Abstract
CD4+ regulatory T cells (Tregs) expressing the transcription factor FoxP3 are highly immune suppressive and play central roles in the maintenance of self-tolerance and immune homeostasis, yet in malignant tumors they promote tumor progression by suppressing effective antitumor immunity. Indeed, higher infiltration by Tregs is observed in tumor tissues, and their depletion augments antitumor immune responses in animal models. Additionally, increased numbers of Tregs and, in particular, decreased ratios of CD8+ T cells to Tregs among tumor-infiltrating lymphocytes are correlated with poor prognosis in various types of human cancers. The recent success of cancer immunotherapy represented by immune checkpoint blockade has provided a new insight in cancer treatment, yet more than half of the treated patients did not experience clinical benefits. Identifying biomarkers that predict clinical responses and developing novel immunotherapies are therefore urgently required. Cancer patients whose tumors contain a large number of neoantigens stemming from gene mutations, which have not been previously recognized by the immune system, provoke strong antitumor T-cell responses associated with clinical responses following immune checkpoint blockade, depending on the resistance to Treg-mediated suppression. Thus, integration of a strategy restricting Treg-mediated immune suppression may expand the therapeutic spectrum of cancer immunotherapy towards patients with a lower number of neoantigens. In this review, we address the current understanding of Treg-mediated immune suppressive mechanisms in cancer, the involvement of Tregs in cancer immunotherapy, and strategies for effective and tolerable Treg-targeted therapy.
Keywords: immune suppression, immune checkpoint inhibitors, Treg-targeted therapy
Introduction
CD4+ regulatory T cells (Tregs) are a highly immune suppressive subset of CD4+ T cells, characterized by expression of the master regulatory transcription factor FoxP3 (1–3). Tregs were originally identified as CD4+CD25+ T cells by Sakaguchi et al. (4) and are proven to play central roles in the maintenance of self-tolerance in healthy individuals (5–9). Treg deficiency due to mutations in the FOXP3 gene results in fatal autoimmune disorders and allergy in both mice and humans (5–7). Tregs are therefore involved in maintaining immune homeostasis: they protect hosts from developing autoimmune diseases and allergy, whereas in malignancies, they promote tumor progression by suppressing effective antitumor immunity (8, 9).
Cancer cells harboring inherent genetic instability form new antigens (so-called neoantigens), which have not been previously recognized by the immune system. To avoid immune surveillance targeting immunogenic cancer antigens including neoantigens, cancers acquire resistance and escape machineries against the immune system by selecting less-immunogenic cells, and establishing an immunosuppressive environment using immunosuppressive elements to become clinically apparent ‘cancers’. In cancer tissues, immune suppressive cytokines, molecules and cells including Tregs constitute the immunosuppressive network to inhibit effective antitumor immunity, thereby promoting cancer progression (10, 11).
Cancer immunotherapy represented by blockade of immune checkpoint molecules such as CTLA-4 and PD-1 has provided remarkable clinical efficacy across multiple cancer types even in patients with advanced cancers (12–27). Long-term follow-up in a pooled meta-analysis of 1861 melanoma patients receiving the anti-CTLA-4 antibody, ipilimumab, in phase II or III trials revealed prolonged survival in approximately 20 percent, in some cases extending to 10 years (28). The cohort of the phase I clinical trial for the anti-PD-1 antibody, nivolumab, in heavily pretreated solid cancers showed overall survival of 9.9, 22.4 and 16.8 months in melanoma, non-small cell lung cancer and renal cell carcinoma, respectively (14).
However, accumulating data have uncovered that these durable responses are only observed in approximately 20–30% of the treated patients (28), indicating the importance of identifying biomarkers to predict clinical responses in addition to developing novel cancer immunotherapies. Clinical efficacy after immune checkpoint blockade is reportedly associated with the somatic mutational burden in the tumor cells (29–32); that is, clinical benefit is limited to those whose cancer cells harbor mutation-derived neoantigens (not present in normal cells) being recognized as ‘non-self’ by the immune system (33, 34). Tregs engaged in self-tolerance favorably control the activation of T cell responses to cancer antigens that are derived from self-constituents (so-called shared antigens), but are less suppressive to T cells recognizing foreign antigens (35). Therefore, it is anticipated that integration of approaches reducing the suppressive activity and/or number of Tregs with approaches blocking immune checkpoint molecules, can broaden the therapeutic spectrum of cancer immunotherapy to cancer patients who have a lower number of neoantigens.
Here, we will review the current understanding of Treg-mediated immune suppressive mechanisms in cancer, the involvement of Tregs in cancer immune therapy, and future therapeutic strategies targeting Tregs.
Natural and induced Tregs
Tregs are separated into natural/thymic and peripherally induced Tregs on the basis of the sites in which they are generated (8, 36). Although not fully clarified in humans, natural/thymic Tregs stem from self-reactive thymocytes present in the thymus (8). A fraction of CD4+CD8– thymocytes receive TCR stimulation by complexes of MHC plus self-peptide and acquire expression of CD25, through which IL-2 transmits signals via STAT5 to express FoxP3, resulting in differentiation into Tregs (37–39). Natural/thymic Tregs reportedly express high levels of Helios (a member of the Ikaros transcription factor family) and Neuropilin-1(a type-1 transmembrane protein). In contrast, Tregs that develop in the periphery often lack or have a low level expression of these molecules.
According to data from animal models, these peripherally induced Tregs are readily converted from conventional T cells by in vitro stimulation with TGF-β or retinoic acid (40). However, in humans, FoxP3+ T cells induced from conventional T cells by in vitro TCR stimulation with TGF-β fail to gain suppressive function and rather produce pro-inflammatory cytokines (41, 42). At present, the function of peripherally induced Tregs such as TGF-β-induced ones in humans is obscure though there are some reports showing that several cytokines or a specific microbiota environment can induce Tregs with a suppressive function from CD4+CD25– T cells (43, 44). Yet it remains to be determined whether these peripherally induced FoxP3+ Tregs are functionally stable in vivo. Therefore, in this review, the Tregs we will refer to are natural/thymic Tregs unless otherwise specified.
Identification and functional classification of human Tregs
FoxP3 is the master regulatory molecule in Tregs, and expression of FoxP3 represents the Treg population in mice. In contrast, to define Tregs definitely in humans causes difficulty due to the upregulation of FoxP3 following activation of naive T cells (42). As CD25 is an activation marker and its expression is not confined to Tregs, additional markers are needed. Although CD4+CD25+ T cells with additional low level expression of CD127 (the α-chain of the IL-7 receptor) were reported to possess FoxP3 expression and suppressive function (45, 46), CD127 is also down-regulated following recent activation of naive T cells that also express a low level of FoxP3 (47), suggesting possible contamination of non-Tregs in the CD127lowCD4+CD25+ T-cell fraction.
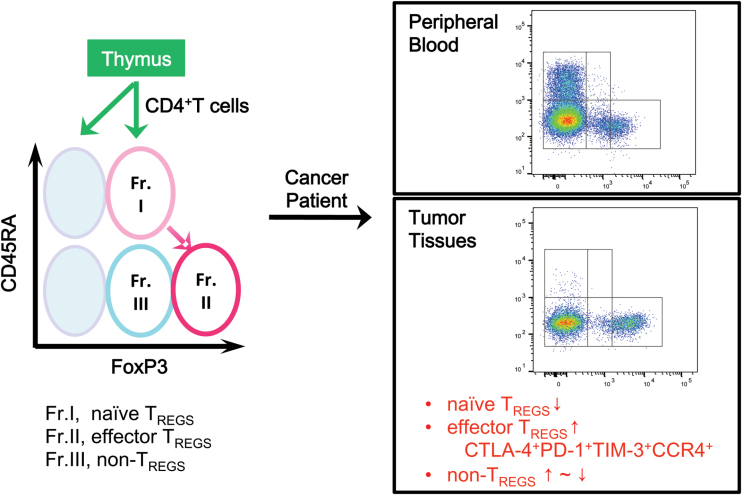
We have therefore proposed a classification of human Tregs based on expression levels of CD45RA and FoxP3 (Fig. 1; Table 1) (8, 11, 48). FoxP3+CD4+ T cells can thus be divided into three fractions: naive Tregs (nTregs: CD45RA+FoxP3lowCD4+); effector Tregs (eTregs: CD45RA–FoxP3highCD4+); and non-Tregs (CD45RA–FoxP3lowCD4+). The nTregs have recently egressed from the thymus, have not yet been activated in the periphery and possess weak suppressive activity. Upon activation with TCR stimulation, nTregs vigorously proliferate and differentiate into highly suppressive eTregs. In contrast, non-Tregs are not immune suppressive but are rather immune stimulatory T cells, producing inflammatory cytokines including IFN-γ and IL-17 (48).
Fig. 1.
Identification of human Tregs. Human Tregs are classified into naive and effector Tregs by the expression levels of a naive marker CD45RA and of FoxP3. In TMEs compared with blood, naive Treg (fraction I, Fr. I) numbers are reduced and highly suppressive effector Treg (fraction II) numbers are increased, expressing CTLA-4, PD-1, TIM-3 and CCR4. The frequency of FoxP3+ non-Treg cells (fraction III) is variable depending on cancer types.
Table 1.
Classification of FoxP3+CD4+ T cells
| Cell subset | Phenotype/cytokines | Characteristics |
|---|---|---|
| Naive Tregs (nTregs): fraction I, resting Tregs CD45RA+FoxP3lowCD4+ |
CTLA-4lowCD25high
CD127low/–Ki-67– |
Weak suppressive activity Differentiate to effector Tregs upon TCR stimulation |
| Effector Tregs (eTregs): fraction II, activated Tregs CD45RA–FoxP3highCD4+ |
CTLA-4highCD25high
Ki-67+, PD-1+, TIM-3+, GITR+, Fas+, IL-10+, TGF-β+ |
Strong suppressive and proliferative activity Prone to apoptosis Tend to increase in peripheral blood with aging |
|
Non-Tregs: fraction III CD45RA–FoxP3lowCD4+ |
IL-2+, IFN-γ+, IL-17+ | Heterogeneous population No suppressive activity |
This classification, based on Treg function, reflects the pathophysiology of autoimmune and inflammatory diseases. Both sarcoidosis patients lacking tuberculin reaction because of an immune suppressive state and systemic lupus erythematosus (SLE) patients with systemic auto-immunity have increased FoxP3+CD4+ T cells in the peripheral blood (48). In our classification with CD45RA and FoxP3 expression, highly suppressive eTregs (CD45RA–FoxP3highCD4+) are the dominant component of FoxP3+CD4+ T cells in the former, whereas FoxP3+ non-Tregs (CD45RA–FoxP3lowCD4+) are increased in the latter (48), clearly demonstrating the clinical state of these patients—an immune suppressive state and a dysregulation of self-tolerance in sarcoidosis and SLE, respectively.
Suppressive mechanisms of Tregs
Tregs exhibit their suppressive activity by numerous cellular and humoral mechanisms (summarized in Table 2) such as suppression of antigen-presenting cells via CTLA-4, secretion of inhibitory cytokines (IL-10, TGF-β and IL-35), expression of granzyme/perforin, consumption of IL-2, and degradation of ATP (reviewed in [8]).
Table 2.
Treg-mediated suppressive mechanisms
| Moleculesa | Ligands | Function |
|---|---|---|
| Contact-dependent suppression | ||
| CTLA-4 | B7-1/B7-2 | Blockade of B7–CD28 costimulatory signals by binding to B7 with greater avidity Inhibition of maturation of antigen-presenting cells (APCs) by physical transfer of B7 on/ in Tregs or transmitting reverse signals to induce IDO in APCs |
| ? | ? | Rendering self-antigen-specific CD8+ T cells to a stable anergic state expressing CCR7 and CTLA-4 |
| CD39, CD73 | A2A
receptor |
Conversion of ATP, an inflammatory molecule and a danger signal, to inhibitory adenosine by CD39/CD73 |
| Granzyme, perforin | Not applicable | Direct cytotoxicity against effector cells |
| Cytokine-mediated suppression | ||
| CD25 (IL-2 receptor α-chain) | IL-2 | Inhibition of differentiation to effector cells by consuming IL-2 |
| TGF-β, IL-10,IL-35 | Not applicable | Inhibition of effector T cells, macrophages, cancer-associated fibroblasts, etc. |
aThe major mechanisms are mediated by CTLA-4 and by CD25.
Among these mechanisms, suppression via CTLA-4 (a co-inhibitory receptor constitutively expressed by Tregs) and IL-2 consumption via CD25 (the IL-2 receptor α-chain, also constitutively expressed by Tregs) appear to play key roles for the following reasons: Treg-specific CTLA-4 deficiency impairs in vitro and in vivo Treg-mediated suppression (49); FoxP3 directly suppresses IL-2 gene transcription and up-regulates CTLA4 and IL2RA (which encodes CD25) gene transcription (2); and high-dose IL-2 neutralizes in vitro Treg-mediated suppression (50, 51). CTLA-4 engages with B7 molecules (i.e. B7-1 and B7-2; CD80 and CD86) on antigen-presenting cells with greater avidity compared with CD28 (52) and provides inhibitory reverse signaling to antigen-presenting cells. In addition, B7 molecules are physically transferred to the surface or the inside of Tregs together with CTLA-4 (52). Then, maturation of antigen-presenting cells (via the co-stimulatory signal from B7 to CD28 on effector cells) is strongly blocked.
Tregs suppress effective antitumor immune responses
In animal models
The involvement of Tregs in tumor immunity was originally reported in 1999 (10, 53). Mice treated with anti-CD25 antibody (which depleted the CD4+CD25+ Tregs) and nude (T cell deficient) mice that were given splenocytes that had been treated with anti-CD25, exhibited tumor rejection and retardation of tumor growth, and interestingly the latter mice simultaneously exhibited autoimmunity in the stomach and the thyroid (10). Another study showed that intra-tumoral injection of anti-CD4 antibody in tumor-bearing mice caused rejection of late-stage tumors by depleting Tregs and altering the cytokine milieu in the tumor microenvironment (TME) (54). In addition, concomitant tumor immunity, which is a phenomenon that tumor-bearing mice can reject the same tumor cells when inoculated at a distant site, is also suppressed by Tregs; mice bearing a poorly immunogenic B16 melanoma, in which concomitant tumor immunity is not evoked, rejected a secondary B16 melanoma challenge when Tregs were depleted by anti-CD4 antibody (55). Taken together, Tregs suppress anti-tumor immunity and promote tumor progression.
In humans
In the TME in melanoma, non-small cell lung, gastric and ovarian cancers, eTregs heavily infiltrate and account for 20–50% of CD4+ T cells, as compared with 5 to 10 percent in the peripheral blood of healthy individuals (8, 11). High infiltration of Tregs in tumors is associated with a poor prognosis in various types of cancers including melanoma, non-small cell lung, gastric, hepatocellular, pancreatic, renal cell, breast and cervical cancers (11, 56). In ovarian cancer, a decreased ratio of CD8+ T cells to Tregs in tumors is related to poor prognosis (57), indicating suppression of effector CD8+ T cells by Tregs. Yet in some cancers such as colorectal, head and neck, and bladder cancers, a higher infiltration of FoxP3+ T cells is reportedly correlated with better prognosis (56).
In fact, in colorectal cancer we have recently shown that FoxP3+ non-Tregs heavily infiltrated a fraction of colorectal cancers containing high levels of inflammatory cytokines such as TGF-β and IL-12 and were associated with a better prognosis (58). The difficulty of distinguishing FoxP3+ non-Tregs from FoxP3high eTregs in tumor tissues would have been a major confounding factor in previous studies evaluating the clinical significance of FOXP3+CD4+ T cells in colorectal cancers using immunohistochemistry. Therefore, although in some cancers controversies do exist regarding the significance of Tregs, Treg-infiltration into a tumor suppresses anti-tumor immunity and generally corresponds to poor prognosis.
Trafficking and characteristics of Tregs in cancer
How and why are activated Tregs present in high numbers in tumor sites? Tregs appear to chemo-attracted to the TME (summarized in Table 3). Although the combination of chemokines and their receptors differs in each cancer—i.e. CCR4 with CCL22 in breast cancer (59); CCR4 with undefined chemokines in colorectal (60) and oral squamous cancers (61) and in Hodgkin lymphoma (62); CCR4 with CCL22, CCR10 with CCL28 and CXCR4 with CXCL12 in ovarian cancer (63–65); and CCR5 with CCL5 in pancreatic cancer (66)—blockade of chemotaxis by antibodies or small molecules may result in a reduction in Treg numbers in tumors (66, 67).
Table 3.
Chemokines and chemokine receptors related to Treg trafficking
| Cancer | Chemokine receptor on Tregs | Chemokine | Origin of chemokines | Ref |
|---|---|---|---|---|
| Human | ||||
| Breast | CCR4 | CCL22 | Tumor cells | (59) |
| Cervical | ND | CXCL12 | Tumor cells | (68) |
| Colorectal | CCR4 | ND | ND | (60) |
| Oral squamous | CCR4 | ND | ND | (61) |
| Ovarian | CCR4 | CCL22 | TAMs | (63) |
| CCR10 | CCL28 | Tumor cells | (64) | |
| CXCR3 | ND | Tumor | (69) | |
| CXCR4 | CXCL12 | Tumor | (65) | |
| Pancreatic | CCR5 | CCL5 | Tumor cells | (66) |
| Hodgkin lymphoma | CCR4 | ND | ND | (62) |
| Mouse | ||||
| Colorectal | CCR6 | CCL20 | TAMs | (70) |
| Melanoma | CCR4 | CCL22 | Tumor | (67) |
| CCR5 | CCL3,4,5 | MDSCs | (71) | |
| Pancreatic | CCR5 | CCL5 | Tumor cells | (66) |
MDSCs, myeloid-derived suppressor cells; ND, not described; TAMs, tumor-associated macrophages.
These Treg-recruiting chemokines are generated in TMEs by macrophages and/or tumor cells. Hypoxia is also reported to induce CCL28 production by ovarian cancer cells and to recruit Tregs (64). Additionally, activated CD8+ T cells infiltrating into the tumor stimulate production of the Treg-recruiting chemokine CCL22 by tumor cells (67). Moreover, in a mouse model with a xenograft of human melanoma, infiltration by Tregs was decreased in the tumor if Tregs were transferred alone compared with tumors where Tregs and CD8+ T cells were co-transferred, suggesting that initial CD8+ T-cell infiltration stimulates CCL22 production by tumors as an escape mechanism (67).
In the TME, highly immune suppressive eTregs with high-level expression of suppression-related molecules such as CTLA-4 and TIGIT are detected with reduced number of nTregs, indicating a highly activated status of tumor-infiltrating Tregs (11, 72). In breast cancer, RANKL-expressing Tregs are reported to promote metastasis of RANK-expressing cancer cells (73) (Fig. 2). One possible mechanism of Treg activation in tumors is that proliferating and dying tumor cells provide a large amount of self-antigens, which Tregs might recognize and be activated by as tumors contains a subset of immature dendritic cells that promote the proliferation/stimulation of Tregs in a TGF-β-dependent manner (74, 75).
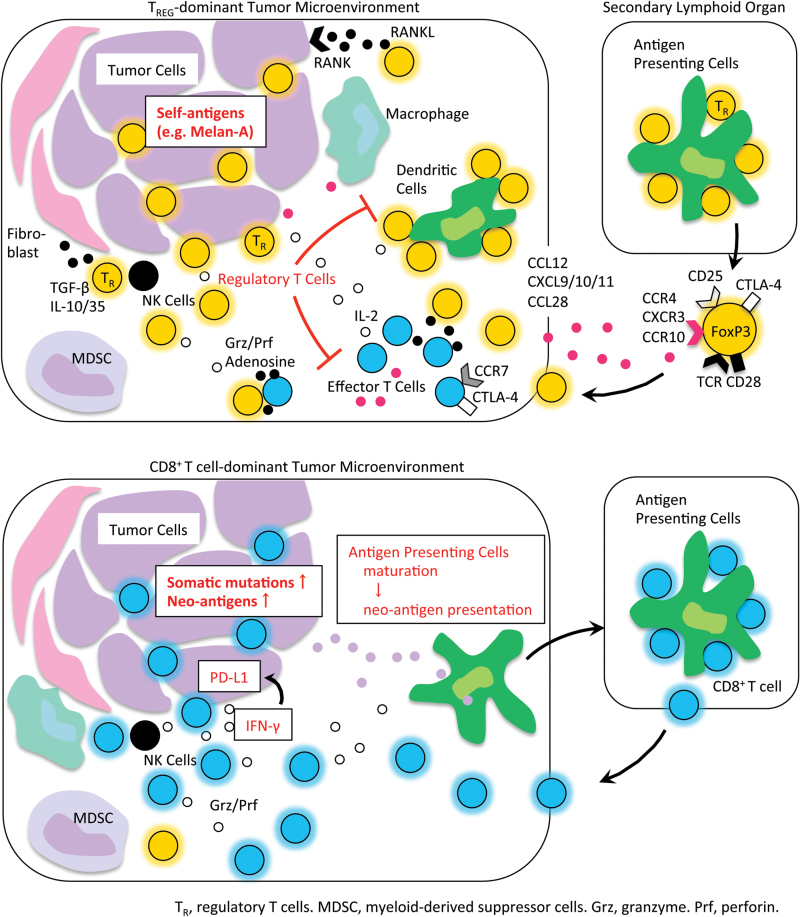
Fig. 2.
Tregs in cancer immunity. In cancer patients with minimal neoantigens (top part of the figure), Tregs appear to be primed at the secondary lymphoid organs and traffic to the TME by chemotaxis. Tregs suppress effective antitumor immunity and/or contribute to tumor progression and metastasis. In contrast, in cancer patients with abundant neoantigens (bottom part of the figure), effector cells including CD8+ T cells are primed and expanded; while they are suppressed in local tumor sites by the immune suppressive network and chronic exposure to cancer antigens in tumors, they are yet on stand-by for tumor killing upon re-stimulation with inhibition of the immune suppressive network, particularly PD-1 signaling. Grz, granzyme; MDSC, myeloid-derived suppressor cell; Prf, perforin; TR, regulatory T cell.
In accordance with this, the TCR repertoire of tumor-infiltrating Tregs is skewed and largely distinct from that of tumor-infiltrating conventional T cells, suggesting that Tregs recognize certain skewed antigens and clonally expand in the TME (76, 77). Indeed, Treg clones established from human melanoma recognize cancer-testis antigens including NY-ESO-1 (78, 79), TRAG-3 (78), LAGE-1 (80) and ARTC1 (antigen recognized by Treg cells) (81), and differentiation/overexpression self-antigens including gp100, TRP1, and survivin (79). Tregs in human colorectal cancer are known to be reactive to Mucin-1, HER2/neu, CEA, telomerase, survivin and EGFR (82). WT1 is also reported to be recognized by leukemia-derived Tregs (83). Yet whether these antigens are exclusively recognized by Tregs or recognition is shared by helper CD4+ T cells is unclear; however, Tregs usually harbor higher affinity TCRs compared with conventional T cells and are predominantly activated in tumors.
Strategies for Treg-targeted therapy
As discussed above, activated eTregs are present at a high frequency in tumors and need to be controlled for the generation/activation of antitumor immunity. Some clinical studies indicated the potential of depleting CD25-expressing lymphocytes to augment anti-tumor immune responses; yet, other similar studies failed to support this. As activated effector T cells in addition to Tregs also express CD25, CD25-based cell depletion may reduce activated effector T cells as well, cancelling the effect of Treg depletion to augment anti-tumor immunity. Additionally, one plausible concern is increased autoimmunity-related toxicities following Treg depletion. In order to secure safety of Treg-targeted therapy, selective depletion of eTregs in tumors rather than the entire Treg population can be exploited to augment anti-tumor immunity without eliciting deleterious autoimmunity (72). Targeting molecules and signals specific for eTregs is being tested in clinical trials as an effective strategy for eTreg depletion.
Humanized IgG1 monoclonal antibody targeting CCR4: mogamulizumab
We showed that CCR4 was specifically expressed by a subset of suppressive eTregs abundant in melanoma, and treatment using anti-CCR4 antibody depleted the melanoma-infiltrating Tregs that expressed CCR4 and efficiently induced/augmented both CD4+ and CD8+ T cells that were specific for cancer-testis antigen (72). Mogamulizumab has been approved in Japan for the treatment of CCR4-expressing adult T-cell leukemia/lymphoma (ATLL). Anti-CCR4 antibody markedly reduced eTregs as well as ATLL cells and augmented ATLL antigen (cancer-testis antigen)-specific CD8+ T-cell responses in an ATLL patient, possibly in association with the prolonged survival of this patient (72).
Based on these preclinical data, multiple early phase clinical trials with mogamulizumab as an eTreg depletion reagent are being conducted as monotherapy (trial numbers NCT02281409 and NCT01929486 (84)) and in combination with anti-PD-1 antibody (NCT02476123 and NCT02705105), anti-PD-L1 (PD-1 ligand 1) antibody or anti-CTLA-4 antibody (NCT02301130) and anti-4-1BB agonistic antibody (NCT02444793) in advanced solid tumors, and in combination with docetaxel in non-small cell lung cancer (NCT02358473).
Anti-OX-40 antibody and anti-GITR antibody
OX-40 and GITR are members of the TNF receptor superfamily and are both co-stimulatory receptors expressed by activated T cells. On Tregs, OX-40 is induced after activation and GITR is constitutively expressed (85–90). These signals reduce the suppressive activity of Tregs as well as enhancing activation of effector T cells.
In mouse models, an anti-OX-40 agonistic antibody augmented anti-tumor immunity in melanoma, colon cancer, glioma, breast cancer, sarcoma, renal cancer and prostate cancer (91). Its effect was mainly dependent on the reduction of Tregs in tumor tissues. A phase I trial of an OX-40 agonist demonstrated anti-tumor activity in melanoma and renal cell cancer (92). Early phase clinical trials evaluating OX-40 agonists in head and neck, breast and prostate cancer and in B cell lymphoma are also being investigated (NCT01862900, NCT02274155, NCT02318394 and NCT02205333). Additionally, combination of an OX-40 fusion protein (MEDI6383) and an anti-PD-L1 antibody, durvalumab, is also being investigated (NCT02221960). In mouse models, an anti-GITR agonistic antibody stimulated strong anti-tumor immunity in fibrosarcoma, colorectal carcinoma and melanoma models by decreasing Treg numbers and converting Treg-mediated resistance to effector T cell activation (93–95). Phase I clinical trials evaluating GITR agonists in solid tumors are being tested (NCT 02583165 and NCT02628574).
Small molecules targeting Treg-specific signals
Tregs are highly dependent on PI3K signals for their maintenance and function. Inactivation of PI3K signals in Tregs activates CD8+ T cells and induces tumor regression (96). Therefore, not only molecules specifically expressed by Tregs, but also signals on which Tregs specifically depend could become targets to control Tregs.
Treg depletion with vaccination
Treg depletion alone may not be sufficient to establish effective antitumor immunity. We have shown that self-antigen (Melan-A, a differentiation antigen of melanocytes)-reactive CD8+ T cells fall into an irreversible anergic state (i.e. hypoproliferative and with low cytokine production) with a unique phenotype (CCR7+CTLA-4+) after Treg-mediated suppression and they cannot be re-activated even in the absence of Tregs (35). Thus, in addition to overcoming Treg-mediated suppression, subsequent re-priming of effector T cells from the naive T-cell population would be necessary. At least two strategies to augment anticancer immunity by depleting Tregs prior to administering cancer vaccines have been evaluated: daclizumab or cyclophosphamide (CPA).
Humanized IgG1 monoclonal antibody targeting CD25: daclizumab.
Since Tregs are enriched in the CD4+CD25+ T cell fraction, Treg-depletion by the CD25-depleting antibody daclizumab has been evaluated in clinical trials. When daclizumab was administered following dendritic cell vaccination in metastatic melanoma (n = 15), not only Tregs but also activated effector cells were depleted and neither antitumor immune responses nor antibody production was observed (97). In contrast, in breast cancer patients, administration of daclizumab followed by vaccination consisting of multiple tumor-associated peptides succeeded in Treg-depletion and demonstrated favorable clinical responses (98). Stable disease was obtained in 6 out of 10 patients. Progression-free survival was 4.8 months (95% Confidence Interval, 3.0–6.5 months). The overall survival (OS) was 27.8 months (19.5–36.1). The 2-year survival was 65.5±17.3% (rate ± SD). No immune related adverse reaction was observed.
Cyclophosphamide.
CPA is an alkylating agent that reportedly depletes Tregs when used in low doses. In a phase II clinical trial, patients with advanced renal cell cancer received therapeutic vaccination of IMA901 consisting of multiple tumor-associated peptides and GM-CSF with or without preceding CPA administration (99). Patients treated with IMA901/GM-CSF/CPA showed Treg reduction with augmented anti-tumor immune responses. The OS tended to be extended in the IMA901/GM-CSF/CPA-treated group (n = 33) compared with the IMA901/GM-CSF-treated group (n = 35) (23.5 months versus 14.8 months). A phase III trial investigating the addition of IMA901/GM-CSF/CPA to the standard care of sunitinib was completed in 2015 and the results are awaited.
Involvement of Tregs in immune checkpoint inhibitors
Immune checkpoint blockade—inhibiting the immunosuppressive signals from co-inhibitory molecules—allows a resurgence in the effector function of tumor-infiltrating T cells and provides clinical success in various types of cancers including malignant melanomas and lung cancers. As immune checkpoint molecules such as CTLA-4 and PD-1 are expressed by both tumor-infiltrating effector T cells and Tregs, current immune checkpoint blocking agents could target Tregs as well. Analyses of anti-CTLA-4 antibodies in mouse models revealed that the antitumor efficacy was dependent on depletion of CTLA-4-expressing Tregs in tumors through the antibody-dependent cellular cytotoxic (ADCC) activity of the anti-CTLA-4 antibody; depletion of Fc function totally abrogated the anti-tumor effect of the anti-CTLA-4 antibody (94, 100–102). Additionally, PD-1-expressing Tregs reportedly possess higher immune suppressive function than Tregs without PD-1 expression in a mouse model (103). Therefore, PD-1-blocking antibodies might act on Tregs to augment anti-tumor immunity as well as reversing the effector function of dysfunctional effector cells.
Yet, more than half of the treated patients did not respond to immune checkpoint blockade therapy, even if combinations of blocking antibodies were used. Immuno-monitoring of biomarkers to properly evaluate immune responses in cancer patients is critical for detecting responders. There are two types of tumor antigens: tumor-specific antigens (TSAs), which are either oncogenic viral proteins or abnormal proteins that stem from somatic mutations (neoantigens); and tumor-associated antigens (TAAs), which are highly or aberrantly expressed normal proteins. It is not yet determined how CD8+ T cells specific for each antigen contribute to clinical tumor regression and whether activation of these CD8+ T cells specific for self-antigens versus non-self-antigens are controlled differently.
In vitro experiments comparing Treg-mediated suppression of self-antigen (Melan-A)-specific CD8+ T cells versus non-self (cytomegalovirus)-specific CD8+ T cells showed that cytomegalovirus-specific CD8+ T cells were resistant to suppression by Tregs (35), indicating that Treg-mediated suppression is more prominent on self-antigen-expressing tumor cells rather than those expressing neoantigens. It is therefore noteworthy that cancers in patients susceptible to immune checkpoint blockade monotherapy contain a large number of neoantigens and that CD8+ T cells specific for the antigens are resistant to Treg-mediated immune suppression. In contrast, cancers with a lower number of neoantigens did not respond to immune checkpoint blockade and CD8+ T cells are under the control of Treg-mediated immune suppression. Thus, integration of Treg-targeting therapies that reduce Treg function and/or number may expand the therapeutic spectrum of cancer immunotherapy.
Conclusion
Tregs, initially found as a key player of self-tolerance, have been revealed to play a critical role in tumor immunity and become a promising therapeutic target of cancer immunology. Yet their contribution in current cancer immunotherapy has not been fully determined and further detailed studies are essential for developing novel effective cancer immunotherapies.
Funding
This study was supported by Grants-in-Aid for Scientific Research (B) (H.N., No. 26290054) and for challenging Exploratory Research (H.N., No. 16K15551) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by The National Cancer Center Research and Development Fund (H.N., No. 28-A-7).
Conflict of interest: The authors have no conflict of interest on this manuscript.
References
- 1. Khattri R. Cox T. Yasayko S. A. and Ramsdell F. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337. [DOI] [PubMed] [Google Scholar]
- 2. Hori S. Nomura T. and Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057. [DOI] [PubMed] [Google Scholar]
- 3. Fontenot J. D. Gavin M. A. and Rudensky A. Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330. [DOI] [PubMed] [Google Scholar]
- 4. Sakaguchi S., Sakaguchi N., Asano M., et al. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes variouss autoimmune diseases. J. Immunol. 155:1151. [PubMed] [Google Scholar]
- 5. Bennett C. L., Christie J., Ramsdell F., et al. 2001. Thse immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20. [DOI] [PubMed] [Google Scholar]
- 6. Wildin R. S., Ramsdell F., Peake J., et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18. [DOI] [PubMed] [Google Scholar]
- 7. Brunkow M. E., Jeffery E. W., Hjerrild K. A., et al. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68. [DOI] [PubMed] [Google Scholar]
- 8. Sakaguchi S. Miyara M. Costantino C. M. and Hafler D. A. 2010. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10:490. [DOI] [PubMed] [Google Scholar]
- 9. Wing K. and Sakaguchi S. 2010. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11:7. [DOI] [PubMed] [Google Scholar]
- 10. Shimizu J. Yamazaki S. and Sakaguchi S. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211. [PubMed] [Google Scholar]
- 11. Nishikawa H. and Sakaguchi S. 2014. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 27:1. [DOI] [PubMed] [Google Scholar]
- 12. Hodi F. S., O’Day S. J., McDermott D. F., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Topalian S. L., Hodi F. S., Brahmer J. R., et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Topalian S. L., Sznol M., McDermott D. F., et al. 2014. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robert C., Long G. V., Brady B., et al. 2015. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372:320. [DOI] [PubMed] [Google Scholar]
- 16. Hamid O., Robert C., Daud A., et al. 2013. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robert C., Schachter J., Long G. V., et al. 2015. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372:2521. [DOI] [PubMed] [Google Scholar]
- 18. Rizvi N. A., Mazières J., Planchard D., et al. 2015. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 16:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brahmer J., Reckamp K. L., Baas P., et al. 2015. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borghaei H., Paz-Ares L., Horn L., et al. 2015. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brahmer J. R., Drake C. G., Wollner I., et al. 2010. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28:3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brahmer J. R., Tykodi S. S., Chow L. Q., et al. 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366:2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Powles T., Eder J. P., Fine G. D., et al. 2014. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558. [DOI] [PubMed] [Google Scholar]
- 24. Ansell S. M., Lesokhin A. M., Borrello I., et al. 2015. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 372:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larkin J., Chiarion-Sileni V., Gonzalez R., et al. 2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chapman P. B. D’Angelo S. P. and Wolchok J. D. 2015. Rapid eradication of a bulky melanoma mass with one dose of immunotherapy. N. Engl. J. Med. 372:2073. [DOI] [PubMed] [Google Scholar]
- 27. Postow M. A., Chesney J., Pavlick A. C., et al. 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372:2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Topalian S. L. Drake C. G. and Pardoll D. M. 2015. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rizvi N. A., Hellmann M. D., Snyder A., et al. 2015. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snyder A., Makarov V., Merghoub T., et al. 2014. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371:2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Allen E. M., Miao D., Schilling B., et al. 2015. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 350:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le D. T., Uram J. N., Wang H., et al. 2015. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 372:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsushita H., Vesely M. D., Koboldt D. C., et al. 2012. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gubin M. M., Zhang X., Schuster H., et al. 2014. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maeda Y., Nishikawa H., Sugiyama D., et al. 2014. Detection of self-reactive CD8⁺ T cells with an anergic phenotype in healthy individuals. Science 346:1536. [DOI] [PubMed] [Google Scholar]
- 36. Adeegbe D. O. and Nishikawa H. 2013. Natural and induced T regulatory cells in cancer. Front. Immunol. 4:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyman O. and Sprent J. 2012. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 12:180. [DOI] [PubMed] [Google Scholar]
- 38. Malchow S., Leventhal D. S., Nishi S., et al. 2013. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jordan M. S., Boesteanu A., Reed A. J., et al. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301. [DOI] [PubMed] [Google Scholar]
- 40. Coombes J. L., Siddiqui K. R., Arancibia-Cárcamo C. V., et al. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204:1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walker M. R. Carson B. D. Nepom G. T. Ziegler S. F. and Buckner J. H. 2005. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25+ cells. Proc. Natl Acad. Sci. USA 102:4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tran D. Q. Ramsey H. and Shevach E. M. 2007. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110:2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ellis G. I. Reneer M. C. Vélez-Ortega A. C. McCool A. and Martí F. 2012. Generation of induced regulatory T cells from primary human naïve and memory T cells. J. Vis. Exp. 62:3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hsu P., Santner-Nanan B., Hu M., et al. 2015. IL-10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J. Immunol. 195:3665. [DOI] [PubMed] [Google Scholar]
- 45. Liu W., Putnam A. L., Xu-Yu Z., et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203:1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seddiki N., Santner-Nanan B., Martinson J., et al. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203:1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mazzucchelli R. and Durum S. K. 2007. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 7:144. [DOI] [PubMed] [Google Scholar]
- 48. Miyara M., Yoshioka Y., Kitoh A., et al. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899. [DOI] [PubMed] [Google Scholar]
- 49. Wing K., Onishi Y., Prieto-Martin P., et al. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271. [DOI] [PubMed] [Google Scholar]
- 50. Takahashi T., Kuniyasu Y., Toda M., et al. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969. [DOI] [PubMed] [Google Scholar]
- 51. Thornton A. M. and Shevach E. M. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walker L. S. and Sansom D. M. 2011. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 11:852. [DOI] [PubMed] [Google Scholar]
- 53. Onizuka S. Tawara I. Shimizu J. Sakaguchi S. Fujita T. and Nakayama E. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 59:3128. [PubMed] [Google Scholar]
- 54. Yu P., Lee Y., Liu W., et al. 2005. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J. Exp. Med. 201:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turk M. J. Guevara-Patiño J. A. Rizzuto G. A. Engelhorn M. E. Sakaguchi S. and Houghton A. N.. 2004Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med. 200:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fridman W. H. Pagès F. Sautès-Fridman C. and Galon J. 2012. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12:298. [DOI] [PubMed] [Google Scholar]
- 57. Sato E., Olson S. H., Ahn J., et al. 2005. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl Acad. Sci. USA 102:18538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saito T., Nishikawa H., Wada H., et al. 2016. Two FOXP3+CD4+ T-cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. in press. [DOI] [PubMed] [Google Scholar]
- 59. Gobert M., Treilleux I., Bendriss-Vermare N., et al. 2009. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 69:2000. [DOI] [PubMed] [Google Scholar]
- 60. Svensson H., Olofsson V., Lundin S., et al. 2012. Accumulation of CCR4⁺CTLA-4hi FOXP3⁺CD25hi regulatory T cells in colon adenocarcinomas correlate to reduced activation of conventional T cells. PLoS One 7:e30695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Watanabe Y., Katou F., Ohtani H., et al. 2010. Tumor-infiltrating lymphocytes, particularly the balance between CD8+ T cells and CCR4+ regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109:744. [DOI] [PubMed] [Google Scholar]
- 62. Ishida T., Ishii T., Inagaki A., et al. 2006. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 66:5716. [DOI] [PubMed] [Google Scholar]
- 63. Curiel, T. J., Coukos, G., Zou, L. et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942. [DOI] [PubMed] [Google Scholar]
- 64. Facciabene A., Peng X., Hagemann I. S., et al. 2011. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475:226. [DOI] [PubMed] [Google Scholar]
- 65. Wei S., Kryczek I., Edwards R. P., et al. 2007. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 67:7487. [DOI] [PubMed] [Google Scholar]
- 66. Tan M. C., Goedegebuure P. S., Belt B. A., et al. 2009. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol. 182:1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spranger S., Spaapen R. M., Zha Y., et al. 2013. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jaafar F., Righi E., Lindstrom V., et al. 2009. Correlation of CXCL12 expression and FoxP3+ cell infiltration with human papillomavirus infection and clinicopathological progression of cervical cancer. Am. J. Pathol. 175:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Redjimi N., Raffin C., Raimbaud I., et al. 2012. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res. 72:4351. [DOI] [PubMed] [Google Scholar]
- 70. Liu J., Zhang N., Li Q., et al. 2011. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One 6:e19495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schlecker E., Stojanovic A., Eisen C., et al. 2012. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. 189:5602. [DOI] [PubMed] [Google Scholar]
- 72. Sugiyama D., Nishikawa H., Maeda Y., et al. 2013. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl Acad. Sci. USA 110:17945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tan W., Zhang W., Strasner A., et al. 2011. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ghiringhelli F., Puig P. E., Roux S., et al. 2005. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 202:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nishikawa H., Kato T., Tawara I., et al. 2005. Definition of target antigens for naturally occurring CD4+ CD25+ regulatory T cells. J. Exp. Med. 201:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hindley J. P., Ferreira C., Jones E., et al. 2011. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 71:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sainz-Perez A. Lim A. Lemercier B. and Leclerc C. 2012. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res. 72:3557. [DOI] [PubMed] [Google Scholar]
- 78. Fourcade J., Sun Z., Kudela P., et al. 2010. Human tumor antigen-specific helper and regulatory T cells share common epitope specificity but exhibit distinct T cell repertoire. J. Immunol. 184:6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vence L., Palucka A. K., Fay J. W., et al. 2007. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 104:20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang H. Y., Lee D. A., Peng G., et al. 2004. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity 20:107. [DOI] [PubMed] [Google Scholar]
- 81. Wang H. Y. Peng G. Guo Z. Shevach E. M. and Wang R. F. 2005. Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J. Immunol. 174:2661. [DOI] [PubMed] [Google Scholar]
- 82. Bonertz A., Weitz J., Pietsch D. H., et al. 2009. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J. Clin. Invest. 119:3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lehe C., Ghebeh H., Al-Sulaiman A., et al. 2008. The Wilms’ tumor antigen is a novel target for human CD4+ regulatory T cells: implications for immunotherapy. Cancer Res. 68:6350. [DOI] [PubMed] [Google Scholar]
- 84. Kurose K., Ohue Y., Wada H., et al. 2015. Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin. Cancer Res. 21:4327. [DOI] [PubMed] [Google Scholar]
- 85. Shimizu, J., Yamazaki, S., Takahashi, T. et al. 2002. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 3:135. [DOI] [PubMed] [Google Scholar]
- 86. Jensen S. M., Maston L. D., Gough M. J., et al. 2010. Signaling through OX40 enhances antitumor immunity. Semin. Oncol. 37:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Griseri T. Asquith M. Thompson C. and Powrie F. 2010. OX40 is required for regulatory T cell-mediated control of colitis. J. Exp. Med. 207:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hirschhorn-Cymerman D., Rizzuto G. A., Merghoub T., et al. 2009. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J. Exp. Med. 206:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schaer D. A. Cohen A. D. and Wolchok J. D. 2010. Anti-GITR antibodies — potential clinical applications for tumor immunotherapy. Curr. Opin. Investig. Drugs 206:1378. [PubMed] [Google Scholar]
- 90. Schaer D. A., Budhu S., Liu C., et al. 2013. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol. Res. 1:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bulliard Y., Jolicoeur R., Zhang J., et al. 2014. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol. Cell Biol. 92:475. [DOI] [PubMed] [Google Scholar]
- 92. Curti B. D., Kovacsovics-Bankowski M., Morris N., et al. 2013. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 73:7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitsui, J., Nishikawa, H., Muraoka, D. et a. 2010. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin. Cancer Res. 16:2781. [DOI] [PubMed] [Google Scholar]
- 94. Bulliard Y., Jolicoeur R., Windman M., et al. 2013. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 210:1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cohen A. D., Schaer D. A., Liu C., et al. 2010. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One 5:e10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ali K., Soond D. R., Piñeiro R., et al. 2014. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 510:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jacobs J. F., Punt C. J., Lesterhuis W. J., et al. 2010. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 16:5067. [DOI] [PubMed] [Google Scholar]
- 98. Rech A. J., Mick R., Martin S., et al. 2012. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci. Transl. Med. 4:134ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Walter S., Weinschenk T., Stenzl A., et al. 2012. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 18:1254. [DOI] [PubMed] [Google Scholar]
- 100. Simpson T. R., Li F., Montalvo-Ortiz W., et al. 2013. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med 210:1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Selby M. J., Engelhardt J. J., Quigley M., et al. 2013. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 1:32. [DOI] [PubMed] [Google Scholar]
- 102. Matheu M. P., Othy S., Greenberg M. L., et al. 2015. Imaging regulatory T cell dynamics and CTLA4-mediated suppression of T cell priming. Nat. Commun. 6:6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Park H. J., Park J. S., Jeong Y. H., et al. 2015. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J. Immunol. 194:5801. [DOI] [PubMed] [Google Scholar]