Abstract
Epidemiological and experimental animal studies show that suboptimal environments in fetal and neonatal life exert a profound influence on physiological function and risk of diseases in adult life. The concepts of the ‘developmental programming’ and Developmental Origins of Health and Diseases (DOHaD) have become well accepted and have been applied across almost all fields of medicine. Adverse intrauterine environments may have programming effects on the crucial functions of the immune system during critical periods of fetal development, which can permanently alter the immune function of offspring. Immune dysfunction may in turn lead offspring to be susceptible to inflammatory and immune diseases in adulthood. These facts suggest that inflammatory and immune disorders might have developmental origins. In recent years, inflammatory and immune disorders have become a growing health problem worldwide. However, there is no systematic report in the literature on the developmental origins of inflammatory and immune diseases and the potential mechanisms involved. Here, we review the impacts of adverse intrauterine environments on the immune function in offspring. This review shows the results from human and different animal species and highlights the underlying mechanisms, including damaged development of cells in the thymus, helper T cell 1/helper T cell 2 balance disturbance, abnormal epigenetic modification, effects of maternal glucocorticoid overexposure on fetal lymphocytes and effects of the fetal hypothalamic–pituitary–adrenal axis on the immune system. Although the phenomena have already been clearly implicated in epidemiologic and experimental studies, new studies investigating the mechanisms of these effects may provide new avenues for exploiting these pathways for disease prevention.
Keywords: adverse intrauterine environment, T cells, thymus, developmental origins, inflammatory and immune diseases
Introduction
Immunity is a human physiological function, which can maintain the health of the body by destroying and rejecting ‘non-self’, such as antigens, damaged cells and tumor cells produced by the body itself. Both as the body's defense against foreign invasion and as a damage response, there is an inseparable relation between inflammation and immunity. During the past decades, inflammatory and immune disorders have become a growing health problem worldwide. An epidemiological study indicated that the prevalence of self-reported food allergy increased significantly among US adults, to 13% in 2010 and 14.9% in 2006 compared with 9.1% in 2001 (Verrill et al., 2015). From 1999 to 2012 in the UK, the incidence of systemic lupus erythematosus increased from 64.99/100 000 to 97.04/100 000 (Rees et al., 2016). There were three cross-sectional surveys in Pisa during 1985–2011, and results showed that there was an increasing trend in prevalence rates of respiratory symptoms/diseases. Currently asthma (1st–3rd prevalence rates: 3.4–7.2%), allergic rhinitis (16.2–37.4%), usual phlegm (8.7–19.5%) and chronic obstructive pulmonary disease (2.1–6.8%) had more than doubled (Maio et al., 2016). Similar tendencies were shown in other inflammatory diseases, such as rheumatoid arthritis and eosinophilic esophagitis (Widdifield et al., 2014; Giriens et al., 2015).
Prenatal and early life events are important determinants for disorders later in life. Based on a large number of investigations and the results of evidence-based research, scholars proposed a concept of the origin of human diseases—‘Developmental Origins of Health and Disease (DOHaD)’. This hypothesis stated that the fetal tissues and organs in the sensitive period of development would show permanent or programming changes in their structure and function because of the adverse intrauterine environment. These changes might significantly increase the susceptibility to a variety of chronic diseases in offspring, such as metabolic syndrome, fatty liver, depressive disorder etc. The development processes of these diseases were accompanied by inflammatory and immune changes (Huizink et al., 2004; Fowden et al., 2006). Thus we speculated whether the occurrence of inflammatory and immune diseases also had developmental origins? Currently, there is no systematic review on this subject. A growing number of studies suggested that the increased risk of adult inflammatory and immune diseases was related to negative prenatal exposure and a developmental deficiency of the immune system in early life (Ege et al., 2006; Schaub et al., 2009). Therefore, in this paper, we will systematically review the growing evidence and mechanisms from human and experimental animal studies supporting the view for the developmental origins of the inflammatory and immune disorders for the first time.
Evidence for the developmental origins of inflammatory and immune disorders
Human research
A number of works in the scientific literature of epidemiological studies suggested that the programming changes of fetal immunity could affect fetal development, such as organ formation and function implementation, which are important determinants for diseases later in life. Previous study showed that maternal psychosocial stress could increase the levels of pro-inflammatory cytokines in maternal serum, which might be related to the increased risk of allergy in infants (Coussons-Read et al., 2007). O'Connor et al. (2013) examined the cell-mediated immune responses in infants at 6 months of age, and found that prenatal maternal anxiety was associated with reduced interferon-G (IFNG) and increased interleukin-4 (IL4) responder cell frequencies. These findings demonstrated that prenatal maternal anxiety could alter adaptive immunity in the infant. In addition, researchers found that a high maternal immunoglobulin E (IgE) level in pregnancy was associated with increased IgE in children at the age of 1 year. Thus, maternal immune status in pregnancy was related to the risk of immune responses and atopy in offspring (Herberth et al., 2011). What is more, researchers also found that maternal allergy was associated with the change of T cell subsets in cord blood at their child's birth, which might predispose one to an increased risk for developing atopic dermatitis during the first 2 year of life (Fu et al., 2013).
Except for the maternal psychological and immune status, prenatal exposure to xenobiotics and maternal nutrition also can influence fetal immune programming. For example, it was reported that exposure to tobacco during pregnancy might affect the development of the fetal lung and immune system, resulting in the susceptibility to asthma and respiratory diseases in newborns and children (Hylkema and Blacquiere, 2009). And prenatal parasitic infection might increase the risk of parasite infection in offspring during the first 30 months of life (Schwarz et al., 2008). It was reported that arsenic exposure in utero might alter the fetal immune system and then lead to immune dysregulation in offspring through decreasing CD45RA+CD4+CD69+ cell counts and increasing CD45RA+CD69−CD294+ cell counts in cord blood (Nadeau et al., 2014). In addition, prenatal arsenic exposure could alter microRNAs involved in innate and adaptive immune signaling in newborn cord blood (Rager et al., 2014). Studies also showed that maternal intake of foods high in fatty acids during pregnancy was associated with the risk of suspected atopic eczema among infants aged 3–4 months (Saito et al., 2010). And excessive supplementation of folic acid during pregnancy or maternal exposure to high levels of traffic particles could lead to an increased risk of asthma and respiratory disorders in children (Haberg et al., 2009; Perera et al., 2009; Whitrow et al., 2009). Other research showed that maternal vitamin K intake during pregnancy might increase the susceptibility to asthma at the age of 18 months and 7 years (Maslova et al., 2014). With a validated diet history questionnaire, researchers found that higher maternal intake of vitamin D during pregnancy might increase the risk of infantile eczema in children aged 23 to 29 months (Miyake et al., 2014).
Adverse birth outcomes resulted from adverse intrauterine environments, such as intrauterine growth retardation (IUGR) and preterm birth, which are often associated with changes of immune function in offspring. It was reported that the IUGR fetus presented a smaller thymic volume compared with that of control (Olearo et al., 2012). Furthermore, the ability of the innate immune system of the IUGR fetus to mount an immune response was weakened after birth. After stimulating the whole cord blood cell cultures with lipopolysaccharides (LPSs), the concentrations of IL6 and IL10 were significantly lower in that from the IUGR fetus, showing that the IUGR infants had a weaker ability to mount an immune response (Troger et al., 2013). Similarly, the peripheral regulatory T cell (Treg) pool in cord blood of preterm infants could be altered by prenatal exposure to inflammation and chorioamnionitis. The changed percentage of Tregs might influence the role of Tregs in peripheral tolerance and the control of immune responses to pathogens (Luciano et al., 2014).
Experimental animal studies
Besides human research, data from animal studies provide further evidence of alterations of immune function by an adverse intrauterine environment, which lead to offspring susceptibility to inflammatory and immune diseases (Xiong and Zhang, 2013). Researchers found that maternal infection had profound impacts on fetal inflammation in their internal organs and skin (Wolfs et al., 2009; Kemp et al., 2011; Kuypers et al., 2012). Kay et al. demonstrated that the immune function of the 2-month-old offspring rats was suppressed due to the noise and light stress for 3 days weekly throughout the pregnancy (Kay et al., 1998). In addition, prenatal cadmium (Cd) exposure could reduce the acquired immune function of offspring at the age of 20 weeks (Holaskova et al., 2012). Tobacco smoke had pro-inflammatory effects, which could not only increase the expression of pro-inflammatory chemokines, but also lead to the apoptosis or necrosis of bronchial epithelial cells. Thus, exposure to tobacco smoke during pregnancy could increase the risk of asthma, allergies and respiratory infections in offspring (Cheraghi and Salvi, 2009). Prenatal exposure to nicotine, moreover, could alert the structure of respiratory and collagen deposition around blood vessels. These effects could increase the susceptibility to respiratory diseases in adult offspring (Hylkema and Blacquiere, 2009).
Other research showed that an adverse intrauterine environment might affect the number and function of immune cells in offspring. For example, ethanol exposure during pregnancy could weaken the phagocytic activity of macrophages (MΦ) in newborn mice (Gauthier et al., 2010). The restraint to the mother during the last third of gestation decreased the response to mitogen stimulation and the number of leukocytes in the blood of newborn pigs, and altered the ratios of CD4+ and CD8+ T cells in blood (Couret et al., 2009). Furthermore, by giving a foot-shock once daily from Day 15 to 19 to pregnant mice, the spreading and phagocytosis of MΦ were decreased in 2-month-old male offspring (Palermo Neto et al., 2001). Upon LPS stimulation of the blood cells from 2-year-old offspring born with maternal stress exposure, the levels of tumor necrosis factor-a (TNFA) and IL6 were significantly lower than those of control (Coe et al., 2002). It was reported that prenatal stress could change the number and proliferation of lymphocytes, and the cytotoxicity of natural killer (NK) cells in the blood of male adult offspring (Gotz and Stefanski, 2007). Prenatally acquired vitamin A deficiency could reduce the number of dendritic cells (DCs) in offspring, which not only inhibited the immunomodulatory effects but also influenced the negative selection of thymocytes, resulting in the inhibition of the development of immune cells (Vlasova et al., 2013). It was reported that carbon black nanoparticle exposure during the critical periods of development increased the total number of thymocytes and the proportion of double negative (DN) and double positive (DP) cells, which seemed to be responsible for the overall high numbers of splenic cells (El-Sayed et al., 2015).
In sum, the above human population and experimental animal studies provided a link between an adverse gestational environment (such as xenobiotics exposure, psychosocial stress, physical stimulation, maternal treatment and nutrition etc.) and a perturbed immune regulation in children that could be related to the development of immune disorders.
Potential biological mechanisms
Impaired cell development in thymus
The thymus is the earliest developed immune organ in the fetus. In the embryo, nearly 95% of immature T cells have undergone apoptosis and only a little could develop into naive T cells after migrating into the thymus. A study reported that prenatal exposure to 2, 3, 7, 8-tetrachlorodibenzo-para-dioxin induced the down-regulation of microRNA (miR23a, miR23b, miR18b and miR98) expression profile in the fetal thymus, which led to increased expression of Fas and Fasl in the surface of thymocytes. These effects promoted thymocyte apoptosis and finally resulted in thymus atrophy in the offspring, which was one of the important mechanisms of damaged immune function in adults (Singh et al., 2012a). Moreover, prenatal exposure to diethylstilbestrol (DES) triggered the up-regulation of Fas and Fasl expression in thymocytes, and caused thymic atrophy and thymocyte apoptosis (Singh et al., 2012b). Similarly, another study reported that perinatal DES exposure-induced thymic atrophy might result from the increased thymocyte apoptosis mediated by a death receptor pathway involving TNF family members (Brown et al., 2006). In addition, betamethasone administration to mothers in the third trimester of pregnancy could induce the apoptosis of DP thymocytes, and then cause the atrophy of the fetal thymus (Diepenbruck et al., 2013). Furthermore, by using the fetal thymus organ culture (FTOC), researchers found that nitric oxide could synergize DP thymocyte apoptosis induced by glucocorticoids (GCs), which affected the function of T cells profoundly (Cohen et al., 2012). Aaron J et al. also took FTOC as a model to investigate the effects of nicotine on the maturity of T cells. They found that nicotine could directly induce the apoptosis of fetal thymocytes and affect the normal development of fetal thymus, which finally could disable the function of the thymus in immune regulation (Middlebrook et al., 2002). And maternal undernutrition during the late pregnancy could modify the maturation of DP thymocytes to reduce their numbers, resulting in the retarded development of the thymus in the IUGR fetus (Liu et al., 2015).
Except for the increased apoptosis of thymocytes, the disturbed differentiation and development of immune cells also can affect immune function. Researchers found that prenatal Cd exposure could cause the dysregulation of the sonic hedgehog and Wnt/β-catenin signaling pathways in the fetal thymus, which are important for thymocyte maturation, and could induce fetal thymic atrophy and immune function disorder (Hanson et al., 2010). The bone morphogenetic protein 2/4 (BMP2/4) signaling pathway is required for thymus morphogenesis and the differentiation of T cells (Hager-Theodorides et al., 2014). Studies showed that prenatal exposure to nicotine could down-regulate the expression of BMP2 to affect the two developmental stages of fetal thymocytes: the transition from DN1 to DN3, and from DN to DP cells, interfering with the normal differentiation of fetal thymocytes (Ma et al., 2011).
Apoptosis is crucial during the development process of thymocytes. Increased apoptosis or impaired differentiation of thymocytes in the fetal stage could reduce the volume and weight of the thymus, also could change the phenotypes of thymocytes. Intrauterine thymus dysplasia often resulted in thymus dysfunction after birth, mainly manifested as a reduced thymocyte output. As a result, the reduction of peripheral lymphocytes might weaken the body's cellular immune response. In addition, the changes of phenotypes of thymocytes could be divided into two categories, including changes of the DN/DP ratio and the CD4+/CD8+ ratio. The changes of the DN/DP ratio suggested the blocked development of thymocytes. And the changes of the CD4+/CD8+ ratio might cause an imbalance of CD4+/CD8+ in the periphery, which could disrupt the body's immune homeostasis. In summary, impaired cell development in the thymus could weaken the immune function of offspring, which could increase susceptibility to inflammatory and immune diseases.
Th1/Th2 balance disturbance
Helper T 1 (Th1) and Th2 cells are both CD4+ T cells, and the cytokines produced by them are known as Th1-type cytokines (IL2, IFNG) and Th2-type cytokines (IL4, 5 and 10), respectively (Saxena and Kaur, 2015). A balance of Th1/Th2 cells is necessary to maintain immune homeostasis: A disruption of this balance could lead to Th1 shift or Th2 shift and then changes in the normal cellular and humoral immune regulation (Hashimoto, 2015).
Many animal studies suggested that the excessive Th2 cell-mediated responses might play an important role in the susceptibility to inflammatory and immune diseases. For example, it was reported that prenatal stress could trigger the clonal expansion of Th2 cells through antigen-presenting cells, and the over-proliferated Th2 cells might break the balance of Th1/Th2 (Pincus-Knackstedt et al., 2006). In addition, researchers demonstrated that prenatal Cd exposure could reduce the population of MΦ and depress Th1-type cytokines to a higher degree than that of Th2-type in offspring at 2 and 7 weeks of age. These offspring would be susceptible to immune diseases resulting from excessive Th2 cell-mediated response (Hanson et al., 2012). Furthermore, maternal exposure to airborne particulates might result in postnatal immune dysfunction by exacerbation of Th1/Th2 deviation, with decreased IFNG and increased IL4 levels in the blood and splenocytes. And this deviation was associated with lowered Tbet and elevated Gata3 mRNA expressions (Hong et al., 2013).
The balance between Th1 and Th2 cells are critical for the control of immune responses and susceptibility/resistance to diseases. TBET and GATA3, the specific transcription factors of Th subsets, play extremely important roles in the differentiation, phenotype maintenance and function of Th cells. Through affecting the production of Th1-type and Th2-type cytokines, and the expression of the corresponding transcription factors, an adverse intrauterine environment could cause a Th2 shift in the offspring. The role of Th2 cells in the pathogenesis of allergic diseases was well clarified. Therefore adverse intrauterine events might lead to susceptibility to inflammatory and immune diseases in offspring by triggering a Th2 skewing.
Abnormal epigenetic modification
Epigenetics is a discipline to study the heritable changes in gene expression without changing the DNA sequences. The epigenetic modifications include DNA methylation, histone modifications (methylation and acetylation), chromatin remodeling and regulation of non-coding RNAs, etc. (Mattick, 2007). Given that these modifications are reversible and sensitive to environmental factors, they provide a mechanistic link among environmental exposures, developmental programming and risk for diseases.
A substantial body of work suggested a link between epigenetic gene regulation, immunity and physiologic development. Tregs are one of the T-cell subsets; they have immunosuppressive effects with a characteristic marker—transcription factor FOXP3—and secrete transforming growth factor b (TGFB), IL10 and other cytokines. The demethylation state of Treg-specific demethylated region (TSDR) in the FOXP3 gene was suggested to be a valuable biomarker that could reliably indicate and quantify stable Tregs in cord blood. Prenatal tobacco smoke exposure could decrease the Treg numbers in cord blood through affecting the demethylation state of the TSDR. Children with lower Treg numbers at birth had a higher risk of developing atopic dermatitis (Hinz et al., 2012). In addition, tobacco smoke exposure could suppress the expression of FOXP3 by increasing the level of CpG methylation within the FOXP3 locus, and then affect the function of Treg (Runyon et al., 2012). It was reported that changes in maternal diet, such as excessive intake of folic acid, might increase the methylation level of FOXP3 in fetal Treg and reduce its transcriptional activity. The decreased expression of FOXP3 represented the reduced generation of Treg, which resulted in the impaired immunosuppressive function of offspring (Klunker et al., 2009). In addition, microRNA also could regulate the Treg numbers. Researchers found that maternal tobacco smoke exposure increased the level of miR223 expression and then lowered Treg numbers in cord blood, which was correlated with subsequent allergy risk in children (Herberth et al., 2014). Studies also indicated that exposure to environment pollutants during pregnancy might increase the methylation of the Ifng gene in CD4+ cells to silence the differentiation pathways of Treg and Th1, which could inhibit Th2 allergic differentiation. Thus, the increased Ifng gene methylation might break the balance of Th1 and Th2, reduce Th1 function and relatively enhance Th2 function (Martino and Prescott, 2010). Furthermore, environmental pollutants during pregnancy could also cause a loss of histone 4 (H4) acetylation in the Ifng promoter, which results in a reduced IFNG level and increased asthma risk in the offspring (Reiprich et al., 2013).
Moreover, adverse intrauterine environments also can affect the immune system in offspring by changing the epigenetic modification of cytokine coding genes. For example, the oxidative stress during pregnancy could decrease the histone deacetylase activity in the lung, which leads to increased acetylation level and activity of nuclear factor κB (NFKB). And NFKB further enhances the expression of inflammatory genes (Il6 and Il8) in the lung, which could cause the susceptibility to respiratory system inflammation (Rahman and Adcock, 2006). Prenatal exposure to particulate matter promoted the production of IgE through the hypomethylation of Ifng and the hypermethylation of Il4 in CD4+ cells (Liu et al., 2008). In addition, prenatal dexamethasone exposure could decrease the levels of active chromatin signs (acetylation of histone H3 lysines, H3K4me1/3 and H3K36me3) in the Tnfa promoter, which regulated Tnfa expression, and resulted in profound and lasting impacts on the developing immune system (Yu et al., 2014).
Some individual differences in human disease and behavior that cannot be accounted for genetically may be due to epigenetic changes in gene expression. Epigenetic changes are propagated at the cellular level, and allow the organism to adapt to changes within a particular environment (Sanders, 2006). The alterations of early immune development resulting from abnormal epigenetic modification mainly include suppression of IFNG production, altered innate immunity and deficient Treg networks. These alterations culminate in a propensity for uncontrolled Th2 immune responses (Martino and Prescott, 2011). Since Th1/Th2 immunity is linked closely to disease and behavior, it is probable that epigenetic changes induced by adverse intrauterine environments may explain the occurrence of disease susceptibility. In summary, prenatal xenobiotic exposure or stress can change the epigenetic modifications of immune-related genes, which regulate the differentiation of Tregs or Th1/Th2 cells, and ultimately induce the immunological dysfunction in offspring.
Effects of maternal GC overexposure on fetal lymphocytes
Different doses or concentrations of GCs produce different effects in adults. Small doses or physiological levels of GCs mainly produce physiological effects, while the large doses exceeding the physiological level can generate immunosuppressive effects. The placenta is a natural barrier, which provides an allograft transplantation immune interface between mother and fetus (Reis et al., 2001). During pregnancy, the majority of GCs cannot enter the fetus because of the inactivation by placental enzyme 11b-hydroxysteroid dehydrogenase type 2 (11BHSD2). Therefore, the fetus will be exposed to a low level of maternal GCs under normal circumstances. But the level will increase because of the excessive increases in maternal GCs or the inhibition of the activity of 11BHSD2 (Staud et al., 2006).
High concentrations of GCs in the fetus could suppress the function of MΦ, reduce lymphocyte proliferation, increase the proportion of CD4+/CD8+ T cells in the blood and decrease the number of T cells in the spleen. Thereby it could attenuate the immune function of the fetus, and lead to susceptibility of the offspring to inflammatory and immune diseases after birth (Kramer et al., 2004; Kapoor et al., 2006). Taking a concrete example, a high level of GCs could inhibit cellular immunity in the fetus, and induce the release of granulocytes in bone marrow. These effects were reported to cause the susceptibility to inflammation (Song et al., 1994). Further, since the fetal immune system is in an immature state, DCs are more sensitive to GC-mediated immune suppression. In vitro, by treating the DCs obtained from the cord blood with dexamethasone, researchers found that neonatal DCs seemed to be especially sensitive to the immunosuppressive effects of dexamethasone as indicated by altered phenotype, endocytic function, ability to stimulate T cells and a cytokine shift favoring Th2. These alterations in DC function caused an increased risk for certain infections and atopic diseases (Mainali et al., 2005). Prenatal betamethasone and endotoxin exposure increased the mRNA level of pro-inflammatory cytokine Il1b in the pulmonary lymphocytes of progeny. This result had implications for lung inflammation in infants exposed to maternal GCs (Kallapur et al., 2003). In addition, researchers found that prenatal stress could increase the protein level and mRNA expression of GC receptor (GR) in lymphoid cells in offspring, which in turn modify the immune homeostasis (such as that of GCs) (Pascuan et al., 2014).
GC-mediated immune suppression had been proved in many in vivo and in vitro studies. Increased level of circulating GCs in the fetus was attributed to a high GC level in maternal blood or inactivated 11BHSD2 in placenta (Kapoor et al., 2006). The changes of the immune system resulting from direct effects of GCs included the decreased numbers of immune cells and the changed CD4+/CD8+ ratio (Parimi et al., 1999), both of which could lead to immune dysfunction in the offspring. In summary, the overexposure to maternal GCs can act directly on fetal immune cells and increase susceptibility to inflammatory and immune diseases in offspring.
Effects of the fetal hypothalamic–pituitary–adrenal axis on the immune system
GCs are secreted by the adrenal glands, the end effector organ of the hypothalamic–pituitary–adrenal (HPA) axis. Apart from the direct influence of the overexposure to maternal GCs on fetal immune cells, the dysregulation of the fetal HPA axis has an indirect influence on the immune system of the fetus exposed to adverse intrauterine environments (Matthews, 2002). Under normal circumstances, besides the effects of GCs, the HPA axis has immunomodulatory effects on the activity and growth of immune cells through growth hormones. The fetal HPA dysfunction caused by prenatal xenobiotics exposure could affect the brain neurotransmitter system and cognitive ability, and change the neural regulation of immune function in offspring. It was reported that stress-induced high level of maternal GCs could stimulate the placenta to secret corticotropin-releasing hormone (CRH) and cause the activation of the fetal HPA axis, which indirectly caused the increased level of circulating GCs in the fetus (Toumi et al., 2013). In addition, like GCs, hypothalamic adrenocorticotropic hormone (ACTH) also has immunosuppressive effects. Prenatal stress could trigger a higher secretion of ACTH through affecting the HPA axis in the fetus, and then caused the susceptibility to immune diseases in adult offspring (Vieau et al., 2007).
In addition, the regulation of GR in the fetus also can influence the immune function through affecting the activation of the fetal HPA axis. Studies suggested that prenatal stress might reduce the mRNA and protein expression of GR in the fetal hippocampus, which might weaken the negative feedback inhibition of the HPA axis, and cause the continuous activation of the fetal HPA axis (Karrow, 2006). Other results also provided the evidence that prenatal stress could affect the activation of the fetal HPA axis and lead to the immunosuppression in offspring because of high circulating GCs (Petropoulos et al., 2014).
There is a complicated two-way relationship between the HPA axis and the immune system, and GCs as an immunosuppressive medium are well known. The excessive activation or attenuated negative feedback of the fetal HPA axis could increase the level of circulating GCs in the fetus. Moreover, as the hormone secreted by the HPA axis, ACTH could directly control thymocyte homeostasis independent of GCs (Talaber et al., 2015). In sum, an adverse intrauterine environment could affect the maturity or activation of the fetal HPA axis, resulting in the increased secretion of GCs, CRH and ACTH, and consequently influence the immune function of offspring.
Protective effects of intrauterine environment on the immune system
Although multiple exposure factors during pregnancy increased the risk of inflammatory and immune diseases in offspring, a few kinds of exposure (such as microbial exposure and higher intake of dairy products) had protective effects. From research on humans, higher maternal intake of total dairy products, cheese, yogurt and calcium during pregnancy might reduce the risk of infantile eczema, asthma and atopic eczema (Miyaka et al., 2014). In addition, maternal exposure to an environment rich in microbial compounds in a German farm might protect against the development of atopic sensitization (Ege et al., 2006). Similar protective effects on offspring were observed in a New Zealand farm environment (Douwes et al., 2008). Animal studies also showed that both pathogenic and non-pathogenic microbial exposure during pregnancy could suppress allergic airway inflammation in offspring (Blumer et al., 2005, 2007). Epigenetically regulated childhood immune development by maternal microbial exposure is likely induced via changes in maternal immune regulation (Jenmalm, 2011). The protective effects might depend on increased expression of Ifng mediated by increased H4 acetylation of the Ifng promoter (Martino and Prescott, 2011). Human studies also showed that allergy protection by in utero microbial exposure was associated with the demethylation of the FOXP3 gene and enhanced neonatal Treg function (Schaub et al., 2009).
It is worth noting that the evidence for the role of vitamin D intake in the development of allergic disease is limited and conflicting. Some data suggested that higher maternal intake of vitamin D during pregnancy might increase the risk of allergic diseases (Miyake et al., 2014), while there was another report that mothers with a higher vitamin D intake might have infants with a reduced risk of wheezing and allergic diseases (Miles and Calder, 2015). The effects of vitamin D on T cells are complex. It not only could enhance innate immunity and Th2 cell differentiation and suppress Th1 cell responses, but also could increase Treg cell populations. Therefore, these effects of vitamin D could not suggest a clear and simple role in allergic disease prevention or amelioration (Mora et al., 2008; Chambers and Hawrylowicz, 2011).
Summary
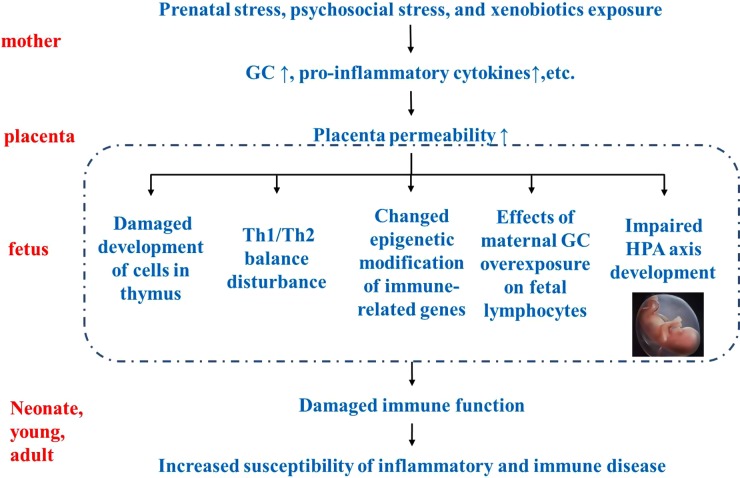
Adverse intrauterine environments during pregnancy have profound impacts on the immune system of offspring. Although maternal microbial exposure and higher intake of dairy products could lead to the protection against allergic diseases, most of environmental factors could increase the risk of inflammatory and immune diseases in offspring. Prenatal stress, psychosocial stress and xenobiotics exposure in mothers can cause increased GCs, pro-inflammatory cytokines and so on. Impaired placental permeability results in an adverse intrauterine environment. The compromised immune function after birth may originate from the impaired development of cells in the fetal thymus, disturbance of Th1/Th2 balance, changed epigenetic modification of immune-related genes, effects of maternal GC overexposure on fetal lymphocytes and impaired HPA axis development in the fetus. As a result, immune dysfunction may increase the susceptibility to inflammatory and immune diseases in offspring, and even in adults (Fig. 1).
Figure 1.
Developmental origins of inflammatory and immune disorders. Prenatal stress, psychosocial stress and xenobiotics exposure may affect the development of the fetal immune system, through affecting immune cells, epigenetic modification of immune-related genes or other physiological systems that regulate immune responses. As a result, immune dysfunction may increase susceptibility to inflammatory and immune diseases in offspring and even into adulthood. GC: glucocorticoid; HPA: hypothalamic–pituitary–adrenal; TH1/2: helper T 1/helper T 2.
Future research
With the rapid progress in genetic technology, more and more diseases will be attributed to genetic inheritance and abnormal embryonic growth and development. By tracing the developmental origins of diseases, scholars want to provide new avenues for early intervention on them. The immune system is very complex, which include nerve immunity and cellular immunity. Moreover, affecting any link during the development of immune organs and immune cells may cause immune dysfunction in offspring. Therefore, the mechanisms of inflammatory and immune diseases are multifaceted. During the recent years, the relationship between early exposure and later diseases is well established, but most of the investigations are limited in either describing the phenomena of immune disorders in later life or the impacts of risks on T cell development in early life. There are few studies on the relationship between intrauterine mechanisms and immune dysfunction after birth, which still rest on the changed morphology and function of the fetal thymus or peripheral lymphoid organs after birth, and immune dysfunction due to T cell imbalance. Furthermore, immune function is inseparable from the assistance of cytokines. Usually, IFNG/IL4 is used to reflect Th1-/Th2- mediated immune responses, but do researchers need to consider the efficiency and strength of the cytokines individually for their role in the immune responses? Except for Th1 and Th2, the roles of other Th cells (such as Th17) also need to be studied. Therefore, the pathogenesis of inflammatory and immune disorders remains to be investigated further.
Authors' roles
T.C. and J.P. contributed to conception, drafting and editing of the manuscript, H.L., H.Y. and D.W. edited the manuscript. All authors approved the final manuscript.
Funding
This work was supported by Grants from the National Natural Science Foundation of China (No. 81173138, 81273107, 30800931), the Outstanding Youth Science Fund of Hubei Province (No. 2012FFA017) and the Youth ChenGuang Project of Wuhan (No. 2014070404010197).
Conflict of interest
None declared.
References
- Blumer N, Herz U, Wegmann M, Renz H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin Exp Allergy 2005;35:397–402. [DOI] [PubMed] [Google Scholar]
- Blumer N, Sel S, Virna S, Patrascan CC, Zimmermann S, Herz U, Renz H, Garn H. Perinatal maternal application of Lactobacillus rhamnosus GG suppresses allergic airway inflammation in mouse offspring. Clin Exp Allergy 2007;37:348–357. [DOI] [PubMed] [Google Scholar]
- Brown N, Nagarkatti M, Nagarkatti PS. Induction of apoptosis in murine fetal thymocytes following perinatal exposure to diethylstilbestrol. Int J Toxicol 2006;25:9–15. [DOI] [PubMed] [Google Scholar]
- Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep 2011;11:29–36. [DOI] [PubMed] [Google Scholar]
- Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr 2009;168:897–905. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab 2002;87:675–681. [DOI] [PubMed] [Google Scholar]
- Cohen O, Ish-Shalom E, Kfir-Erenfeld S, Herr I, Yefenof E. Nitric oxide and glucocorticoids synergize in inducing apoptosis of CD4(+)8(+) thymocytes: implications for ‘Death by Neglect’ and T-cell function. Int Immunol 2012;24:783–791. [DOI] [PubMed] [Google Scholar]
- Couret D, Jamin A, Kuntz-Simon G, Prunier A, Merlot E. Maternal stress during late gestation has moderate but long-lasting effects on the immune system of the piglets. Vet Immunol Immunopathol 2009;131:17–24. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun 2007;21:343–350. [DOI] [PubMed] [Google Scholar]
- Diepenbruck I, Much CC, Krumbholz A, Kolster M, Thieme R, Thieme D, Diepenbruck S, Solano ME, Arck PC, Tolosa E. Effect of prenatal steroid treatment on the developing immune system. J Mol Med 2013;91:1293–1302. [DOI] [PubMed] [Google Scholar]
- Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, Cunningham C, Le Gros G, von Mutius E, Pearce N. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J 2008;32:603–611. [DOI] [PubMed] [Google Scholar]
- Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, Schram-Bijkerk D, Brunekreef B, van Hage M, Scheynius A et al. . Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol 2006;117:817–823. [DOI] [PubMed] [Google Scholar]
- El-Sayed YS, Shimizu R, Onoda A, Takeda K, Umezawa M. Carbon black nanoparticle exposure during middle and late fetal development induces immune activation in male offspring mice. Toxicology 2015;327:53–61. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology 2006;21:29–37. [DOI] [PubMed] [Google Scholar]
- Fu Y, Lou H, Wang C, Lou W, Wang Y, Zheng T, Zhang L. T cell subsets in cord blood are influenced by maternal allergy and associated with atopic dermatitis. Pediatr Allergy Immunol 2013;24:178–186. [DOI] [PubMed] [Google Scholar]
- Gauthier TW, Ping XD, Gabelaia L, Brown LA. Delayed neonatal lung macrophage differentiation in a mouse model of in utero ethanol exposure. Am J Physiol Lung Cell Mol Physiol 2010;299:L8–L16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giriens B, Yan P, Safroneeva E, Zwahlen M, Reinhard A, Nydegger A, Vavricka S, Sempoux C, Straumann A, Schoepfer AM. Escalating incidence of eosinophilic esophagitis in Canton of Vaud, Switzerland, 1993–2013: a population-based study. Allergy 2015;70:1633–1639. [DOI] [PubMed] [Google Scholar]
- Gotz AA, Stefanski V. Psychosocial maternal stress during pregnancy affects serum corticosterone, blood immune parameters and anxiety behaviour in adult male rat offspring. Physiol Behav 2007;90:108–115. [DOI] [PubMed] [Google Scholar]
- Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child 2009;94:180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager-Theodorides AL, Ross SE, Sahni H, Mishina Y, Furmanski AL, Crompton T. Direct BMP2/4 signaling through BMP receptor IA regulates fetal thymocyte progenitor homeostasis and differentiation to CD4+CD8+ double-positive cell. Cell Cycle 2014;13:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ML, Brundage KM, Schafer R, Tou JC, Barnett JB. Prenatal cadmium exposure dysregulates sonic hedgehog and Wnt/beta-catenin signaling in the thymus resulting in altered thymocyte development. Toxicol Appl Pharmacol 2010;242:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ML, Holaskova I, Elliott M, Brundage KM, Schafer R, Barnett JB. Prenatal cadmium exposure alters postnatal immune cell development and function. Toxicol Appl Pharmacol 2012;261:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Inflammatory biomarkers as differential predictors of antidepressant response. Int J Mol Sci 2015;16:7796–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberth G, Hinz D, Roder S, Schlink U, Sack U, Diez U, Borte M, Lehmann I. Maternal immune status in pregnancy is related to offspring's immune responses and atopy risk. Allergy 2011;66:1065–1074. [DOI] [PubMed] [Google Scholar]
- Herberth G, Bauer M, Gasch M, Hinz D, Roder S, Olek S, Kohajda T, Rolle-Kampczyk U, von Bergen M, Sack U et al. . Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clin Immunol 2014;133:543–550. [DOI] [PubMed] [Google Scholar]
- Hinz D, Bauer M, Roder S, Olek S, Huehn J, Sack U, Borte M, Simon JC, Lehmann I, Herberth G et al. . Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy 2012;67:380–389. [DOI] [PubMed] [Google Scholar]
- Holaskova I, Elliott M, Hanson ML, Schafer R, Barnett JB. Prenatal cadmium exposure produces persistent changes to thymus and spleen cell phenotypic repertoire as well as the acquired immune response. Toxicol Appl Pharmacol 2012;265:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Liu C, Chen X, Song Y, Wang Q, Wang P, Hu D. Maternal exposure to airborne particulate matter causes postnatal immunological dysfunction in mice offspring. Toxicology 2013;306:59–67. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Buitelaar JK. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility. Psychol Bull 2004;130:115–142. [DOI] [PubMed] [Google Scholar]
- Hylkema MN, Blacquiere MJ. Intrauterine effects of maternal smoking on sensitization, asthma, and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:660–662. [DOI] [PubMed] [Google Scholar]
- Jenmalm MC. Childhood immune maturation and allergy development: regulation by maternal immunity and microbial exposure. Am J Reprod Immunol 2011;66 Suppl 1:75–80. [DOI] [PubMed] [Google Scholar]
- Kallapur SG, Kramer BW, Moss TJ, Newnham JP, Jobe AH, Ikegami M, Bachurski CJ. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 2013;284:L633–L642. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol 2006;572:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrow NA. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine-immune axis during fetal and neonatal development: lessons learned from the model inflammagen, lipopolysaccharide. Brain Behav Immun 2006;20:144–158. [DOI] [PubMed] [Google Scholar]
- Kay G, Tarcic N, Poltyrev T, Weinstock M. Prenatal stress depresses immune function in rats. Physiol Behav 1998;63:397–402. [DOI] [PubMed] [Google Scholar]
- Kemp MW, Saito M, Nitsos I, Jobe AH, Kallapur SG, Newnham JP. Exposure to in utero lipopolysaccharide induces inflammation in the fetal ovine skin. Reprod Sci 2011;18:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunker S, Chong MM, Mantel PY, Palomares O, Bassin C, Ziegler M, Ruckert B, Meiler F, Akdis M, Littman DR et al. . Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med 2009;206:2701–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res 2004;55:764–768. [DOI] [PubMed] [Google Scholar]
- Kuypers E, Collins JJ, Kramer BW, Ofman G, Nitsos I, Pillow JJ, Polglase GR, Kemp MW, Newnham JP, Gavilanes AW et al. . Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 2012;302:L380–L389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ballaney M, Al-alem U, Quan C, Jin X, Perera F, Chen LC, Miller RL. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci 2008;102:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, He S, Zhang Y, Xia W, Li M, Zhang C, Gao F. Effects of intrauterine growth restriction during late pregnancy on the development of the ovine fetal thymus and the T-lymphocyte subpopulation. Am J Reprod Immunol 2015;74:26–37. [DOI] [PubMed] [Google Scholar]
- Luciano AA, Arbona-Ramirez IM, Ruiz R, Llorens-Bonilla BJ, Martinez-Lopez DG, Funderburg N, Dorsey MJ. Alterations in regulatory T cell subpopulations seen in preterm infants. PLoS One 2014;9:e95867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zwahlen RA, Zheng LW, Sham MH. Influence of nicotine on the biological activity of rabbit osteoblasts. Clin Oral Implants Res 2011;22:338–342. [DOI] [PubMed] [Google Scholar]
- Mainali ES, Kikuchi T, Tew JG. Dexamethasone inhibits maturation and alters function of monocyte-derived dendritic cells from cord blood. Pediatr Res 2005;58:125–131. [DOI] [PubMed] [Google Scholar]
- Maio S, Baldacci S, Carrozzi L, Pistelli F, Angino A, Simoni M, Sarno G, Cerrai S, Martini F, Fresta M et al. . Respiratory symptoms/diseases prevalence is still increasing: a 25-yr population study. Respir Med 2016;110:58–65. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Prescott SL. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy 2010;65:7–15. [DOI] [PubMed] [Google Scholar]
- Martino D, Prescott S. Epigenetics and prenatal influences on asthma and allergic airways disease. Chest 2011;139:640–647. [DOI] [PubMed] [Google Scholar]
- Maslova E, Hansen S, Strom M, Halldorsson TI, Olsen SF. Maternal intake of vitamins A, E and K in pregnancy and child allergic disease: a longitudinal study from the Danish National Birth Cohort. Br J Nutr 2014;111:1096–1108. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab 2002;13:373–380. [DOI] [PubMed] [Google Scholar]
- Mattick JS. A new paradigm for developmental biology. J Exp Biol 2007;210:1526–1547. [DOI] [PubMed] [Google Scholar]
- Middlebrook AJ, Martina C, Chang Y, Lukas RJ, DeLuca D. Effects of nicotine exposure on T cell development in fetal thymus organ culture: arrest of T cell maturation. J Immunol 2002;169:2915–2924. [DOI] [PubMed] [Google Scholar]
- Miles EA, Calder PC. Maternal diet and its influence on the development of allergic disease. Clin Exp Allergy 2015;45:63–74. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal consumption of dairy products, calcium, and vitamin D during pregnancy and infantile allergic disorders. Ann Allergy Asthma Immunol 2014;113:82–87. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau KC, Li Z, Farzan S, Koestler D, Robbins D, Fei DL, Malipatlolla M, Maecker H, Enelow R, Korrick S et al. . In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin Immunol 2014;155:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Winter MA, Hunn J, Carnahan J, Pressman EK, Glover V, Robertson-Blackmore E, Moynihan JA, Lee FE, Caserta MT. Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain Behav Immun 2013;32:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olearo E, Oberto M, Ogge G, Botta G, Pace C, Gaglioti P, Todros T. Thymic volume in healthy, small for gestational age and growth restricted fetuses. Prenat Diagn 2012;32:662–667. [DOI] [PubMed] [Google Scholar]
- Palermo Neto J, Massoco CO, Favare RC. Effects of maternal stress on anxiety levels, macrophage activity, and Ehrlich tumor growth. Neurotoxicol Teratol 2001;23:497–507. [DOI] [PubMed] [Google Scholar]
- Parimi PS, Birnkrant DJ, Rao LV, Diaz G, Moore JJ. Effect of dexamethasone on lymphocyte subpopulations in premature infants with bronchopulmonary dysplasia. J Perinatol 1999;19:347–351. [DOI] [PubMed] [Google Scholar]
- Pascuan CG, Rubinstein MR, Palumbo ML, Genaro AM. Prenatal stress induces up-regulation of glucocorticoid receptors on lymphoid cells modifying the T-cell response after acute stress exposure in the adult life. Physiol Behav 2014;128:141–147. [DOI] [PubMed] [Google Scholar]
- Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, Ho SM. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One 2009;4:e4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos S, Matthews SG, Szyf M. Adult glucocorticoid exposure leads to transcriptional and DNA methylation changes in nuclear steroid receptors in the hippocampus and kidney of mouse male offspring. Biol Reprod 2014;90:43. [DOI] [PubMed] [Google Scholar]
- Pincus-Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF, Wahn U, Hamelmann E, Arck PC. Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. J Immunol 2006;177:8484–8492. [DOI] [PubMed] [Google Scholar]
- Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, Drobna Z, Currier J, Douillet C, Olshan AF et al. . Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen 2014;55:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219–242. [DOI] [PubMed] [Google Scholar]
- Rees F, Doherty M, Grainge M, Davenport G, Lanyon P, Zhang W. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann Rheum Dis 2016;75:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiprich M, Rudzok S, Schutze N, Simon JC, Lehmann I, Trump S, Polte T. Inhibition of endotoxin-induced perinatal asthma protection by pollutants in an experimental mouse model. Allergy 2013;68:481–489. [DOI] [PubMed] [Google Scholar]
- Reis FM, Florio P, Cobellis L, Luisi S, Severi FM, Bocchi C, Picciolini E, Centini G, Petraglia F. Human placenta as a source of neuroendocrine factors. Biol Neonate 2001;79:150–156. [DOI] [PubMed] [Google Scholar]
- Runyon RS, Cachola LM, Rajeshuni N, Hunter T, Garcia M, Ahn R, Lurmann F, Krasnow R, Jack LM, Miller RL et al. . Asthma discordance in twins is linked to epigenetic modifications of T cells. PLoS One 2012;7:e48796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Yokoyama T, Miyake Y, Sasaki S, Tanaka K, Ohya Y, Hirota Y. Maternal meat and fat consumption during pregnancy and suspected atopic eczema in Japanese infants aged 3–4 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol 2010;21:38–46. [DOI] [PubMed] [Google Scholar]
- Sanders VM. Epigenetic regulation of Th1 and Th2 cell development. Brain Behav Immun 2006;20:317–324. [DOI] [PubMed] [Google Scholar]
- Saxena R, Kaur J. Th1/Th2 cytokines and their genotypes as predictors of hepatitis B virus related hepatocellular carcinoma. World J Hepatol 2015;7:1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub B, Liu J, Hoppler S, Schleich I, Huehn J, Olek S, Wieczorek G, Illi S, von Mutius E. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 2009;123:774–782 e775. [DOI] [PubMed] [Google Scholar]
- Schwarz NG, Adegnika AA, Breitling LP, Gabor J, Agnandji ST, Newman RD, Lell B, Issifou S, Yazdanbakhsh M, Luty AJ et al. . Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis 2008;47:1017–1025. [DOI] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Guan H, Nagarkatti P, Nagarkatti M. Prenatal exposure to TCDD triggers significant modulation of microRNA expression profile in the thymus that affects consequent gene expression. PLoS One 2012a;7:e45054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Nagarkatti PS, Nagarkatti M. Prenatal exposure of mice to diethylstilbestrol disrupts T-cell differentiation by regulating Fas/Fas ligand expression through estrogen receptor element and nuclear factor-kappaB motifs. J Pharmacol Exp Ther 2012b;343:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Kelly JP, Leonard BE. The effect of stressful behavioral exposure on endocrine and immune parameters in the rat. Stress Med 1994;10:239–245. [Google Scholar]
- Staud F, Mazancova K, Miksik I, Pavek P, Fendrich Z, Pacha J. Corticosterone transfer and metabolism in the dually perfused rat placenta: effect of 11beta-hydroxysteroid dehydrogenase type 2. Placenta 2006;27:171–180. [DOI] [PubMed] [Google Scholar]
- Talaber G, Tuckermann JP, Okret S. ACTH controls thymocyte homeostasis independent of glucocorticoids. FASEB J 2015;29:2526–2534. [DOI] [PubMed] [Google Scholar]
- Toumi ML, Merzoug S, Baudin B, Tahraoui A. Quercetin alleviates predator stress-induced anxiety-like and brain oxidative signs in pregnant rats and immune count disturbance in their offspring. Pharmacol Biochem Behav 2013;107:1–10. [DOI] [PubMed] [Google Scholar]
- Troger B, Muller T, Faust K, Bendiks M, Bohlmann MK, Thonnissen S, Herting E, Gopel W, Hartel C. Intrauterine growth restriction and the innate immune system in preterm infants of ≤32 weeks gestation. Neonatology 2013;103:199–204. [DOI] [PubMed] [Google Scholar]
- Verrill L, Bruns R, Luccioli S. Prevalence of self-reported food allergy in U.S. adults: 2001, 2006, and 2010. Allergy Asthma Proc 2015;36:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieau D, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Molendi-Coste O, Laborie C, Breton C, Deloof S, Lesage J. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology 2007;32 Suppl 1:S16–S20. [DOI] [PubMed] [Google Scholar]
- Vlasova AN, Chattha KS, Kandasamy S, Siegismund CS, Saif LJ. Prenatally acquired vitamin A deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J Immunol 2013;190:4742–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol 2009;170:1486–1493. [DOI] [PubMed] [Google Scholar]
- Widdifield J, Paterson JM, Bernatsky S, Tu K, Tomlinson G, Kuriya B, Thorne JC, Bombardier C. The epidemiology of rheumatoid arthritis in Ontario, Canada. Arthritis Rheumatol 2014;66:786–793. [DOI] [PubMed] [Google Scholar]
- Wolfs TG, Buurman WA, Zoer B, Moonen RM, Derikx JP, Thuijls G, Villamor E, Gantert M, Garnier Y, Zimmermann LJ et al. . Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PLoS One 2009;4:e5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol 2013;34:27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HR, Kuo HC, Chen CC, Sheen JM, Tiao MM, Chen YC, Chang KA, Tain YL, Huang LT. Prenatal dexamethasone exposure in rats results in long-term epigenetic histone modifications and tumour necrosis factor-alpha production decrease. Immunology 2014;143:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]