Abstract
Peritoneal metastasis is a common sign of advanced tumor stage, tumor progression or tumor recurrence in patients with colorectal cancer. Due to the improvement of systemic chemotherapy, the development of targeted therapy and the introduction of additive treatment options such as cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), the therapeutic approach to peritoneal metastatic colorectal cancer (pmCRC) has changed over recent decades, and patient survival has improved. Moreover, in contrast to palliative systemic chemotherapy or best supportive care, the inclusion of CRS and HIPEC as inherent components of a multidisciplinary treatment regimen provides a therapeutic approach with curative intent. Although CRS and HIPEC are increasingly accepted as the standard of care for selected patients and have become part of numerous national and international guidelines, the individual role, optimal timing and ideal sequence of the different systemic, local and surgical treatment options remains a matter of debate. Ongoing and future randomized controlled clinical trials may help clarify the impact of the different components, allow for further improvement of patient selection and support the standardization of oncologic treatment regimens for pmCRC. The addition of further therapeutic options such as neoadjuvant intraperitoneal chemotherapy or pressurized intraperitoneal aerosol chemotherapy, should be investigated to optimize therapeutic regimens and further improve the oncological outcome.
Keywords: Peritoneal metastasis, Colorectal cancer, Systemic chemotherapy, Intraperitoneal chemotherapy, Cytoreductive surgery
Core tip: Beyond diverse systemic, interventional and surgical palliative treatment options for peritoneal metastasis arising from colorectal cancer, the combination of systemic chemotherapy, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy provides a therapeutic approach with curative intent for selected patients. Nevertheless, the treatment regimens, the sequence of therapy and the impact of the different components of the multidisciplinary treatment concept on clinical and oncological outcomes remain a matter of debate. Moreover, the addition of further therapeutic options to the existing treatment regimens might allow for higher complete resection rates and improved survival rates.
INTRODUCTION
Colorectal cancer (CRC) remains one of the leading causes of cancer-related death worldwide[1]. Peritoneal metastasis (PM) is common in patients with advanced stage primary and recurrent colorectal cancer[2,3]. The natural course of this disease is associated with poor prognosis and led to a mean overall survival of 5.2 mo in the prospective European multicenter EVOCAPE I study (n = 118)[4]. A retrospective analysis of 3000 patients with pmCRC reported a median survival of 7 mo without specific treatment[5]. Although peritoneal metastases develop avascular tumor nodules within the abdominal cavity that often cannot be efficiently addressed by systemic chemotherapy[6], advances in the development of cytostatic agents, targeted therapy and combined treatment regimens has led to significant improvement in survival rates. Moreover, additive treatment options such as cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) might be performed with curative intent in selected patients[7]. Thus, pmCRC currently requires a multidisciplinary treatment approach that considers the available treatment options and modalities (Figure 1).
Figure 1.
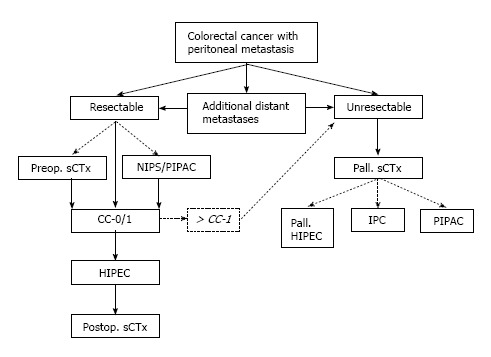
Proposed algorithm for treating peritoneal metastatic colorectal cancer. CC: Completeness of cytoreduction; CC-0/1: Complete macroscopic cytoreduction; IPC: Intraperitoneal chemotherapy; NIPS: Neoadjuvant intraperitoneal/systemic chemotherapy; PIPAC: Pressurized intraperitoneal aerosol chemotherapy; sCTx: Systemic chemotherapy; scattered lines indicate additional therapeutic options; HIPEC: Hyperthermic intraperitoneal chemotherapy.
STAGING SYSTEMS FOR PM
The estimation of the extent of peritoneal tumor dissemination plays an important role in choosing therapeutic options for patients with pmCRC. Different staging systems allow for the standardization of PM classification and facilitate prognosis estimation. The most commonly used classification system for peritoneal tumor dissemination is the peritoneal cancer index (PCI). This numerical score combines the lesion size (LS) and tumor localization in 13 abdomino-pelvic regions including four small bowel regions (region 0-12) and ranges from 0 to 39[8]. The PCI was initially introduced for intraoperative determination of the extent of peritoneal carcinomatosis but the extent can also be determined by staging laparoscopy or diagnostic imaging. Elias et al[9] showed that the PCI is easy to use and reproducible with high inter-surgeon concordance. Although the PCI is underestimated by computed tomography compared to intraoperative findings, the clinical impact of the inaccuracies of CT-PCI is modest[10]. Thus, the (CT-)PCI is a helpful tool to determine and communicate the extent of peritoneal disease and to select patients for different therapeutic options. Moreover, the PCI correlates with overall and progression-free survival in patients with pm CRC[11-14]. Nevertheless, the predictive value is limited with respect to PM and does not include other prognostic factors. Therefore, prognostic scores for patients with pm CRC have been developed. The Peritoneal Surface Disease Severity Score (PSDSS) is based on the following three important prognostic indicators: (1) clinical symptoms; (2) PCI; and (3) tumor histopathology. The PSDSS ranges from 2 to 22 and divides patients into four prognostic groups (stage I = PSDSS 2-4, stage II = PSDSS 4-7, stage III = PSDSS 8-10 and stage IV = PSDSS > 10)[15]. Several retrospective analyses show a high correlation between PSDSS and the survival of patients with pmCRC. The score might be helpful for determining survival probability and resectability of peritoneal disease in the context of therapeutic decision-making[16-18]. Another recently developed prognostic score for patients with pmCRC is the Colorectal Peritoneal Score (COREP). COREP includes signet cell histology, hemoglobin, white blood cell count and the value and status of serum tumor markers and ranges from 0 to 18. The cut-off value for the poor-prognosis group is COREP > 6. In the first published evaluation of 77 patients the predictive value of COREP for open/close-procedure, R1 resection and one-year survival was superior to that of PSDSS[19]. Based on the Japanese classification of pmCRC, which divides peritoneal tumor dissemination into four groups (P0: no PM, P1: local PM, P2: limited distant, PM and P3: extended distant PM)[20,21] Noura et al[22] proposed a new simple classification system that includes the colorectal liver metastases (CLM) status. Patients without CLM and local (P1) or limited distant PM (P2) are classified as Grade A and Grade B, respectively. Patients with extended distant PM and all patients with CLM have been defined as Grade C. Initial data shows significant stratification of the survival and R0 resection rates[22]. However, new scores considering different histological and clinical factors might be helpful for decision-making and allow for further improvement of the selection of appropriate therapeutic options within a multidisciplinary treatment approach.
SYSTEMIC CHEMOTHERAPY FOR pmCRC
Although there are multiple prospective randomized trials and retrospective analyses about systemic chemotherapy in patients with advanced stage and metastatic CRC, data regarding the subgroup of patients with pmCRC are limited. Franko et al[23] analyzed 364 patients with PM out of 2095 patients enrolled in the two prospective randomized NCCTG phase III trials N9741 and N9841 and showed a 30% relative reduction in overall survival in this subgroup. The 5-year OS rates were 4.1% and 6% and the median survival was 12.7 mo and 17.6 mo in the pmCRC and the non-pmCRC group, respectively. In this analysis infusional oxaliplatin-based chemotherapy was superior to irinotecan-based regimens irrespective of the PM status[23]. The subgroup analysis of patients with pmCRC enrolled in the prospective randomized CAIRO and CAIRO2 trials showed a significant impairment in the overall survival of these patients. Klaver et al[24] published a median OS of 10.4 and 17.3 mo in the CAIRO trial and 15.2 and 20.7 mo in the CAIRO2 trial. An Asian prospective single-arm phase II study investigating FOLFOX-4 in patients with pmCRC reported median overall survival of 21.5 mo. The median time to progression was 4.4 mo[25]. Consistent with these reported survival rates, Elias et al[26] reported a median OS of 23.9 mo under modern multidrug systemic chemotherapy in 48 patients with pmCRC from the French registry.
Considering the promising results of the first line treatment of patients with metastatic colorectal cancer using systemic polychemotherapy plus targeted therapy with median OS ranging from 25 mo to 41.3 mo[27-30] these regimens have also been used for treating pmCRC. In a retrospective analysis of 65 consecutive patients with pmCRC, Adachi et al[31] reported an improvement in the survival rate in response to systemic chemotherapy after incomplete cytoreduction. The oxaliplatin-based regimen and addition of targeted therapy was superior to irinotecan-based chemotherapy[31]. Razenberg et al[32] analyzed 1235 patients treated with palliative systemic chemotherapy for pmCRC. In 436 patients (35%) bevacizumab has been added to the treatment regimen. The median OS was 7.5 mo vs 11 mo in the bevacizumab group[32]. In a population-based study patients with metachronous colorectal PM were analyzed with respect to their treatment as follows: 94 patients received palliative systemic chemotherapy, 36 patients had the addition of bevacizumab and 92 did not receive therapy and the median survival was 13 mo, 20.3 mo and 3.4 mo, respectively[33]. Comparable results are reported by van Oudheusden in 82 patients who underwent open/close procedures for unresectable colorectal PM. The median OS was 11.2 mo with palliative systemic chemotherapy and 2.7 mo with best supportive care[34].
These data demonstrate the efficacy of modern systemic chemotherapy regimens with or without targeted therapy in improving the survival of patients with unresectable pmCRC. Based on these findings systemic chemotherapy should be considered the standard of care in patients with unresectable pmCRC and should be the backbone of a multimodal treatment regimen in patients who qualify for a multidisciplinary therapeutic approach. In the absence of contraindications, infusional oxaliplatinbased regimens, such as FOLFOX with or without monoclonal antibodies like bevacizumab, cetuximab or panitumumab, might be preferred as first-line therapies for these patients. Moreover, based on the results of the RAISE trial, ramucirumab might also be considered for the second-line treatment of patients with pmCRC[35]. Nevertheless, reliable data for this subgroup are not available.
SURGERY FOR pmCRC
CRS
In contrast to palliative surgery, such as fecal diversion, intestinal bypass, primary tumor resection, etc., CRS followed by HIPEC provides an additive treatment option for selected colorectal PM patients with a curative intent. Although disease recurrence is common[36], cure rates between 16% and 28% are reported after complete CRS and HIPEC[37,38]. The aim of surgical cytoreduction, which may consist of multiple peritonectomy procedures and visceral resections is the removal of all visible tumor deposits within the abdominal cavity[8,39,40]. Despite extensive and aggressive surgery, most patients return to baseline in terms of their quality of life within 6 mo after surgery[41-43]. The success of surgery is classified according to the completeness of cytoreduction (CC) score[13,44]. Complete macroscopic cytoreduction (CC-0/1), defined as no visible tumor or single tumor nodules < 2.5 mm, is a precondition for the efficient application of HIPEC. Therefore, consistent preoperative patient selection is crucial for the efficacy of the multimodal treatment concept. A PCI > 20 might be considered a relative contraindication for CRS and HIPEC[45]. Da Silva et al[11] reported a median OS of 41 mo in patients with PCI < 20 and 16 mo in patients with PCI > 20 after complete macroscopic cytoreduction. Comparable results are published by Hompes et al[12] for patients with a PCI higher or lower than 15. A recently published analysis of 180 patients defined a cut-off PCI value of 17[14].
CRS in patients with additional CLM
There are limited published data regarding cytoreductive surgery in patients with additional resectable CLM. In a retrospective matched-pair analysis, hepatobiliary procedures during CRS and HIPEC did not lead to increased perioperative complication rates and/or overall mortality[46]. According to the Milan consensus statement of the Peritoneal Surface Malignancy Group International cytoreductive surgery (and HIPEC) should not be routinely recommended in patients with more than three peripheral resectable liver metastases[45]. However, two retrospective studies demonstrated median survival rates of approximately 36 mo after CRS, including mostly minor liver resections followed by HIPEC[47,48]. As expected, liver involvement is associated with decreased overall survival rates. Berger et al[49] reported a median overall survival of 45.1 mo in 108 patients with additional liver involvement and 73.5 mo in 166 patients with isolated PM after CRS and HIPEC. Nevertheless, patients with malignancies other than CRC were included in the analysis. There was no significant difference regarding the morbidity and mortality between the two groups[49]. Allard et al[50] reported a median survival of 42 mo in patients who underwent complete resection of CLM and unexpected limited CPM with a median PCI of 2. In a multivariate analysis Delhorme et al[51] identified the size of liver metastasis and grade II/III toxicity of preoperative chemotherapy as poor prognostic factors. Response to preoperative chemotherapy significantly increased overall survival. These data are supported by a recently published meta-analysis that identified concurrent CLM as an independent negative prognostic factor for overall survival in patients with pmCRC after CRS and HIPEC[52]. Noura et al[22] showed that the presence of CLM impairs survival and R0 resection rates. The 5-year overall survival rates of patients without CLM and local or limited distant PM were 25.6% and 12.0%, respectively. The 5-year survival rate of patients with extended distant PM and/or additional CLM was 5.6%. R0 resection rates were 65.9%, 44.6% and 8.1%[22]. However, the combination of extended liver surgery for CLM and extended cytoreductive surgery in patients with high PCI should be avoided because of the impaired clinical and oncological outcomes. Moreover, there are no reliable data on patients with pmCRC and additional isolated resectable lung metastases. Therefore, lung metastasis should be considered a contraindication of CRS and HIPEC.
HIPEC
The aim of intraoperative HIPEC is to consolidate complete surgical resection by destroying scattered (and residual) tumor cells within the abdominal cavity. In a prospective randomized phase III trial comparing CRS and HIPEC plus systemic chemotherapy with 5-FU/FA to systemic chemotherapy with 5-FU/FA in selected patients with pmCRC there was a significant survival benefit for the treatment group. The median survival was 22 and 12.6 mo for the treatment and non-treatment groups. In the subgroup of patients with complete macroscopic cytoreduction (CC-0/1), the median survival reached 42.9 mo. The low survival rates might be explained by the use of 5-FU-based systemic chemotherapy in both groups in the pre-oxaliplatin era[53,54].
Elias et al[26] reported a median survival of 62.7 mo and a 5-year survival rate of 51% after complete macroscopic cytoreduction and bidirectional oxaliplatin-based HIPEC. All patients additionally received modern systemic chemotherapy[26]. A prospective phase II study investigating complete macroscopic cytoreduction and bidirectional oxaliplatin-based HIPEC showed a 2-year overall survival rate of 88.7% and a median disease-free survival (DFS) of 19.8 mo[12]. Based on the promising results of the FOLFOXIRI protocol in the systemic treatment of mCRC, irinotecan has been added to the bidirectional oxaliplatin-based HIPEC regimen, leading to increased morbidity without improving the survival. Quenet et al[55] reported a median overall survival of 47 mo and a 5-year survival rate of 42.4%. Goéré et al[37] reported a cure rate, defined as the 5-year disease-free survival, of 16% after CRS and HIPEC in 107 patients with pmCRC. Another retrospective analysis of 342 patients with pmCRC from a prospective database showed a 10-year recurrence-free survival rate of 10% after CRS and HIPEC[56].
Although there are only few prospective RCTs, several studies and retrospective analyses show that the integration of CRS and HIPEC into a multidisciplinary treatment approach that includes systemic chemotherapy can improve the survival of selected patients with pmCRC[7,57]. Nevertheless, the exact role of the HIPEC procedure and components remains unclear. A comparative analysis published by Hompes et al[58] investigated different HIPEC regimens and their effects on patient survival. There was no statistically significant difference between bidirectional oxaliplatin-based HIPEC and MMC-based HIPEC after complete macroscopic cytoreduction. The median RFS was 12.2 mo in the oxaliplatin-group and 13.8 mo in the MMC group (P = 0.87). The median OS was 37.1 mo in the oxaliplatin group and 26.5 mo in the MMC group (P = 0.45)[58]. A matched-pair analysis showed no significant differences in morbidity and mortality by HIPEC regimen. The grade 3/4 morbidity rates according to CTCAE were 42.5% in the OX group and 37.5% in the MMC group (P = 0.648) and the mortality rates of the OX and MMC groups were 2.5% and 0%, respectively[59]. Consistent with these findings the American Society of Peritoneal Surface Malignancies reported an OS of 32.7 mo in patients with MMC-based HIPEC and 31.4 mo for oxaliplatin-based HIPEC in 539 patients with pm CRC after complete macroscopic cytoreduction (P = 0.925). After stratification to PSDSS there was a statistically significant survival benefit for the MMC-subgroup with PSDSS I/II (P = 0.012)[60]. A retrospective analysis of a limited number of patients compared bidirectional oxaliplatin-based HIPEC to bidirectional irinotecan-based HIPEC. The 3-year survival rates were 65.0% in the OX group vs 41.7% in the IRI group (P = 0.295)[61].
In a recently published retrospective analysis of 50 consecutive patients with pmCRC, Désolneux et al[62] reported a median survival of 34.2 mo and a 5-year survival rate of 29.6% after complete macroscopic cytoreduction and systemic chemotherapy alone. These findings are supported by a retrospective Japanese multicenter database analysis of 564 patients who underwent surgery without HIPEC for pmCRC. In patients with R0 resection, the median overall survival was 30 mo and 5-year survival rate was 32.4%. The 5-year survival rate after R0 resection and adjuvant chemotherapy was 31.7% compared to 24.6% without adjuvant treatment. R0 resection and adjuvant chemotherapy were independent positive prognostic factors for survival[63]. This concept and the role of HIPEC is investigated by the French prospective randomized PRODIGE 7 trial that compares CRS and HIPEC plus systemic chemotherapy with CRS alone plus systemic chemotherapy. However, survival data are not yet available. Cashin et al[64] published survival data of a prematurely terminated prospective randomized trial evaluating CRS followed by normothermic intraperitoneal chemotherapy (IPC) with 5-FU vs CRS followed by systemic oxaliplatin-based chemotherapy. Both treatments were continued for 6 mo. The median overall survival times were 25 mo vs 18 mo (P = 0.04) and the 2-year survival rates were 54% vs 38% (P = 0.04)[64]. However, the optimal therapeutic regimen of IPC after complete CRS remains a matter of debate[65].
Prophylactic and palliative HIPEC
Another therapeutic concept that is evaluated by the ongoing French ProphyloCHIP trial is the prophylactic application of HIPEC in patients with CRC and high risk of developing PM, such as tumor perforation, isolated ovarian metastases or removal of localized PM during resection of primary tumor resection. The enrolled patients were randomized eight months after adjuvant chemotherapy to the control arm with follow-up or to the treatment arm with explorative laparotomy and prophylactic HIPEC (NCT01226394). The COLOPEC trial evaluates the effect of adjuvant HIPEC during or shortly after resection of primary CRC with a high risk of metachronous PM. A risk reduction from 25% to 10% and, therefore, improvement in the long-term survival is assumed[66].
HIPEC without cytoreductive surgery, also applied by the laparoscopic approach, might be considered in patients with unresectable PM (Figure 2) and symptomatic therapy for refractory malignant ascites. Several retrospective studies showed significant reduction of ascites production and efficient symptom control after HIPEC. Nevertheless, the number of reported patients and procedures is limited and data from prospective randomized trials are not available[67-69].
Figure 2.
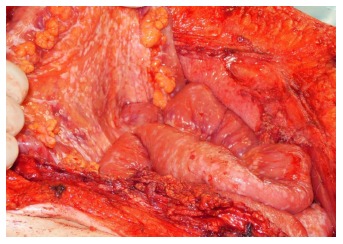
Diffuse peritoneal tumor dissemination.
PERIOPERATIVE SYSTEMIC CHEMOTHERAPY
The importance of systemic chemotherapy in the context of CRS and HIPEC has been demonstrated. Postoperative systemic chemotherapy has been shown to be an independent positive prognostic marker in all registries and retrospective analyses[13,70]. In a recently published database analysis of 5516 patients with PM arising from colorectal adenocarcinoma, mucinous adenocarcinoma and signet ring cell carcinoma, Simkens et al[71] showed that systemic chemotherapy improved survival independent of the histological subtype. In contrast to these findings, a multicenter study, including 221 patients with pmCRC reported no significant difference in the OS after CRS and HIPEC between postoperative systemic chemotherapy and surveillance. The median OS was 43.3 mo. Nevertheless, during the first year the rates of progression and recurrence were significantly lower in the chemotherapy group[72]. However, the optimal sequence of the therapeutic modalities remains a matter of investigation. Elias et al[13] reported no significant impact on the prognosis of neoadjuvant systemic chemotherapy in patients undergoing CRS and HIPEC for pmCRC. Passot et al[73] showed an overall response rate of 36% and a disease progression rate of 21% in patients who received different regimens of modern neoadjuvant systemic chemotherapy before CRS and HIPEC. Interestingly, the response to neoadjuvant treatment was not a significant prognostic factor, therefore, it might not be considered a contraindication for CRS and HIPEC. The median survival of patients with disease progression was 31.4 mo[73]. Further analysis of different preoperative chemotherapy regimens consisting of 5-FU, oxaliplatin, irinotecan and/or monoclonal antibodies showed a 9.7% complete response rate, 20.2% major response and 70.1% rate of minor or no response. In the multivariate analysis the pathohistological response was an independent predictor of survival (P = 0.01)[74]. Devilee et al[75] compared patients with pmCRC who received neoadjuvant systemic chemotherapy before CRS and HIPEC with patients who were treated with adjuvant systemic chemotherapy after CRS and HIPEC. All patients underwent complete or nearly complete macroscopic cytoreduction. The 3-year survival rates were 89% and 50% for the neoadjuvant and adjuvant groups. Although the PCI was lower and operation time was shorter for patients who received preoperative chemotherapy, neoadjuvant treatment was still independently associated with improved survival after correcting for other significant prognostic factors[75]. Kuijpers et al[76] analyzed a prospective database regarding the effect of systemic chemotherapy on survival of patients with lymph-node positive pm CRC undergoing CRS and HIPEC. There was a statistically significant increase in the median PFS (15 mo vs 4 mo, P = 0.024) and median OS (30 mo vs 14 mo, P = 0.015) in patients who received perioperative systemic chemotherapy. Interestingly, the timing of systemic chemotherapy had no influence on survival[76]. The prospective multicenter phase II COMBATAC study evaluates CRS and bidirectional oxaliplatin-based HIPEC plus perioperative cetuximab-containing polychemotherapy[77]. The first safety data showed no increase in the morbidity or mortality when using the perioperative treatment approach[78]. There is another ongoing prospective phase II study (BEV-IP) evaluating perioperative systemic chemotherapy plus bevacizumab in combination with CRS and oxaliplatin-based HIPEC[79]. However, survival data from both studies are not yet available.
IPC AND PRESSURIZED INTRAPERITONEAL AEROSOL CHEMOTHERAPY
Except for the case of early postoperative IPC (EPIC) instead of or in addition to HIPEC after cytoreductive surgery there are no reliable published data for normothermic IPC without cytoreductive surgery for local treatment of pmCRC. The concept of sequential intraperitoneal treatment, which could be applied over a peritoneal port system, has been demostrated for ovarian cancer[80]. Yonemura et al[81] developed a protocol consisting of neoadjuvant systemic and intraperitoneal chemotherapy (NIPS) for gastric cancer. Clinical trials are needed to evaluate the potential role of IPC in patients with pmCRC, especially in the neoadjuvant setting. Preoperative IPC or NIPS may allow for higher rates of CC-0/1 resection and may further improve the outcome after CRS and HIPEC. Moreover, sequential IPC with or without palliative systemic chemotherapy might improve response rates and local tumor control in patients with unresectable PM arising from CRC.
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a new technique for the local application of cytostatics as aerosol under pressure that allows for improved drug distribution and tumor tissue penetration. The feasibility and safety of the procedure has been demosntrated[82,83]. Most data published for ovarian cancer show local anticancer activity after sequential application of PIPAC[84]. A recently published retrospective analysis of 48 applications of PIPAC given every six weeks in 17 patients with pretreated pmCRC reported a median OS of 15.7 mo. The overall response rate was 71%[85]. Quality of life analysis accessed by the EORTC-QLQ30 questionnaire in 48 patients with PM arising from different tumor entities (PCI: 16 ± 10) that received at least two PIPAC applications showed an impairment of the global physical score and pain score after the first treatment improved after the second PIPAC application. Gastrointestinal symptoms remained stable with PIPAC therapy[86]. Based on the promising preliminary data, PIPAC might become an additional therapeutic option for the palliative local treatment of pmCRC in the future. Moreover, it might be interesting as a neoadjuvant local treatment with or without the addition of systemic chemotherapy beyond CRS and HIPEC. Several prospective clinical trials evaluating this therapy approach are ongoing. The results may help to determine the role of PIPAC within a multidisciplinary treatment concept and allow for further improvement of patient selection.
CONCLUSION
The therapeutic approach to PM of colorectal cancer has changed in recent decades. There are multiple treatment options for patients with pmCRC that must be integrated in an individualized multidisciplinary treatment approach (Figure 1). Consistent diagnostics and patient selection are crucial to obtaining optimal oncologic outcome. Thus, the therapeutic approach should be discussed by an interdisciplinary tumor board, and, if necessary, patients should be referred to specialized treatment centers. In addition to multiple palliative treatment options, CRS and HIPEC provide an additive treatment modality with curative intent for selected patients with pmCRC. The integration of further treatment options such as repeated preoperative intraperitoneal chemotherapy or PIPAC in current treatment regimens should be discussed and evaluated in randomized controlled clinical trials. Prognostic factors, such as peritoneal tumor distribution, lymph node status, hematogenous metastasis, histology, tumor mutation status, tumor immunology, numerous patient-related factors and the resection status must be considered during patient selection and should be further investigated. The development and clinical use of the prognostic scores may help tailor individual treatment regimens that consider all available therapeutic options. Further prospective randomized trials focussed on patients with pmCRC are highly recommended to optimize and standardize the multimodal treatment regimens.
Footnotes
Conflict-of-interest statement: All authors declare that they have no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Germany
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: April 4, 2016
First decision: May 17, 2016
Article in press: July 13, 2016
P- Reviewer: Kulaylat MN, Wang GY S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Russell AH, Tong D, Dawson LE, Wisbeck W. Adenocarcinoma of the proximal colon. Sites of initial dissemination and patterns of recurrence following surgery alone. Cancer. 1984;53:360–367. doi: 10.1002/1097-0142(19840115)53:2<360::aid-cncr2820530232>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev. 2003;22:465–472. doi: 10.1023/a:1023791229361. [DOI] [PubMed] [Google Scholar]
- 4.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 6.Stewart JH, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol. 2005;12:765–777. doi: 10.1245/ASO.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2009;7:5. doi: 10.1186/1477-7819-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 9.Elias D, Souadka A, Fayard F, Mauguen A, Dumont F, Honore C, Goere D. Variation in the peritoneal cancer index scores between surgeons and according to when they are determined (before or after cytoreductive surgery) Eur J Surg Oncol. 2012;38:503–508. doi: 10.1016/j.ejso.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Esquivel J, Chua TC, Stojadinovic A, Melero JT, Levine EA, Gutman M, Howard R, Piso P, Nissan A, Gomez-Portilla A, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol. 2010;102:565–570. doi: 10.1002/jso.21601. [DOI] [PubMed] [Google Scholar]
- 11.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203:878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Hompes D, D’Hoore A, Van Cutsem E, Fieuws S, Ceelen W, Peeters M, Van der Speeten K, Bertrand C, Legendre H, Kerger J. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol. 2012;19:2186–2194. doi: 10.1245/s10434-012-2264-z. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 14.Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, Dumont F, Elias D. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22:2958–2964. doi: 10.1245/s10434-015-4387-5. [DOI] [PubMed] [Google Scholar]
- 15.Pelz JO, Chua TC, Esquivel J, Stojadinovic A, Doerfer J, Morris DL, Maeder U, Germer CT, Kerscher AG. Evaluation of best supportive care and systemic chemotherapy as treatment stratified according to the retrospective peritoneal surface disease severity score (PSDSS) for peritoneal carcinomatosis of colorectal origin. BMC Cancer. 2010;10:689. doi: 10.1186/1471-2407-10-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esquivel J, Garcia SS, Hicken W, Seibel J, Shekitka K, Trout R. Evaluation of a new staging classification and a Peritoneal Surface Disease Severity Score (PSDSS) in 229 patients with mucinous appendiceal neoplasms with or without peritoneal dissemination. J Surg Oncol. 2014;110:656–660. doi: 10.1002/jso.23679. [DOI] [PubMed] [Google Scholar]
- 17.Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, Deraco M, et al. The American Society of Peritoneal Surface Malignancies (ASPSM) Multiinstitution Evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 Patients with Colorectal Cancer with Peritoneal Carcinomatosis. Ann Surg Oncol. 2014;21:4195–4201. doi: 10.1245/s10434-014-3798-z. [DOI] [PubMed] [Google Scholar]
- 18.Yoon W, Alame A, Berri R. Peritoneal Surface Disease Severity Score as a predictor of resectability in the treatment of peritoneal surface malignancies. Am J Surg. 2014;207:403–407 discussion 406-407. doi: 10.1016/j.amjsurg.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Cashin PH, Graf W, Nygren P, Mahteme H. Comparison of prognostic scores for patients with colorectal cancer peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20:4183–4189. doi: 10.1245/s10434-013-3204-2. [DOI] [PubMed] [Google Scholar]
- 20.Rectum JSfCotCa. Tokyo: Kanehara & Co., Ltd; 2009. Japanese classification of colorectal carcinoma. [Google Scholar]
- 21.Kobayashi H, Enomoto M, Higuchi T, Uetake H, Iida S, Ishikawa T, Ishiguro M, Sugihara K. Validation and clinical use of the Japanese classification of colorectal carcinomatosis: benefit of surgical cytoreduction even without hyperthermic intraperitoneal chemotherapy. Dig Surg. 2010;27:473–480. doi: 10.1159/000320460. [DOI] [PubMed] [Google Scholar]
- 22.Noura S, Ohue M, Ito Y, Miyoshi N, Kobayashi H, Kotake K, Sugihara K. New Staging System for Colorectal Cancer Patients with Synchronous Peritoneal Metastasis in Accordance with the Japanese Classification of Colorectal Carcinoma: A Multi-Institutional Study. Dig Surg. 2016;33:66–73. doi: 10.1159/000442027. [DOI] [PubMed] [Google Scholar]
- 23.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klaver YL, Simkens LH, Lemmens VE, Koopman M, Teerenstra S, Bleichrodt RP, de Hingh IH, Punt CJ. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol. 2012;38:617–623. doi: 10.1016/j.ejso.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Lee DH, Oh SY, Lee YR, Huh SJ, Yoon HH, Kim SH, Lee S, Lee JH, Kim Y, Kim HJ, et al. A Phase II Study of Modified FOLFOX4 for Colorectal Cancer Patients with Peritoneal Carcinomatosis. Cancer Res Treat. 2011;43:225–230. doi: 10.4143/crt.2011.43.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 27.Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 28.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–2247. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 30.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Mahoney MR, O’Neil BH, Shaw JE, Polite BN, Hochster HS, Atkins JN, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) J Clin Oncol. 2014;32:LBA3. [Google Scholar]
- 31.Adachi T, Hinoi T, Egi H, Shimomura M, Ohdan H. Oxaliplatin and molecular-targeted drug therapies improved the overall survival in colorectal cancer patients with synchronous peritoneal carcinomatosis undergoing incomplete cytoreductive surgery. Surg Today. 2015;45:986–992. doi: 10.1007/s00595-014-1017-y. [DOI] [PubMed] [Google Scholar]
- 32.Razenberg LG, van Gestel YR, Lemmens VE, de Hingh IH, Creemers GJ. Bevacizumab in Addition to Palliative Chemotherapy for Patients With Peritoneal Carcinomatosis of Colorectal Origin: A Nationwide Population-Based Study. Clin Colorectal Cancer. 2016;15:e41–e46. doi: 10.1016/j.clcc.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 33.van Oudheusden TR, Razenberg LG, van Gestel YR, Creemers GJ, Lemmens VE, de Hingh IH. Systemic treatment of patients with metachronous peritoneal carcinomatosis of colorectal origin. Sci Rep. 2015;5:18632. doi: 10.1038/srep18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Oudheusden TR, Braam HJ, Luyer MD, Wiezer MJ, van Ramshorst B, Nienhuijs SW, de Hingh IH. Peritoneal cancer patients not suitable for cytoreductive surgery and HIPEC during explorative surgery: risk factors, treatment options, and prognosis. Ann Surg Oncol. 2015;22:1236–1242. doi: 10.1245/s10434-014-4148-x. [DOI] [PubMed] [Google Scholar]
- 35.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 36.van Oudheusden TR, Nienhuijs SW, Luyer MD, Nieuwenhuijzen GA, Lemmens VE, Rutten HJ, de Hingh IH. Incidence and treatment of recurrent disease after cytoreductive surgery and intraperitoneal chemotherapy for peritoneally metastasized colorectal cancer: A systematic review. Eur J Surg Oncol. 2015;41:1269–1277. doi: 10.1016/j.ejso.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Goéré D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, Maggiori L, Dumont F, Ducreux M, Elias D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257:1065–1071. doi: 10.1097/SLA.0b013e31827e9289. [DOI] [PubMed] [Google Scholar]
- 38.Cashin PH, Dranichnikov F, Mahteme H. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy treatment of colorectal peritoneal metastases: cohort analysis of high volume disease and cure rate. J Surg Oncol. 2014;110:203–206. doi: 10.1002/jso.23610. [DOI] [PubMed] [Google Scholar]
- 39.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran BJ, Mukherjee A, Sexton R. Operability and early outcome in 100 consecutive laparotomies for peritoneal malignancy. Br J Surg. 2006;93:100–104. doi: 10.1002/bjs.5210. [DOI] [PubMed] [Google Scholar]
- 41.McQuellon RP, Loggie BW, Fleming RA, Russell GB, Lehman AB, Rambo TD. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65–73. doi: 10.1053/ejso.2000.1033. [DOI] [PubMed] [Google Scholar]
- 42.McQuellon RP, Loggie BW, Lehman AB, Russell GB, Fleming RA, Shen P, Levine EA. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10:155–162. doi: 10.1245/aso.2003.03.067. [DOI] [PubMed] [Google Scholar]
- 43.Chia CS, Tan GH, Lim C, Soo KC, Teo MC. Prospective Quality of Life Study for Colorectal Cancer Patients with Peritoneal Carcinomatosis Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5203-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, Baratti D, Bartlett D, Barone R, Barrios P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14:128–133. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 46.Glockzin G, Renner P, Popp FC, Dahlke MH, von Breitenbuch P, Schlitt HJ, Piso P. Hepatobiliary procedures in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2011;18:1052–1059. doi: 10.1245/s10434-010-1415-3. [DOI] [PubMed] [Google Scholar]
- 47.Kianmanesh R, Scaringi S, Sabate JM, Castel B, Pons-Kerjean N, Coffin B, Hay JM, Flamant Y, Msika S. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597–603. doi: 10.1097/01.sla.0000255561.87771.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chua TC, Yan TD, Zhao J, Morris DL. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. Eur J Surg Oncol. 2009;35:1299–1305. doi: 10.1016/j.ejso.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Berger Y, Aycart S, Tabrizian P, Agmon Y, Mandeli J, Heskel M, Hiotis S, Sarpel U, Labow DM. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with liver involvement. J Surg Oncol. 2016;113:432–437. doi: 10.1002/jso.24153. [DOI] [PubMed] [Google Scholar]
- 50.Allard MA, Adam R, Ruiz A, Vibert E, Paule B, Levi F, Sebagh M, Guettier C, Azoulay D, Castaing D. Is unexpected peritoneal carcinomatosis still a contraindication for resection of colorectal liver metastases? Combined resection of colorectal liver metastases with peritoneal deposits discovered intra-operatively. Eur J Surg Oncol. 2013;39:981–987. doi: 10.1016/j.ejso.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Delhorme JB, Dupont-Kazma L, Addeo P, Lefebvre F, Triki E, Romain B, Meyer N, Bachellier P, Rohr S, Brigand C. Peritoneal carcinomatosis with synchronous liver metastases from colorectal cancer: Who will benefit from complete cytoreductive surgery? Int J Surg. 2016;25:98–105. doi: 10.1016/j.ijsu.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 52.Kwakman R, Schrama AM, van Olmen JP, Otten RH, de Lange-de Klerk ES, de Cuba EM, Kazemier G, Te Velde EA. Clinicopathological Parameters in Patient Selection for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer Metastases: A Meta-analysis. Ann Surg. 2016;263:1102–1111. doi: 10.1097/SLA.0000000000001593. [DOI] [PubMed] [Google Scholar]
- 53.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 54.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 55.Quenet F, Goéré D, Mehta SS, Roca L, Dumont F, Hessissen M, Saint-Aubert B, Elias D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254:294–301. doi: 10.1097/SLA.0b013e3182263933. [DOI] [PubMed] [Google Scholar]
- 56.Passot G, Vaudoyer D, Villeneuve L, Kepenekian V, Beaujard AC, Bakrin N, Cotte E, Gilly FN, Glehen O. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: A 25-year experience with 1,125 procedures. J Surg Oncol. 2016;113:796–803. doi: 10.1002/jso.24248. [DOI] [PubMed] [Google Scholar]
- 57.Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 58.Hompes D, D’Hoore A, Wolthuis A, Fieuws S, Mirck B, Bruin S, Verwaal V. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol. 2014;109:527–532. doi: 10.1002/jso.23546. [DOI] [PubMed] [Google Scholar]
- 59.Glockzin G, von Breitenbuch P, Schlitt HJ, Piso P. Treatment-related morbidity and toxicity of CRS and oxaliplatin-based HIPEC compared to a mitomycin and doxorubicin-based HIPEC protocol in patients with peritoneal carcinomatosis: a matched-pair analysis. J Surg Oncol. 2013;107:574–578. doi: 10.1002/jso.23228. [DOI] [PubMed] [Google Scholar]
- 60.Prada-Villaverde A, Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J Surg Oncol. 2014;110:779–785. doi: 10.1002/jso.23728. [DOI] [PubMed] [Google Scholar]
- 61.Glockzin G, Gerken M, Lang SA, Klinkhammer-Schalke M, Piso P, Schlitt HJ. Oxaliplatin-based versus irinotecan-based hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis from appendiceal and colorectal cancer: a retrospective analysis. BMC Cancer. 2014;14:807. doi: 10.1186/1471-2407-14-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Désolneux G, Mazière C, Vara J, Brouste V, Fonck M, Béchade D, Bécouarn Y, Evrard S. Cytoreductive surgery of colorectal peritoneal metastases: outcomes after complete cytoreductive surgery and systemic chemotherapy only. PLoS One. 2015;10:e0122816. doi: 10.1371/journal.pone.0122816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi H, Kotake K, Funahashi K, Hase K, Hirata K, Iiai T, Kameoka S, Kanemitsu Y, Maeda K, Murata A, et al. Clinical benefit of surgery for stage IV colorectal cancer with synchronous peritoneal metastasis. J Gastroenterol. 2014;49:646–654. doi: 10.1007/s00535-013-0820-3. [DOI] [PubMed] [Google Scholar]
- 64.Cashin PH, Mahteme H, Spång N, Syk I, Frödin JE, Torkzad M, Glimelius B, Graf W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur J Cancer. 2016;53:155–162. doi: 10.1016/j.ejca.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 65.Sugarbaker PH, Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol. 2016;7:29–44. doi: 10.3978/j.issn.2078-6891.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klaver CE, Musters GD, Bemelman WA, Punt CJ, Verwaal VJ, Dijkgraaf MG, Aalbers AG, van der Bilt JD, Boerma D, Bremers AJ, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer. 2015;15:428. doi: 10.1186/s12885-015-1430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garofalo A, Valle M, Garcia J, Sugarbaker PH. Laparoscopic intraperitoneal hyperthermic chemotherapy for palliation of debilitating malignant ascites. Eur J Surg Oncol. 2006;32:682–685. doi: 10.1016/j.ejso.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 68.Valle SJ, Alzahrani NA, Alzahrani SE, Liauw W, Morris DL. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for refractory malignant ascites in patients unsuitable for cytoreductive surgery. Int J Surg. 2015;23:176–180. doi: 10.1016/j.ijsu.2015.09.074. [DOI] [PubMed] [Google Scholar]
- 69.Cavazzoni E, Bugiantella W, Graziosi L, Franceschini MS, Donini A. Malignant ascites: pathophysiology and treatment. Int J Clin Oncol. 2013;18:1–9. doi: 10.1007/s10147-012-0396-6. [DOI] [PubMed] [Google Scholar]
- 70.Glehen O, Cotte E, Schreiber V, Sayag-Beaujard AC, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:747–754. doi: 10.1002/bjs.4473. [DOI] [PubMed] [Google Scholar]
- 71.Simkens GA, Razenberg LG, Lemmens VE, Rutten HJ, Creemers GJ, de Hingh IH. Histological subtype and systemic metastases strongly influence treatment and survival in patients with synchronous colorectal peritoneal metastases. Eur J Surg Oncol. 2016;42:794–800. doi: 10.1016/j.ejso.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Maillet M, Glehen O, Lambert J, Goere D, Pocard M, Msika S, Passot G, Elias D, Eveno C, Sabaté JM, et al. Early Postoperative Chemotherapy After Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Isolated Peritoneal Carcinomatosis of Colon Cancer: A Multicenter Study. Ann Surg Oncol. 2016;23:863–869. doi: 10.1245/s10434-015-4914-4. [DOI] [PubMed] [Google Scholar]
- 73.Passot G, Vaudoyer D, Cotte E, You B, Isaac S, Noël Gilly F, Mohamed F, Glehen O. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256:125–129. doi: 10.1097/SLA.0b013e318255486a. [DOI] [PubMed] [Google Scholar]
- 74.Passot G, You B, Boschetti G, Fontaine J, Isaac S, Decullier E, Maurice C, Vaudoyer D, Gilly FN, Cotte E, et al. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21:2608–2614. doi: 10.1245/s10434-014-3647-0. [DOI] [PubMed] [Google Scholar]
- 75.Devilee RA, Simkens GA, van Oudheusden TR, Rutten HJ, Creemers GJ, Ten Tije AJ, de Hingh IH. Increased Survival of Patients with Synchronous Colorectal Peritoneal Metastases Receiving Preoperative Chemotherapy Before Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5214-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 76.Kuijpers AM, Mehta AM, Boot H, van Leerdam ME, Hauptmann M, Aalbers AG, Verwaal VJ. Perioperative systemic chemotherapy in peritoneal carcinomatosis of lymph node positive colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Oncol. 2014;25:864–869. doi: 10.1093/annonc/mdu031. [DOI] [PubMed] [Google Scholar]
- 77.Glockzin G, Rochon J, Arnold D, Lang SA, Klebl F, Zeman F, Koller M, Schlitt HJ, Piso P. A prospective multicenter phase II study evaluating multimodality treatment of patients with peritoneal carcinomatosis arising from appendiceal and colorectal cancer: the COMBATAC trial. BMC Cancer. 2013;13:67. doi: 10.1186/1471-2407-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piso P, Koller M, Arnold D, Schlitt HJ, Glockzin G. Multimodality treatment of colorectal peritoneal metastasis with perioperative systemic chemotherapy, cytoreductive surgery (CRS), and HIPEC: First safety results of the COMBATAC trial. J Clin Oncol. 2014;32:LBA382. [Google Scholar]
- 79.Willaert W, Van Der Speeten K, Liberale G, Ceelen W. BEV-IP: Perioperative chemotherapy with bevacizumab in patients undergoing cytoreduction and intraperitoneal chemoperfusion for colorectal carcinomatosis. BMC Cancer. 2015;15:980. doi: 10.1186/s12885-015-1954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016;(1):CD005340. doi: 10.1002/14651858.CD005340.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yonemura Y, Elnemr A, Endou Y, Ishibashi H, Mizumoto A, Miura M, Li Y. Effects of neoadjuvant intraperitoneal/systemic chemotherapy (bidirectional chemotherapy) for the treatment of patients with peritoneal metastasis from gastric cancer. Int J Surg Oncol. 2012;2012:148420. doi: 10.1155/2012/148420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solass W, Giger-Pabst U, Zieren J, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol. 2013;20:3504–3511. doi: 10.1245/s10434-013-3039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol. 2016;14:128. doi: 10.1186/s12957-016-0892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tempfer CB, Solass W, Reymond MA. Pressurized intraperitoneal chemotherapy (PIPAC) in women with gynecologic malignancies: a review. Wien Med Wochenschr. 2014;164:519–528. doi: 10.1007/s10354-014-0312-y. [DOI] [PubMed] [Google Scholar]
- 85.Demtröder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis. 2016;18:364–371. doi: 10.1111/codi.13130. [DOI] [PubMed] [Google Scholar]
- 86.Odendahl K, Solass W, Demtröder C, Giger-Pabst U, Zieren J, Tempfer C, Reymond MA. Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) Eur J Surg Oncol. 2015;41:1379–1385. doi: 10.1016/j.ejso.2015.06.001. [DOI] [PubMed] [Google Scholar]


