber and colleagues has shown that tumors established in an immune-competent host will be less immunogenic compared with tumors established in hosts lacking an adaptive immune system (24, 47). Loss of immunogenicity can be established through multiple molecular mechanisms, which include down-regulation of MHC class I molecules and reduced expression of the immunogenic antigens, either through genetic or epigenetic alterations of the tumor cells (25, 48).
Further it has been postulated that less-immunogenic tumor cells are not eliminated by the immune system and will eventually escape and grow progressively as edited tumors (Fig. 2, lower right); this is summarized in the Three E hypothesis (elimination, equilibrium, and escape) (48). More recent work has shown that edited tumors respond to checkpoint blockade and therefore indicated that up-regulation of immune inhibitory mechanisms is part of this immune-escape (49). In sum it can be assumed that T-cell-inflamed tumors are being edited by the host immune system to have reduced immunogenic antigen-expression and -presentation but at the same time immune inhibitory mechanisms are being up-regulated. The latter are part of a natural response to protect the host cells from destruction by the immune system. Nonetheless, the up-regulation of immune inhibitory pathways, including CTLA-4 and PD-1–PD-L1, makes those tumors sensitive to immunotherapeutic interventions, in particular checkpoint blockade.
Escape through preventing T-cell-infiltration into the TME
In sharp contrast to the T-cell-inflamed TME, the non-T-cell-inflamed TME lacks not only T cells but also the up-regulation of immune inhibitory mechanisms (11). Therefore, it is not surprising that checkpoint blockade is not effective in this sub-group of patients (5). Two essential steps in the induction of a potent anti-tumor immunity are (i) recruitment and activation of APC, most importantly Batf3-expressing DC; and (ii) recruitment of activated effector T cells into the TME. Recent studies have provided evidence that tumor-cell-intrinsic alterations can mediate either lack of DC infiltration or lack of effector cell recruitment. Additionally, multiple other studies have provided evidence that tumor-cell-intrinsic signaling impacts the local anti-tumor immune response.
Escape through a lack of innate immune sensing
The comparison of gene expression combined with mutational analysis (using TCGA) between T-cell-inflamed and non-T-cell-inflamed melanoma samples revealed an activation of the WNT–β-catenin signaling pathway in the non-T-cell-inflamed cohort (23). Molecularly this could be attributed to a stabilization mutation in the β-catenin gene (CTNNB1), loss-of-function mutations in β-catenin inhibitors (adenomatous polyposis coli and Axin1) or enhanced gene expression of pathway-activating genes. In sum, activation of the β-catenin pathway was observed in 48% of all non-T-cell-inflamed metastatic melanomas. Interestingly, analysis of β-catenin target genes showed an inverse correlation with CD8α and PD-L1 expression in the tumors and strongly indicated a significant correlation between the β-catenin pathway activation and a non-T-cell-inflamed TME (23).
Mechanistic studies were performed using genetically engineered mice (GEM) containing a tamoxifen-regulated Cre driven by the tyrosinase promoter combined with active Braf (BrafV600E) and conditional PTEN deletion (PTEN–/–), with or without a conditional active β-catenin mutant (CAT-STA) (50–52). Melanomas arising from BrafV600E/PTEN–/– mice included a modest T-cell infiltrate as analyzed by flow cytometry and immunohistochemistry. However, melanomas with active β-catenin signaling (BrafV600E/CAT-STA or BrafV600E/PTEN–/–/CAT-STA ) completely lacked a T-cell infiltrate. Those observations directly indicate that activating β-catenin signaling within melanoma tumor cells can exclude T cells from the TME.
Further analysis of the immune response was obtained by combining the GEM with Cre-inducible expression of the model antigen SIY (SIYRYYGL) (53). This strategy allowed determination of T-cell activation after adoptive transfer of SIY-specific 2C TCR-transgenic T cells. Indeed, in mice with T-cell-inflamed tumors (BrafV600E/PTEN–/–), a spontaneous activation of 2C T cells was observed, which could not be observed in mice bearing non-T-cell-inflamed tumors (BrafV600E/PTEN–/–/CAT-STA).
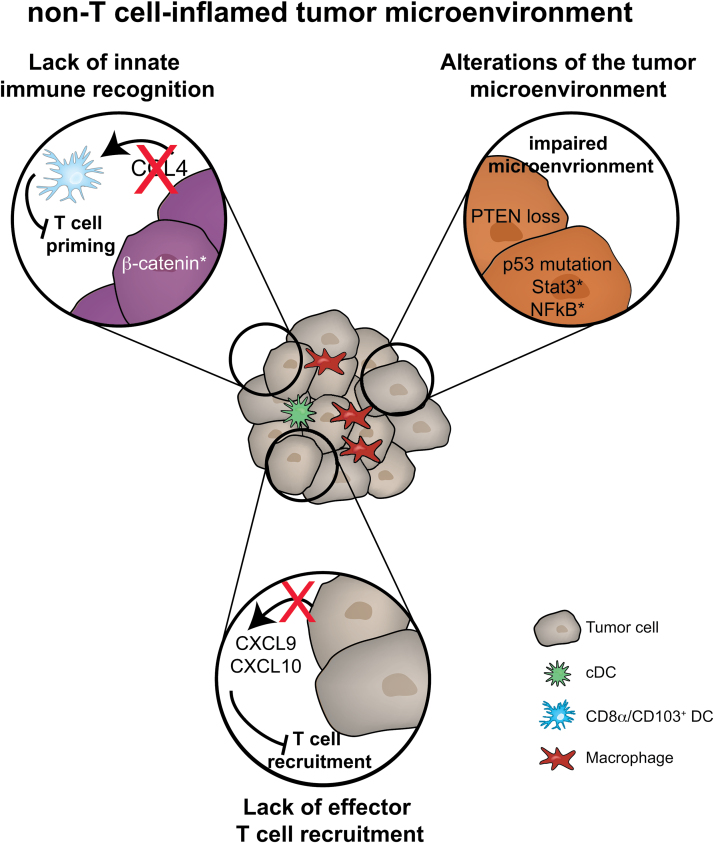
Importantly, the difference in baseline T-cell infiltration impacted on the ability of the mice to respond in vivo to immunotherapy. Whereas the combination of anti-CTLA-4 plus anti-PD-L1 mAbs slowed tumor growth in inducible T-cell-inflamed (BrafV600E/PTEN–/–) tumor-bearing mice, there was no therapeutic effect observed on non-T-cell-inflamed tumors (BrafV600E/PTEN–/–/CAT-STA). This observation indicates a defect in early immune priming, likely through alterations in DC activation. Detailed analysis of the APC compartment in the tumors revealed the lack of Batf3-driven DC subtypes, CD103+ and CD8α+ DC. The lack of DC recruitment was further associated with a reduction in CCL4 chemokine expression by the melanoma cells (Fig. 3).
Fig. 3.
Immune escape in a non-T-cell-inflamed tumor microenvironment. The figure depicts the three main mechanisms of immune escape through potent T-cell exclusion from the tumor microenvironment: a lack of innate immune cell recruitment, which results in a block of T-cell activation; oncogenic pathways that alter the tumor microenvironment in thus far ill-defined mechanisms but which exclude T cells; and a lack of effector T-cell recruitment due to loss of effector chemokine production.
Collectively, these data have provided evidence that the Wnt–β-catenin pathway, as the first tumor-cell-intrinsic signaling pathway, prevents the T-cell-inflamed TME and generates resistance to checkpoint blockade therapy (23).
Escape through a lack of effector T-cell recruitment
Besides inhibition of innate immune sensing, multiple clinical studies have observed that expression of chemokines involved in effector T-cell recruitment is significantly reduced in tumors lacking a CD8+ T-cell infiltrate (22). The predominant chemokine receptor expressed and responsible for effector T-cell infiltration into the tumor is CXCR3, with its ligands CXCL9 and CXCL10 (Fig. 3) (21).
Mechanistically, it is conceivable that those chemokines are derived from the tumor cells themselves or from stroma/immune cells. Some studies support the notion that enhanced expression of CXCL9 and CXCL10 might be due to genetic amplification of the genes and an elegant study by Peng et al. has shown that methylation of the genetic loci for both chemokines is associated with reduced infiltration of effector T cells (54); methylation, catalyzed by DNA methyltransferase 1 (DNMT-1), can be associated with decreased gene expression. Further, the authors have proven that the lack of effector T-cell infiltration was associated with a loss of therapeutic efficacy for checkpoint blockade, which could be restored by administration of the DNMT-1 inhibitor, 5-aza-2′-deoxycytidine (5-AZA-dC).
Since not all T-cell-inflamed tumors will include modifications targeting this genetic locus, it is also a conceivable concept that immune components of the tumor stroma might contribute to the effector T-cell recruitment. CXCL9 and CXCL10 are in fact interferon-responsive genes, known to be activated in DC upon type I interferon activation, e.g. through Sting activation in DC (16). The observation that the absence of certain DC is associated with the lack of effector T-cell recruitment could suggest that DC within the tumor might also contribute to the effector T-cell recruiting signature.
Escape through alterations in the TME
Besides the already discussed effect of the Wnt–β-catenin pathway on T-cell infiltration in melanoma, additional molecular perturbations might function to limit host immunity in the non-T-cell-inflamed melanomas and in other cancer types as well.
The PI3K–PTEN–AKT pathway is another strong candidate to be considered that could impact on host immune responses. Studies focusing on models for inflammation-induced cancer progression have associated active PI3K signaling with an immune-suppressive microenvironment mediated by increased accumulation of tumor-associated macrophages (55, 56). Accumulation of macrophages was associated with increased production of TNF, IL-6, CSF-1, VEGF-A and IL-8 by the tumor cells themselves, contributing to recruitment of M2-like macrophages, which can be immunosuppressive (57).
Consistent with these observations in a poorly immunogenic environment, a recent study by Hwu and coworkers has elegantly proven that loss of PTEN in melanoma cells is associated with a reduced response towards therapeutic interventions. By using a combination of pre-clinical mouse models and human patient samples, a functional link between lack of T-cell-infiltration and loss of PTEN associated with AKT activation could be identified (58). Although the molecular mechanism remains to be determined, the gross similarity between the β-catenin-mediated and PI3K/PTEN-mediated phenotypes suggests similar defects in innate immune sensing and T-cell activation but interestingly the β-catenin defects and PI3K/PTEN defects occur in non-overlapping patient cohorts (Fig. 3).
Similarly to the observation regarding PTEN deletion, mutations of the tumor-suppressor p53 have also been suggested to be associated with immune-modulatory effects. In fact, wild-type p53 signaling has been correlated with recruitment and activation of immune cells (59). For instance, re-activation of the p53 pathway resulted in tumor regression a murine liver carcinoma model, an observation associated with increased expression of pro-inflammatory chemokines. Additional studies revealed that the observed p53-mediated tumor regression was associated with activation and recruitment of natural killer (NK) cells into the TME (60). The recruitment of NK cells was dependent on p53-induced CCL2 production. Clinically, a recent study correlated wild-type p53 signaling to the T-cell-inflamed microenvironment in triple-negative breast cancer (i.e. lacking ER, HR and Her2/neu) (61). In sum, steady-state p53 signaling could contribute to recruitment of innate and adaptive immune cells, whereas mutation of p53 results in the reduction of innate immune activation and thereby a lack of T-cell infiltration (Fig. 3).
Additional oncogenic pathways potentially impacting on host immune responses are the NFκB signaling pathway and Stat3 signaling. Activation of both pathways in cancer cells has been associated with tumor progression (62, 63). Augmented NFκB signaling has been associated with immune-derived TNF signaling and increased expression of tumor-derived chemokines, which could have positive or negative immune-modulatory effects (64–66). The impact of tumor-intrinsic NFκB signaling on the immune response might highly depend on the cellular context, and whether tumor-promoting inflammatory cells are associated with cancer progression. Additional studies will be needed to determine if tumor-cell-intrinsic NFκB signaling is enhancing or dampening the local anti-tumor immune response.
Studies using transplantable tumors with active STAT3 signaling have indicated a decreased expression of pro-inflammatory mediators, whereas expression of a dominant-negative STAT3 variant resulted in increased expression of pro-inflammatory molecules (67, 68), including CCL5 and CXCL10. Additional evidence for this mechanism has been provided through a conditional knockout model for STAT3; an increased anti-tumor immune response was observed in the absence of STAT3 signaling. This phenotype was associated with increased T-cell accumulation and T-cell function within the TME (69, 70). Thus, STAT3 may represent another immune inhibitory mechanism and, based on the currently available data, this might be due to reduced recruitment of both DC and T cells (Fig. 3).
Overcoming the lack of T-cell infiltration can be considered the next big hurdle to overcome resistance toward immunotherapy. Based on the current knowledge, inhibition of inhibitory molecules (PD-1, Lag-3) or engagement of activating molecules (OX-40, 4-1BB) will have no or only limited effects in this cohort of patients. It is conceivable that combination therapies of checkpoint blockade therapy with either small-molecule inhibitors (e.g. Braf-inhibitors), chemotherapy or radiation therapy would be synergistic through mechanisms that restore T-cell infiltration. A recent study using a genetically engineered mouse model for lung cancer elegantly showed that chemotherapy, using oxaliplatin and mafoslamide, enhances the amount of CD8+ T-cell infiltration and this treatment synergized with checkpoint blockade (71). The authors speculated that lack of T-cell infiltration was due to loss of p53 expression and therefore those data provide a strong notion that immunogenic cell death induced through systemic chemotherapy could overcome lack of T-cell infiltration. Pre-clinical and clinical observations have given indication that inhibition of Braf-signaling in melanoma or radiation therapy can increase the efficacy of checkpoint blockage (72, 73). It remains to be determined though if this enhanced infiltration of T cells following those treatment also results in the induction of T-cell infiltration in the context of a non-T-cell-inflamed TME.
Conclusion
The above discussed escape mechanisms either in the T-cell-inflamed or in the non-T-cell-inflamed TME mediate immune suppression and escape against an endogenous anti-tumor immune response. In particular, immune suppression in the T-cell-inflamed TME is currently being harnessed to activate the endogenous T-cell response through blocking of immune inhibitory mechanisms. Checkpoint blockade activates the immune response in an extremely potent fashion and it needs to be determined if such a strong anti-tumor immune response can result in the development of newly adapted immune escape strategies.
An emerging hypothesis is that alterations in tumor-cell-intrinsic signaling, e.g. activation of β-catenin, can mediate direct immune evasion from strong anti-tumor immune responses through direct tumor immune-avoidance. This process could theoretically occur at any given time in tumor development. In concordance with the concept of immune evasion (the Three E hypothesis) (24), we propose that tumor escape might occur through selection of tumor cells that possess altered signaling pathways, including activation of the Wnt–β-catenin pathway. This mechanism would lead to exclusion of immune cells from the TME, resulting in a conversion of T-cell-inflamed tumors into non-T-cell-inflamed lesions.
Pre-clinical and clinical studies should be conducted that allow the detailed mechanistic analysis of secondary resistance following immunotherapeutic interventions in order to develop strategies to avoid or reverse secondary resistance.
Funding
Cancer Research Institute Irvington Postdoctoral Fellowship (FP057884-01 to S.S.).
Conflict of interest statement: The author has no conflict of interest to declare.
References
- 1. Hodi F. S., O’Day S. J., McDermott D. F., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topalian S. L., Sznol M., McDermott D. F., et al. 2014. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Topalian S. L., Hodi F. S., Brahmer J. R., et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larkin J., Chiarion-Sileni V., Gonzalez R., et al. 2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tumeh P. C., Harview C. L., Yearley J. H., et al. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galon J., Costes A., Sanchez-Cabo F., et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960. [DOI] [PubMed] [Google Scholar]
- 7. Ropponen K. M. Eskelinen M. J. Lipponen P. K. Alhava E. and Kosma V. M. 1997. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J. Pathol. 182:318. [DOI] [PubMed] [Google Scholar]
- 8. Naito Y., Saito K., Shiiba K., et al. 1998. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 58:3491. [PubMed] [Google Scholar]
- 9. Harlin H., Meng Y., Peterson A. C., et al. 2009. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 69:3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salerno E. P. Olson W. C. McSkimming C. Shea S. and Slingluff C. L. Jr. 2014. T cells in the human metastatic melanoma microenvironment express site-specific homing receptors and retention integrins. Int. J. Cancer 134:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spranger S., Spaapen R. M., Zha Y., et al. 2013. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hildner K., Edelson B. T., Purtha W. E., et al. 2008. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engelhardt J. J., Boldajipour B., Beemiller P., et al. 2012. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell 21:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuertes M. B., Kacha A. K., Kline J., et al. 2011. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 208:2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broz M. L., Binnewies M., Boldajipour B., et al. 2014. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woo S. R., Fuertes M. B., Corrales L., et al. 2014. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corrales L., Glickman L. H., McWhirter S. M., et al. 2015. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 11:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snyder A., Makarov V., Merghoub T., et al. 2014. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371:2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rizvi N. A., Hellmann M. D., Snyder A., et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gajewski T., Zha Y., Hernandez K., et al. 2015. Density of immunogenic antigens and presence or absence of the T cell-inflamed tumor microenvironment in metastatic melanoma. J. Clin. Oncol. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mikucki M. E., Fisher D. T., Matsuzaki J., et al. 2015. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 6:7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bedognetti D., Spivey T. L., Zhao Y., et al. 2013. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin-2. Br. J. Cancer 109:2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spranger S. Bao R. Y. and Gajewski T. F. 2015. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523:231. [DOI] [PubMed] [Google Scholar]
- 24. Dunn G. P. Bruce A. T. Ikeda H. Old L. J. and Schreiber R. D. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3:991. [DOI] [PubMed] [Google Scholar]
- 25. DuPage M., Cheung A. F., Mazumdar C., et al. 2011. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell 19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Munn D. H., Zhou M., Attwood J. T., et al. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281:1191. [DOI] [PubMed] [Google Scholar]
- 27. Gajewski T. F. 2007. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin. Cancer Res. 13(Pt 1):5256. [DOI] [PubMed] [Google Scholar]
- 28. Yeung O. W., Lo C. M., Ling C. C., et al. 2015. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J. Hepatol. 62:607. [DOI] [PubMed] [Google Scholar]
- 29. Pandiyan P. Zheng L. Ishihara S. Reed J. and Lenardo M. J. 2007. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 8:1353. [DOI] [PubMed] [Google Scholar]
- 30. Read S., Greenwald R., Izcue A., et al. 2006. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 177:4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wing K., Onishi Y., Prieto-Martin P., et al. 2008CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271. [DOI] [PubMed] [Google Scholar]
- 32. Kobie J. J. Shah P. R. Yang L. Rebhahn J. A. Fowell D. J. and Mosmann T. R. 2006. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5’-adenosine monophosphate to adenosine. J. Immunol. 177:6780. [DOI] [PubMed] [Google Scholar]
- 33. Deaglio S., Dwyer K. M., Gao W., et al. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bauer C. A., Kim E. Y., Marangoni F., et al. 2014. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J. Clin. Invest. 124:2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marangoni F., Murooka T. T., Manzo T., et al. 2013. The transcription factor NFAT exhibits signal memory during serial T cell interactions with antigen-presenting cells. Immunity 38:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tadokoro C. E., Shakhar G., Shen S., et al. 2006. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 203:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taube J. M., Anders R. A., Young G. D., et al. 2012. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 4:127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keir M. E. Butte M. J. Freeman G. J. and Sharpe A. H. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wherry E. J. 2011. T cell exhaustion. Nat. Immunol. 12:492. [DOI] [PubMed] [Google Scholar]
- 40. Freeman G. J., Long A. J., Iwai Y., et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baitsch L., Baumgaertner P., Devêvre E., et al. 2011. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121:2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gajewski T. F. Schreiber H. and Fu Y. X. 2013. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spranger S. Koblish H. K. Horton B. Scherle P. A. Newton R. and Gajewski T. F. 2014. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J. Immunother. Cancer 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Postow M. A., Chesney J., Pavlick A. C., et al. 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372:2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Curran M. A. Kim M. Montalvo W. Al-Shamkhani A. and Allison J. P. 2011. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One 6:e19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sakuishi K. Apetoh L. Sullivan J. M. Blazar B. R. Kuchroo V. K. and Anderson A. C. 2010. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207:2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsushita H., Vesely M. D., Koboldt D. C., et al. 2012. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schreiber R. D. Old L. J. and Smyth M. J. 2011. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565. [DOI] [PubMed] [Google Scholar]
- 49. Gubin M. M., Zhang X., Schuster H., et al. 2014. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bosenberg M., Muthusamy V., Curley D. P., et al. 2006. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis 44:262. [DOI] [PubMed] [Google Scholar]
- 51. Damsky W. E., Curley D. P., Santhanakrishnan M., et al. 2011. β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell 20:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dankort D., Curley D. P., Cartlidge R. A., et al. 2009. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 41:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cheung A. F. Dupage M. J. Dong H. K. Chen J. and Jacks T. 2008. Regulated expression of a tumor-associated antigen reveals multiple levels of T-cell tolerance in a mouse model of lung cancer. Cancer Res. 68:9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peng D., Kryczek I., Nagarsheth N., et al. 2015. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmid M. C., Avraamides C. J., Dippold H. C., et al. 2011. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kγ, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 19:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coussens L. M. Zitvogel L. and Palucka A. K. 2013. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 339:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bronte V. and Murray P. J. 2015. Understanding local macrophage phenotypes in disease: modulating macrophage function to treat cancer. Nat. Med. 21:117. [DOI] [PubMed] [Google Scholar]
- 58. Peng W., Chen J. Q., Liu C., et al. 2016. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 6:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xue W., Zender L., Miething C., et al. 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iannello A. Thompson T. W. Ardolino M. Lowe S. W. and Raulet D. H. 2013. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 210:2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Quigley D., Silwal-Pandit L., Dannenfelser R., et al. 2015. Lymphocyte Invasion in IC10/Basal-Like Breast Tumors Is Associated with Wild-Type TP53. Mol. Cancer Res. 13:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bassères D. S. and Baldwin A. S. 2006. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 25:6817. [DOI] [PubMed] [Google Scholar]
- 63. Baldwin A. S. 2012. Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunol. Rev. 246:327. [DOI] [PubMed] [Google Scholar]
- 64. Pikarsky E., Porat R. M., Stein I., et al. 2004. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431:461. [DOI] [PubMed] [Google Scholar]
- 65. Greten F. R., Eckmann L., Greten T. F., et al. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285. [DOI] [PubMed] [Google Scholar]
- 66. Muthuswamy R., Berk E., Junecko B. F., et al. 2012. NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res. 72:3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang T., Niu G., Kortylewski M., et al. 2004. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 10:48. [DOI] [PubMed] [Google Scholar]
- 68. Burdelya L., Kujawski M., Niu G., et al. 2005. Stat3 activity in melanoma cells affects migration of immune effector cells and nitric oxide-mediated antitumor effects. J. Immunol. 174:3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Toso A., Revandkar A., Di Mitri D., et al. 2014. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9:75. [DOI] [PubMed] [Google Scholar]
- 70. Ihara S., Kida H., Arase H., et al. 2012. Inhibitory roles of signal transducer and activator of transcription 3 in antitumor immunity during carcinogen-induced lung tumorigenesis. Cancer Res. 72:2990. [DOI] [PubMed] [Google Scholar]
- 71. Pfirschke C., Engblom C., Rickelt S., et al. 2016. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 44:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu C., Peng W., Xu C., et al. 2013. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin. Cancer Res. 19:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Twyman-Saint Victor C., Rech A. J., Maity A., et al. 2015. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373. [DOI] [PMC free article] [PubMed] [Google Scholar]