Abstract
The American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC) tumor, nodes, metastasis (TNM) classification system based on tumor features is used for prognosis estimation and treatment recommendations in most cancers. However, the clinical outcome can vary significantly among patients within the same tumor stage and TNM classification does not predict response to therapy. Therefore, many efforts have been focused on the identification of new markers. Multiple tumor cell-based approaches have been proposed but very few have been translated into the clinic. The recent demonstration of the essential role of the immune system in tumor progression has allowed great advances in the understanding of this complex disease and in the design of novel therapies. The analysis of the immune infiltrate by imaging techniques in large patient cohorts highlighted the prognostic impact of the in situ immune cell infiltrate in tumors. Moreover, the characterization of the immune infiltrates (e.g. type, density, distribution within the tumor, phenotype, activation status) in patients treated with checkpoint-blockade strategies could provide information to predict the disease outcome. In colorectal cancer, we have developed a prognostic score (‘Immunoscore’) that takes into account the distribution of the density of both CD3+ lymphocytes and CD8+ cytotoxic T cells in the tumor core and the invasive margin that could outperform TNM staging. Currently, an international retrospective study is under way to validate the Immunoscore prognostic performance in patients with colon cancer. The use of Immunoscore in clinical practice could improve the patients’ prognostic assessment and therapeutic management.
Keywords: colorectal cancer, immune response, lymphocyte, T cells, tumor microenvironment
Introduction: from a tumor cell-oriented model to the integration of the tumor microenvironment
The many biological discoveries and the derived concepts that dominated the 20th century led to a strictly cell-based view of cancer (1). The resulting theory of cancer origin, referred to as the somatic mutation theory, defined cancer as a cell disease caused by DNA damage. In this ‘cell-oriented’ model, the cancer cells are autonomous and operate independently from their microenvironment. Advances in the knowledge of cancer molecular biology have gradually revealed the limits of this cell-oriented model (2). Indeed, the extreme complexity of the genome, the diversity and sheer number of genomic alterations observed in cancer cells and genomic instability prevent any structuring vision of cancer (3).
On the basis of these observations, Hanahan and Weinberg (4) proposed, at the end of the second millennium, to modify the cell-oriented model; cancer was then defined by the acquisition of six major behavioral traits (hallmarks) secondary to genomic changes. In 2011, two new features were added to the previous major hallmarks: reprogramming of energy metabolism (the Warburg effect) and immune surveillance escape (5). Beyond the recognition of the essential role of the immune system in tumor initiation and progression, this article marked a conceptual breakthrough. The ‘tumor cell-oriented’ paradigm is replaced by a holistic vision including the tumor environment as a major player in the formation and development of cancer (6).
The microenvironment is composed of a set of cellular compartments comprising vascular, neuroendocrine, stromal, epithelial and immune cells. These compartments constitute a heterogeneous and dynamic set, where all the players communicate with each other directly by cell contact or through secreted molecules (5). The tumor environment definition is likely to evolve further by integrating novel components such as the microbiota (7, 8).
The natural immune response: from prognosis to therapy
Cancer natural history involves interactions between tumor and host defense mechanisms. The concept that the immune system can recognize and eliminate primary developing tumors has been postulated for nearly 100 years (9). The validity of this concept, named ‘cancer immunosurveillance’ (10), has been difficult to establish but is now demonstrated with a considerable amount of data from animal models and human patients (11). This was later integrated into the theory of ‘cancer immunoediting’ that takes into account the interactions between cancer and immune cells, each one influencing and changing the behavior of the other (12). When the tumor elimination is incomplete, a temporary state of equilibrium occurs. The selective pressure exerted by the immune cells induces a selection of tumor cell variants that leads to the escape phase. During the escape phase, the immune system is no longer able to contain tumor growth leading to clinically detectable malignant tumors (11).
Despite the immune escape, human solid tumors are commonly infiltrated by cells from the innate immune system (innate lymphoid cells, NK cells, NK-T cells, γδ T cells, macrophages, neutrophils, eosinophils and mast cells) and adaptive immunity (T lymphocytes, B lymphocytes and dendritic cells). For over 30 years, several publications have evaluated, with an increasing level of precision, the quality, the density and the functional orientation of T-cells infiltrating human tumors. There is now accumulative evidence showing a positive association between the density of intratumoral lymphocyte infiltrates in solid tumors and increased patient survival (13). The studies involved thousands of human solid tumor samples. A beneficial in situ immune reaction is not restricted to patients with minimal tumor invasion, indicating that the in situ immunologic forces may persist along with tumor progression.
This corpus of data provides strong support for the existence of a natural anti-tumor immune response in immunocompetent individuals. Strikingly, this immune response influences the course of the disease despite the apparent insensitivity of the tumor cells at the primary site to the immune attack. The search of the mechanisms involved in T-cell dysfunction has revealed that exhaustion of T cells, originally identified in CD8+ T cells during chronic infection (e.g. by HIV, hepatitis C virus and hepatitis B virus), also occurred in cancer (14). Exhausted T cells overexpress multiple inhibitory receptors including programmed cell death 1 (PD-1), cytotoxic T-lymphocyte antigen 4 (CTLA-4) and others (15). Importantly, antibodies targeting and blocking these inhibitory ‘checkpoint’ molecules have recently been shown to be effective in the treatment of human solid tumors (16). These therapies have begun to revolutionize the current standard cancer treatment in multiple cancer types.
Effective clinical responses have revealed that it is possible to augment the function of endogenous anti-tumor T-cell responses. Thus, CD8+ T cells of the tumor microenvironment might not be terminally dysfunctional and could be reinvigorated (17). Unfortunately, most patients do not experience complete responses and some do not respond at all. Thus, identifying predictive markers of the efficacy to checkpoint blockade strategies is needed. Analysis of pretreatment tumors has recently shown that the in situ natural immune response may be explored to predict and monitor response to checkpoint blockade (18, 19).
Hence, a biological test assessing the tumor immune infiltrate (e.g. type, density, distribution within the tumor and phenotype activation status) could become a central biomarker that is predictive for prognosis and response to (immuno)therapy. In order to satisfy this expectation, a methodology named the ‘Immunoscore’ has been defined to quantify the in situ immune infiltrate. This review aims to summarize (i) the most convincing evidence from cohort studies of the prognostic and predictive roles of the immune infiltrate in cancer patients and (ii) the performance of the Immunoscore and the state of advancement of the international Immunoscore program.
The prognostic value of tumor-infiltrating lymphocytes and the Th1 immune orientation at the tumor site
In 1931, MacCarty observed, by histological analysis of colon cancer sections stained with hematoxylin and eosin (H&E), that a strong intratumoral immune infiltrate conferred an advantage in terms of survival (20). This pioneer observation was later confirmed in colorectal (21, 22), melanoma (23, 24), breast (25) and others cancers. In 1986, Jass (26) demonstrated for the first time that high lymphocyte density evaluated on histological sections in the invasive margin (IM) of rectal tumors was the only variable to be accepted in a multivariate prognostic model along with the tumor, nodes, metastasis (TNM) classification. This observation was of paramount importance, since it revealed that the in situ adaptive immune reaction was a critical variable influencing overall survival times independently of the influence of the tumor extension, challenging our understanding of the natural history of cancer.
The subsequent identification of specific markers and transcriptional profiles allowed the quantification of the immune sub-populations and determination of their functional orientation. It has been demonstrated in a large number of solid tumors (ovarian (27), head and neck (28, 29), bladder (30), breast (31), liver (32), prostate (33, 34), melanoma (35), lung (36, 37), esophagus (38) and colorectal (39–45) cancers) that the presence of T cells expressing CD3 with CD8 or CD4 and memory T cells expressing CD45RO were associated with good prognosis. However, discordant results have been reported in clear-cell renal cell carcinoma (46, 47), Hodgkin lymphoma or uveal melanoma (48).
Th1 cells play a key role by linking innate immunity and adaptive immunity (49). Th1 cells facilitate the development of tumor-specific CD8+ cytotoxic T cells induced via cross-presentation of antigenic tumor peptides on MHC class I molecules presented by dendritic cells (50, 51). Further studies have demonstrated that the Th1 cytokine IFN-γ has antiproliferative and/or proapoptotic effects on tumor cells (52, 53) and the capacity to enhance tumor cell immunogenicity by upregulating components of the MHC class I antigen-processing and antigen-presentation pathway (54, 55). Moreover IFN-γ can promote the production of tumoricidal molecules (e.g. nitric oxide and superoxide) by macrophages (56, 57) and NK cells activated by IFN-γ can kill tumor cells (58). In colorectal cancers, we have observed a correlation between cell densities of intratumoral T cytotoxic CD8+, T memory CD45RO+ and Th1 cells that express the transcription factor T-box transcription factor (T-bet; A. Kirilovsky et al., unpublished data)
Moreover, transcriptomic analyses have confirmed a strong association between the cytotoxic/memory tumor-infiltrating profile and the expression of genes involved in Th1 orientation such as transcription factors T-bet (TBX21), IFN-regulatory factor 1 (IRF1) and signal transducer and activator of transcription 1 (STAT1), cytokines IFN-γ (IFNG) and IL-12 (IL12), chemokines (CXCL9, CXCL10, CCL5, CX3CL1 and CCL2), adhesion molecules (intercellular adhesion molecule 1 (ICAM1), mucosal vascular addressin cell adhesion molecule 1 (MADCAM1) and vascular cell adhesion molecule 1 VCAM1) and the cytotoxic factors granzymes (GZMs), perforin 1 (PRF1) and granulysin (GNLY) (59, 60). Thus, the local presence of IL-12 and IFN-γ, chemoattractants and adhesion molecules could attract T cells with a Th1, cytotoxic and memory profiles at tumor site.
Importantly, Th1 gene signatures were correlated with good prognosis in colorectal cancer (59–63) but also in breast (64, 65), ovarian (27, 66) and melanoma (67) tumors. These observations are in accordance with many mouse models showing that deficiency in genes involved in the Th1-oriented response (e.g. IFN-γ, IFN-γR and IL-12) increased the frequency of spontaneous or carcinogen-induced tumors (11).
Prognostic value of other immune orientations at the tumor site
Data on the impact of Th2 immune orientation in cancers are still controversial. Components of Th2-oriented immunity such as Th2 cytokines (IL-4 and IL-13) and B cells might have dual effects on tumor progression (68–70). Moreover, eosinophils have been shown to decrease tumor growth and initiate anti-tumor activity, (71) and the immunosuppressive cytokine IL-10 is traditionally considered to favor tumor growth (68, 69). The prognosis of Th2 immune orientation was assessed in a small number of studies and results are also contradictory. Thus, in ovarian (72), pancreatic (73) and gastric (74) cancers, Th2 responses are associated with poor prognosis. Conversely, in breast cancer (75) and follicular or Hodgkin lymphoma, (76) there is a beneficial association between immune Th2 infiltrate and survival. Finally, in colorectal cancer, a Th2 gene signature did not correlate with the clinical outcome (63).
Th17 cells are currently recognized as an independent T-cell lineage from Th1 and Th2 (77, 78). Th17 cells producing IL-17 and IFN-γ induce inflammation (79) and can therefore promote inflammation-dependent tumor cell growth. Th17 cells have been detected in several cancers, but the prognostic value associated with this infiltrate varies according to the cancer type. For instance, Th17 cells have been associated with poor prognosis in colorectal (63), lung (80) and hepatocellular (81) carcinoma and with a good prognosis in ovarian (82) and esophageal (83) cancers. Overall, a strong intratumoral Th17 cell infiltration is associated with a slower tumor progression of prostate cancer, (84) whereas the opposite effect is observed in hormone-refractory prostate cancer (85). These opposite results could be related to a plasticity of Th17 cells; those cells having the ability to redifferentiate into suppressive Treg cells or alternatively into Th1-like pro-inflammatory cells capable of activating cytotoxic immune effectors (86, 87).
Finally, Treg cells are the fourth major subset of CD4+ T cells, characterized by the expression of the α chain of the IL-2R (CD25) and the transcription factor Forkhead box P3 (FoxP3) (88). Treg cells can suppress the function of effector T cells and antigen-presenting cells (APCs) by either cell–cell interactions or by the release of TGF-β and IL-10, two suppressive cytokines (89). The prognostic value of Treg cell density at the tumor site is still debated. A pioneering publication by Curiel et al. (90) positively correlated the presence of a high number of FoxP3+ cells in ovarian carcinoma ascites with the degree of tumor extension and reduced survival. This negative prognostic value was also reported in other solid cancers such as pancreatic (91), liver (92) and breast (93) tumors.
Based on these results, it was hypothesized that the presence of natural or induced Treg cells at the tumor site could be a major mechanism of tumor escape from the cytotoxic immune response. Since then, conflicting evidence has been complicating the picture. Thus, a favorable prognosis associated with a high density of FoxP3+ intratumoral cells was reported in follicular and Hodgkin lymphomas (94, 95), head and neck cancers (29) and colorectal cancers (63, 96–98). Additional studies are required because the phenotypic and functional markers currently used to identify Treg cells are not fully specific (99, 100).
Overall, analysis of data from the literature on the prognostic effect of different immune T populations reveals that cytotoxic CD8+ T cells and memory T cells associated with a Th1-oriented immune reaction strongly correlate with good clinical course in most studied cancer types (13, 101). On the other hand, the prognostic value of Th2, Th17 or Treg cell populations is inconsistent and varies depending on the cancer type and stage (13).
Toward a clinical application in solid tumors: the Immunoscore
In colorectal tumors, immune cells are present within the tumor glands, in the surrounding stroma, at a distance within the IM as well as in newly formed tertiary lymphoid islets located in the tumor vicinity (102). As the immune infiltration in tumors is heterogeneous, we hypothesized that the analysis of each tumor region could provide information on the tumor pathophysiology and possibly prognosis. Thus, we measured the density of immune cells and their distribution in the tumor core (CT) and the IM in three independent retrospectives cohort studies of colorectal cancer (n = 609 patients) (61). We performed an in situ quantification of T lymphocytes (CD3+), memory T cells (CD45RO+), cytotoxic T lymphocytes (CD8+) and their cytotoxic molecules (granzyme B) using a dedicated image analysis program after immunostaining with specific antibodies.
We found a significant correlation between the density of immune cell populations in the two tumor regions (CT and IM) and the patients’ clinical outcome, in terms of disease-free survival (DFS) and overall survival (OS). Moreover, when the CT and IM cell densities were considered together, the outcome differences between groups of patients with a high immune cell density in both tumor regions compared with patients with a low immune density in both areas further increased. Unexpectedly, multivariate analysis indicated that the ‘weight’ of the immune density parameter was independent from, and larger than, that provided by the pathology-based prognostic evaluation (i.e. the TNM staging) (61, 103).
Thus, the immune ‘contexture’—defined as the type, functional orientation, density and location of adaptive immune cells within distinct tumor regions (61, 104)—appears to be the strongest prognostic factor for DFS and OS in colorectal cancer. The statistical preponderance of the immune assessment could be explained by the observation that the immune density is inversely correlated with tumor extension (T stage) but is constantly low in patients whose tumors will relapse, even at early stages of tumor progression (103). This information cannot be provided by the simple assessment of the TNM staging. Altogether, these results strongly suggest that tumor behavior should now be considered as the result of a balance between the invasive tumor process and a coordinated immune reaction of the host.
To evaluate the prognostic performance of the immune contexture, we then focused on patients with clinically localized colorectal cancers (stage I–II), among whom 25% will experience a relapse after surgery. We combined the analysis of CD8 and CD45RO staining in tumor regions (CT and IM) in a large retrospective cohort (n = 602 patients) (59). Five years after diagnosis, only 4.8% of patients with a strong infiltrate had relapsed and 13.8% had died. Conversely, 75% of patients with a low immune infiltrate had a relapse and 72.5% had died (log-rank test P < 0.0001).
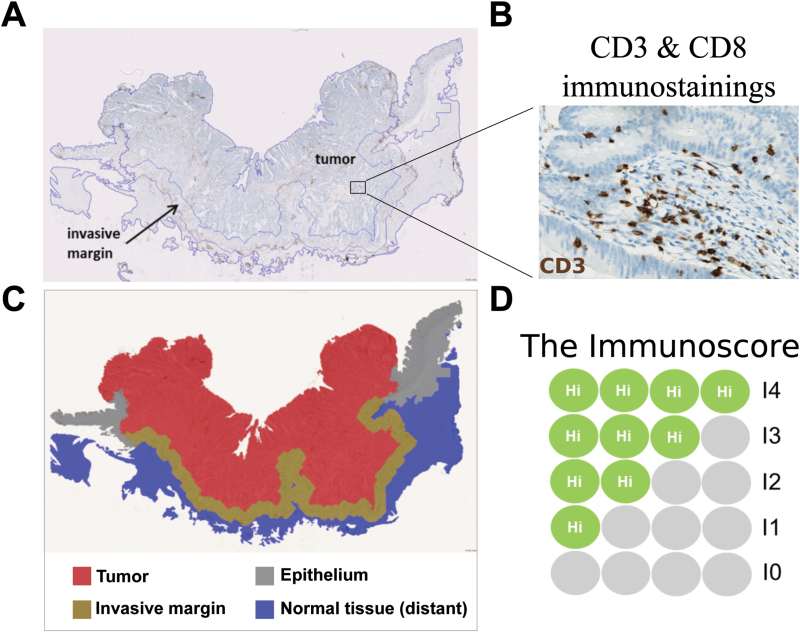
We then derived a simple test named Immunoscore to facilitate the transfer of this discovery to the clinic (http://www.Immunoscore.org; Fig. 1). The Immunoscore is based on the numeration of two lymphocyte populations, CD3+ and CD8+, in the CT and in IM regions. CD45RO+ memory T-cell density is highly overlapping with CD3+ T-cell density and because of background staining and rapid loss of antigenicity after cutting tumor slides, CD45RO was excluded from the final test. The density of CD3+ cells and CD8+ cells is quantified using a dedicated image analysis workstation.
Fig. 1.
(A) A section of colonic cancer immunostained for CD3, showing the regions of interest (the CT and the IM). (B) An enlargement showing CD3+ cells (stained brown) in the stroma and within tumor glands (original magnification ×300). (C) The tumor (shown in red) and the IM (shown in brown) are selected to determine the Immunoscore. (D) The Immunoscore is based on the numeration of CD3+ and of CD8+ cells in the tumor and the IM. The densities of stained cells are determined using an image analysis workstation. The immune densities are categorized into Hi (high) or Lo (low) in each tumor region, according to a predetermined cutoff value. Patients are stratified according to a score ranging from I0 to I4, depending on the total number of high densities observed (the two markers CD3 and CD8 are assessed in the CT and the two markers are assessed in the IM).
Each tumor is categorized into high or low density for each marker in each tumor region, according to an optimal cutoff value determined using the minimum P value approach (61). Patients are stratified according to a score ranging from I0 to I4 (103, 105), depending on the total number of high densities observed (two markers assessed in CT and two markers assessed in IM). For example, I4 refers to a tumor with high densities of CD3+ and CD8+ cells in CT and IM regions of the tumor. I3 refers to tumors with three high densities. Patients with low densities of CD3 and CD8 in both tumor regions (0 high density) is classified I0 (Fig. 1).
We built an immunomonitoring platform at the Hospital Européen Georges Pompidou in Paris to perform the Immunoscore in routine settings on large cohorts and to achieve multiple quality controls and we coordinated an international retrospective study, under the supervision of the Society for Immunotherapy of Cancer (SITC), to validate the prognostic performance of the Immunoscore in patients with colonic cancer (105). The international retrospective study involved 23 centers in 17 countries worldwide. Thousands of colonic tumors have been evaluated on whole slide sections by international expert pathologists and immunologists of each center.
The study is now completed. Analyses are currently being performed by external statisticians according to a predetermined workplan. Results will be presented at the next American Society of Clinical Oncology (ASCO) annual meeting (June 3–7, 2016; Chicago, IL, USA). Rectal tumors were excluded from the Immunoscore SITC study because of distinct clinicopathologic features and treatment regimens. We have conducted an ancillary study to evaluate the prognostic performance of the Immunoscore on localized rectal cancers (106). The Immunoscore classified nearly 50% of the patients with very distinct behaviors: 35% with very a good outcome (score I4) as opposed to 12% with a poor outcome (score I0 or I1). Cox multivariate analysis supported the advantage of the Immunoscore compared with TNM staging in predicting recurrence and survival.
This methodology is still under investigation in several other types of cancer. Thus, in hepatocellular carcinoma, the Immunoscore has been already strongly associated with cancer outcome (107). Even in brain metastasis, one of the most common complications from cancer and which has a very poor prognosis, the Immunoscore was significantly correlated with survival prognosis and was independent from other prognostic parameters at multivariable analysis (108). Hopefully, these initiatives will result in the implementation of the Immunoscore as a new component for the classification of cancer: TNM-I (TNM-immune).
Prediction of the response to chemotherapy and radiotherapy
The innate and adaptive immune responses elicited by anthracyclines, oxaliplatin and ionizing irradiation are required for an optimal response of these anticancer treatments. The main mechanisms involve the release of tumor antigen, ATP and the purinergic receptor and the induction of an immunogenic cell death with the exposure of calreticulin, facilitating the engulfment of dying cells by APCs and the release of high mobility group box 1 (HMGB1) that binds TLR4 and stimulates antigen presentation (109).
In breast cancer, anthracycline-based neoadjuvant therapy is more effective when the tumor is infiltrated by T cells before the beginning of chemotherapy (110). It induces a significant influx of CD8+ T cells into the tumor bed and a decreased density of immunosuppressive cells—Treg cells and monocytic myeloid-derived suppressor cells (MDSCs) (111). A meta-analysis has recently shown that higher values of total tumor-infiltrating lymphocytes (TILs) predicted a better response to neoadjuvant chemotherapy in most breast cancers, except hormone-receptor negative ones (112). Moreover, intratumoral lymphocytes with a concomitant up-regulation of CD3D and CXCL9 were independent predictors of complete response (110). In rectal cancers, we observed a significant correlation between densities of CD3+ and CD8+ cells and the pathological response to neoadjuvant radiochemotherapy (106). Association of CD3+ and CD8+ TILs with good response after neoadjuvant treatments was confirmed in two studies (113, 114).
Prediction of the response to immunotherapy
The clinical response to immunotherapies could be influenced by the quality of the natural immune response observed at the tumor site (Table 1). The data from the first immunotherapy trials with anti-CTLA-4 in melanoma have confirmed this hypothesis. Indeed, patients with tumors expressing a higher level of genes involved in Th1-oriented (IFN-γ) and cytotoxic responses (perforin, granzyme and granulysin) or chemoattraction (CCR5, CCL4, CCL5, CXCR3, CXCL9, CXCL10 and CXCL11) were more likely to respond favorably to ipilimumab (monoclonal anti-CTLA-4 antibody) treatment (18, 118, 119). Moreover, these immune reaction markers were significantly increased in tumors after treatment, and this increase was higher in responding tumors (18, 118). Fully activated CD4+ and CD8+ T cells with evidence of induction/potentiation of a memory phenotype (CD45RO+) were observed following on treatment (115).
Table 1.
Predictive, treatment response and surrogate markers for immune checkpoint inhibitors in cancer immunotherapies
| Checkpoint inhibitor | Method | Marker | Predictive marker(baseline) | Biomarker(treatment response) | Surrogate marker(clinical response) | References |
|---|---|---|---|---|---|---|
| Anti-CTLA-4 | Staining (H&E) | TIL | - | NT | + | (18) |
| Immunohistochemistry | CD8 | - | + | + | (18, 115, 116) | |
| FoxP3 | + | NT | + (inverse) | (18) | ||
| IDO | + | NT | + (inverse) | (18) | ||
| PD-L1 (tumor cells) | NT | NT | + (inverse) | (117) | ||
| Cytometry | Activated CD4/CD8 T cells | NT | + | NT | (115) | |
| Memory CD8 T cells | NT | + | NT | (115) | ||
| MDSCs | NT | NT | + (inverse) | (115) | ||
| Transcriptomics | T cell signature | + | + | + | (18, 118) | |
| Cytotoxicity signature | + | + | + | (18, 118, 119) | ||
| Th1 signature | + | + | + | (18, 118) | ||
| CXCR3/CXCL9–11 Pathway | + | + | + | (18, 118) | ||
| CCR5/CCL3–5 Pathway | + | + | NT | (18, 118) | ||
| MHC-II | + | + | NT | (18, 118) | ||
| CXCR6 | + | + | + | (18, 118) | ||
| CTLA-4/PD-L2 | + | NT | NT | (119) | ||
| Whole-exome sequencing | Mutational load | + | NT | NT | (119) | |
| Neoantigen load | + | NT | NT | (119) | ||
| Anti-PD-1 | Immunohistochemistry | PD-L1 (tumor cells) | + | NT | NT | (120–124) |
| PD-L1 (immune & tumor cells) | + | NT | NT | (19, 120) | ||
| CD8 | + | NT | + | (19) | ||
| CD8 + Ki67 | NT | NT | + | (19) | ||
| Granzyme B | NT | NT | + | (19) | ||
| pSTAT1 (IM) | + | NT | + | (19) | ||
| PD-1 (immune cells) | + | NT | NT | (19) | ||
| CD4 | - | NT | NT | (125) | ||
| MHC-II (tumor cells) | + | NT | NT | (125) | ||
| Cytometry | T cells (CD3+) | NT | + | + | (126) | |
| B cells (CD19+ or CD20+) | NT | + | NT | (126) | ||
| MDSCs | - | + | NT | (126) | ||
| % CD8 Tem | NT | + | + | (126) | ||
| % CD4 Tem | NT | + (inverse) | NT | (126) | ||
| % CD4 T effector T-cell–like | NT | + | + (inverse) | (126) | ||
| Next-generation sequencing | TCR clonality | + (inverse) | NT | + | (19) | |
| Whole-exome sequencing | Mutational load | + | NT | NT | (127) | |
| Neoantigen load | + | NT | NT | (127) | ||
| Anti-PD-L1 | Immunohistochemistry | PD-L1 (immune cells) | + | NT | + | (128, 129) |
| PD-L1 (tumor cells) | - | NT | NT | (128, 129) | ||
| CD8 | - | NT | + | (128) | ||
| Transcriptomics | CX3CL1 (fractalkine) | + (inverse) | NT | NT | (128) | |
| IFN-γ | NT | NT | NT | (128) | ||
| CTLA-4 | + | NT | + | (128) | ||
| IDO1 | + | NT | NT | (128) | ||
| CXCL9 | + | NT | NT | (128) | ||
| CXCL10 | NT | NT | + | (128) | ||
| Cytotoxicity signature | NT | NT | + | (128) | ||
| EOMES | NT | NT | + | (128) |
Association between markers and events are depicted as follows:
+ is a significant positive correlation.
+ (inverse) is an inverse correlation between marker presence and event.
- is an absence of significant correlation.
The abbreviations used are: TIL, Tumor infiltrating lymphocytes; FoxP3, Forkhead box P3; IDO, Indoleamine 2,3-dioxygenase; PD-1, Programmed cell death 1; PD-L, Programmed cell death ligand; MDSCs, monocytic myeloid-derived suppressor cells; Th1, T helper cell type 1; MHC-II, Major histocompatibility complex class II; pSTAT, phosphorylated signal transducers and activator of transcription; CTLA-4, Cytotoxic T lymphocyte-associated protein 4; Tem, T effector memory; IFN, interferon; TNF, Tumor necrosis factor, EOMES, eomesodermin; IM, Invasive margin; NT, not tested.
Interestingly, clinical activity was correlated with a higher baseline expression of FoxP3 and indoleamine 2,3-dioxygenase (IDO) proteins in tumors and a decreased expression after treatment in responding tumors (18). In a limited number of regionally advanced melanomas monitored by flow cytometry (115), Treg cell levels tended to be higher at week 6 in the disease progression group, whereas the opposite was observed in the clinical benefit group. And a significant decrease in tumor MDSCs was associated with improved progression-free survival at 1 year.
Tumor expression of PD-1 ligand 1 (PD-L1) in melanoma is associated with the presence of TILs and a strong expression of IFN-γ transcripts, suggesting an adaptive tumor resistance mechanism (130). A recent study (120) on several types of solid cancers including melanoma and non-squamous non–small cell lung carcinoma treated by anti-PD-1 showed that PD-L1 tumoral expression was geographically associated with the presence of an immune infiltration. Furthermore, PD-L1 expression by both the tumor and the immune infiltrate was associated with PD-1 expression on lymphocytes. In this study, these parameters were associated with a good clinical response. However, in another study considering squamous non-small cell lung carcinoma, PD-L1 status was not correlated with a survival increase suggesting the importance of histological types and subtypes and should therefore be taken into account while considering predictive markers (131).
Consistent with these observations, Tumeh et al. (19) recently reported in patients with stage III melanoma treated with anti-PD-1 that the most strongly predictive marker of clinical response was not PD-1 or PD-L1, but the density of CD8+ T cells in the IM as well as in the CT. T-cell clonality in the tumor was also more pronounced in responders. In addition, the density of T CD8+ cells increased during treatment in responders whereas it remained weak in nonresponders (19).
Thus, the existence of a natural T CD8+ cytotoxic immune reaction at the tumor site seems to be a prerequisite necessary to induce/reinvigorate an anti-tumor immune response with anti-PD-1. The presence of T CD8+ cytotoxic cells with tumor cells expressing PD-L1 in a context of IFN-γ production could be the most favorable ground for anti-PD-1 immunotherapies. Recent reports further indicate that the presence of CD8+ T cells expressing PD-L1 could predict a response to anti-PD-L1 treatment, and a global increase of the CD8+ T-cell density was observed after treatment in responding tumors (128, 129).
Conclusion
Despite a partial exhaustion of the anti-tumor immune response, the intratumoral immune reaction is an important parameter influencing the natural course of the disease. The presence of cytotoxic T cells and a Th1 immune reaction in tumor microenvironment is almost constantly associated with an increase in the patient’s survival. In addition, there is increasing evidence supporting the hypothesis that an immune-active tumor microenvironment correlates with improved patient response to immune checkpoint inhibitors.
However, there are unresolved issues regarding measuring levels of the immune infiltrate. The essential parameters defining the immune populations have been grouped according to a concept of contexture that considers immune cell type, the functional orientation, density and location of adaptive immune cells in different tumor regions. For routine evaluation, a simple test called Immunoscore has been established and could improve patients’ care. The result of the international validation of the Immunoscore will be presented shortly at the annual ASCO meeting. The Immunoscore could address difficulties and be sufficiently convenient to use in a clinical setting to provide an accurate prediction of a patient’s prognosis and clinical response.
Funding
The work performed in the Laboratory of Integrative Cancer Immunology and the immunomonitoring platform of the HEGP hospital was supported by grants from the Institut National du Cancer, France (INCa), Assistance Publique-Hôpitaux de Paris, the National PHRC programme IMMUCOL, the Canceropole Ile de France, INSERM and the European Commission (7FP, Geninca Consortium; grant no. 202230), Cancer Research for Personalized Medicine (CARPEM) and the LabEx Immuno-oncology.
Acknowledgements
We acknowledge all the scientists who made contributions to the area of research reviewed here that were not cited due to space constraints. The authors wish to acknowledge the following organizations for supporting the Immunoscore following the WIC meeting: Society for Immunotherapy of Cancer (SITC); the European Academy of Tumour Immunology (EATI); La Fondazione Melanoma Onlus; National Cancer Institute, USA (NCI); Biotherapy Development Association (BDA); Canadian Cancer Immunotherapy Consortium (CCIC); Cancer Immunotherapy Consortium (CIC); Cancer Research Institute (CRI); Association for Cancer Immunotherapy (CIMT); Committee for Tumour Immunology and Biotherapy (TIBT); European Society for Cancer Immunology and Immunotherapy (ESCII); Italian Network for Tumour Biotherapy (NIBIT); Japanese Association of Cancer Immunology (JACI); Nordic Centre for Development of Antitumour Vaccines (NCV-Network); Progress in Vaccination Against Cancer (PIVAC); Adoptive engineered T cell Targeting to Activate Cancer Killing (ATTACK); Tumour Vaccine and Cell Therapy Working Group (TVACT); and Institut National du Cancer, France.
Conflict of interest statement: F.P. and J.G. own patents on the Immunoscore and Immune contexture. The research is found by the company HalioDx which licensed the Immunoscore patent.
References
- 1. Vogelstein B., Fearon E. R., Hamilton S. R., et al. 1988. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319:525. [DOI] [PubMed] [Google Scholar]
- 2. Folkman J. Hahnfeldt P. and Hlatky L. 2000. Cancer: looking outside the genome. Nat. Rev. Mol. Cell Biol. 1:76. [DOI] [PubMed] [Google Scholar]
- 3. Vogelstein B. Papadopoulos N. Velculescu V. E. Zhou S. Diaz L. A. Jr and Kinzler K. W. 2013. Cancer genome landscapes. Science 339:1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanahan D. and Weinberg R. A. 2000. The hallmarks of cancer. Cell 100:57. [DOI] [PubMed] [Google Scholar]
- 5. Hanahan D. and Weinberg R. A. 2011. Hallmarks of cancer: the next generation. Cell 144:646. [DOI] [PubMed] [Google Scholar]
- 6. Sonnenschein C. and Soto A. M. 2011. The death of the cancer cell. Cancer Res. 71:4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maynard C. L. Elson C. O. Hatton R. D. and Weaver C. T. 2012. Reciprocal interactions of the intestinal microbiota and immune system. Nature 489:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vétizou M., Pitt J. M., Daillère R., et al. 2015. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehrlich P. 1909. Über den jetzigen Stand der Karzinomforschung. Ned Tijdschr Geneeskd 5:273. [Google Scholar]
- 10. Burnet F. M. 1970. The concept of immunological surveillance. Prog. Exp. Tumor Res. 13:1. [DOI] [PubMed] [Google Scholar]
- 11. Dunn G. P. Old L. J. and Schreiber R. D. 2004. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22:329. [DOI] [PubMed] [Google Scholar]
- 12. Schreiber R. D. Old L. J. and Smyth M. J. 2011. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565. [DOI] [PubMed] [Google Scholar]
- 13. Fridman W. H. Pagès F. Sautès-Fridman C. and Galon J. 2012. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12:298. [DOI] [PubMed] [Google Scholar]
- 14. Lee P. P., Yee C., Savage P. A., et al. 1999. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5:677. [DOI] [PubMed] [Google Scholar]
- 15. Wherry E. J. 2011. T cell exhaustion. Nat. Immunol. 12:492. [DOI] [PubMed] [Google Scholar]
- 16. Postow M. A. Callahan M. K. and Wolchok J. D. 2015. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 33:1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pauken K. E. and Wherry E. J. 2015. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamid O., Schmidt H., Nissan A., et al. 2011. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J. Transl. Med. 9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tumeh P. C., Harview C. L., Yearley J. H., et al. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacCarty W. C. 1931. Principles of prognosis in cancer. JAMA 96:30. [Google Scholar]
- 21. Svennevig J. L. Lunde O. C. Holter J. and Bjørgsvik D. 1984. Lymphoid infiltration and prognosis in colorectal carcinoma. Br. J. Cancer 49:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. House A. K. and Watt A. G. 1979. Survival and the immune response in patients with carcinoma of the colorectum. Gut 20:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clark W. H., Jr, Elder D. E., Guerry D., 4th, et al. 1989. Model predicting survival in stage I melanoma based on tumor progression. J. Natl Cancer Inst. 81:1893. [DOI] [PubMed] [Google Scholar]
- 24. Clemente C. G. Mihm M. C. Jr Bufalino R. Zurrida S. Collini P. and Cascinelli N. 1996. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77:1303. [DOI] [PubMed] [Google Scholar]
- 25. Aaltomaa S., Lipponen P., Eskelinen M., et al. 1992. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur. J. Cancer 28A:859. [DOI] [PubMed] [Google Scholar]
- 26. Jass J. R. 1986. Lymphocytic infiltration and survival in rectal cancer. J. Clin. Pathol. 39:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L., Conejo-Garcia J. R., Katsaros D., et al. 2003. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348:203. [DOI] [PubMed] [Google Scholar]
- 28. Le Q-T. 2005. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J. Clin. Oncol. 23:8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Badoual C., Hans S., Rodriguez J., et al. 2006. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin. Cancer Res. 12:465. [DOI] [PubMed] [Google Scholar]
- 30. Nakakubo Y., Miyamoto M., Cho Y., et al. 2003. Clinical significance of immune cell infiltration within gallbladder cancer. Br. J. Cancer 89:1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahmoud S. M., Paish E. C., Powe D. G., et al. 2011. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 29:1949. [DOI] [PubMed] [Google Scholar]
- 32. Wada Y. Nakashima O. Kutami R. Yamamoto O. and Kojiro M. 1998. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 27:407. [DOI] [PubMed] [Google Scholar]
- 33. Fukunaga A., Miyamoto M., Cho Y., et al. 2004. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 28:e26. [DOI] [PubMed] [Google Scholar]
- 34. Kärjä V. Aaltomaa S. Lipponen P. Isotalo T. Talja M. and Mokka R. 2005. Tumour-infiltrating lymphocytes: a prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 25:4435. [PubMed] [Google Scholar]
- 35. Piras F., Colombari R., Minerba L., et al. 2005. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 104:1246. [DOI] [PubMed] [Google Scholar]
- 36. Hiraoka K., Miyamoto M., Cho Y., et al. 2006. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br. J. Cancer 94:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dieu-Nosjean M. C., Antoine M., Danel C., et al. 2008. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26:4410. [DOI] [PubMed] [Google Scholar]
- 38. Schumacher K. Haensch W. Röefzaad C. and Schlag P. M. 2001. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 61:3932. [PubMed] [Google Scholar]
- 39. Naito Y., Saito K., Shiiba K., et al. 1998. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 58:3491. [PubMed] [Google Scholar]
- 40. Nagtegaal I. D., Marijnen C. A., Kranenbarg E. K., et al. 2001. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect—a histopathological and immunohistochemical study. BMC Cancer 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Funada Y. Noguchi T. Kikuchi R. Takeno S. Uchida Y. and Gabbert H. E. 2003. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol. Rep. 10:309. [PubMed] [Google Scholar]
- 42. Chiba T., Ohtani H., Mizoi T., et al. 2004. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br. J. Cancer. 91:1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prall F. 2004. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum. Pathol. 35:808. [DOI] [PubMed] [Google Scholar]
- 44. Ali A. A. McMillan D. C. Matalka I. I. McNicol A. M. and McArdle C. S. 2004. Tumour T-lymphocyte subset infiltration and tumour recurrence following curative resection for colorectal cancer. Eur. J. Surg. Oncol. 30:292. [DOI] [PubMed] [Google Scholar]
- 45. Pagès F., Berger A., Camus M., et al. 2005. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353:2654. [DOI] [PubMed] [Google Scholar]
- 46. Nakano O., Sato M., Naito Y., et al. 2001. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 61:5132. [PubMed] [Google Scholar]
- 47. Giraldo N. A., Becht E., Pagès F., et al. 2015. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin. Cancer Res. 21:3031. [DOI] [PubMed] [Google Scholar]
- 48. Becht E. Giraldo N. A. Dieu-Nosjean M. C. Sautès-Fridman C. and Fridman W. H. 2016. Cancer immune contexture and immunotherapy. Curr. Opin. Immunol. 39:7. [DOI] [PubMed] [Google Scholar]
- 49. Abbas A. K. Murphy K. M. and Sher A. 1996. Functional diversity of helper T lymphocytes. Nature 383:787. [DOI] [PubMed] [Google Scholar]
- 50. Albert M. L. Sauter B. and Bhardwaj N. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86. [DOI] [PubMed] [Google Scholar]
- 51. Yu P. Spiotto M. T. Lee Y. Schreiber H. and Fu Y. X. 2003. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J. Exp. Med. 197:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bromberg J. F. Horvath C. M. Wen Z. Schreiber R. D. and Darnell J. E. Jr. 1996. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc. Natl Acad. Sci. USA 93:7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chin Y. E. Kitagawa M. Kuida K. Flavell R. A. and Fu X. Y. 1997. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol. Cell. Biol. 17:5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pfizenmaier K., Bartsch H., Scheurich P., et al. 1985. Differential gamma-interferon response of human colon carcinoma cells: inhibition of proliferation and modulation of immunogenicity as independent effects of gamma-interferon on tumor cell growth. Cancer Res. 45:3503. [PubMed] [Google Scholar]
- 55. Shankaran V., Ikeda H., Bruce A. T., et al. 2001. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410:1107. [DOI] [PubMed] [Google Scholar]
- 56. Nathan C. F. Murray H. W. Wiebe M. E. and Rubin B. Y. 1983. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hung K. Hayashi R. Lafond-Walker A. Lowenstein C. Pardoll D. and Levitsky H. 1998. The central role of CD4(+) T cells in the antitumor immune response. J. Exp. Med. 188:2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smyth M. J., Cretney E., Takeda K., et al. 2001. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J. Exp. Med. 193:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pagès F., Kirilovsky A., Mlecnik B., et al. 2009. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 27:5944. [DOI] [PubMed] [Google Scholar]
- 60. Mlecnik B., Tosolini M., Charoentong P., et al. 2010. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology 138:1429. [DOI] [PubMed] [Google Scholar]
- 61. Galon J., Costes A., Sanchez-Cabo F., et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960. [DOI] [PubMed] [Google Scholar]
- 62. Camus M., Tosolini M., Mlecnik B., et al. 2009. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 69:2685. [DOI] [PubMed] [Google Scholar]
- 63. Tosolini M., Kirilovsky A., Mlecnik B., et al. 2011. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 71:1263. [DOI] [PubMed] [Google Scholar]
- 64. Ascierto M. L., Kmieciak M., Idowu M. O., et al. 2012. A signature of immune function genes associated with recurrence-free survival in breast cancer patients. Breast Cancer Res. Treat. 131:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Curtis C., Shah S. P., Chin S-F., et al. 2012. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Leffers N., Fehrmann R. S., Gooden M. J., et al. 2010. Identification of genes and pathways associated with cytotoxic T lymphocyte infiltration of serous ovarian cancer. Br. J. Cancer 103:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mann G. J., Pupo G. M., Campain A. E., et al. 2013. BRAF mutation, NRAS mutation, and the absence of an immune-related expressed gene profile predict poor outcome in patients with stage III melanoma. J. Invest. Dermatol. 133:509. [DOI] [PubMed] [Google Scholar]
- 68. Ellyard J. I. Simson L. and Parish C. R. 2007. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens 70:1. [DOI] [PubMed] [Google Scholar]
- 69. Li Z. Chen L. and Qin Z. 2009. Paradoxical roles of IL-4 in tumor immunity. Cell. Mol. Immunol. 6:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Germain C. Gnjatic S. and Dieu-Nosjean M. C. 2015. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front. Immunol. 6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tepper R. I. Coffman R. L. and Leder P. 1992. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257:548. [DOI] [PubMed] [Google Scholar]
- 72. Kusuda T. Shigemasa K. Arihiro K. Fujii T. Nagai N. and Ohama K. 2005. Relative expression levels of Th1 and Th2 cytokine mRNA are independent prognostic factors in patients with ovarian cancer. Oncol. Rep. 13:1153. [PubMed] [Google Scholar]
- 73. De Monte L., Reni M., Tassi E., et al. 2011. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 208:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ubukata H. Motohashi G. Tabuchi T. Nagata H. Konishi S. and Tabuchi T. 2010. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J. Surg. Oncol. 102:742. [DOI] [PubMed] [Google Scholar]
- 75. Yoon N. K., Maresh E. L., Shen D., et al. 2010. Higher levels of GATA3 predict better survival in women with breast cancer. Hum. Pathol. 41:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schreck S., Friebel D., Buettner M., et al. 2009. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol. Oncol. 27:31. [DOI] [PubMed] [Google Scholar]
- 77. Harrington L. E., Hatton R. D., Mangan P. R., et al. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123. [DOI] [PubMed] [Google Scholar]
- 78. Park H., Li Z., Yang X. O., et al. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ouyang W. Kolls J. K. and Zheng Y. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen X., Wan J., Liu J., et al. 2010. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer 69:348. [DOI] [PubMed] [Google Scholar]
- 81. Liao R., Sun J., Wu H., et al. 2013. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 32:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kryczek I., Banerjee M., Cheng P., et al. 2009. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lv L., Pan K., Li X. D., et al. 2011. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One 6:e18219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sfanos K. S., Bruno T. C., Maris C. H., et al. 2008. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res. 14:3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Derhovanessian E., Adams V., Hähnel K., et al. 2009. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int. J. Cancer 125:1372. [DOI] [PubMed] [Google Scholar]
- 86. Mucida D., Park Y., Kim G., et al. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317:256. [DOI] [PubMed] [Google Scholar]
- 87. Basu R. Hatton R. D. and Weaver C. T. 2013. The Th17 family: flexibility follows function. Immunol. Rev. 252:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Josefowicz S. Z. and Rudensky A. 2009. Control of regulatory T cell lineage commitment and maintenance. Immunity 30:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sakaguchi S. Miyara M. Costantino C. M. and Hafler D. A. 2010. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10:490. [DOI] [PubMed] [Google Scholar]
- 90. Curiel T. J., Coukos G., Zou L., et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942. [DOI] [PubMed] [Google Scholar]
- 91. Hiraoka N. Onozato K. Kosuge T. and Hirohashi S. 2006. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res. 12:5423. [DOI] [PubMed] [Google Scholar]
- 92. Fu J., Xu D., Liu Z., et al. 2007. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132:2328. [DOI] [PubMed] [Google Scholar]
- 93. Merlo A., Casalini P., Carcangiu M. L., et al. 2009. FOXP3 expression and overall survival in breast cancer. J. Clin. Oncol. 27:1746. [DOI] [PubMed] [Google Scholar]
- 94. Carreras J., Lopez-Guillermo A., Fox B. C., et al. 2006. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 108:2957. [DOI] [PubMed] [Google Scholar]
- 95. Tzankov A. Meier C. Hirschmann P. Went P. Pileri S. A. and Dirnhofer S. 2008. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 93:193. [DOI] [PubMed] [Google Scholar]
- 96. Le Gouvello S., Bastuji-Garin S., Aloulou N., et al. 2008. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut 57:772. [DOI] [PubMed] [Google Scholar]
- 97. Michel S., Benner A., Tariverdian M., et al. 2008. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br. J. Cancer 99:1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Salama P., Phillips M., Grieu F., et al. 2009. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 27:186. [DOI] [PubMed] [Google Scholar]
- 99. Miyara M., Yoshioka Y., Kitoh A., et al. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899. [DOI] [PubMed] [Google Scholar]
- 100. Miyara M., Chader D., Sage E., et al. 2015. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc. Natl Acad. Sci. USA 112:7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pagès F. Galon J. Dieu-Nosjean M. C. Tartour E. Sautès-Fridman C. and Fridman W. H. 2010. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29:1093. [DOI] [PubMed] [Google Scholar]
- 102. Bindea G., Mlecnik B., Tosolini M., et al. 2013. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39:782. [DOI] [PubMed] [Google Scholar]
- 103. Mlecnik B., Tosolini M., Kirilovsky A., et al. 2011. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 29:610. [DOI] [PubMed] [Google Scholar]
- 104. Galon J. Angell H. K. Bedognetti D. and Marincola F. M. 2013. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39:11. [DOI] [PubMed] [Google Scholar]
- 105. Galon J., Pagès F., Marincola F. M., et al. 2012. Cancer classification using the Immunoscore: a worldwide task force. J. Transl. Med. 10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Anitei M. G., Zeitoun G., Mlecnik B., et al. 2014. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin. Cancer Res. 20:1891. [DOI] [PubMed] [Google Scholar]
- 107. Sun C., Xu J., Song J., et al. 2015. The predictive value of centre tumour CD8+ T cells in patients with hepatocellular carcinoma: comparison with Immunoscore. Oncotarget 6:35602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Berghoff A. S., Fuchs E., Ricken G., et al. 2016. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 5:e1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kroemer G. Galluzzi L. Kepp O. and Zitvogel L. 2013. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31:51. [DOI] [PubMed] [Google Scholar]
- 110. Denkert C., Loibl S., Noske A., et al. 2010. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 28:105. [DOI] [PubMed] [Google Scholar]
- 111. Ladoire S., Mignot G., Dabakuyo S., et al. 2011. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J. Pathol. 224:389. [DOI] [PubMed] [Google Scholar]
- 112. Yu X. Zhang Z. Wang Z. Wu P. Qiu F. and Huang J. 2016. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin. Transl. Oncol. 18:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Teng F., Mu D., Meng X., et al. 2015. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am. J. Cancer Res. 5:2064. [PMC free article] [PubMed] [Google Scholar]
- 114. Teng F., Meng X., Kong L., et al. 2015. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl. Res. J. Lab. Clin. Med. 166:721. [DOI] [PubMed] [Google Scholar]
- 115. Tarhini A. A., Edington H., Butterfield L. H., et al. 2014. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 9:e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Klein O., Ebert L. M., Nicholaou T., et al. 2009. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin. Cancer Res. 15:2507. [DOI] [PubMed] [Google Scholar]
- 117. Twyman-Saint Victor C., Rech A. J., Maity A., et al. 2015. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ji R. R., Chasalow S. D., Wang L., et al. 2012. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol. Immunother. 61:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Van Allen E. M., Miao D., Schilling B., et al. 2015. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Taube J. M., Klein A., Brahmer J. R., et al. 2014. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20:5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Brahmer J. R., Drake C. G., Wollner I., et al. 2010. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28:3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Topalian S. L., Hodi F. S., Brahmer J. R., et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Garon E. B., Rizvi N. A., Hui R., et al. 2015. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 372:2018. [DOI] [PubMed] [Google Scholar]
- 124. Borghaei H., Paz-Ares L., Horn L., et al. 2015. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Johnson D. B., Estrada M. V., Salgado R., et al. 2016. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat. Commun. 7:10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ribas A., Shin D. S., Zaretsky J., et al. 2016. PD-1 blockade expands intratumoral T memory cells. Cancer Immunol. Res. 4:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rizvi N. A., Hellmann M. D., Snyder A., et al. 2015. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 348:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Herbst R. S., Soria J. C., Kowanetz M., et al. 2014. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Powles T., Eder J. P., Fine G. D., et al. 2014. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558. [DOI] [PubMed] [Google Scholar]
- 130. Taube J. M., Anders R. A., Young G. D., et al. 2012. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 4:127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Brahmer J., Reckamp K. L., Baas P., et al. 2015. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373:123. [DOI] [PMC free article] [PubMed] [Google Scholar]