Abstract
Introduction There is an immense need for scaling-up neuropsychiatric care in low-income countries. Contextualized cost-effectiveness analyses (CEAs) provide relevant information for local policies. The aim of this study is to perform a contextualized CEA of neuropsychiatric interventions in Ethiopia and to illustrate expected population health and budget impacts across neuropsychiatric disorders.
Methods A mathematical population model (PopMod) was used to estimate intervention costs and effectiveness. Existing variables from a previous WHO-CHOICE regional CEA model were substantially revised. Treatments for depression, schizophrenia, bipolar disorder and epilepsy were analysed. The best available local data on epidemiology, intervention efficacy, current and target coverage, resource prices and salaries were used. Data were obtained from expert opinion, local hospital information systems, the Ministry of Health and literature reviews.
Results Treatment of epilepsy with a first generation antiepileptic drug is the most cost-effective treatment (US$ 321 per DALY adverted). Treatments for depression have mid-range values compared with other interventions (US$ 457–1026 per DALY adverted). Treatments for schizophrenia and bipolar disorders are least cost-effective (US$ 1168–3739 per DALY adverted).
Conclusion This analysis gives the Ethiopian government a comprehensive overview of the expected costs, effectiveness and cost-effectiveness of introducing basic neuropsychiatric interventions.
Keywords: Cost-effectiveness, ethics, mental health, neuropsychiatric disorders
Key Message
A cost-effectiveness analysis of basic neuropsychiatric interventions in an Ethiopian setting based on a proposed set of interventions from the Ethiopian Ministry of Health. Depending on the willingness-to-pay several interventions can be viewed as cost-effective. A discussion on how to weight cost-effectiveness compared with other fairness concerns.
Introduction
Mental and behavioral disorders contribute to around 19% of all years of life lost due to disability (YLD) in Eastern Sub-Saharan Africa (Vos et al. 2012). Even though effective treatment exists, major depression is the second leading cause of YLD both globally and in the sub-region of Eastern Sub-Saharan Africa. Mental illness greatly influences the patients’ overall health, economic situation and social integration. The prevalence and severity of other conditions, such as cardiovascular diseases, communicable diseases and intentional/unintentional injuries, are associated with poor mental health (Prince et al. 2007). In addition, mental disorders are a financial risk factor and can substantially influence ability to work and household prosperity. Poor populations are also at higher risk for mental illnesses (Lund et al. 2010). The mentally ill are a vulnerable and often stigmatized group. Ethiopian studies have shown that mental illness is associated with a high degree of stigma (Shibre et al. 2001; Assefa et al. 2012).
Scaling up mental health services in Ethiopia is critical due to the shortage of neuropsychiatric treatment and the massive lack of trained personnel in all mental health professions, as in most low-income countries (Saxena et al. 2007; mhGAP-Ethiopia Working Group 2010; Bruckner et al. 2011; Federal Democratic Republic of Ethiopia Ministry of Health 2012). This is recognized in the current National Mental Health Strategy, which has an ambitious goal of addressing the mental health needs of the entire Ethiopian population (Federal Democratic Republic of Ethiopia Ministry of Health 2012). The National Mental Health Strategy explicitly states that efficiency and cost-effectiveness are important in the scale-up of mental health interventions. The basic scale-up scenario in the National Mental Health Strategy targets treatment for depression, psychosis, bipolar disorder and epilepsy, which are key interventions in the WHO mental health Gap Action Programme (mh-GAP) (mhGAP-Ethiopia Working Group 2010). The strategy identifies low budgets as a weakness and indicates that these are a threat to implementing mental health services (Federal Democratic Republic of Ethiopia Ministry of Health 2012). Evidence on the cost-effectiveness of the basic scale-up scenario is therefore important. However, the cost-effectiveness of these interventions in the Ethiopian context has not been evaluated. Evidence from the latest regional cost-effectiveness analysis (CEA) on neuropsychiatric disorders (Chisholm and Saxena 2012) is not necessarily sensitive to local variations; there are significant variations in results between regional and national CEAs (Reinap et al. 2005; World Health Organization 2006; Gureje et al. 2007; Ayuso-Mateos et al. 2008; Chisholm et al. 2008; Salomon et al. 2012a). A contextualized CEA can provide important information for prioritizing decisions as the mental health policy is being scaled up in Ethiopia. The aim of this study is: (1) to do a contextualized CEA for an Ethiopian setting examining a range of interventions for depression, schizophrenia, bipolar disorder and epilepsy; and (2) to illustrate the differences in expected population health and budget impact across these disorders.
Materials and methods
Study design and population
This is a generalized CEA, which has been performed in an Ethiopian setting (Box 1) from a health provider perspective. All costs and health benefits were discounted at 3% and no age weights were applied. Assumptions and variables in existing CEA models from WHO-CHOICE on neuropsychiatric disorders were revised. The best available local data on epidemiology, intervention efficacy, current coverage, item prices, salaries and quantity assumptions replaced the existing assumptions in the model (see Box 1, Tables 2 and 3 and Appendix). Specific data from Ethiopia have been used when available; however, the availability of data is scarce and we have therefore supplemented it with regional data and estimates based on the calculation framework from WHO-CHOICE.
Box 1.Overview of key characteristics of Ethiopia
| Characteristics | Year | Source | ||
|---|---|---|---|---|
| Demography | Total national population | 94,100,756 | 2013 | World Bank Data |
| Proportion below poverty line of Int$1.25 | 30.7% | 2011 | World Bank Data | |
| Proportion below poverty line of Int$2 | 66.0% | 2011 | World Bank Data | |
| Expenditure on health (USD/capita) | 17.6 | 2012 | World Bank Data | |
| Human resources | Psychiatrists* | 40 | 2010 | MoH Ethiopia |
| Psychiatric nurses | 461 | 2010 | MoH Ethiopia | |
| Psychologists** | 14 | 2010 | MoH Ethiopia | |
| Social Workers | 3 | 2010 | MoH Ethiopia | |
| Occupational therapists | 0 | 2010 | MoH Ethiopia | |
| Psychiatric beds | 326 | 2010 | mhGAP-Ethiopian Working Group | |
| Epidemiology | Prevalence depression | 2.2% | 2010 | Fekadu et al. (2007) |
| Prevalence schizophrenia | 0.5% | 2010 | mhGAP-Ethiopian Working Group | |
| Prevalence bipolar disorder | 0.5% | 2010 | mhGAP-Ethiopian Working Group | |
| Prevalence epilepsy | 1% | 2010 | mhGAP-Ethiopian Working Group | |
| Prevalence alcohol drinking problem | 2.2–3.7% | 2010 | mhGAP-Ethiopian Working Group | |
| Current coverage | Depression | <1% | 2010 | mhGAP-Ethiopian Working Group |
| Schizophrenia | <1% | 2010 | mhGAP-Ethiopian Working Group | |
| Bipolar Disorder | 1% | 2010 | mhGAP-Ethiopian Working Group | |
| Epilepsy | 5% | 2010 | mhGAP-Ethiopian Working Group |
*30 of the 40 psychiatrists are located in Addis Abeba.
**none with training as clinical psychologists.
Table 2.
Effectiveness assumptions
| Treatment | Outcome | Efficacy | Drug/other treatment | Source |
|---|---|---|---|---|
| Depression | ||||
| Treatment with older Antidepressants (TCA) | Remission | 29% | Amtriptyline (100 mg daily) | Arroll et al. (2005); Andrews et al. (2000) |
| Disability | −31% | |||
| Treatment with newer Antidepressants (SSRI) | Remission | 28% | Fluoxetine (20 mg daily) | Arroll et al. (2005); Andrews et al. (2000) |
| Disability | −31% | |||
| Psychotherapy | Remission | 28% | Cognitive therapy (16–20 h) | de Maat et al. (2007); Andrews et al. (2000) |
| Disability | −31% | |||
| Comibnation of AD and psychotherapy | Remission | 38% | Amtriptyline/Fluoxetine, cognitive therapy | de Maat et al. (2007); Andrews et al. (2000) |
| Disability | −31% | |||
| Proactive care | Remission | 38% | Amtriptyline/Fluoxetine, cognitive therapy | de Maat et al. (2007); Andrews et al. (2000); Geddes et al. (2003) |
| Disability | −31% | |||
| Incidence | −35% | |||
| Schizophrenia | ||||
| Older antipsychotics | Disability | −13% | Chlorpromazine 300 mg daily/Fluphenazine deconate 25 mg daily | Leutch et al. (1999) |
| Newer antipsychotics | Disability | −14% | Risperidone 4 mg daily | Leutch et al. (1999) |
| Older antipsychotics + psychosocial treatment | Disability | −23% | Chlorpromazine 100 mg daily/Fluphenazine deconate 25 mg daily, supportive psychosocial treatment | Mojtabai et al. (1998); Leutch et al. (1999) |
| Newer antipsychotics + psychosocial treatment | Disability | −24% | Risperidone 4 mg daily, supportive psychosocial treatment | Mojtabai et al. (1998); Leutch et al. (1999) |
| Case-management with older antipsychotics | Disability | −24% | Chlorpromazine 300 mg daily/Fluphenazine deconate 25 mg daily | Mojtabai et al. (1998); Leutch et al. (1999) |
| Case-management with newer antipsychotics | Disability | −25% | Risperidone 4 mg daily | Mojtabai et al. (1998); Leutch et al. (1999) |
| Bipolar disorder | ||||
| Older mood stabilizer | Disability | −70% | Lithium Carbonate | Goodwin et al. (1990); Angst et al. (2000); Bowden et al. (2000); Tondo et al. (2001) |
| Case-fatality | −65% | |||
| Newer mood stabilizer | Disability | −65% | Sodium Valproate | Goodwin et al. (1990); Bowden et al 1994; Angst et al. (2000); Bowden et al. (2000); Tondo et al. (2001) |
| Case-fatality | −65% | |||
| Older Mood Stabilizer + psychosocial | Disability | −70% | Lithium Carbonate, supportive psychososial treatment | Goodwin et al. (1990); Angst et al. (2000); Bowden et al. (2000); Tondo et al. (2001); Lam et al. (2009) |
| Case-fatality | −65% | |||
| Newer mood stabilizer + psychosocial | Disability | −65% | Sodium Valproate, supportive psychosocial treatment | Goodwin et al. (1990); Bowden et al. (1994, 2000); Angst et al. (2000); Tondo et al. (2001); Lam et al (2009) |
| Case-fatality | −65% | |||
| Epilepsy | ||||
| Older antiepileptic | Remission | 60% | Phenobarbital 100 mg daily | Annegers et al. (1979); Goodridge, et al. (1983); Placenica et al. (1994); Cockerell et al. (1995) Tudur Smith et al. (2009) |
| Disability | −43% | |||
| Newer antiepileptic | Remission | 60% | Carbamazepine 600 mg daily | Annegers et al. (1979); Goodridge et al. (1983); Placenica et al. (1994); Cockerell et al. (1995) Tudur Smith et al. (2009) |
| Disability | −43 % | |||
Table 3.
Cost assumptions
| Salaries | (Source: Amanuel Psychiatric Hospital, Black Lion Hospital, Ministry of Health Ethiopia) | |
|---|---|---|
| Yearly salary (ETB) | ||
| Medical director | 91 428 | |
| Psychiatric specialist | 83 988–91 428 | |
| General practitionar | 40 968 | |
| Nurse | 19 998–35 868 | |
| Public health worker | 27 000–35 868 | |
| Health worker | 17 124 | |
| Occupational therapists | 31 200 | |
| Psychologist | 29 112 | |
| Social worker | 31 224 | |
| Pharmacist | 18 426–40 968 | |
| Labratory technichian | 30 168 | |
| Secretary | 19 578 | |
| Lab tests | (Source: Amanuel Psychiatric Hospital, Black Lion Hospital) | |
| Unit Price ETB (per test) | ||
| Complete blood count package | 45 | WBC, RBC, Hb, hct, Plt and three differnitals |
| Hb | 10 | |
| Iron | 20 | |
| Kidney function test | 15 | Creatinine |
| Electolytes | 75 | Includes K+, Na+, Cl-, Ca+2 |
| Liver function tests | 75 | Bil-tot, bil-dir, ALAT, ASAT, ALP |
| Thyroid tests | 180 | Includes TSH and FT4 |
| Glucose | 15 | |
| Lithium level | 51 | |
| Drugs | (Source: http://erc.msh.org Nov 2013) | |
| Unit price US$ (per tabl/amp) | ||
| Amtriptyline 25 mg tablet | 0.0036 | |
| Fluoxtine 20 mg capsule | 0.0353 | |
| Haldolperidol 5 mg tablet | 0.0140 | |
| Fluphenazine Decanoate 25 mg/1 ml injection | 0.5958 | |
| Risperidone 2 mg tablet | 0.0085 | |
| Triexiphenidyl Hcl 5 mg tablet | 0.0063 | |
| Lithium Carbonate 300 mg tablet | 0.8000 | Amanuel Psychiatric Hospital |
| Sodium Valproate 200 mg tablet | 0.0315 | |
| Phenobarbital 100 mg tablet | 0.0041 | |
| Carbamazapine 200 mg tablet | 0.0148 | |
| Insitutional costs, training costs and Programme costs | (Based on WHO-CHOICE framework) | |
| GDP per capita (Int$) | 1,354 | World Bank 2013 |
| GDP per capita (US$) | 498 | World Bank 2013 |
| PPP conversion factor (ETB per Int$) | 6.9 | World Bank 2013 |
| Offical exchange rate (ETB per US$) | 17.7 | World Bank 2012 |
| % of population that are urban | 17.5 % | World Bank 2013 |
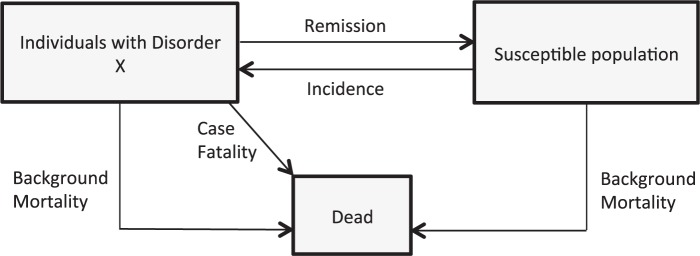
The software used in the analysis was population model (PopMod), which is a population-based multi-state analytical health economic tool developed by WHO-CHOICE. A detailed description of the method can be found elsewhere (Edejer et al. 2012). The model is based on three health states: condition X, susceptible without condition X and death. In the first year, the total Ethiopian population (exact age groups) is distributed into the three health states. Jumps between health states are determined by disease-specific incidence rates, remission rates, case fatality rates and age-specific mortality rates for susceptible groups (Figure 1).
Figure 1.
Model explanation.
Each model-run is one year and the model runs for 100 years, but interventions are only applied for the first 10-year period. Health benefits of the programme are assessed over a 100-year period in order to include the long-term benefits of prevention or treatment late in life.
The newest age-specific demographic distribution in Ethiopia from UN populations 2010 was used (UN Population Devision). Epidemiological data on prevalence, incidence, case-fatality, health state valuation, disability weights, birth rates and mortality have been collected from the latest available update of the Global Burden of Disease (GBD-2010) study (Salomon et al. 2012b). Where country-specific data on epidemiological factors was available, this has been used; where this has not been available, we have used data from the East African sub-region. The epidemiological input was compared with and validated for Ethiopian epidemiological studies (Tekle-Haimanot et al. 1997; Awas et al. 1999; Kebede and Alem 1999a,b; Kebede et al. 1999, 2003, 2006; Fekadu et al. 2007; Negash et al. 2005; Almu et al. 2006). The GBD data have been supplemented with information collected from experts at Amanuel Psychiatric Hospital and the Ministry of Health in Ethiopia, and from a literature review. The mh-GAP implementation project estimated the coverage level of the different mental health interventions in Ethiopia in 2010; these estimates were used as the current coverage levels in the model (mhGAP-Ethiopia Working Group 2010).
Selection of disorders and interventions
A complete model of care was used, and the scale-up scenarios are close to what is described in the National Mental Health Strategy in Ethiopia and the WHO-supported Ethiopia mh-GAP programme. Treatment is provided primarily by generalists in a primary-care setting (mhGAP-Ethiopia Working Group 2010; Federal Democratic Republic of Ethiopia Ministry of Health 2012). An overview of the interventions included in the analysis is presented in Table 1. The interventions in this analysis have been analysed in the regional CEA from 2012 (Chisholm and Saxena 2012); however, the interventions included in this analysis target disorders in the basic scenario of the National Mental Health Strategy. The analysis was not limited to interventions in the basic scenario of the National Mental Health Strategy. Analyses of newer medications for bipolar disorder and schizophrenia and psychotherapy for depression were added, since these are considered in treatment guidelines from higher-income settings like the UK (National Institute of Health and Clinical Excellence 2006; National Institute of Health and Clinical Excellence 2009a,b). The National Mental Health Strategy suggests treating bipolar disorders using mainly antipsychotics. This option was not included in the analysis due to the very limited evidence on long-term treatment effects (Geddes and Miklowitz 2013). The target coverage varies between the disease categories and reflects the targets set in the basic scale-up scenario (Federal Democratic Republic of Ethiopia Ministry of Health 2012). We adhere to the ICD-10 definitions of disorders.
Table 1.
Overview of interventions
| Disorder | Intervention number | Intervention description | Coverage |
|---|---|---|---|
| Depression | DEP1 | Older antidepressants (TCA) | 30% |
| DEP2 | Newer antidepressants (SSRI) | 30% | |
| DEP3 | Psychotherapy | 30% | |
| DEP4 | Older antidepressants (TCA) and psychotherapy | 30% | |
| DEP5 | Newer antidepressants (SSRI) and psychotherapy | 30% | |
| DEP6 | Maintenance: Older antidepressants (TCA) and psychotherapy | 30% | |
| DEP7 | Maintenance: Newer antidepressants (SSRI) and psychotherapy | 30% | |
| Schizophrenia | SCZ1 | Typical antipsychotics | 75% |
| SCZ2 | Atypical antipsychotics | 75% | |
| SCZ3 | Typical antipsychotics + psychosocial treatment | 75% | |
| SCZ4 | Atypical antipsychotics + psychosocial treatment | 75% | |
| SCZ5 | Case id + manager: Typical antipsychotics and psychosocial treatment | 75% | |
| SCZ6 | Case id + manager: Atypical antipsychotics and psychosocial treatment | 75% | |
| Bipolar disorder | BIP1 | Older mood stabilizer (Lithium) | 50% |
| BIP2 | Newer mood stabilizer (Valproate) | 50% | |
| BIP3 | Older mood stabilizer (Lithium) and psychosocial treatment | 50% | |
| BIP4 | Newer mood stabilizer (Valproate) and psychosocial treatment | 50% | |
| Epilepsy | EPI1 | Older antiepileptic treatment (Phenobarbital) | 75% |
| EPI2 | Newer antiepileptic treatment (Carbomazepine) | 75% |
2.3 Estimation of intervention effectiveness
Health benefits are measured in disability adjusted life years (DALYs). A null scenario is estimated first, where the health state of the Ethiopian population is calculated if no interventions were available (the model ‘rewinds’ the demography by downscaling the current coverage of interventions to zero). The null scenario serves as a baseline for assessing the incremental effects of increasing intervention coverage. When compared with the null scenario, the effect of treatments is incremental reductions in disability weight values, fatality rates and incidence of mental illness or increasing remission rates (see Table 2 and Appendix). In addition, effects are adjusted by treatment coverage and adherence to treatment. Estimates of the efficacy of interventions have been updated with results from systematic reviews, meta-analyses and Randomized Controlled Trials (Table 2). Evidence from Ethiopia was considered most valuable, but such evidence was not available.
Estimation of intervention costs
Unit prices and quantities needed at different levels of the health system (e.g. drugs, salaries, hospital bed-day costs etc.) are used to calculate costs. Facility costs (or direct patient costs), programme costs and training costs are included in total costs (see appendix). The patient out-of-pocket expenditures related to help-seeking and the productivity impact for the patient, household and families are not included in the analysis. Finally, total 10-year costs are combined with the respective effectiveness of the interventions.
Amanuel Psychiatric Hospital was the main source for local unit prices of laboratory tests and salaries (see Table 3). This information was cross-verified with data from Black Lion Hospital and the Ministry of Health in Ethiopia. Amanuel Psychiatric Hospital is a specialist psychiatry hospital and Black Lion Hospital is a general teaching hospital in Addis Ababa. Ethical clearance from the Institutional Review Board at the Medical Faculty of Addis Ababa University was obtained for collection of costs data at the institutions. Unit prices were coherent across all of these governmental institutions. Default unit cost estimates for drugs were taken from the International Drug Price Indicator Guide (http://erc.msh.org), where the lowest ‘supplier price’ could be identified. The costs for drugs from this source were compared with Amanuel Psychiatric Hospital’s prices for drugs for patients. When Amanuel Psychiatric Hospital provided a lower price for a drug, this price was used. Average unit costs of bed-days and outpatient visits at primary and secondary health care levels were estimated using the WHO-CHOICE econometric analysis (Adam et al. 2003; Adam and Evans 2006). Unit costs for bed-days and outpatient visits include all cost components except drugs and laboratory tests. See Appendix for more details on cost and quantity assumption.
Programme costs not directly linked to the providing health facility were analysed separately for each intervention. These costs include planning and administration, training of staff and monitoring and evaluation at a national, provincial and district level. Programme quantities are based on WHO-CHOICE expert estimates.
Estimation of cost-effectiveness
Cost-effectiveness was assessed by calculating incremental cost-effectiveness ratios (ICERs). The ICER was calculated in comparison to the null scenario. The current situation has been excluded from the analysis, as it is close to the modelled null scenarios: the Ministry of Health in Ethiopia has estimated that the current coverage of many basic interventions is only about 1% (mhGAP-Ethiopia Working Group 2010). WHO recommends using a willingness-to-pay threshold defined by the nation’s GDP. Where interventions falling under one GDP per capita are considered highly cost-effective and interventions with an ICER below three GDP per capita are considered cost-effective (Commision on Macroeconomics and Health 2001). However, Revill et al. (2014) states ‘the use of WHO-recommended cost-effectiveness benchmarks of one and three times GDP per capita lacks a theoretical or empirical basis’ (Revill et al. 2014). We will present the results according to three possible willingness-to-pay thresholds: (1) US$ 100; (2) one times GDP—US$ 500; (3) three times GDP—US$ 1500.
Uncertainty analysis
To perform the uncertainty analysis, we used the WHO-CHOICE software programme Monte Carlo (MC) League (Hutubessy et al. 2001; Baltussen et al. 2002). The MC League represents uncertainty regarding costs and effects in the form of stochastic league tables and the analysis allows for covariance between the costs and effectiveness. We conducted a probabilistic MC uncertainty analysis of the results with 1000 simulation runs and a truncated normal distribution to assess the certainty of the results. We used baseline results together with variation coefficients ranging between 15 and 25%. The results of the analysis are presented as willingness-to-pay curves and scatter plots of the uncertainty range for both costs and effects.
Results
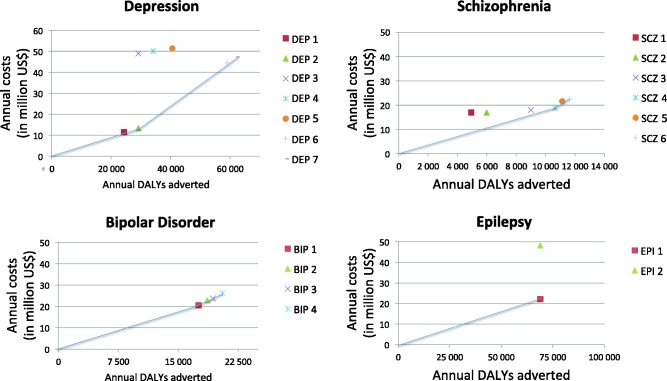
Table 4 provides an overview of the costs, effectiveness and cost-effectivenessratios for the interventions suggested in the National Mental Health Strategy. Figure 2 shows expansion paths for all disorders, suggesting how to scale up the interventions according to variations in societal willingness-to-pay for mental health care.
Table 4.
Cost-effectiveness overview
| Disease Category |
Intervention number | Annual costs (in million US$) | Annual DALYs adverteda | ACER | ICER |
|---|---|---|---|---|---|
| Depression | DEP1 | 11.44 | 24 340 | 470 | Db |
| DEP2 | 13.31 | 29 136 | 457 | 457 | |
| DEP3 | 48.90 | 29 136 | 1678 | D | |
| DEP4 | 49.97 | 34 075 | 1467 | D | |
| DEP5 | 51.24 | 40 576 | 1263 | D | |
| DEP6 | 44.55 | 58 926 | 756 | D | |
| DEP7 | 47.21 | 62 193 | 759 | 1026 | |
| Schizophrenia | SCZ1 | 16.96 | 4925 | 3444 | D |
| SCZ2 | 16.96 | 5992 | 2831 | D | |
| SCZ3 | 17.98 | 8988 | 2001 | D | |
| SCZ4 | 18.84 | 10 650 | 1769 | 1769 | |
| SCZ5 | 21.60 | 11 126 | 1941 | D | |
| SCZ6 | 22.46 | 11 617 | 1933 | 3739 | |
| Bipolar disorder | BIP1 | 20.50 | 17 552 | 1168 | 1168 |
| BIP2 | 22.90 | 18 636 | 1229 | D | |
| BIP3 | 23.71 | 19 333 | 1227 | 1807 | |
| BIP4 | 26.14 | 20 530 | 1273 | 2023 | |
| Epilepsy | EPI1 | 22.16 | 68 935 | 321 | 321 |
| EPI2 | 48.20 | 68 935 | 699 | D |
ACER, average cost-effectiveness ratio (US$ per DALY adverted); ICER, incremental cost-effectiveness ratio (US$ per DALYs adverted)
aDiscounted, not age-weigthed.
bD, dominated (intervention is more costly and/or less effective than other more efficient interventions).
Figure 2.
Expansion path of interventions.
We can consider the results depending on three willingness-to-pay thresholds: (1) US$ 100; (2) US$ 500 (one GDP); (3) US$ 1500 (three GDP). If the willingness-to-pay threshold is set to US$ 100 none of the interventions targeting neuropsychiatric disorders would be implemented. Implementing alternative 2, a threshold of one GDP (US$ 500), a basic scenario package would include treatment of epilepsy with older anti-epileptic (US$ 321 per DALY adverted) and newer antidepressants and psychotherapy, with maintenance treatment for depression (US$ 457 per DALY adverted). Treatments for schizophrenia and bipolar disorder would be excluded. A scale-up of these interventions will cost US$ 35.5 million annually. The new health budget would then be US$ 18.0 per capita, a 2.1% increase compared with the 2012 health budget (World Bank). The expected health gain this scale up is an additional 98 000 healthy life years.
Implementing Alternative 3, threshold of three GDP (US$1500), a basic scenario package would include treatment of epilepsy with older anti-epileptic (US$ 321 per DALY adverted), treatment for depression with newer antidepressants and psychotherapy with maintenance treatment (US$ 1026 per DALY adverted) and treatment with Lithium and psychosocial treatment for bipolar disorder (US$ 1168 per DALY adverted). The most cost-effective intervention for schizophrenia—risperidone with psychosocial supportive therapy (US$ 1769 per DALY adverted)—would be excluded. A scale-up of these will cost US$ 89.9 million annually. The new health budget would then be US$ 18.6 per capita, a 5.4% increase compared with the 2012 health budget (World Bank). The expected health gain this scale up is an additional 149 000 healthy life years.
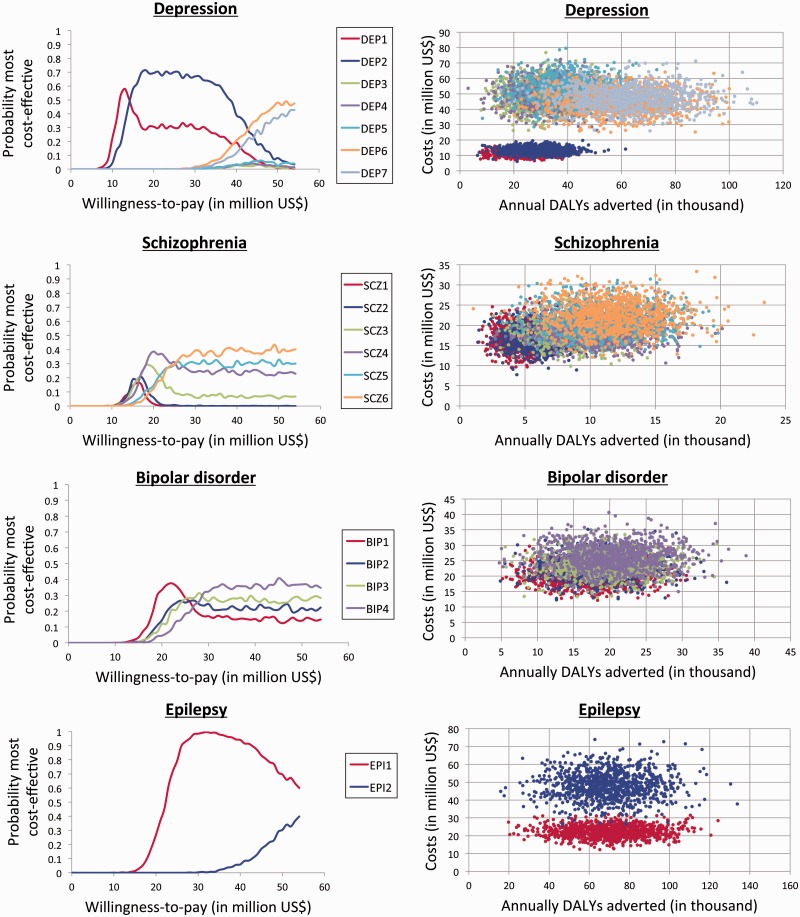
The size of the health budget, or willingness to pay for a treatment, has substantial impact on the certainty of the results (see Figure 3). For depression, the probability curves for intervention DEP1 and DEP2 are roughly within the same range and it is therefore difficult to conclude whether newer or older antidepressants are most cost-effective. Due to lack of evidence, the uncertainty of the results is noteworthy, especially for interventions targeting schizophrenia and bipolar disorder.
Figure 3.
Sensitivity analysis.
Discussion
This analysis assesses the cost-effectiveness of different eligible interventions targeting depression, schizophrenia, bipolar disorder and epilepsy. The three presented scale-up scenarios assume three different willingness-to-pay thresholds. Applying the lowest threshold of US$ 100 for a year of healthy life (Alternative 1) would result in there being no mental health care interventions selected for scale-up in Ethiopia (since all studied strategies exceed this level of efficiency). By including cost-effective neuropsychiatric intervention strategies under either Alternative 2 or 3, the total health budget, would increase by 2.1 and 5.4%, respectively. Even at the highest threshold used in Alternative 3 (US$ 1500 per health life year), treatment for schizophrenia would be excluded if a strict health-maximizing approach was pursued since even the most cost-effective strategy—treatment with newer antipsychotic medication (generically produced risperidone) plus psychosocial therapy—has a cost-effectiveness ratio above this fixed point.
Although it is evidently important to establish efficient pathways towards universal coverage, concerns regarding fairness also need to be taken into account in the formation of public health priorities (Norheim et al. 2013). This is often seen in actual policy decision-making, where policies often deviate from crude cost-effectiveness rankings (Dixon and Welch 1991). In particular, financial protection and disease severity are relevant fairness concerns that are not taken into account by crude ICER rankings (Norheim et al. 2013). Severity of disease was considered the leading priority in a survey conducted among key stakeholders in Uganda (Kapiriri et al. 2004; Kapiriri and Norheim 2004) and is a principle that has been theoretically well argued for (Nord 1993; Ottersen 2013). Schizophrenia, e.g. is highly disabling, occurs at a young age and pushes households into poverty; it can therefore be considered as a more severe condition than others under consideration (Daniels and Sabin 2002). If we introduce severity as an additional criterion, it will likely shift the ranking for treatment of schizophrenia. As of today, there are few methods available for explicit handling of such trade-offs between health maximization and concerns such as severity of disease and financial protection in economic evaluations regarding health care. Thus, there is a need to develop methods to incorporate cost-effectiveness with other relevant criteria in a priority-setting analytical framework.
In the search for efficiency, it is also crucial not to jeopardize the quality of care or service user outcomes. In the care model used in this analysis, we scale up mental health care in a primary care setting with referral as needed to specialized inpatient care. For conditions like schizophrenia, inpatient care remains a key cost driver (as can be seen in Supplementary Table S2 of the appendix). The National Mental Health strategy in Ethiopia suggests task shifting as an important strategy to reduce personnel costs (Federal Democratic Republic of Ethiopia Ministry of Health 2012). They suggest low- and middle-level health workers can undertake certain responsibilities carried out to date by specialists and primary care doctors. However, there is currently lack of evidence on how such task shifting could impact the quality of care. There are now studies being conducted in rural Ethiopia on task shifting of schizophrenia care (Lund et al. 2015). If quality of care and health outcomes can be maintained through such an approach, the cost-effectiveness of schizophrenia care may be substantially reduced.
We have chosen to use PopMod for this analysis, as this model has been used for similar studies in other low-income countries. It allows us to compare the results to previous studies as well as other disease categories. However, the method has limitations. It is a mathematical model and, therefore, is not as context-specific as an empirically based trial. The parameter assumptions are drawn from international as well as national data. The major limitation of this study is the limited availability of local data. There are few primary studies on specific costs or the efficacy of neuropsychiatric treatments in the Ethiopian healthcare system. Further, the costs data gathered in Ethiopia are mainly from Amanuel Psychiatric Hospital, Black Lion Hospital and the Ethiopian Ministry of Health. The hospitals are both located in Addis Ababa, while 81% of the population lives in rural areas. At the time of data collection, Amanuel Psychiatric Hospital was the only psychiatric hospital in the country. We have therefore relied on these three sources and estimates in the WHO-CHOICE model to calculate costs. The interventions in the analysis are focused on community-based treatment. In the model, we have estimated that there will be extra logistic expenditures due to transportation of, e.g. drugs. The salaries will not differ, as they are based on the Ethiopian Ministry of Health national tariffs.
The analysis takes a health provider perspective; costs do not include out-of-pocket costs related to help-seeking or loss of income due to loss in productivity. The health expenditures of patients need to be assessed, as it is clear that neuropsychiatric disorders have a large impact on all aspects of life, both for the patient and his/her family. The impact of the interventions on the patients’ ability to work, caretakers’ responsibility for the patient as well as the ability to have a job and costs in seeking help from both traditional and western health care providers are aspects that are not studied in this analysis. As an addition to this analysis, an extended CEA could be performed, such that the expected non-health benefits and financial protection for the respective interventions can be evaluated (Verguet et al. 2014).
Direct comparisons of many of the results in this contextualization and the regional CEA on mental health care (Chisholm and Saxena 2012) are complicated by the extensive revisions of local input assumptions as well as by differences in reporting year and currency, changing drug processes and age-weighting procedures. However, by comparing the results of this analysis to an earlier regional analysis that also removed age weights and reported in US$ (Hyman et al. 2006), it becomes apparent that many of the interventions studied at the national level have a lower budget impact than the regional study. This is in line with other WHO-CHOICE contextualization studies in Chile, Estonia, Mexico, Nigeria, Spain, Sri Lanka and Thailand (Reinap et al. 2005; World Health Organization 2006; Gureje et al. 2007; Ayuso-Mateos et al. 2008; Chisholm et al. 2008; Salomon et al. 2012a), which have likewise indicated that regional studies are not always that sensitive to local variations in epidemiology, costs and coverage. However, the major trends are similar between regional, global and contextualized CEAs; e.g. all of these analyses estimate that interventions for schizophrenia are the least cost effective. In addition, it is important to adjust for fluctuations in drug prices, as they are constantly changing and thus influencing the costs and cost-effectiveness of the interventions. Drug prices are often major contributors to the total costs of interventions in low- and middle-income countries. This was seen in a CEA in Nigeria where the prices of atypical antipsychotics were very high at the time of the analysis in Nigeria, and this drove the cost-effectiveness rates higher compared with the regional CEA (Gureje et al. 2007).
Conclusions
Neuropsychiatric conditions contribute to a major burden of disease in Ethiopia, and the current coverage of neuropsychiatric interventions is low in Ethiopia. Our analysis provides an overview of the expected costs, effectiveness and cost-effectiveness ratios for different possible interventions targeting depression, schizophrenia, bipolar disorder and epilepsy. The limit the Ethiopian governments set for willingness-to-pay will determine which neuropsychiatric interventions could be provided for the Ethiopian people under a health maximization approach. Decisions on how to best scale up the Ethiopian health system should be made comparing interventions across disorders and medical disciplines. Ultimately, the decision should be based on both CEAs and an ethical discussion on how to weight cost-effectiveness estimates and severity of disease.
Supplementary Material
Acknowledgements
We thank Tshesome Shibre for all his help in facilitating contact with key people in Ethiopia. We are grateful to staff at Amanuel Psychiatric Hospital for help in collecting data from various offices at the hospital. We thank the Ethiopian Ministry of Health for sharing information and plans. The research group in Priority Setting in Health at the Institute of Global Health and Public Health at University of Bergen has been very helpful in giving constructive feedback on the analysis and the article. This project was made possible through funding from the Medical Student Research Programme at the University of Bergen.
Conflict of interest statement. K.B.S., A.F. and K.A.J. have nothing to disclose. D.C. is an employee of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization. K.B.S. recieved funding from the University of Bergen as part of the Medical Student PhD Track Programme.
References
- Adam T, Evans DB. 2006. Determinants of variation in the cost of inpatient stays versus outpatient visits in hospitals: a multi-country analysis. Social Science and Medicine 63: 1700–10. [DOI] [PubMed] [Google Scholar]
- Adam T, Evans DB, Murray CJ. 2003. Econometric estimation of country-specific hospital costs. Cost Effectiveness and Resource Allocation 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almu S, Tadesse Z, Cooper P, Hackett R. 2006. The prevalence of epilepsy in the Zay Society, Ethiopia–an area of high prevalence. Seizure 15: 211–3. [DOI] [PubMed] [Google Scholar]
- Andrews G, Sanderson K, Corry J, Lapsley HM. 2000. Using epidemiological data to model efficiency in reducing the burden of depression. The journal of mental health policy and economics 3(4): 175–286. [DOI] [PubMed] [Google Scholar]
- Angst J, Sellaro R. 2000. Historical perspectives and natural history of bipolar disorder. Biological psychiatry 48(6): 445–457. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Elveback LR. 1979. Remision of seizures and relapse in patients with epilepsy. Epilepsia 20(6): 729–737. [DOI] [PubMed] [Google Scholar]
- Arroll B, Macgillivray S, Ogston S, et al. 2005. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: A meta-analysis. The Annals of Family Medicine 3(5): 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa D, Shibre T, Asher L, Fekadu A. 2012. Internalized stigma among patients with schizophrenia in Ethiopia: a cross-sectional facility-based study. BMC Psychiatry 12: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awas M, Kebede D, Alem A. 1999. Major mental disorders in Butajira, southern Ethiopia. Acta Psychiatrica Scandinavica Supplement 397: 56–64. [DOI] [PubMed] [Google Scholar]
- Ayuso-Mateos JL, Recacha PG, Haro JM, et al. 2008. Reducing the Burden of Mental Illness in Spain: Population-Level Impact and Cost-Effectiveness of Treatments in Depression and Schizophrenia. Fundación BBVA. [Google Scholar]
- Baltussen RM, Hutubessy RC, Evans DB, Murray CJ. 2002. Uncertainty in cost-effectiveness analysis. Probabilistic uncertainty analysis and stochastic league tables. International Journal of Technology Assessessment in Health Care 18: 112–9. [PubMed] [Google Scholar]
- Bowden CL, Brugger AM, Swamm AC, et al. 1994. Efficacy of divalproex vs lithium and placebo in the treatment of mania. Jama 271(12): 918–924. [PubMed] [Google Scholar]
- Bowden CL, Calabrese JR, McElroy SL, et al. 2000. A randomized, placebo-controlled 12-month trial of divalproex and lithium of outpatients with bipolar I disorder. Archives of general psychiatry. 57(5): 481–489 [DOI] [PubMed] [Google Scholar]
- Bruckner TA, Scheffler RM, Shen G, et al. 2011. The mental health workforce gap in low- and middle-income countries: a needs based approach. Bulletin of the World Health Organization 89: 184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm D, Gureje O, Saldivia S, et al. 2008. Schizophrenia treatment in the developing world: An interregional and multinational cost-effectiveness analysis. Bulletin of the World Health Organization 86: 542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm D, Saxena S. 2012. Cost effectiveness of strategies to combat neuropsychiatric conditions in sub-Saharan Africa and South East Asia: mathematical modelling study. Br Med J 344: e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerell OC, Sander J, Hart YM, et al. 1995. Remission of epilepsy: results from the National General Pratice Study of Epilepsy. The Lancet. 346(8968): 140–144. [DOI] [PubMed] [Google Scholar]
- Commision on Macroeconomics and Health. 2001. Macroeconomics and Health: Investing in Health for Economic Development. Geneva: World Health Organization. [Google Scholar]
- Daniels N, Sabin JE. 2002. Setting Limits Fairly: Can We Learn to Share Medical Resources? Oxford: OUP Catalogue. [Google Scholar]
- Dixon J, Welch HG. 1991. Priority setting: Lessons from Oregon. The Lancet 337: 891–4. [DOI] [PubMed] [Google Scholar]
- Edejer TT-T, Baltussen R, Adam T, et al. 2012. WHO Guide to Cost-Effectiveness Analysis. Geneva: WHO. [Google Scholar]
- Federal Democratic Republic of Ethiopia Ministry of Health. 2012. National Mental Health Strategy 2012/13–2015/16. In: Health Federal Democratic Republic of Ethiopia Ministry of Health (ed). Ethiopia: Addis Abeba. [Google Scholar]
- Fekadu A, Alem A, Medhin G, Shibre T, Cleare A, et al. 2007. Utility of the concept of minor depressive disorder: evidence from a large rural community sample in a developing country setting. J Affect Disord 104: 111–118. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Miklowitz DJ. 2013. Treatment of bipolar disorder. The Lancet 381: 1672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JR, Carney SM, Davis C, et al. 2003. Relaps prevention with antidepressant drug treatment in depressive disorders: a systematic review. The Lancet. 361(9358) 653–661. [DOI] [PubMed] [Google Scholar]
- Goodridge DM, Shorvon SD. 1983. Epileptic seizures in a population of 6000. I: Demography, diagnosis and classification, and the role of the hospital services. BMJ 287(6393) 641–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. 1990. Manic. Depressive Illness. New York, NY: Oxford University Press. [Google Scholar]
- Gureje O, Chisholm D, Kola L, Lasebikan V, Saxena S. 2007. Cost-effectiveness of an essential mental health intervention package in Nigeria. World Psychiatry 6: 42–8. [PMC free article] [PubMed] [Google Scholar]
- Hutubessy RC, Baltussen RM, Evans DB, Barendregt JJ, Murray CJ. 2001. Stochastic league tables: Communicating cost-effectiveness results to decision-makers. Health Economics 10: 473–7. [DOI] [PubMed] [Google Scholar]
- Hyman S, Chisholm D, Kessler R, Patel V, Whiteford H. 2006. Mental disorders. In: Disease Control Priorities Related to Mental, Neurological, Developmental and Substance Abuse Disorders. Geneva: World Health Organization, 1–20. [Google Scholar]
- Kapiriri L, Arnesen T, Norheim OF. 2004. Is cost-effectiveness analysis preferred to severity of disease as the main guiding principle in priority setting in resource poor settings? The case of Uganda. Cost Effectiveness and Resource Allocation 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapiriri L, Norheim OF. 2004. Criteria for priority-setting in health care in Uganda: Exploration of stakeholders’ values. Bulletin of the World Health Organization 82: 172–9. [PMC free article] [PubMed] [Google Scholar]
- Kebede D, Alem A. 1999a. Major mental disorders in Addis Ababa, Ethiopia. I. Schizophrenia, schizoaffective and cognitive disorders. Acta Psychiatrica Scandinavica Supplement 397: 11–7. [DOI] [PubMed] [Google Scholar]
- Kebede D, Alem A. 1999b. Major mental disorders in Addis Ababa, Ethiopia. II. Affective disorders. Acta Psychiatrica Scandinavica Supplement 397: 18–23. [DOI] [PubMed] [Google Scholar]
- Kebede D, Alem A, Rashid E. 1999. The prevalence and socio-demographic correlates of mental distress in Addis Ababa, Ethiopia. Acta Psychiatrica Scandinavica Supplement 397: 5–10. [DOI] [PubMed] [Google Scholar]
- Kebede D, Alem A, Shibire T, et al. 2006. Symptomatic and functional outcome of bipolar disorder in Butajira, Ethiopia. Journal of Affective Disorders 90: 239–49. [DOI] [PubMed] [Google Scholar]
- Kebede D, Alem A, Shibre T, et al. 2003. Onset and clinical course of schizophrenia in Butajira-Ethiopia. Social Psychiatry and Psychiatric Epidemiology, 38: 625–31. [DOI] [PubMed] [Google Scholar]
- Lam DH, Burbeck R, Wright K, Pilling S. 2009. Psychological therapies in bipolar disorder: the effect of illness history on relapse prevention - a systematic review. Bipolar Disorder. 11(5): 474–482. [DOI] [PubMed] [Google Scholar]
- Leutch S, Pitschel-Walz G, Abraham D, Kissling W. 1999. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapin, risperidone and sertindole compared to conventional antipsychotics and placebo: A meta-analysis of randomized controlled trials. Schizophrenia research. 35(1): 51–68 [DOI] [PubMed] [Google Scholar]
- Lund C, Alem A, Schneider M, et al. 2015. Generating evidence to narrow the treatment gap for mental disorders in sub-Saharan Africa: Rationale, overview and methods of AFFIRM. Epidemiology and Psychiatric Sciences 24: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C, Breen A, Flisher AJ, et al. 2010. Poverty and common mental disorders in low and middle income countries: A systematic review. Social Science and Medicine 71: 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maat SM, Dekker J, Schoevers RA, de Jonghe F. 2007. Relative efficacy of psychotherapy and combined therapy in the treatment of depression: a meta-analysis. European Psychiatry. 22(1): 1–8 [DOI] [PubMed] [Google Scholar]
- mhGAP-Ethiopia Working Group. 2010. Mental Health Gap Action Programme Ethiopia: Final document. In: Health FDRoEMo (ed). Ethiopia: Addis Abeba. [Google Scholar]
- Mojtabai R, Nicholoson RA, Carpenter BN. 1998. Role of psychosocial treatments in management of schizophrenia: A meta-analytic review of controlled outcome studies. Schizophrenia Bullitin. 24(4): 569. [DOI] [PubMed] [Google Scholar]
- National Institute of Health and Clinical Excellence. 2006. Bipolar Disorder: The Management of Bipolar Disorder in Adults, Children and Adolescents, in Primary and Secondary Care. London: National Institute of Health and Clinical Excellence. [Google Scholar]
- National Institute of Health and Clinical Excellence. 2009a. Core Interventions in the Treatment and Management of Schizophrenia in Adults in Primary and Secondary Care. London: National Institute of Health and Clinical Excellence. [Google Scholar]
- National Institute of Health and Clinical Excellence. 2009b. Depression in Adults: NICE Guidelines. London: National Institute of Health and Clinical Excellence. [Google Scholar]
- Negash A, Alem A, Kebede D, et al. 2005. Prevalence and clinical characteristics of bipolar I disorder in Butajira, Ethiopia: a community-based study. Journal of Affective Disorders 87: 193–201. [DOI] [PubMed] [Google Scholar]
- Nord E. 1993. The trade-off between severity of illness and treatment effect in cost-value analysis of health care. Health Policy, 24: 227–38. [DOI] [PubMed] [Google Scholar]
- Norheim OF, Johri M, Chisholm D, et al. 2013. Guidance on Priority Setting in Health Care (GPS Health): Fairness and Equity Criteria not Adequateky Captured by Cost-Effectiveness Analysis. Geneva: World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen T. 2013. Lifetime QALY prioritarianism in priority setting. Journal of Medical Ethics 39: 175–80. [DOI] [PubMed] [Google Scholar]
- Placenica M, Sander JW, Roman M, et al. 1994. The caracteristics of epilepsy in a largely untreated population in rural Ecuador. Journal of Neurology, Neurosurgery & Psychiatry. 57(3): 320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Patel V, Saxena S, et al. 2007. No health without mental health. Lancet 370: 859–77. [DOI] [PubMed] [Google Scholar]
- Reinap M, Lai T, Janno S, Tamme T, Tamm M. 2005. Cost-Effectiveness of Mental Health Interventions in Estonia. Tallinn: Healthcare Society in Estonia. [Google Scholar]
- Revill P, Walker S, Madan J, et al. 2014. Using Cost-Effectiveness Thresholds to Determine Value for Money in Low-and Middle-Income Country Healthcare Systems: Are Current International Norms Fit for Purpose? UK: University of York. [Google Scholar]
- Salomon JA, Carvalho N, Gutierrez-Delgado C, et al. 2012a. Intervention strategies to reduce the burden of non-communicable diseases in Mexico: Cost effectiveness analysis. Br Med J 344: e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon JA, Vos T, Hogan DR, et al. 2012b. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 380: 2129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Thornicroft G, Knapp M, Whiteford H. 2007. Resources for mental health: scarcity, inequity, and inefficiency. Lancet 370: 878–89. [DOI] [PubMed] [Google Scholar]
- Shibre T, Negash A, Kullgren G, et al. 2001. Perception of stigma among family members of individuals with schizophrenia and major affective disorders in rural Ethiopia. Social Psychiatry and Psychiatric Epidemiology 36: 299–303. [DOI] [PubMed] [Google Scholar]
- Tekle-Haimanot R, Forsgren L, Ekstedt J. 1997. Incidence of epilepsy in rural central Ethiopia. Epilepsia 38: 541–6. [DOI] [PubMed] [Google Scholar]
- Tondo L, Hennen J, Baldessarini R. 2001. Lower suicide risk with long-term lithium treatmnet in major affective illness: a meta-analysis. Acta Psychiatrica Scandinavica. 1043: 163–172 [DOI] [PubMed] [Google Scholar]
- Tudur Smith C, Marson AG, Williamson PR. 2003. Carbamazepine versus phenobarbitone monotherapy for epilepsy. The Cochrane Library [DOI] [PubMed] [Google Scholar]
- United Nations, Depatment of Economics and Social Affairs, Population Devision. 2011. Population database.
- Verguet S, Laxminarayan R, Jamison DT. 2014. Universal public finance of tuberculosis treatment in India: An extended cost-effectiveness analysis. Health Economics 24: 318–32. [DOI] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, et al. 2012. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. 2014. Data retrieved June 15 2014 from World Development Indicators Online (WDI) database.
- World Health Organization. 2006. Dollars, DALYs and Decisions: Economic Aspects of Mental Health Systems. Geneva: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.