Abstract
Introduction
To investigate whether there was a protective effect of melatonin on apoptotic mechanisms after an acute unilateral obstruction of the kidney.
Material and methods
A total of 25 rats consisting of five groups were used in the study, designated as follows: Group 1: control, Group 2: sham, Group 3: unilateral ureteral obstruction treated with only saline, Group 4: unilateral ureteral obstruction treated with melatonin immediately, and Group 5: unilateral obstruction treated with melatonin one day after obstruction. Melatonin was administered as a 10 mg/kg dose intraperitoneally. The kidneys were evaluated according to the apoptotic index and Ki-67 scores.
Results
Comparison of all obstruction groups (Group 3, 4, and 5), revealed that the apoptotic index was significantly higher in Groups 1 and 2. Despite melatonin reduced apoptotic mechanisms in Groups 4 and 5, there was no significant difference between Groups 4 and 5 in terms of the reduction of apoptosis. However, the reduction of apoptosis in the melatonin treated group did not decrease to the level of Groups 1 and 2.
Conclusions
Despite melatonin administration, which significantly reduces the apoptotic index occurring after acute unilateral ureteral obstruction, the present study did not observe a return to normal renal histology in the obstruction groups.
Keywords: melatonin, kidney, obstruction, apoptosis, hypoxia, antioxidant
INTRODUCTION
Acute obstructive uropathy occurs mainly due to urinary tract stones that are mostly encountered in all age groups in the daily practice of urology and it is often reversible [1]. Unilateral ureteral obstruction (UUO) is one of the most common etiological factors [2]. Obstruction causes histopathological changes (massive tubular dilatation, apoptotic tubular cell deletion, and progressive tubulointerstitial fibrosis) in the kidney [3]. The pathophysiological changes in UUO such as decreased blood flow and glomerular filtration rates, elevated intra-pelvic pressure, and vasoactive and inflammatory responses are very well established in animal models [3]. The impairment effect of hypoxia and ischemia in many organs such as the liver, brain, heart, and kidney has been well known for many years [4]. Hypoxia increases the reactive oxygen species (ROS) and this may contribute to the abnormal cellular dysfunction, thus starting the cascade of apoptosis in the damaged tissue [5].
Melatonin (N-acetyl-5-methoxytryptamine) secreted from the pineal gland is a widespread physiological mediator that acts to synchronize the biological clock and has antioxidant activity and anticancer activity [6, 7]. It has been shown that melatonin is also secreted from other tissues including the ovary, testes, bone narrow, gut, placenta, and liver [8]. Melatonin's role in reducing oxidative stress and lipid peroxidation has been evaluated in experimental animal models [9]. Its antioxidant effect is seen in scavenging both the reactive oxygen species (ROS) and the reactive nitrogen species (RNS) [10]. However, there are only a few reports about the protective effect of melatonin on UUO promoted nephropathy. The aim of this study was to investigate whether there was a protective effect of melatonin on apoptotic mechanisms after an acute unilateral obstruction of the kidney.
MATERIAL AND METHODS
Animals
Twenty-five male Wistar rats (150–200 grams) were used. The animals were housed at 18–22°C under a 12 hour light/12 hour dark cycle. The rats had free access to a diet of standard pellets and tap water (ad libitum feeding) throughout the study. All protocols were approved by the institutional animal ethics committee.
The animals were divided into 5 groups. Melatonin (M5250, Sigma, Rehovot, Israel) 10 mg/kg/day was prepared in 5 ml saline injected intraperitoneally. Group 1 (control, n = 5, no drug administration without obstruction), Group 2 (n = 5, sham operated without obstruction), Group 3 (n = 5, unilateral ureteral obstruction without melatonin administration, only saline administered), Group 4 (n = 5, melatonin 10 mg/kg per day intra-peritoneal route for one week at the time of unilateral ureteral obstruction) and Group 5 (n = 5, melatonin 10 mg/kg per day intra-peritoneal route for one week started one day after the unilateral ureteral obstruction).
Surgery and experimental protocol
The rats in groups 2 to 5 were anaesthetized with an intramuscular injection of xylazine (10 mg/kg) and ketamine (70 mg/kg). The abdominal area was prepared with betadine, and sterile drapes were applied. A midline incision was made and the left ureter was exposed on the left psoas muscle. The ureter was embedded into the psoas muscle with a 3/0 non-absorbable silk suture. The abdomen was then closed in two layers. The animals received 50 ml/kg of warm saline instilled into the abdominal cavity during the entire procedure. The animals were then allowed to recover. Seven days after the obstruction, all animals in each group were anaesthetized with xylazine (10 mg/kg) and ketamine (50 mg/kg) and the abdominal wall was re-opened. Left nephrectomies were carried out immediately and the left kidney was stored in 10% formalin for the histological examination. The abdomen was then closed in two layers. The animals received 50 ml/kg of warm saline instilled into the abdominal cavity during the entire procedure.
Histopathological examination
Kidney tissues were fixed in a 10% neutral phosphate-buffered formalin solution in order to be evaluated under light microscopy. Following the dehydration process in an ascending series of ethanol (70%, 80%, 96%, 100%), tissue samples were washed in xylene and then embedded in paraffin. Tissue sections of 4 µm were stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). H&E and periodic acid-Schiff (PAS)-stained sections were observed under light microscopy in order to establish the glomerular, tubular, interstitial, and vascular lesions.
The ApopTagPlus Peroxidase in situ apoptosis detection kit (Chemicon, S7101) was used in order to evaluate the apoptotic nuclei. The sections were dewaxed and treated with proteinase K (20 µg/ml) for 15 minutes at room temperature (RT), then incubated with an equilibration buffer for 10 minutes, followed by incubation with a working-strength TdT enzyme solution at 37°C for 60 minutes. The reaction was terminated by incubating the samples in the working-strength stop/wash buffer for 10 minutes at RT. Furthermore, the sections were incubated with anti-digoxigenin conjugate for 30 minutes at RT and then incubated with diaminobenzidine (DAB) for 5 min at RT. The sections were counterstained with hematoxylin and examined under the light microscope. As a negative control, sections were incubated in the absence of TdT enzymes. Germinal centers of hyperplastic lymph nodes served as a positive control. The number of TUNEL-positive tubular cells was counted in 50 non-overlapped fields in each section under 400× magnification (∼0.1 cm2). Apoptosis was also evaluated by using morphological criteria, which include the eosinophilic cytoplasm, cytoplasmic shrinkage, nuclear fragmentation, nuclear chromatin condensation, and formation of apoptotic bodies [11].
Dako's Ki-67 antibody (clone MIB-1, M7240) at a 1:100 dilution was used in order to investigate proliferative activity of the renal tubular cells. Tissue sections were cut at 5 µm, dewaxed, and hydrated in xylene and ethanol before the antigen retrieval. Antigen retrieval was performed by placing the slides in a 0.01M citrate buffer (pH 6.0) and heating in a microwave at 750W for 4 to 5 minutes to obtain the optimal antigen exposure. Endogenous peroxidase was quenched with a 3% solution of hydrogen peroxide in distilled water for 5 minutes. The slides were then incubated with the primary antibody at RT for 60 minutes, and the immunoreactivity was demonstrated by using the LSAB2 kit (Dako, K0675, Glostrup, Denmark), followed by incubation in the 3-3’-diaminobenzidine–hydrogen peroxide solution. The slides were counterstained with hematoxylin, dehydrated, and mounted. Cells that exhibited immunoreactivity were expressed as a percentage of the total number of counted tubular cells. The results of the Ki-67 staining were recorded as the percentage of 1000 epithelial cells displaying positive nuclear staining. Apoptotic Index (AI) was calculated, i.e. the number of apoptotic cells as a percentage of the total number of cells.
Statistical analysis
All statistical analyses were performed by using the SPSS for Windows, version 16 (Chicago, IL, USA) and a probability value of less than 0.05 was accepted as statistically significant. Data were expressed as mean standard deviation (SD) or ranges. The Kruskal–Wallis one-way analysis of variance by ranks was used for a simultaneous statistical test of the pathologic score for all obstruction groups. When the null hypothesis could be rejected, the comparisons between the two groups were made by using the Mann–Whitney U non-parametric test for independent samples.
RESULTS
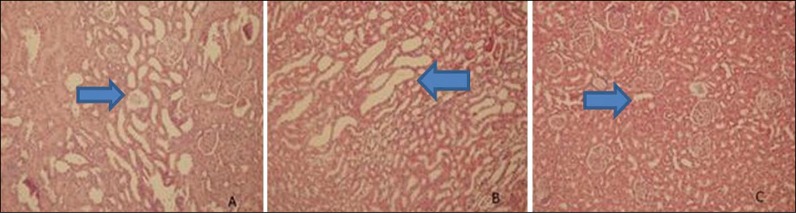
A moderate dilatation of the collecting ducts and distal tubules was observed in obstructed kidney sections in histopathological examination (Figure 1A). Dilated renal tubules were lined by flattened tubular epithelial cells. Distal tubules and collecting ducts were also dilated in melatonin treated groups (Figure 1B). We did not detect any unusual histopathological features in the renal tissues of animals in both the control and sham-operated groups (Figure 1C). Glomerular pathology was not observed in any section. Scanty lymphocytes were present in the interstitium. Vascular structures were normal in all samples.
Figure 1.
Histopathological examination of renal tubules and collecting ducts. A – tubular dilatation at obstructed kidneys; B – tubular dilatation at melatonin treated obstructed kidneys; C – normal tubules and collecting ducts at control and sham groups.
The TUNEL assay was performed in paraffin-embedded sections in order to determine the renal tubular apoptosis. The number of apoptotic tubular cells, as determined with the TUNEL-assay, markedly increased after 24 hours of UUO.
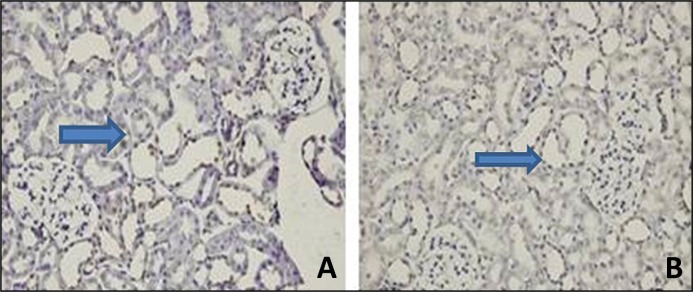
Melatonin treatment significantly decreased the number of TUNEL-positive cells in the obstructed kidney (Figure 2).
Figure 2.
Effect of melatonin pretreatment on apoptosis induced by unilateral ureteral obstruction. Apoptosis was evaluated by TUNEL staining. Positive cells are show as a dark brown nuclear stain. Light photomicrographs (magnification, x40) of kidney sections from A the obstruction alone groups. B – melatonin treated obstructed groups.
All obstructive groups, including the drug-administered groups, had a higher apoptotic index compared to Groups 1 and 2 (Table 1). Melatonin also reduced the apoptotic index in Groups 4 and 5. However, the reduction of apoptosis due to melatonin in Groups 4 and 5 was not statistically significant. Furthermore, when compared to Groups 1 and 2, melatonin failed to attain normal renal histology in the obstruction treated groups. Melatonin decreased apoptosis, even when given synchronously or after the ureteral obstruction.
Table 1.
Comparison of apoptotic index (AI) and Ki-67 scores with other groups
| n | Apoptotic index | Ki-67 | |
|---|---|---|---|
| Control | 5 | 42 (25-58) | 3 (2-5) |
| Sham | 5 | 42 (28-54) | 3 (2-4) |
| Obstruction | 5 | 273 (187-378) | 2 (1-3) |
| Obs + melatonin (at the obstruction time) | 5 | 116 (68-195) | 3 (2-5) |
| Obs - > melatonin (one day after obstruction) | 5 | 143.5 (101-195) | 10 (8-13) |
Statistic tests performed by Mann-Whitney test, p < 0.001
Obstruction alone or with melatonin administration does not affect Ki-67. Additionally, Ki-67 was found to be higher in the melatonin administered after obstruction group when compared with the other groups. Also, Ki-67 was significantly higher in Group 5 compared to Groups 3 and 4. Statistical comparison results of the apoptotic index (AI) and Ki-67 of each group are shown in Table 2.
Table 2.
Statistical comparison of apoptotic index (AI) and Ki-67 regarding in each groups
| Groups | Apoptosis | Ki-67 |
|---|---|---|
| 1-2 | 0.841 | 0.421 |
| 1-3 | 0.003 | 0.106 |
| 1-4 | 0.002 | 0.435 |
| 1-5 | 0.002 | 0.002 |
| 2-3 | 0.003 | 0.432 |
| 2-4 | 0.002 | 1.000 |
| 2-5 | 0.002 | 0.002 |
| 3-4 | 0.001 | 0.336 |
| 3-5 | 0.001 | 0.000 |
| 4-5 | 0.195 | 0.000 |
Statistic tests performed by Mann-Whitney test, p < 0.001
DISCUSSION
UUO is one of the most common problems in daily urology practice [1]. Renal function decreases and interstitial fibrosis and inflammation, tubular apoptosis, and leukocyte infiltration increases in UUO [12]. Chung et al. have reported that UUO decreased renal blood flow, and increased renal vascular resistance and reactive oxygen species (ROS) [1]. Gillenwater et al. have reported a 20–70% decrease in glomerular filtration rates (GFR) and a 25% decrease in renal blood flow (RBF) compared to normal levels [13]. Although these hemodynamic changes have not been precisely described, many studies proved that monocystic infiltration of interstitial compartments was associated with renal injury [14]. Macrophage infiltration in obstructive nephropathy was capable of releasing different products such as proteolytic enzymes, ROS, platelet derived growth factor, cyclooxygenase, and lipooxygenase products that play an active role in the establishment of interstitial fibrosis [14].
Apoptosis is influenced by both intrinsic and extrinsic factors, and free radicals and radiation are thought to trigger apoptosis via the intrinsic pathway [15]. The imbalance between oxidants and antioxidants in favor of the oxidants is termed ‘oxidative stress’ [4]. Oxygen radicals account for most major free radicals that are formed in the cell [15].
Ischemia-reperfusion (I/R) injuries in kidneys are well known in the surgical era, but I/R injury in obstructive nephropathy has not yet been extensively studied. Recent reports have indicated that high amounts of ROS were produced after the resolution of renal obstruction [16]. Moreover, the reactive oxygen species, which increases after UUO, are known to reduce the threshold of tissues that die due to apoptosis [17]. Malik et al. have also shown that the stretching of the renal tubular cells by transmission of high hydrostatic pressure and the accompanying ischemia can provide a powerful stimulus for apoptosis in obstructed neonatal rat kidneys [18]. Recently, the apoptotic effect of the reactive free oxygen radicals on the renal tubular cells have been shown in a rabbit model [19]. Similar to our previous studies, we again preferred rat models to investigate the effects of UUO on kidney tissues.
Melatonin (N-acetyl-5-methoxytryptamine) is a well-known physiological mediator that exists in most organisms ranging from bacteria to humans [20]. It is secreted from the pineal gland of vertebrates, but it is also known to be produced in many other tissues [20]. It has been shown that despite the diminishing levels of the melatonin in the elderly, exogenous administration of melatonin reduces lipid peroxidation [8]. Melatonin shows its effect in both direct and indirect ways. It directly acts as a scavenger against free radicals and related products [21]. Importantly, melatonin shows an indirect antioxidative action via stimulation of the cellular antioxidant defense system by increasing mRNA levels and activating several important antioxidant enzymes, including superoxide dismutase and glutathione. By doing so, melatonin reduces the activity of the pro-oxidative enzyme nitric oxide synthase and diminishes the free radical formation at the mitochondrial level by reducing the leakage of electrons from the electron transport chain [20]. We administered melatonin to Group 4 at the time of the obstruction and to Group 5 one day after the obstruction in order to compare the effect of melatonin on immediate and delayed administration.
Ki-67 is a monoclonal antibody that is associated with cell proliferation and was first described by Gerdes [22]. The presence of Ki-67 in all phases of cell division except G0 makes it an excellent marker for determining cell growth in target cells, especially in cancer cells [23]. The Ki-67 staining and tubule histology suggest a substantial diminution in tubule regeneration in mice and suppression of macrophages during their repair phase [24]. Thus, the AI is an indicator of the rate of toxin build-up at the cellular level. A high AI indicated that the cellular environment required frequent ‘housekeeping’. A low AI would, therefore, indicate that the rate of cellular toxin build-up was low [25]. In our study, Ki-67 was elevated only when melatonin was administered to the obstruction group one day later. All other four groups showed no statistically different effect on Ki-67 (p <0.05). Melatonin decreased the apoptosis but not in the control/sham groups’ level (p <0.05).
As a conclusion, the melatonin administration significantly reduces the apoptotic mechanisms after an acute unilateral ureteral obstruction. Despite this, melatonin has failed to restore normal renal hystopathology in obstruction treated groups.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Yeh CH, Chiang HS, Lai TY, Chien CT. Unilateral ureteral obstruction evokes renal tubular apoptosis via the enhanced oxidative stress and endoplasmic reticulum stress in the rat. Neurourol Urodyn. 2011;30:472–479. doi: 10.1002/nau.20855. [DOI] [PubMed] [Google Scholar]
- 2.Wen JG, Frøkiaer J, Jørgensen TM, Djurhuus JC. Obstructive nephropathy: An update of the experimental research. Urol Res. 1999;27:29–39. doi: 10.1007/s002400050086. [DOI] [PubMed] [Google Scholar]
- 3.Gao X, Mae H, Ayabe N, Takai T, Oshima K, Hattori M. Hepatocyte growth factor gene therapy retards the progression of chronic obstructive nephropathy. Kidney Int. 2002;62:1238–1248. doi: 10.1111/j.1523-1755.2002.kid579.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 2006;69:2029–2036. doi: 10.1038/sj.ki.5000161. [DOI] [PubMed] [Google Scholar]
- 5.Chien CT, Lee PH, Chen CF, Ma MC, Lai MK, Hsu SM. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J Am Soc Nephrol. 2001;12:973–985. doi: 10.1681/ASN.V125973. [DOI] [PubMed] [Google Scholar]
- 6.Dun-Xian Tan, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- 7.Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology. 2014;29:325–333. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 8.Garcia JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res. 2014;56:225–237. doi: 10.1111/jpi.12128. [DOI] [PubMed] [Google Scholar]
- 9.Atılgan D, Parlaktas BS, Uluocak N, Erdemir FE, Firat FF, Erkorkmaz UE. Effects of melatonin on partial unilateral ureteral obstruction induced oxidative injury in rat kidney. Urol Ann. 2012;4:89–93. doi: 10.4103/0974-7796.95552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J Pineal Res. 2013;54:245–257. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 11.Labat-Moleur F, Guillermet C, Lorimier P, et al. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. J Histochem Cytochem. 1998;46:327–334. doi: 10.1177/002215549804600306. [DOI] [PubMed] [Google Scholar]
- 12.Sakai T, Kawamura T, Shirasawa T. Mizoribine improves renal tubulointerstitial fibrosis in unilateral ureteral obstruction (UUO)-treated rat by inhibiting the infiltration of macrophages and the expression of alpha-smooth muscle actin. J Urol. 1997;158:2316–2322. doi: 10.1016/s0022-5347(01)68242-9. [DOI] [PubMed] [Google Scholar]
- 13.Gillenwater JY. The pathophysiology of urinary tract obstruction. In: Walsh PC, Retik AB, Stamey TA, Vaughn ED Jr, editors. Campbell's Urology. 8th ed. Philadelphia: WB Saunders; 2002. pp. 499–505. [Google Scholar]
- 14.Harris KP, Schreiner GF, Klahr S. Effect of leukocyte depletion on the function of the postobstructed kidney in the rat. Kidney Int. 1989;36:210–215. doi: 10.1038/ki.1989.181. [DOI] [PubMed] [Google Scholar]
- 15.Koç A, Ünal D, Cimentepe E, et al. The effects of antioxidants on testicular apoptosis andoxidative stress produced by cell phones. Turk J Med Sci. 2013;43:131–137. [Google Scholar]
- 16.Hardeland R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–130. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 17.Bayrak O, Cimentepe E, Karatas OF, et al. Ovarian apoptosis after shock wave lithotripsy for distal ureteral stones. Urol Res. 2009;37:69–74. doi: 10.1007/s00240-009-0172-x. [DOI] [PubMed] [Google Scholar]
- 18.Malik RK, Thornhill BA, Chang AY, Kiley SC, Chevalier RL. Renal apoptosis parallels ceramide content after prolonged ureteral obstruction in the neonatal rat. Am J Physiol Renal Physiol. 2001;281:F56–61. doi: 10.1152/ajprenal.2001.281.1.F56. [DOI] [PubMed] [Google Scholar]
- 19.Cimentepe E, Eroglu M, Ozturk U, et al. Renal apoptosis after shockwave application in rabbit model. J Endourol. 2006;20:1091–1095. doi: 10.1089/end.2006.20.1091. [DOI] [PubMed] [Google Scholar]
- 20.Acuna-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC. Extrapineal melatonin: sources, regulations and potential functions. Cell Mo Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez AB, Nogales G, Marchena JM, Ortega E, Barriga C. Suppression of both basal and antigen-induced lipid peroxidation in ring dove heterophils by melatonin. Biochem Pharmaco. 1999;58:1301–1306. doi: 10.1016/s0006-2952(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 22.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 23.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 24.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am SocNephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen BL, Kiecolt-Glaser JK, Glaser R. Abiobehavioral model of cancer stress and disease course. Am Psychol. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]