Abstract
Introduction
Vitamin D controls calcium and phosphate homeostasis. Additionally, it has been proven that vitamin D is an important modulator of cellular differentiation and proliferation in a number of normal and malignant cells. Vitamin D can regulate proliferation, apoptosis, and cell adhesion at the tumor cell level. It also modifies tumor angiogenesis, invasion, and metastasis and also decreases oxidative DNA damage.
Material and methods
The Medline and Web of Science databases were searched without time limit on October 2015 using the terms ‘vitamin D’ in conjunction with ‘kidney cancer’, ‘bladder cancer’, ‘prostate cancer’, and ‘testis cancer’. Autoalerts in Medline were also run and reference lists of original articles, review articles, and book chapters were searched for further eligible articles.
Results
In recent years, vitamin D has received vast attention due to suggestions that it may have a crucial role in the prevention and therapy of various cancers. Many epidemiologic studies have reported the impact of VD3 on preventing several cancers and other pathologies. Assuming that vitamin D status changes cancer risk, enough vitamin D supply would be an easy, economical, and safe cancer incidence and mortality reduction method. However, despite numerous researches, the role of vitamin D in cancer incidence and therapy remains unclear.
Conclusions
The impact of vitamin D is well described in breast, colon, and prostate cancer; yet, there is only little published about other malignancies.
Keywords: cancer, urology, vitamin D, vitamin D receptor
INTRODUCTION
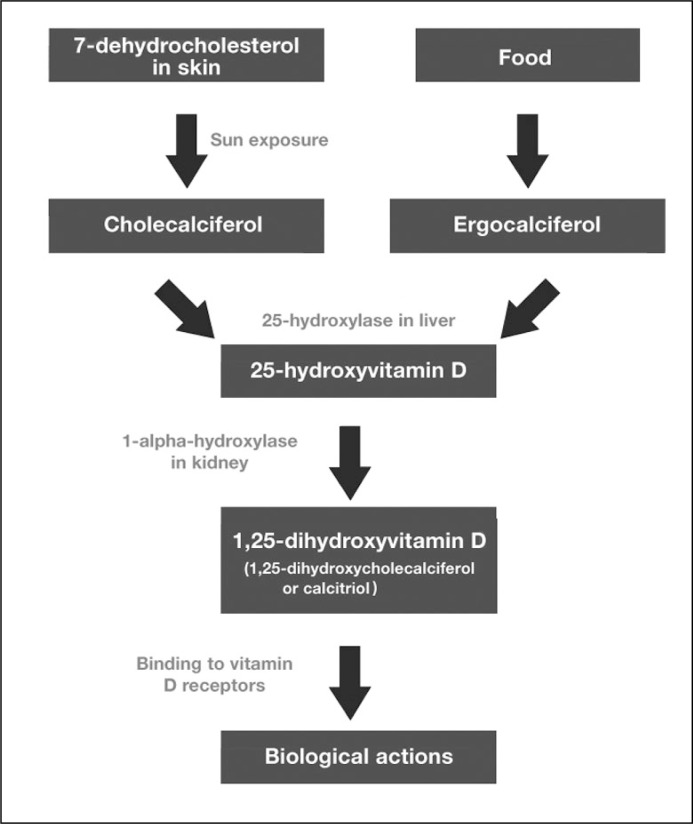
The term vitamin D (VD) refers to a group of fat-soluble secosteroids mediating numerous actions in many tissues of the human body. Cholecalciferol, a member of the VD family, can be both synthesized in skin, which is exposed to sunlight ultraviolet radiation from 7-dehydrocholesterol, or ingested with food (Figure 1). Ergocalciferol, the other VD family representative, is obtained by dietary intake from vegetable sources or oral supplements. Dietary absorbed or dermally synthetized cholecalciferol and ergocalciferol are biologically inactive and require enzymatic hydroxylation in the liver and kidney. Both compounds are hydroxylated to 25-hydroxycholecalciferol (calcidiol or 25(OH)D) in the liver. Calcidiol is then transported by α-globulin to the proximal tubules of the kidneys, where it is hydroxylated at the 1-α position by 25-hydroxyvitamin D3 1-alpha-hydroxylase to the biologically active calcitriol (1,25(OH)2D3 or VD3) [1, 2].
Figure 1.
Vitamin D pathways.
25-hydroxyvitamin D can be measured by enzyme immunoassay (EIA) and radioimmunoassay (RIA), or chemiluminescence assay, which provide a total 25-hydroxyvitamin D concentration. Additionally, high-performance liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LC/MS), and liquid chromatography-tandem mass spectrometry (LC/MS/MS) directly detect 25-hydroxyvitamin D concentrations. The latter methods can separate both 25-hydroxyvitamin D2 and D3, and hence can be useful when vitamin D2 therapy is used.
Calcitriol binds to nuclear VD receptor (VDR). VDR is a member of the steroid–thyroid–retinoid receptor family of ligand-activated transcription factors. VDR is present in most cells in the body and calcitriol directly or indirectly regulates as much as 3–5% of the human genome [3]. Apart from the influence of VD on calcium and phosphate homeostasis, it has been proven that VD is an important modulator of cellular differentiation and proliferation in a number of normal and malignant cells. In tumor cels, VD can regulate proliferation, apoptosis, and cell adhesion at the cell level. It also modifies tumor angiogenesis, invasion, and metastasis and also decreases oxidative DNA damage [4–7]. Calcitriol demonstrates antiproliferative effects through the increase of cyclin-dependent kinase (CDK) inhibitors p21 and p27 expression, and decrease in CDK activity, leading directly to G0/G1cell cycle arrest [8, 9, 10]. It also modulates the intracellular kinase pathways, such as p38 MAPK, ERK, and PI3K [3]. Calcitriol and its analogues inhibit the high telomerase activity that is seen in human cancer cells through telomerase reverse transcriptase (TERT) mRNA expression [11]. Calcitriol also induces apoptosis, mainly through the stimulation of pro-apoptotic gene BAX and suppression of anti-apoptotic genes, such as BCL2 [12]. Another important biological role is anti-inflammatory action – the key mechanisms include inhibition of prostaglandin synthesis (via cyclooxygenase 2 suppression), prostaglandin signalling (through decrease of prostaglandin receptors expression) [13], and Nf-KB signaling pathway [14]. In response to calcitriol, some malignant cells acquire a more mature phenotype, which suggests its pro-differentiating effect. The mechanism of action includes regulation of β-catenin, PI3K, and JUNN-terminal kinase signaling pathways. By the control of matrix metalloproteinases (MMPs) activity, calcitriol inhibits tumors’ invasion and metastasis [15].
Multiple recent reviews have been published about VD and cancer incidence [4, 16, 17]. Some observational, preclinical, and clinical studies suggest that VD deficiency increases the risk of developing multiple malignancies [3]. In addition, VD and its metabolites can be used in anti-tumor therapy.
MATERIAL AND METHODS
The Medline and Web of Science databases were searched without time limit on October 2015 using the terms ‘vitamin D’ in conjunction with ‘kidney cancer’, ‘bladder cancer’, ‘prostate cancer’, and ‘testis cancer’. Boolean operators (NOT, AND, OR) were also used in succession to narrow and broaden the search. Autoalerts in Medline were also run, and reference lists of original articles, review articles, and book chapters were searched for further eligible articles. The search was limited to the English, Polish, German, and Spanish literature. Articles that did not address the topics were excluded, and the full text of the remaining articles was reviewed.
This paper presents a review of the current knowledge on the effects of vitamin D on the pathogenesis and treatment options in prostate, kidney, bladder, and testicular cancers.
Renal cancer
Several biological links might support the VD influence on renal cell cancer (RCC) risk and RCC survival. It was shown in in-vitro and in-vivo studies that VD inhibits RCC cells proliferation, angiogenesis, clonogenicity, and metastasis, induces cell differentiation, and prolongs survival [18, 19, 20]. Moreover, VD modifies hypertension, diabetes, and obesity, all possible risk factors for RCC [21, 22]. Some ecologic studies have shown an inverse association between levels of solar ultraviolet irradiance and RCC incidence [23].
It was demonstrated 30 years ago that VD3 inhibits the growth of the RCC cell line [20]. Those finding were confirmed in more recent studies on both in-vitro cell lines and in-vivo in animal models [24, 25]. Dormoy et. al. have shown that VD3 inhibited cell proliferation and cell growth in cell lines, independently of VHL expression. In the same paper, it was demonstrated in the animal model that tumor growth was almost completely abolished in the group treated with VD3 injections. Some animals showed a complete tumor regression. It was also proven that cholecalciferol treatment of mice did not induce calcification or calcium reabsorption, and thus, was not toxic [26].
To date, a few big studies on circulating VD and the risk of RCC were conducted [27, 27, 29]. Gallicchio et al. combined data from 8 prospective cohort studies and did not support the hypothesis that higher circulating VD level measured in prediagnostic blood specimens was associated with a decreased risk of RCC overall or with RCC specifically [27]. In contrast, Afzal et. al. and Muller et. al. showed that low concentrations of VD were related with higher risk of RCC, as well as lower all-cause mortality among RCC cases [28]. Additionally, Muller proved that high concentrations of VD might also be associated with increased risk of all-cause mortality among RCC cases [29].
Other authors have focused on VD intake. Studies from Central and Eastern Europe, Italy, Finland, and the United States found no associations between VD intake and RCC risk [30, 31, 32, 33].
In another paper, in stigators tried to predict plasma VD levels on the basis of race, UVB flux, physical activity, BMI value, vitamin D intake, alcohol consumption, and postmenopausal hormone use. They observed that higher predicted plasma VD levels were associated with a statistically significantly lower risk of RCC in men and women. Yet, they found no association between VD intake and RCC incidence [34].
Further issues concern vitamin D-binding protein (DBP) and the risk of RCC. It is suggested that DBP may have a direct impact on carcinogenesis through its non-VD related biological functions, including being a member of the extracellular actin scavenger system and by playing a role in chemotaxis, macrophage activation, apoptosis, and angiogenesis. It has been observed in prospective analysis that men with higher serum concentrations of DBP experienced lower risk of RCC [35].
Another problem is related to the existing evidence that indicates that VDR gene polymorphisms are associated with RCC. Several studies have been conducted so far, yet it is not possible to make an authoritative declaration on the role of VDR polymorphisms in renal cancer development and prognosis because of conflicting studies results. However, some polymorphisms have been found to be strongly associated with RCC [36, 37, 38].
Lambert et al. compared the in vitro and in vivo growth-inhibitory properties of 1,25(OH)2D3-3-BE (VDR-alkylating derivative of 1,25(OH)2D3) to VD3 in human RCC cells. They observed that 1,25(OH)2D3-3-BE strongly suppresses growth of kidney cancer cells in vitro and tumor growth in vivo [25].
Prostatic cancer
Prostate cancer (PCa) accounts for the most frequently diagnosed malignancy in men and the second-leading cause of men's death from cancer worldwide [39]. Many epidemiological studies examining the VD and cancer association have been conducted; however, the relationship between PCa and VD still remain not entirely understood [40].
PCa cells can express VD metabolizing enzymes and the VDR. Moreover, it is proven that VD3 affects prostate cell differentiation and proliferation [41].
Preclinical and epidemiologic data suggest that VD deficiency may be of great importance in the pathogenesis and progression of PCa [42]. The possible explanation of this hypothesis is the fact that the vitamin D endocrine system regulates biological processes like prostate growth, cell proliferation, differentiation, and apoptosis [43].
A beneficial relationship between VD intake and PCa in clinical populations is difficult to demonstrate. Gilbert et al. in a meta-analysis study involving 14,174 men with prostate cancer found no association between VD intake and the risk of aggressive PCa [44]. Also, the meta-analysis of 26,769 cases conducted by Huncharek did not reveal a clear relationship between VD intake and PCa risk [45]. Interestingly, in one study, there was a 40% reduction in PCa risk in the group of men supplementing more than 600 IU of VD compared with men who had no supplementation. This association did not differ by tumor aggressiveness. However, according to the authors’ revelations, no similar result was obtained for dietary VD intake [46].
The results of two big and well designed SELECT and PCPT trials indicate a protective role of circulating VD on PCa risk [47, 48]. The effect was clearer in the PCPT study, which proved that VD levels were associated with a linear decrease in risk of Gleason 8–10 PCa [49]. Furthermore, the protective role of VD was related more with high-grade than with low-grade PCa. These findings are in agreement with the hypothesis that vitamin D inhibits the development of clinically significant, but not clinically insignificant, PCa [50]. Furthermore, Gilbert et al. showed that lower total VD concentrations were associated with more aggressive cancers; however, there was no association with overall PCa risk [51]. In a prospective study, Shui et al. proved that increased plasma VD levels were associated with a 57% reduction in the risk of lethal PCa, finding also no statistically significant association of plasma VD levels with overall PCa incidence [52].
On the contrary, there was some evidence indicating an increasing PCa risk with rising vitamin D levels [53].
Studies provided by Xu et al. revealed a significant 17% elevation in risk of PCa for individuals with higher level of VD, and no publication bias was found in the calculations [54]. Another study by Meyer indicated a positive relationship between an increased VD concentration and PCa risk in a big study of 2,106 cases and 2,106 healthy controls [55].
Other studies showed no VD and PCa association. A recent meta-analysis of 25 papers demonstrate little evidence that circulating concentrations of VD were significantly associated with risk of PCa. Additionally, there was only weak evidence that increased concentrations of circulating VD3 were associated with a decreased risk of aggressive PCa [44].
In another cohort report, Shui et al. did not find any evidence to support associations of circulating VD or common variations in key vitamin D pathway genes with the risk of fatal PCa [56].
Moreover, Yin et al revealed in a meta-analysis of 11 studies that PCa is not associated with an increased VD level [57]. Gandini et al. gained similar results [58].
Recently, some attention has been put to investigate single nucleotide polymorphisms (SNPs) in the vitamin D pathway genes, particularly in VDR genes.
A genome-wide association study designed to identify genes associated with vitamin D deficiency was conducted. As a result, several genes were defined to contain the SNPs, which are reliable predictors of circulating 25-hydroxvitamin D levels [59].
Shui et al. carried out the first study to investigate associations between a number of SNPs in key genes involved in vitamin D metabolism and signalling pathways, and between levels of circulating 25-hydroxyvitamin D with respect to fatal PCa [56]. The strongest evidence for effect modification was found in SNPs-carrying alleles of CYP2R1 (encoding Vitamin D 25-hydroxylase) and GC (the vitamin D binding protein), that is the genes that have been observed to influence circulating 25-hydroxyvitamin D levels [59]. The other SNPs were mostly located in CYP24A1, an enzyme crucial for the catabolism of vitamin D which overexpression has been shown to be related with worse outcomes in several solid tumors, including prostate tumors [60].
Recent findings suggest that some VDR gene polymorphisms may not only be related with PCa risk, but also significantly associated with PCa-related risk factors, including PSA level, Gleason score, and tumor stage [61]. Results obtained in this paper provided evidence for an association between two VDR sequence variants and PCa risk.
Moreover, Gilbert et al. examined the associations of sixteen vitamin D pathway polymorphisms with PSA-detected PCa risk along with other parameters, such as stage and Gleason grade [62]. There was evidence that two SNPs in vitamin D-binding protein, representing decreased 25-hydroxyvitamin levels, were associated with higher prostate cancer risk. Importantly, the study has shown the relationship between a score measuring metabolism of 25-hydroxyvitamin D (indicating low 25(OH)D levels) and its component variants, and high Gleason score.
Furthermore, a meta-analysis investigating the association between VDR polymorphisms (BsmI and FokI) and PCa risk reported no relationship with PCa risk [63]. However, a recent genetic association study and meta-analysis of 13 papers revealed an association between three VDR polymorphisms (Bsm I, Apa I, and Taq I) and PCa grade. Apa I and Bsm I increased the risk of a high grade, while Taq I was associated with a low risk for a high Gleason score [64]. However, none of these was related to cancer stage. There is a strong assumption that these discrepancies may be conditioned by ethnicity and geographic location, mainly because of influence on VDR functions through gene to gene interactions [65, 66, 67].
The next issue concerns the relation between serum DBP concentrations and PCa. It has been shown that DBP status was not directly associated with PCa overall risk. Moreover, when 25(OH)D concentrations were lower, high DBP levels were related with significantly decreased PCa risk [68].
Recent clinical studies have underlined the therapeutic potential of vitamin D and its analogues when administered alone [42, 69, 70, 71] or in combination with cytostatic agents [72, 73, 74]. Furthermore, these studies have ensured the rationale for a calcitriol-taxanes combined therapy in patients with PCa [75, 76]. Based on these findings, a few clinical studies have been carried out in androgen independent PCa patients, where VD was frequently combined with standard cancer therapies resulting in promising effects [75, 77, 78]. Nevertheless, despite initially presented in ASCENT I (clinical trial with high dose VD3 and docetaxol) promising results in the treatment of castration resistance PCa, this success was not repeated in a larger trial (ASCENT II). Thus, the clinical evidence remains in opposite to VD beneficial role prevention/treatment of prostate cancer, which is in accordance with previous reports [79].
Additionally, the inherent calcemic toxicity of VD, particularly in pharmaceutical doses, impedes its general use as an anticancer agent [73, 75].
Bladder cancer
It was shown in in-vitro and in-vivo studies in animal models that calcitriol inhibits proliferation and induces apoptosis in human bladder cancer (BC) [80]. In other reports, authors proved VD3's role in BC pathogenesis and showed that VD3 levels are positively associated with fibroblast growth factor receptor 3 (FGFR3) expression in the tumor. Because FGFR3 mutation and overexpression are markers of better outcome, authors suggested that patients with low levels of plasma VD might be at high risk of more aggressive forms of BC [81].
Various studies have examined BC incidence and serum level of VD. Some papers do not support the relationship of VD status and BC [82]. In another paper, Afzal et al. proved that lower plasma VD was associated with higher risk of tobacco-related cancers, including BC [28]. Mondul et al. observed that men with lower VD3 serum concentrations were at an increased risk of bladder cancer compared to men with higher serum levels [83]. Those findings were confirmed by a recent meta-analysis [84].
The results of studies concerning vitamin D intake and BC risk are contradictory and vary between each study and between subgroups of the studied groups [85, 86].
Circulating DBP was not associated with BC risk; however, in the same paper, it was shown that the inverse association between total serum VD3 and BC risk appeared limited to men with lower DBP levels. Additionally, authors observed an inverse association between free circulating VD3 and BC. These findings suggest that higher concentrations of free circulating VD3 may be more biologically relevant to BC risk than total VD3 [87].
Superficial transitional cell carcinoma of the bladder expresses VDRs, and their polymorphisms were examined in BC [88]. Mittal et al. reported that BC risk is higher among patients with VDR rs10735810 polymorphism (‘Fok1’), which is known to decrease the receptor's activity [89].
Only a few studies on the use of VD3 in BC therapy were conducted.
Ma et al. showed that VD3 enhances the antitumor activity of gemcitabine and cisplatin in-vitro and in-vivo in animal model. The addition of VD3 to the gemcitabine-cisplatin (GC) regimen may inhibit growth of cancer cells and increase the chemosensitivity of bladder cancer resulting in better response to the GC combination [90].
In another paper on using VD as a treatment option, Hsu et al. demonstrated in an animal model that VD and BCG immunotherapy combination therapy leads to a better treatment efficacy than BCG monotherapy. The study suggested that it is possible to prolong survival and reduce BCG concentration by administrating combined VD and BCG intravesically [91].
Testicular cancer, Testicular germ cell tumors TGCT
There are only few reports concerning the connection between testicular cancer and vitamin D. It was shown that VDR is present in various normal testicular cells (smooth muscle of the epididymis, spermatogonia and Sertoli's cells) [92]. VDR expression was also found in almost every type of TGCT [92, 93].
Significant antiproliferative VD3 effect on TGCT cells was proved in in-vitro studies [93]. It was reported that VD3 directly induces and increases transcription of GADD45 protein in TGCT cells, which has antiproliferative effects in tumor cells, acts directly proapototically and affects DNA repair [93, 94]. Also, exposure of cancer cells for VD3 significantly increases the expression of p21, p27, FOXO1, p53 and p73 [95]. Yet, the exact mechanism of VD3 influence on TGCT is complex and not fully understood.
In the study investigating the influence of VD3 on cisplatin sensitivity in TGCT cell lines, the authors demonstrated that co-treatment with VD3 and cisplatin caused a significantly lower viability of cancer cells compared to cisplatin treatment alone. However, in an animal model, co-treatment with either VD3 or cholecalciferol with cisplatin did not result in a significantly different antitumor response, compared to cisplatin treatment alone [95]. In another paper, it was proven that VD3 is capable of inducing a partial differentiation of embryonal carcinoma cells in vitro and in vivo. However, the differentiation inducing VD3 effects caused no significant inhibition of tumor growth in xenograft tumors in animal model [96].
DISCUSSION
In recent years, VD has received vast attention because of suggestions that it may have a crucial role in the prevention and therapy of various cancers. Many epidemiologic studies have reported the impact of VD on preventing several cancers and other pathologies. Assuming that VD status changes cancer risk, enough VD supply would be an easy, economical, and safe cancer incidence and mortality reduction method. However, despite numerous researches, the VD role in cancer incidence and therapy remains unclear.
Studies assessing VD status often give conflicting results. Presented studies differed methodologically, and, additionally were based mainly on one-time blood collection. It is possible that a single measurement in adulthood does not reflect exposure to VD in an etiologically relevant period. What is also worth mentioning that VD status is measured by blood concentration of both free VD and VD that is bound to DBP. However, very little VD circulates in a free form [97]. Pike et al. suggested in the ‘free hormone hypothesis’ that only free, unbound hormones can have biological effects on tissues [98]. This association was observed in pancreas and bladder cancers [87, 99].
Furthermore, some studies have reported higher total risk of death for patients with low VD levels. This fact implies that the relation between VD and cancer may not be specific to cancer survival, but rather a reflection of a general occurrence [100]. Other bias-introducing factor may be difference in the severity of disease at diagnosis.
Moreover, a lot of studies show a positive correlation between VD status and cancer. Those findings are not consistent with common expectations of increased VD status beneficial role. Such meaningful outcomes dictate caution in interpreting the effect of VD and in VD supplements recommendation.
When concerning the VD intake, conflicts may arise from the fact that VD intake is a small contributor to circulating VD levels. It was shown that there is only 3 ng/mL differences in measured plasma VD between extreme categories of dietary VD intake (<100 vs. ≥400 IU/day) [101]. Additionally, blood VD3 levels can be affected by substrate availability through adiposity sequestration, skin pigmentation, physical activity, and the consumption of dietary factors such as genistein and folate [102].
Moreover, some authors state that VDR and all the VD3-metabolizing enzymes are expressed in the normal tissue, but their expression is lost or diminished during the malignant transformation [18]. Therefore, the effect of VD3 on tumor growth may be VDR independent. The antitumor activity may be attributed to inhibition of tumor cell proliferation and induction of tumor cell apoptosis as observed in cultured cells [26].
CONCLUSIONS
The link between VD and various urological cancers remains unclear. Further studies with good methodology are needed to draw conclusions about the use of VD in cancer prevention and therapy.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523:30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33:456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pols HA, Birkenhager JC, Foekens JA, van Leeuwen JP. Vitamin D: a modulator of cell proliferation and differentiation. J Steroid Biochem Mol Biol. 1990;37:873–876. doi: 10.1016/0960-0760(90)90435-n. [DOI] [PubMed] [Google Scholar]
- 6.Khadzkou K, Buchwald P, Westin G, Dralle H, Akerstrom G, Hellman P. 25-hydroxyvitamin D3 1alpha-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J Histochem Cytochem. 2006;54:355–361. doi: 10.1369/jhc.5A6734.2005. [DOI] [PubMed] [Google Scholar]
- 7.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19:468–483. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Blutt SE, Allegretto EA, Pike JW, Weigel NL. 1,25-dihydroxyvitamin D3 and 9-cis-retinoic acid act synergistically to inhibit the growth of LNCaP prostate cells and cause accumulation of cells in G1. Endocrinology. 1997;138:1491–1497. doi: 10.1210/endo.138.4.5063. [DOI] [PubMed] [Google Scholar]
- 9.Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J. Inhibitory effects of 1alpha, 25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol. 2001;15:1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- 10.Flores O, Wang Z, Knudsen KE, Burnstein KL. Nuclear targeting of cyclin-dependent kinase 2 reveals essential roles of cyclin-dependent kinase 2 localization and cyclin E in vitamin D-mediated growth inhibition. Endocrinology. 2010;151:896–908. doi: 10.1210/en.2009-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hisatake J, Kubota T, Hisatake Y, Uskokovic M, Tomoyasu S, Koeffler HP. 5,6-trans-16-ene-vitamin D3: a new class of potent inhibitors of proliferation of prostate, breast, and myeloid leukemic cells. Cancer Res. 1999;59:4023–4029. [PubMed] [Google Scholar]
- 12.Blutt SE, McDonnell TJ, Polek TC, Weigel NL. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology. 2000;141:10–17. doi: 10.1210/endo.141.1.7289. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D. Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology. 2010;151:32–42. doi: 10.1210/en.2009-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao BY, Yao J, Lee YF. 1alpha, 25-dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate cancer cell angiogenesis. Carcinogenesis. 2006;27:1883–1893. doi: 10.1093/carcin/bgl041. [DOI] [PubMed] [Google Scholar]
- 15.Koli K, Keski-Oja J. 1alpha,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11:221–229. [PubMed] [Google Scholar]
- 16.Cheung FS, Lovicu FJ, Reichardt JK. Current progress in using vitamin D and its analogs for cancer prevention and treatment. Expert Rev Anticancer Ther. 2012;12:811–837. doi: 10.1586/era.12.53. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan AV, Trump DL, Johnson CS, Feldman D. The role of vitamin D in cancer prevention and treatment. Endocrinol Metab Clin North Am. 2010;39:401–418. doi: 10.1016/j.ecl.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blomberg Jensen M, Andersen CB, Nielsen JE, et al. Expression of the vitamin D receptor, 25-hydroxylases, 1alpha-hydroxylase and 24-hydroxylase in the human kidney and renal clear cell cancer. J Steroid Biochem Mol Biol. 2010;121:376–382. doi: 10.1016/j.jsbmb.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 19.Fujioka T, Suzuki Y, Okamoto T, Mastushita N, Hasegawa M, Omori S. Prevention of renal cell carcinoma by active vitamin D3. World J Surg. 2000;24:1205–1210. doi: 10.1007/s002680010206. [DOI] [PubMed] [Google Scholar]
- 20.Nagakura K, Abe E, Suda T, Hayakawa M, Nakamura H, Tazaki H. Inhibitory effect of 1 alpha,25-dihydroxyvitamin D3 on the growth of the renal carcinoma cell line. Kidney Int. 1986;29:834–840. doi: 10.1038/ki.1986.74. [DOI] [PubMed] [Google Scholar]
- 21.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 22.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr SB, Gorham ED, Garland CF, Grant WB, Garland FC. Are low ultraviolet B and high animal protein intake associated with risk of renal cancer? Int J Cancer. 2006;119:2705–2709. doi: 10.1002/ijc.22213. [DOI] [PubMed] [Google Scholar]
- 24.Fujioka T, Hasegawa M, Ishikura K, Matsushita Y, Sato M, Tanji S. Inhibition of tumor growth and angiogenesis by vitamin D3 agents in murine renal cell carcinoma. J Urol. 1998;160:247–251. [PubMed] [Google Scholar]
- 25.Lambert JR, Eddy VJ, Young CD, et al. A vitamin D receptor-alkylating derivative of 1alpha,25-dihydroxyvitamin D3 inhibits growth of human kidney cancer cells and suppresses tumor growth. Cancer Prev Res (Phila). 2010;3:1596–1607. doi: 10.1158/1940-6207.CAPR-10-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dormoy V, Beraud C, Lindner V, et al. Vitamin D3 triggers antitumor activity through targeting hedgehog signaling in human renal cell carcinoma. Carcinogenesis. 2012;33:2084–2093. doi: 10.1093/carcin/bgs255. [DOI] [PubMed] [Google Scholar]
- 27.Gallicchio L, Moore LE, Stevens VL, et al. Circulating 25-hydroxyvitamin D and risk of kidney cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:47–57. doi: 10.1093/aje/kwq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afzal S, Bojesen SE, Nordestgaard BG. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin Chem. 2013;59:771–780. doi: 10.1373/clinchem.2012.201939. [DOI] [PubMed] [Google Scholar]
- 29.Muller DC, Fanidi A, Midttun O, et al. Circulating 25-hydroxyvitamin D3 in relation to renal cell carcinoma incidence and survival in the EPIC cohort. Am J Epidemiol. 2014;180:810–820. doi: 10.1093/aje/kwu204. [DOI] [PubMed] [Google Scholar]
- 30.Karami S, Brennan P, Navratilova M, et al. Vitamin d pathway genes, diet, and risk of renal cell carcinoma. Int J Endocrinol. 2010;2010:879362. doi: 10.1155/2010/879362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosetti C, Scotti L, Maso LD, et al. Micronutrients and the risk of renal cell cancer: a case-control study from Italy. Int J Cancer. 2007;120:892–896. doi: 10.1002/ijc.22374. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RT, Wang J, Chinchilli V, et al. Fish, vitamin D, and flavonoids in relation to renal cell cancer among smokers. Am J Epidemiol. 2009;170:717–729. doi: 10.1093/aje/kwp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prineas RJ, Folsom AR, Zhang ZM, Sellers TA, Potter J. Nutrition and other risk factors for renal cell carcinoma in postmenopausal women. Epidemiology. 1997;8:31–36. doi: 10.1097/00001648-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Joh HK, Giovannucci EL, Bertrand KA, Lim S, Cho E. Predicted plasma 25-hydroxyvitamin D and risk of renal cell cancer. J Natl Cancer Inst. 2013;105:726–732. doi: 10.1093/jnci/djt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mondul AM, Weinstein SJ, Moy KA, Mannisto S, Albanes D. Vitamin D-binding protein, circulating vitamin D and risk of renal cell carcinoma. Int J Cancer. 2014;134:2699–2706. doi: 10.1002/ijc.28596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ou C, Zhao HL, Zhu B, Huang LS, Li PZ, Lao M. Association of vitamin D receptor gene polymorphism with the risk of renal cell carcinoma: a meta-analysis. J Recept Signal Transduct Res. 2014;34:463–468. doi: 10.3109/10799893.2014.919593. [DOI] [PubMed] [Google Scholar]
- 37.Khan MI, Bielecka ZF, Najm MZ, et al. Vitamin D receptor gene polymorphisms in breast and renal cancer: current state and future approaches (review) Int J Oncol. 2014;44:349–363. doi: 10.3892/ijo.2013.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng F, Ma P, Sui C, et al. The association between VDR polymorphisms and renal cell carcinoma susceptibility: a meta-analysis. Tumour Biol. 2014;35:6065–6072. doi: 10.1007/s13277-014-1803-6. [DOI] [PubMed] [Google Scholar]
- 39.Tabayoyong W, Abouassaly R. Prostate Cancer Screening and the Associated Controversy. Surg Clin North Am. 2015;95:1023–1039. doi: 10.1016/j.suc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 40.van der Rhee HJ, de Vries E, Coebergh JW. Does sunlight prevent cancer? A systematic review. Eur J Cancer. 2006;42:2222–2232. doi: 10.1016/j.ejca.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Whitlatch LW, Young MV, Schwartz GG, et al. 25-Hydroxyvitamin D-1alpha-hydroxylase activity is diminished in human prostate cancer cells and is enhanced by gene transfer. J Steroid Biochem Mol Biol. 2002;81:135–140. doi: 10.1016/s0960-0760(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 42.Swami S, Krishnan AV, Feldman D. Vitamin D metabolism and action in the prostate: implications for health and disease. Mol Cell Endocrinol. 2011;347:61–69. doi: 10.1016/j.mce.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol Metab. 2003;14:423–430. doi: 10.1016/j.tem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert R, Martin RM, Beynon R, et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control. 2011;22:319–340. doi: 10.1007/s10552-010-9706-3. [DOI] [PubMed] [Google Scholar]
- 45.Huncharek M, Muscat J, Kupelnick B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: a meta-analysis of 26,769 cases from 45 observational studies. Nutr Cancer. 2008;60:421–441. doi: 10.1080/01635580801911779. [DOI] [PubMed] [Google Scholar]
- 46.Ahn J, Albanes D, Peters U, et al. Dairy products, calcium intake, and risk of prostate cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2007;16:2623–2630. doi: 10.1158/1055-9965.EPI-07-0601. [DOI] [PubMed] [Google Scholar]
- 47.Kristal AR, Till C, Song X, et al. Plasma vitamin D and prostate cancer risk: results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23:1494–1504. doi: 10.1158/1055-9965.EPI-14-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23:1484–1493. doi: 10.1158/1055-9965.EPI-13-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz GG. Vitamin D in blood and risk of prostate cancer: lessons from the Selenium and Vitamin E Cancer Prevention Trial and the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23:1447–1449. doi: 10.1158/1055-9965.EPI-14-0520. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–1311. [PubMed] [Google Scholar]
- 51.Gilbert R, Metcalfe C, Fraser WD, et al. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer. 2012;131:1187–1196. doi: 10.1002/ijc.27327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shui IM, Mucci LA, Kraft P, et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study. J Natl Cancer Inst. 2012;104:690–699. doi: 10.1093/jnci/djs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandstedt J, Almquist M, Manjer J, Malm J. Vitamin D, PTH, and calcium and the risk of prostate cancer: a prospective nested case-control study. Cancer Causes Control. 2012;23:1377–1385. doi: 10.1007/s10552-012-9948-3. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Shao X, Yao Y, et al. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. J Cancer Res Clin Oncol. 2014;140:1465–1477. doi: 10.1007/s00432-014-1706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer HE, Robsahm TE, Bjorge T, Brustad M, Blomhoff R. Vitamin D, season, and risk of prostate cancer: a nested case-control study within Norwegian health studies. Am J Clin Nutr. 2013;97:147–154. doi: 10.3945/ajcn.112.039222. [DOI] [PubMed] [Google Scholar]
- 56.Shui IM, Mondul AM, Lindstrom S, et al. Circulating vitamin D, vitamin D-related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer. 2015;121:1949–1956. doi: 10.1002/cncr.29320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin L, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis of longitudinal studies: Serum vitamin D and prostate cancer risk. Cancer Epidemiol. 2009;33:435–445. doi: 10.1016/j.canep.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 59.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tannour-Louet M, Lewis SK, Louet JF, et al. Increased expression of CYP24A1 correlates with advanced stages of prostate cancer and can cause resistance to vitamin D3-based therapies. FASEB J. 2014;28:364–372. doi: 10.1096/fj.13-236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh JJ, Byun SS, Lee SE, et al. Genetic variations in VDR associated with prostate cancer risk and progression in a Korean population. Gene. 2014;533:86–93. doi: 10.1016/j.gene.2013.09.119. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert R, Bonilla C, Metcalfe C, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control. 2015;26:205–218. doi: 10.1007/s10552-014-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Z, Wen J, Kan Q, et al. Lack of association between vitamin D receptor gene FokI and BsmI polymorphisms and prostate cancer risk: an updated meta-analysis involving 21,756 subjects. Tumour Biol. 2013;34:3189–3200. doi: 10.1007/s13277-013-0889-6. [DOI] [PubMed] [Google Scholar]
- 64.Chen L, Davey Smith G, Evans DM, et al. Genetic variants in the vitamin d receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18:2874–2881. doi: 10.1158/1055-9965.EPI-09-0544. [DOI] [PubMed] [Google Scholar]
- 65.Holt SK, Kwon EM, Peters U, Ostrander EA, Stanford JL. Vitamin D pathway gene variants and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1929–1933. doi: 10.1158/1055-9965.EPI-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bai Y, Yu Y, Yu B, et al. Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet. 2009;10:125. doi: 10.1186/1471-2350-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torkko KC, van Bokhoven A, Mai P, et al. VDR and SRD5A2 polymorphisms combine to increase risk for prostate cancer in both non-Hispanic White and Hispanic White men. Clin Cancer Res. 2008;14:3223–3229. doi: 10.1158/1078-0432.CCR-07-4894. [DOI] [PubMed] [Google Scholar]
- 68.Weinstein SJ, Mondul AM, Kopp W, Rager H, Virtamo J, Albanes D. Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of prostate cancer. Int J Cancer. 2013;132:2940–2947. doi: 10.1002/ijc.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 70.Ajibade AA, Kirk JS, Karasik E, et al. Early growth inhibition is followed by increased metastatic disease with vitamin D (calcitriol) treatment in the TRAMP model of prostate cancer. PLoS One. 2014;9:e89555. doi: 10.1371/journal.pone.0089555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berkovich L, Sintov AC, Ben-Shabat S. Inhibition of cancer growth and induction of apoptosis by BGP-13 and BGP-15, new calcipotriene-derived vitamin D3 analogs, in-vitro and in-vivo studies. Invest New Drugs. 2013;31:247–255. doi: 10.1007/s10637-012-9839-1. [DOI] [PubMed] [Google Scholar]
- 72.Ma Y, Trump DL, Johnson CS. Vitamin D in combination cancer treatment. J Cancer. 2010;1:101–107. doi: 10.7150/jca.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muindi JR, Johnson CS, Trump DL, Christy R, Engler KL, Fakih MG. A phase I and pharmacokinetics study of intravenous calcitriol in combination with oral dexamethasone and gefitinib in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;65:33–40. doi: 10.1007/s00280-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Bella G, Mascia F, Colori B. The Di Bella Method (DBM) in the treatment of prostate cancer: a preliminary retrospective study of 16 patients and a review of the literature. Neuro Endocrinol Lett. 2013;34:523–528. [PubMed] [Google Scholar]
- 75.Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer. 2006;106:2136–2142. doi: 10.1002/cncr.21890. [DOI] [PubMed] [Google Scholar]
- 76.Medioni J, Deplanque G, Ferrero JM, et al. Phase I safety and pharmacodynamic of inecalcitol, a novel VDR agonist with docetaxel in metastatic castration-resistant prostate cancer patients. Clin Cancer Res. 2014;20:4471–4477. doi: 10.1158/1078-0432.CCR-13-3247. [DOI] [PubMed] [Google Scholar]
- 77.Beer TM, Ryan CW, Venner PM, et al. Intermittent chemotherapy in patients with metastatic androgen-independent prostate cancer: results from ASCENT, a double-blinded, randomized comparison of high-dose calcitriol plus docetaxel with placebo plus docetaxel. Cancer. 2008;112:326–330. doi: 10.1002/cncr.23163. [DOI] [PubMed] [Google Scholar]
- 78.Okamoto R, Delansorne R, Wakimoto N, et al. Inecalcitol, an analog of 1alpha, 25(OH)(2) D(3), induces growth arrest of androgen-dependent prostate cancer cells. Int J Cancer. 2012;130:2464–2473. doi: 10.1002/ijc.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morris MJ, Smaletz O, Solit D, et al. High-dose calcitriol, zoledronate, and dexamethasone for the treatment of progressive prostate carcinoma. Cancer. 2004;100:1868–1875. doi: 10.1002/cncr.20185. [DOI] [PubMed] [Google Scholar]
- 80.Konety BR, Lavelle JP, Pirtskalaishvili G, et al. Effects of vitamin D (calcitriol) on transitional cell carcinoma of the bladder in vitro and in vivo. J Urol. 2001;165:253–258. doi: 10.1097/00005392-200101000-00074. [DOI] [PubMed] [Google Scholar]
- 81.Amaral AF, Mendez-Pertuz M, Munoz A, et al. Plasma 25-hydroxyvitamin D(3) and bladder cancer risk according to tumor stage and FGFR3 status: a mechanism-based epidemiological study. J Natl Cancer Inst. 2012;104:1897–1904. doi: 10.1093/jnci/djs444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mondul AM, Weinstein SJ, Horst RL, Purdue M, Albanes D. Serum vitamin D and risk of bladder cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening trial. Cancer Epidemiol Biomarkers Prev. 2012;21:1222–1225. doi: 10.1158/1055-9965.EPI-12-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mondul AM, Weinstein SJ, Mannisto S, et al. Serum vitamin D and risk of bladder cancer. Cancer Res. 2010;70:9218–9223. doi: 10.1158/0008-5472.CAN-10-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liao Y, Huang JL, Qiu MX, Ma ZW. Impact of serum vitamin D level on risk of bladder cancer: a systemic review and meta-analysis. Tumour Biol. 2015;36:1567–1572. doi: 10.1007/s13277-014-2728-9. [DOI] [PubMed] [Google Scholar]
- 85.Brinkman MT, Karagas MR, Zens MS, Schned A, Reulen RC, Zeegers MP. Minerals and vitamins and the risk of bladder cancer: results from the New Hampshire Study. Cancer Causes Control. 2010;21:609–619. doi: 10.1007/s10552-009-9490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci E. Prospective study of dietary supplements, macronutrients, micronutrients, and risk of bladder cancer in US men. Am J Epidemiol. 2000;152:1145–1153. doi: 10.1093/aje/152.12.1145. [DOI] [PubMed] [Google Scholar]
- 87.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Influence of vitamin D binding protein on the association between circulating vitamin D and risk of bladder cancer. Br J Cancer. 2012;107:1589–1594. doi: 10.1038/bjc.2012.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sahin MO, Canda AE, Yorukoglu K, Mungan MU, Sade M, Kirkali Z. 1,25 Dihydroxyvitamin D(3) receptor expression in superficial transitional cell carcinoma of the bladder: a possible prognostic factor? Eur Urol. 2005;47:52–57. doi: 10.1016/j.eururo.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Mittal RD, Manchanda PK, Bhat S, Bid HK. Association of vitamin-D receptor (Fok-I) gene polymorphism with bladder cancer in an Indian population. BJU Int. 2007;99:933–937. doi: 10.1111/j.1464-410X.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 90.Ma Y, Yu WD, Trump DL, Johnson CS. 1,25D3 enhances antitumor activity of gemcitabine and cisplatin in human bladder cancer models. Cancer. 2010;116:3294–3303. doi: 10.1002/cncr.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsu JW, Yin PN, Wood R, Messing J, Messing E, Lee YF. 1 alpha, 25-dihydroxylvitamin D3 promotes Bacillus Calmette-Guerin immunotherapy of bladder cancer. Oncotarget. 2013;4:2397–2406. doi: 10.18632/oncotarget.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nangia AK, Hill O, Waterman MD, Schwender CE, Memoli V. Testicular maturation arrest to testis cancer: spectrum of expression of the vitamin D receptor and vitamin D treatment in vitro. J Urol. 2007;178:1092–1096. doi: 10.1016/j.juro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 93.Bremmer F, Thelen P, Pottek T, Behnes CL, Radzun HJ, Schweyer S. Expression and function of the vitamin D receptor in malignant germ cell tumour of the testis. Anticancer Res. 2012;32:341–349. [PubMed] [Google Scholar]
- 94.Tront JS, Hoffman B, Liebermann DA. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 2006;66:8448–8454. doi: 10.1158/0008-5472.CAN-06-2013. [DOI] [PubMed] [Google Scholar]
- 95.Jorgensen A, Blomberg Jensen M, Nielsen JE, Juul A, Rajpert-De Meyts E. Influence of vitamin D on cisplatin sensitivity in testicular germ cell cancer-derived cell lines and in a NTera2 xenograft model. J Steroid Biochem Mol Biol. 2013;136:238–246. doi: 10.1016/j.jsbmb.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Blomberg Jensen M, Jorgensen A, Nielsen JE, et al. Vitamin D metabolism and effects on pluripotency genes and cell differentiation in testicular germ cell tumors in vitro and in vivo. Neoplasia. 2012;14:952–963. doi: 10.1593/neo.121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372:33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 98.Pike JW, Feldman D, Glorieux FH. Vitamin D. vol. San Diego. San Diego: Academic Press; 1997. [Google Scholar]
- 99.Weinstein SJ, Stolzenberg-Solomon RZ, Kopp W, Rager H, Virtamo J, Albanes D. Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: a nested case-control study. Cancer Res. 2012;72:1190–1198. doi: 10.1158/0008-5472.CAN-11-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schottker B, Haug U, Schomburg L, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97:782–793. doi: 10.3945/ajcn.112.047712. [DOI] [PubMed] [Google Scholar]
- 101.Bertrand KA, Giovannucci E, Liu Y, et al. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br J Nutr. 2012;108:1889–1896. doi: 10.1017/S0007114511007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Donkena KV, Young CY. Vitamin D, sunlight and prostate cancer risk. Adv Prev Med. 2011;2011:281863. doi: 10.4061/2011/281863. [DOI] [PMC free article] [PubMed] [Google Scholar]