Abstract
STUDY HYPOTHESIS
Myometrial explants represent a superior model compared with cell culture models for the study of human myometrial progesterone (P4) signalling in parturition.
STUDY FINDING
Gene expression analysis showed myometrial explants closely resemble the in vivo condition and the anti-inflammatory action of P4 is not lost with labour onset.
WHAT IS KNOWN ALREADY
Circulating P4 levels decline before the onset of parturition in most animals, but not in humans. This has led to the suggestion that there is a functional withdrawal of P4 action at the myometrial level prior to labour onset. However, to date, no evidence of a loss of P4 function has been provided, with studies hampered by a lack of a physiologically relevant model.
STUDY DESIGN, SAMPLES/MATERIALS, METHODS
Myometrial biopsies obtained at Caesarean section were dissected into explants after a portion was immediately snap frozen (t = 0). Microarray analysis was used to compare gene expression of t = 0 with paired (i) explants, (ii) passage 4 myometrial cell cultures or (iii) the hTERT myometrial cell line. Western blotting and chemokine/cytokine assays were used to study P4 signalling in myometrial explants.
MAIN RESULTS AND THE ROLE OF CHANCE
Gene expression comparison of t = 0 to the three models demonstrated that explants more closely resemble the in vivo status. At the protein level, explants maintain both P4 receptor (PR) and glucocorticoid receptor (GR) levels versus t = 0 whereas cells only maintain GR levels. Additionally, treatment with 1 µM P4 led to a reduction in interleukin-1 (IL-1) β-driven cyclooxygenase-2 in explants but not in cells. P4 signalling in explants was PR-mediated and associated with a repression of p65 and c-Jun phosphorylation. Furthermore, the anti-inflammatory action of P4 was maintained after labour onset.
LIMITATIONS/REASONS FOR CAUTION
There is evidence of basal inflammation in the myometrial explant model.
WIDER IMPLICATIONS OF THE FINDINGS
Myometrial explants constitute a novel model to study P4 signalling in the myometrium and can be used to further elucidate the mechanisms of P4 action in human labour.
LARGE SCALE DATA
Data deposited at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=gvmpggkurbgxfqf&acc=GSE77830.
STUDY FUNDING AND COMPETING INTEREST
This work was supported by grants from the Joint Research Committee of the Westminster Medical School Research Trust, Borne (No. 1067412-7; a sub-charity of the Chelsea and Westminster Health Charity) and the Imperial NIHR Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS or the Department of Health. The authors have no conflict of interest.
Keywords: progesterone withdrawal, nuclear receptors, myometrial explants, smooth muscle, parturition, myometrial cells, hTERT
Introduction
The seminal papers of Csapo et al. and Frydman et al. demonstrated that progesterone (P4) is essential for human pregnancy, the former showing that P4 is responsible for the maintenance of early pregnancy and that its removal results in miscarriage (Csapo et al., 1973) and the latter, that blocking P4 can result in the onset of labour (Frydman et al., 1991). However, while in most animals labour follows a precipitous fall in peripheral P4 levels, no such fall occurs in humans and non-human primates. This has led to the concept of a myometrial functional P4 withdrawal and several theories have been proposed to explain it. The most widely accepted is that there is a change in the balance of expression of the P4 receptor (PR), which is comprised of two main isoforms: PR-B, which mediates the effects of P4, and PR-A, which antagonises PR-B-mediated P4 signalling, but is also transcriptionally active in its own right. An increase in the PR-A:PR-B ratio at the time of labour onset has been demonstrated in myometrial samples obtained at the time of labour at the mRNA and protein level (Mesiano et al., 2002; Merlino et al., 2007). Furthermore, in PR-A-dominant myometrial cells, P4 enhances pro-inflammatory gene expression (Tan et al., 2012). Another theory suggests that uterine quiescence is maintained throughout pregnancy by a PR-mediated inhibition of the actions of the pro-inflammatory transcription factor NF-κB (Kalkhoven et al., 1996), possibly via the NF-κB inhibitor IκBα (Hardy et al., 2006), but that with the onset of labour, inflammation-induced NF-κB represses PR action bringing about a uterine switch to a contractile phenotype (Allport et al., 2011). A third theory suggests that changes to PR co-regulator expression may cause labour onset (Condon et al., 2003). All three mechanisms have the common end result of a loss of myometrial sensitivity to P4 action and the onset of labour. Efforts to use a mouse model to study this question have been limited, as PR-A knock-out mice are infertile, while no apparent change in ovarian and uterine function was observed in PR-B knock-out mice (Mulac-Jericevic et al., 2000, 2003).
Although increasingly controversial (O'Brien et al., 2007; Norman et al., 2016), supplementation with P4 or 17 alpha-hydroxyprogesterone caproate has been shown, at least in some studies, to reduce the risk of preterm labour in high-risk singleton pregnancies (da Fonseca et al., 2003; Meis et al., 2003). The mechanism involved is uncertain, but since labour is widely accepted to be an inflammatory event (Bollapragada et al., 2009), it is assumed that P4 acts to maintain pregnancy by repressing inflammation. On that basis, the ability of P4 to repress inflammation-induced cyclooxygenase-2 (COX-2) expression has been widely used as a model of P4 action. COX-2 expression is driven by the inflammatory transcription factors NF-κB and activator protein-1 (AP-1) (Soloff et al., 2004; Khanjani et al., 2011, 2012; Lim and Lappas, 2014). Several studies have investigated whether P4 inhibits NF-κB and AP-1 activation to repress COX-2 expression, but these have typically been performed in primary cell cultures or cell lines and have involved the over-expression of PR, NF-κB and/or AP-1 (Bamberger et al., 1996; Kalkhoven et al., 1996; Hardy et al., 2006). Primary cultures of uterine smooth muscle cells have been shown to maintain structural and functional characteristics (Lee et al., 2012; Mosher et al., 2013), but are possibly not an optimal model for the study of P4 action as high doses of P4 (10 µM) are required to bring about a reduction in IL-1β-driven COX-2 (Lei et al., 2012). Previous work by our group using this model has demonstrated that P4 signals via the glucocorticoid receptor (GR) to reduce COX-2 (Lei et al., 2012) via MAPK phosphatase-1 (MKP-1) (Lei et al., 2015a); however, this may be because PR levels are lower in primary cells compared with tissue.
To overcome the limitations of myometrial cell culture, we have developed an explant-based model for the ex vivo study of myometrial function. We compared gene expression in tissue snap frozen at the time of Caesarean section (t = 0) to myometrial explants, passage 4 myometrial cells and the hTERT myometrial cell line. We subsequently compared PR protein levels in t = 0, myometrial explants and passage 4 myometrial cells before using the explant model to study P4 action in myometrial samples before and after the onset of labour to test the hypothesis that a functional withdrawal of P4 action occurs with the onset of labour.
Materials and Methods
Ethical approval
The Brompton and Harefield Research Ethics Committee approved this project.
Myometrial biopsies
Myometrial biopsies were obtained from women at term (≥37 weeks) following informed consent at the time of planned or emergency Caesarean section. Women with multiple pregnancy, gestational diabetes mellitus, pre-eclampsia and obstetric cholestasis were excluded. In addition, labouring women were recruited to the study if labouring spontaneously and requiring an emergency Caesarean section due to fetal distress or a breech presentation. Cervical dilatation was used to categorise labour into early (≤3 cm) or established (>3 cm). Biopsies were collected into sterile universal bottles containing phosphate-buffered saline (PBS) and were processed immediately. Samples used were as follows: term no-labour (TNL): n = 35; term early labour (TEaL): n = 8 and term established labour (TEsL): n = 8.
Explant culture
Biopsies were dissected into 3 × 3 × 3 mm3 pieces (explants) with any obvious vasculature excised. They were placed in Dulbecco's Modified Eagle Medium (DMEM) (Sigma-Aldrich Ltd, Dorset, UK) supplemented with penicillin–streptomycin (Sigma-Aldrich Ltd) or immediately snap-frozen in liquid nitrogen (t = 0). Depending on the experimental protocol, explants were either untreated or immediately treated for 6 h with vehicle control (ethanol ± DMSO), progesterone (100, 500 nM, 1, 5 and 10 μM; Sigma-Aldrich Ltd), mifepristone (RU486, 1 μM; Sigma-Aldrich Ltd) or onapristone (ZK299, 1 μM; Arno Therapeutics, Flemington, NJ, USA). They were subsequently treated for a further 24 h with interleukin-1 (IL-1)β (1, 10, 20, 50 and 100 ng/ml; Sigma-Aldrich Ltd), at which point all tissues were snap frozen in liquid nitrogen and stored at −80°C. The media in which explants were cultured were also stored at −80°C.
Cell culture
Biopsies were digested in a mixture of collagenases as previously described (Sooranna et al., 2004) and passaged by trypsinization in 0.25% (w/v) trypsin containing 0.02% (w/v) EDTA (Sigma-Aldrich Ltd) when confluent. Once confluent at passage 4, cells were incubated overnight in 1% (v/v) charcoal and dextran-stripped fetal calf serum (1% DCC-FCS) supplemented with penicillin–streptomycin. They were then cultured and treated using the same protocol as explant cultures and were placed at −80°C once the experiment was completed. Supernatants were stored at −80°C.
The myometrial hTERT cell line was cultured in the same conditions as myometrial cells with overnight incubation in 1% DCC-FCS once cells reached confluence. Once thawed, cells were not passaged beyond passage 5.
RNA extraction
Total RNA was extracted using a Trizol® Plus RNA Purification kit (Thermo Fisher Scientific, Ambion, Abgene Ltd, West Sussex, UK) with on-column DNase treatment prior to elution, all as per the manufacturer's protocol. Bead homogenization in Precellys® tubes (Stretton Scientific Ltd, Derbyshire, UK) was used for tissue lysis with two 20 s cycles at 5000 rpm; cells were lysed directly with Trizol® added to the culture plate. The concentration and purity of RNA was determined by spectrophotometry and integrity was confirmed using an Agilent 2100 Bioanalyzer with an RNA 6000 Nano Kit (Agilent Technologies, Palo Alto, CA, USA).
Microarray analysis
Whole-genome transcriptome analysis was conducted by hybridizing six biological samples of total RNA per condition to Affymetrix Human Gene 2.1 ST Arrays Strips (Affymetrix, Santa Clara, CA, USA). A minimum RIN score of 8 was used as cut-off for inclusion in the microarray analysis. All steps were conducted at the Nottingham Arabidopsis Stock Centre. Gene expression data were analysed using Partek Genomics Suite 6.6 software (Partek Incorporated, St Louis, MO, USA). The raw CEL files were normalized using the RMA background correction with quantile normalization, log base 2 transformation and mean probe-set summarization with adjustment for GC content. Differentially expressed genes (DEG) were identified by a two-way ANOVA, and P-values were adjusted using the FDR (false-discovery rate) method to correct for multiple comparisons. DEG were considered significant if P-value was ≤0.05 at a fold change (FC) of >2 with FDR <0.05.
Quantitative RT–PCR
Following quantification, 1 µg RNA was reverse transcribed with oligo dT random primers using MuLV reverse transcriptase (Life Technologies Ltd, Paisley, UK). Primer sets were designed and obtained from Invitrogen (Table I). Quantitative PCR was performed using SYBR Green (Roche Diagnostics Ltd, West Sussex, UK) using the previously described cycling protocol (Lei et al., 2015a) and amplicon yield was monitored during cycling in a RotorGene Sequence Detector (Qiagen Ltd, West Sussex, UK). The abundance of mRNA for sequences of interest was expressed relative to the constitutively expressed GAPDH and 18S rRNA. Here we present the data normalized to GAPDH.
Table I.
Primer sets used for quantitative RT–PCR.
| Name | Forward (F) and Reverse (R) primer sequence (5′-3′) | Genbank/EMBL Accession no. | Product size (bp) |
|---|---|---|---|
| GAPDH | F: tgatgacatcaagaaggtggtgaag | BC014085 | 240 |
| R: tccttggaggccatgtaggccat | |||
| 18S rRNA | F: aaacggctaccacatccaag | X03205.1 | 155 |
| R: cctccaatggatcctcgtta | |||
| PTGS2 | F: tgtgcaacacttgagtggct | AY151286 | 297 |
| R: actttctgtactgcgggtgg | |||
| IL8 | F: gccttcctgattttgcagc | NM_000584 | 150 |
| R: cgcagtgtggtccactctca | |||
| OXTR | F: agaagcactcgcgcctctt | NM_000916 | 102 |
| R: aggtgatgtcccacagcaact | |||
| PGR | F: agcccacaatacagcttcgag | NM_000926 | 253 |
| R: tttcgacctccaaggaccat | |||
| IL1B | F: gctgaggaagatgctggttc | NM_000576 | 240 |
| R: tccatatcctgtccctggag | |||
| NR3C1 | F: cttccagaaccatggtagcc | NM_002425 | 166 |
| R: tacgaaactccacccaaagg | |||
| TPM1 | F: ccacgctctcaacgatatga | NM_000366 | 215 |
| R: cagtgtgtgcctggctctaa | |||
| TNFAIP3 | F: aagggtgtctgagcaggaga | NM_001270507 | 163 |
| R: tgcttggtaggagaggagga | |||
| IL6 | F: agtgaggaacaagccagagc | NM_000600 | 246 |
| R: gaggtgcccatgctacattt | |||
| ACTA2 | F: ttcaatgtcccagccatgta | NM_001141945 | 222 |
| R: gaaggaatagccacgctcag | |||
| MYLK | F: ggccacgatgaaacagattt | NM_005965 | 172 |
| R: ggcatgattgatgcacattt | |||
| PTGER3 | F: cgccatgtcttcatcacatc | NM_000957 | 199 |
| R: atgtgatcctggcagaaagg | |||
| GJA1 | F: aattcagacaaggcccacag | NM_000165 | 214 |
| R: catggcttgattccctgact | |||
| ESR1 | F: tccaactgcatttcctttcc | NM_000125 | 201 |
| R: ttggaacatggcagcattta | |||
| IL1A | F: aatgacgccctcaatcaaag | NM_000575.4 | 226 |
| R: tgggtatctcaggcatctcc |
Protein extraction and western blotting
Protein was extracted from explants using bead homogenization in pre-cooled Precellys® tubes (Stretton Scientific Ltd) containing lysis buffer (New England Biolabs, Hertfordshire, UK) supplemented with protease (Roche Diagnostics Ltd) and phosphatase inhibitors (Thermo Fisher Scientific, Abgene Ltd, Epsom, UK). Tissues were immediately homogenized by mechanical disruption using two 20 s cycles at 5000 rpm. Protein from cells was extracted via direct lysis using the same lysis buffer mixture as for explants. The supernatant was separated from tissue debris by centrifugation at 15 890g for 10 min at 4°C. Protein concentrations were determined by DC protein assay (Bio-Rad laboratories Ltd, Hertfordshire, UK) and bovine serum albumin (Sigma-Aldrich Ltd) was used for reference standards.
Samples in NuPAGE® LDS Sample Buffer (Life Technologies Ltd) were denatured at 75°C for 10 min and 20 μg of total protein for each sample was electrophoresed through a 4–20% polyacrylamide gel (Bio-Rad laboratories Ltd). Transfer was carried out onto a polyvinylidene fluoride membrane (Bio-Rad laboratories Ltd) using the Trans-Blot® Turbo Transfer system (Bio-Rad laboratories Ltd), followed by blocking in 5% non-fat dried milk powder (AppliChem GmbH, Germany) dissolved in 0.1% Tween–Tris buffered saline (TBS–T) for 1 h at room temperature. The membrane was incubated overnight at 4°C with primary antibody followed by incubation for 2 h at room temperature with secondary antibody (Table II). Clarity Western ECL substrate (Bio-Rad laboratories Ltd) was used for detection. Protein band size was determined using Precision Plus Protein Standards ladder (Bio-Rad laboratories Ltd). All protein abundance data were expressed relative to the amount of constitutively expressed GAPDH after 1 h incubation at room temperature (Table II).
Table II.
Primary and secondary antibody table.
| Peptide/protein target | Name of antibody | Manufacturer, Catalog Number | Species raised in; monoclonal or polyclonal | Dilution used |
|---|---|---|---|---|
| COX-2 | COX-2 (C20) | Santa Cruz Biotechnology sc-1745 | Goat polyclonal | 1:2000 |
| PR | Progesterone Receptor Clone 16 | Leica Biosystems, NCL-L-PGR-312 | Mouse monoclonal | 1:500 |
| GR | GR (E-20) | Santa Cruz Biotechnology, sc-1003 | Rabbit polyclonal | 1:1000 |
| Phosphorylated (p)-p65 (Ser536) | Phospho-NF-κB p65 | Cell Signaling, 3031 | Rabbit polyclonal | 1:1000 |
| p-cJun (Ser63) | p-c-Jun (KM-1) | Santa Cruz Biotechnology, sc-822 | Mouse monoclonal | 1:5000 |
| p-c-Fos (Ser32) | Phospho-c-Fos (D82C12) | Cell Signaling, 5348 | Rabbit monoclonal | 1:1000 |
| p-ERK1/2 (Thr202/Thr204) | Phospho-p44/p42 MAPK (Erk1/2) | Cell Signaling, 9101 | Rabbit polyclonal | 1:5000 |
| p-p38 (Thr180/Tyr182) | Phospho-p38 MAP Kinase | Cell Signaling, 9211 | Rabbit polyclonal | 1:1000 |
| p-JNK (Thr183/Tyr185) | Phospho-SAPK/JNK | Cell Signaling, 9251 | Rabbit polyclonal | 1:1000 |
| p65 | NFκB p65 (F-6) | Santa Cruz Biotechnology, sc-8008 | Mouse monoclonal | 1:1000 |
| c-Jun | c-Jun (60A8) | Cell Signaling, 9165 | Rabbit monoclonal | 1:1000 |
| c-Fos | c-Fos | Cell Signaling, 4384 | Rabbit, polyclonal | 1:1000 |
| ERK1/2 | p44/42 MAPK (Erk1/2) | Cell Signaling, 9102 | Rabbit, polyclonal | 1:1000 |
| p38 | p38 MAPK | Cell Signaling, 9212 | Rabbit, polyclonal | 1:1000 |
| JNK | SAPK/JNK | Cell Signaling, 9252 | Rabbit, polyclonal | 1:1000 |
| IκBα | IκBα (C-21) | Santa Cruz Biotechnology, sc-371 | Rabbit polyclonal | 1:500 |
| MKP-1 | MKP-1 (C-19) | Santa Cruz Biotechnology, sc-370 | Rabbit polyclonal | 1:500 |
| GAPDH | Anti-glyceraldehyde-3-phosphate dehydrogenase | Millipore, MAB374 | Mouse monoclonal | 1:20 000 |
| Rabbit IgG | Anti-rabbit IgG, HRP-linked antibody | Cell Signaling, 7074 | Goat polyclonal | 1:2000 |
| Mouse IgG | Anti-mouse IgG, HRP-linked antibody | Cell Signaling, 7076 | Horse polyclonal | 1:2000 |
Chemokine/cytokine assays
Human Bio-Plex© Pro™ chemokine/cytokine assays (Bio-Rad laboratories Ltd) were used to measure the concentrations of IL-1α, IL-1β, IL-6, IL-8, C–C motif chemokine 2 (CCL2), CCL5, CCL11, CCL20, C–X–C motif chemokine 1 (CXCL1), CXCL2, intercellular adhesion molecule 1 (ICAM) and leukaemia inhibitory factor (LIF) in explant culture media. These were performed according to the manufacturer's instructions and read using a Bio-Plex© 200 reader and Bio-Plex Manager© v6.1 software (Bio-Rad laboratories Ltd). Data were normalized to tissue weights.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Normality was determined via a Kolmogorov–Smirnov test for up to six replicates or a Shapiro Wilks test for more than six replicates. Normally distributed data were subsequently analysed using a paired t test for the comparison of two groups or an ANOVA followed by Bonferroni's multiple comparison test post hoc testing for three groups or more. Data that were not normally distributed were analysed using a Wilcoxon matched pairs test or a Kruskal–Wallis followed by Dunn's multiple comparisons post hoc testing for three groups or more. P < 0.05 was considered statistically significant.
Results
The myometrial explant gene expression profile closely resembles the in vivo status
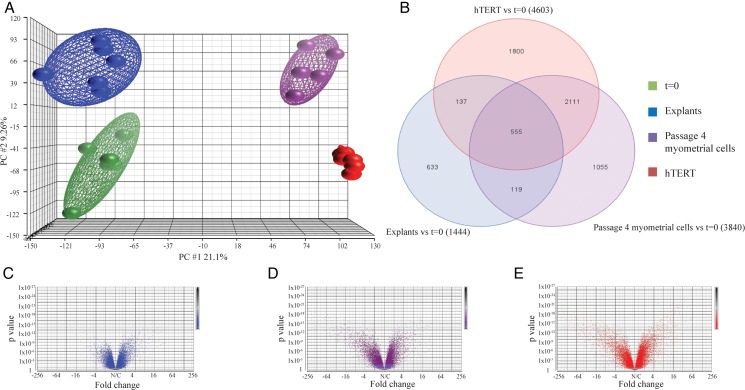
Biopsies obtained from non-labouring women at elective Caesarean section at term were divided into three: (i) dissected and immediately snap frozen (t = 0), (ii) dissected for myometrial explants and (iii) processed for cell culture. Explants, myometrial cells at passage 4 (the typical passage our group uses for experiments) and the hTERT cell line were cultured for a period of 30 h without treatment. Gene expression analysis via microarray demonstrated that explants most closely resemble t = 0 (Fig. 1A). Upon direct comparison between explants and t = 0, 1444 genes varied significantly whereas the corresponding number for passage 4 myometrial cells was 3840 and for hTERT 4603 (Fig. 1B). A total of 555 genes varied commonly upon comparing all 3 groups to t = 0 with gene ontology analysis demonstrating higher enrichment scores for functions including ‘immune response’, ‘inflammatory response’ and ‘leukocyte migration’ (Supplementary Table SI). Furthermore, explants shared 119 genes exclusively with passage 4 myometrial cells and 137 genes with hTERT; the equivalent figure for the passage 4 myometrial cells and hTERT overlap was 2111 (Fig. 1B). Of the 633 genes uniquely up-regulated in the explant group, the most common gene ontology groups pertained to glucose metabolism including ‘glycolysis’, ‘gluconeogenesis’ and ‘glucose metabolic process’ (Supplementary Table SII). Overall, the degree of variability on comparing t = 0 to each of the three groups was least for explants (Fig. 1C) followed by passage 4 myometrial cells (Fig. 1D) and hTERT (Fig. 1E).
Figure 1.
The myometrial explant gene expression profile most closely resembles the physiological status. Myometrial tissue obtained from term non-labouring women was divided into three portions: (i) snap frozen at the time Caesarean section (t = 0), (ii) finely dissected into 3 × 3 × 3 mm3 explants and (iii) digested with a collagenase mixture to isolate cells for myometrial cell culture. Explants were cultured in Dulbecco's Modified Eagle Medium without treatment for 30 h. After overnight incubation with 1% (v/v) charcoal and dextran-stripped fetal calf serum, the media of myometrial cells at passage 4 and hTERT cells was refreshed and cells incubated for a further 30 h without treatment. RNA was extracted as described in Methods. Whole-genome transcriptome analysis was conducted by hybridization to Affymetrix Human Gene 2.1 ST array strips and analysed using Partek Genomics Suite 6.6 software. DEG was identified by two-way ANOVA, and P-values were adjusted using the FDR (false-discovery rate) method to correct for multiple comparisons. DEG were considered significant if P-value was P ≤ 0.05 at a fold change of >2 with FDR <0.5, n = 6. (A) principal component analysis (PCA) plot, (B) Venn diagram of genes varying significantly versus t = 0 that are common and unique to each model, (C) Volcano plot of myometrial explants, (D) Volcano plot of passage 4 myometrial cells (E) Volcano plot of hTERT.
A second set of biopsies and hTERT cultures were used to validate the microarray results via quantitative RT–PCR. Microarray trends were preserved for a panel of genes of interest including those associated with reproductive function (PTGS2, OXTR, PGR, GJA1) and smooth muscle phenotype (ACTA2, MYLK) (Table III). Overall, 15 genes of interest were chosen for validation of microarray results with 3 comparisons performed per gene (explants versus t = 0, passage 4 myometrial cells versus t = 0, hTERT versus t = 0); microarray and RT–PCR data followed the same trend in 39 of 45 cases (86.7%).
Table III.
Quantitative RT–PCR validation of selected genes.
| Gene name | Model | Quantitative RT–PCR fold change | Quantitative RT–PCR direction of change | Microarray fold change | Microarray direction of change |
|---|---|---|---|---|---|
| PTGS2 | Explants | 7.02 ± 3.34 | ↑ | 24.55 ± 0.45 | ↑ |
| Myometrial cells | 2.43 ± 0.65 | ↑ | 2.21 ± 0.41 | ↑ | |
| hTERT | 1.73 ± 0.29 | ↑ | 2.47 ± 0.02 | ↑ | |
| IL8 | Explants | 200.53 ± 43.89 | ↑ | 28.53 ± 0.45 | ↑ |
| Myometrial cells | −4.51 ± 0.07 | ↓ | −1.22 ± 0.14 | ↓ | |
| hTERT | 14.26 ± 0.05 | ↑ | 9.92 ± 0.46 | ↑ | |
| OXTR | Explants | −11.93 ± 0.05 | ↓ | −1.23 ± 0.21 | ↓ |
| Myometrial cells | −34.74 ± 0.01 | ↓ | −7.74 ± 0.42 | ↓ | |
| hTERT | −9.09 ± 0.02 | ↓ | −3.31 ± 0.20 | ↓ | |
| PGR | Explants | −2.89 ± 0.04 | ↓ | −1.76 ± 0.08 | ↓ |
| Myometrial cells | −4.43 ± 0.04 | ↓ | −3.35 ± 0.45 | ↓ | |
| hTERT | −18.47 ± 0.00 | ↓ | −8.21 ± 0.08 | ↓ | |
| IL1B | Explants | 2.53 ± 0.09 | ↑ | 2.64 ± 0.40 | ↑ |
| Myometrial cells | −35.06 ± 0.01 | ↓ | −1.31 ± 0.11 | ↓ | |
| hTERT | 18.90 ± 0.80 | ↑ | 18.44 ± 0.36 | ↑ | |
| NR3C1 | Explants | −2.19 ± 0.04 | ↓ | 1.39 ± 0.03 | ↑ |
| Myometrial cells | −1.64 ± 0.08 | ↓ | 1.28 ± 0.14 | ↑ | |
| hTERT | −1.23 ± 0.04 | ↓ | 1.81 ± 0.04 | ↑ | |
| TPM1 | Explants | −1.37 ± 0.10 | ↓ | −4.40 ± 0.05 | ↓ |
| Myometrial cells | −3.05 ± 0.04 | ↓ | −2.77 ± 0.16 | ↓ | |
| hTERT | 1.33 ± 0.06 | ↑ | −1.48 ± 0.08 | ↓ | |
| TNFAIP3 | Explants | 4.09 ± 1.30 | ↑ | 6.44 ± 0.19 | ↑ |
| Myometrial cells | −13.44 ± 0.01 | ↓ | −5.92 ± 0.12 | ↓ | |
| hTERT | −2.99 ± 0.04 | ↓ | −1.08 ± 0.12 | ↓ | |
| IL6 | Explants | 20.12 ± 4.27 | ↑ | 15.21 ± 0.21 | ↑ |
| Myometrial cells | 1.66 ± 0.46 | ↑ | 2.03 ± 0.31 | ↑ | |
| hTERT | 8.32 ± 1.59 | ↑ | 9.47 ± 0.11 | ↑ | |
| ACTA2 | Explants | −10.48 ± 0.03 | ↓ | −1.79 ± 0.14 | ↓ |
| Myometrial cells | −15.08 ± 0.02 | ↓ | −1.49 ± 0.24 | ↓ | |
| hTERT | −44.47 ± 0.00 | ↓ | −2.83 ± 0.07 | ↓ | |
| MYLK | Explants | −8.98 ± 0.02 | ↓ | −1.40 ± 0.03 | ↓ |
| Myometrial cells | −13.14 ± 0.01 | ↓ | −1.14 ± 0.19 | ↓ | |
| hTERT | −124.56 ± 0.00 | ↓ | −3.58 ± 0.06 | ↓ | |
| PTGER3 | Explants | −3.59 ± 0.09 | ↓ | −1.10 ± 0.08 | ↓ |
| Myometrial cells | −10.37 ± 0.03 | ↓ | −5.57 ± 0.21 | ↓ | |
| hTERT | −653.11 ± 0.00 | ↓ | −24.47 ± 0.04 | ↓ | |
| GJA1 | Explants | 1.06 ± 0.18 | ↑ | 1.58 ± 0.10 | ↑ |
| Myometrial cells | 2.10 ± 0.19 | ↑ | 2.56 ± 0.11 | ↑ | |
| hTERT | 1.49 ± 0.11 | ↑ | 1.39 ± 0.03 | ↑ | |
| ESR1 | Explants | −1.58 ± 0.10 | ↓ | 1.30 ± 0.17 | ↑ |
| Myometrial cells | 1.41 ± 0.23 | ↑ | −1.01 ± 0.13 | ↓ | |
| hTERT | −3.56 ± 0.02 | ↓ | −1.32 ± 0.08 | ↓ | |
| IL1A | Explants | 3.36 ± 0.83 | ↑ | 2.71 ± 0.35 | ↑ |
| Myometrial cells | −1.55 ± 0.04 | ↓ | −1.26 ± 0.10 | ↓ | |
| hTERT | 18.24 ± 3.60 | ↑ | 17.09 ± 0.19 | ↑ |
Myometrial tissue obtained from non-labouring women at term was snap frozen at the time Caesarean section (t = 0), finely dissected into 3 × 3 × 3 mm3 explants or digested with a collagenase mixture to isolate the myometrial cells. Explants were left in culture without treatment for 30 h. After overnight incubation with 1% charcoal and dextran-stripped fetal calf serum, the media of myometrial cells at passage 4 and hTERT myometrial cells was refreshed and cells were left in culture for 30 h without treatment. RNA was extracted, cDNA was synthesized and quantitative RT–PCR was performed as described in Methods. Data presented as mean fold change ± SEM; n = 6.
Nuclear receptor levels
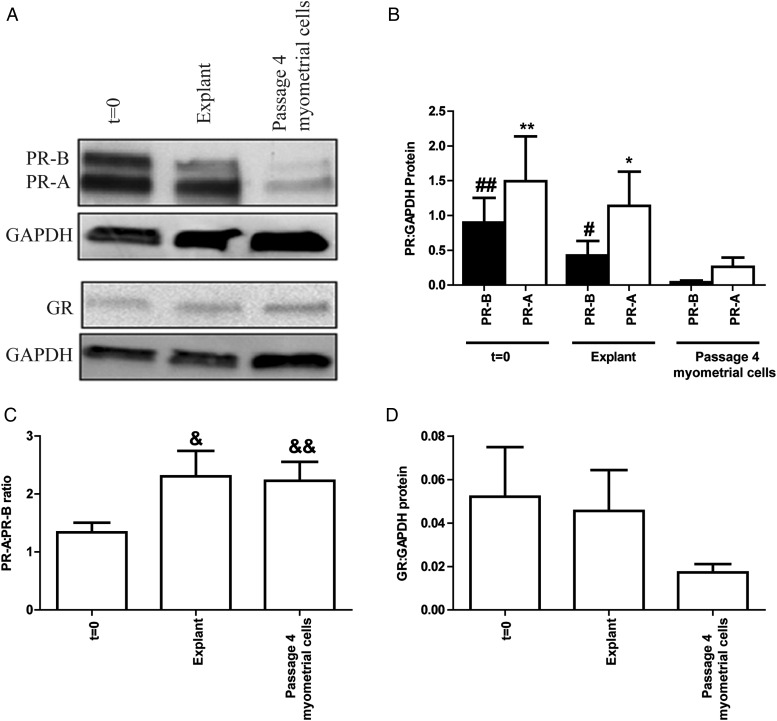
Subsequent experiments focused on comparing explants and passage 4 myometrial cells to t = 0, as the same biopsy could be utilized in matched experiments and these two groups most closely resembled t = 0 based on the microarray results. A separate set of biopsies obtained from non-labouring women were divided into (i) t = 0, (ii) myometrial explants and (iii) myometrial cells, and utilized to assess key nuclear receptor levels on the protein level. Although the level of both PR isoforms tended to decline, there was no significant difference between t = 0 and explants. However, the levels of PR were observed to be significantly lower in myometrial cells at passage 4 than in either t = 0 tissue or explants (Fig. 2A and B). The PR-A:PR-B ratio was similar in explants and cells although this was significantly raised compared with t = 0 (P = 0.0327 and P = 0.0067, respectively) (Fig. 2C). No statistically significant difference was observed in GR levels between t = 0, explants and cells (Fig. 2A and D).
Figure 2.
Myometrial explants maintain nuclear receptor levels in culture. Myometrial tissue obtained from term non-labouring women was divided into three portions: (i) snap frozen (t = 0), (ii) finely dissected into 3 × 3 × 3 mm3 explants and (iii) digested with a collagenase mixture to isolate the cells for myometrial cell culture. Explants were treated immediately with ethanol vehicle for 30 h. After overnight incubation with 1% (v/v) charcoal and dextran-stripped fetal calf serum, myometrial cells at passage 4 were treated with ethanol vehicle for 30 h. Protein was extracted and quantified. Western blotting for the progesterone receptor (PR) isoforms (PR-A and PR-B) and glucocorticoid receptor (GR) was performed as described in Methods. (A) Representative western blots of PR and GR levels, (B) densitometric analysis of PR levels, (C) analysis of PR-A:PR-B ratio, (D) densitometric analysis of GR levels. The data are expressed as mean + SEM. Normality was tested using a Shapiro–Wilks test followed by the Wilcoxon-signed rank testing. #P < 0.05 cells versus explants for PR-B; ##P < 0.01 cells versus t = 0 for PR-B; *P < 0.05 cells versus explants for PR-A; **P < 0.01 cells versus t = 0 for PR-A; &P < 0.05 t = 0 versus explants; &&P < 0.01 t = 0 versus cells, n = 8–9.
Progesterone-repression of IL-1β-induced COX-2
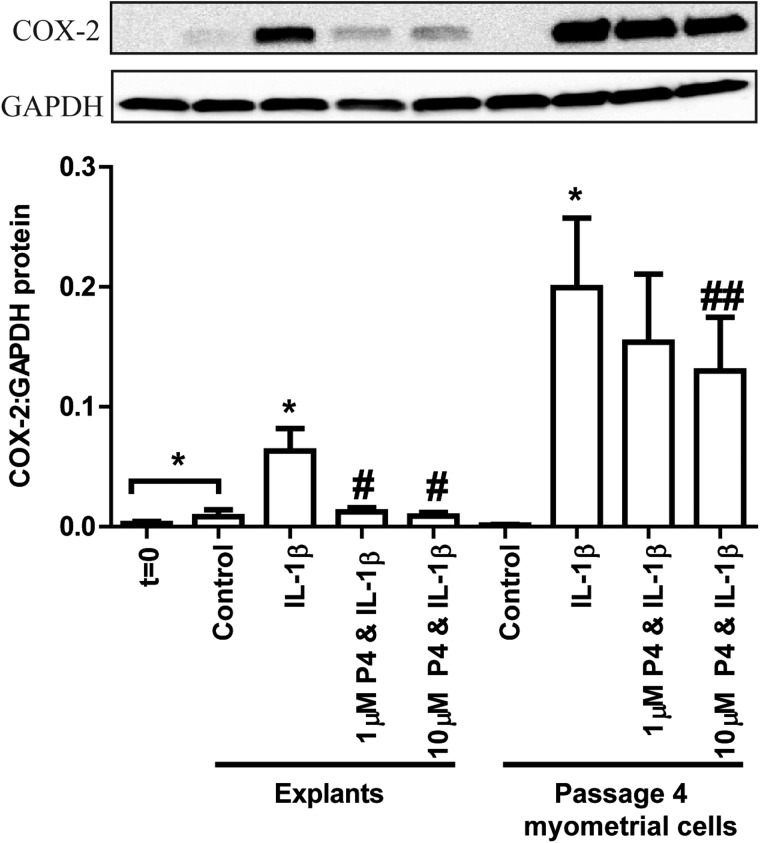
Previous group data demonstrated that 10 µM was the minimum P4 dose causing a significant reduction in IL-1β-driven COX-2 in passage 4 myometrial cells. Based on dose–response experiments, the myometrial explant IL-1β EC50 was defined as 10 ng/ml and the P4 IC50 P4 was 1 µM (Supplementary Fig. S1).
In order to directly compare P4 sensitivity, explants and cells originating from the same biopsy were treated with 10 ng/ml IL-1β ± 1 or 10 µM P4. Compared t = 0, basal COX-2 levels were significantly raised in explants (P = 0.0313) and not in cells. Although not significant, the addition of IL-1β showed a trend towards a greater increase in COX-2 levels in myometrial cells than in explants (P = 0.07; Fig. 3). The IL-1β-induced increase in COX-2 protein levels was statistically significantly reduced by pre-incubation with 1 µM P4 in myometrial explants and 10 µM P4 in cell cultures (Fig. 3).
Figure 3.
Explants respond to 1 µM progesterone (P4) in paired biopsies whereas myometrial cells do not. Myometrial tissue obtained from term non-labouring women was divided into three portions: (i) snap frozen (t = 0), (ii) finely dissected into 3 × 3 × 3 mm3 explants and (iii) digested with a collagenase mixture to isolate cells for myometrial cell culture. Explants were immediately pre-treated for 6 h with ethanol vehicle, 1 or 10 µM P4 followed by a 24 h treatment with IL-1β (10 ng/ml). After overnight incubation with 1% (v/v) charcoal and dextran-stripped fetal calf serum, myometrial cells at passage 4 were pre-treated for 6 h with ethanol vehicle, 1 or 10 µM P4 followed by a 24 h treatment with IL-1β (10 ng/ml). Protein was extracted and quantified. Western blotting for cyclooxygenase-2 (COX-2) was performed as described in Methods. A representative western blot is shown at the top of the figure with densitometric analysis below. The data are expressed as mean + SEM. Normality was tested using a Kolmogorov–Smirnov test followed by comparison of control versus IL-1β by the Wilcoxon-signed rank testing or paired t testing depending on the data distribution; *P < 0.05 versus control in that group. The IL-1β, 1 µM P4 & IL-1β and 10 µM P4 & IL-1β conditions in each group were compared by ANOVA followed by Bonferroni's Multiple Comparison Test; #P < 0.05 versus IL-1β in that group; ##P < 0.01 versus IL-1β in that group, n = 4–8.
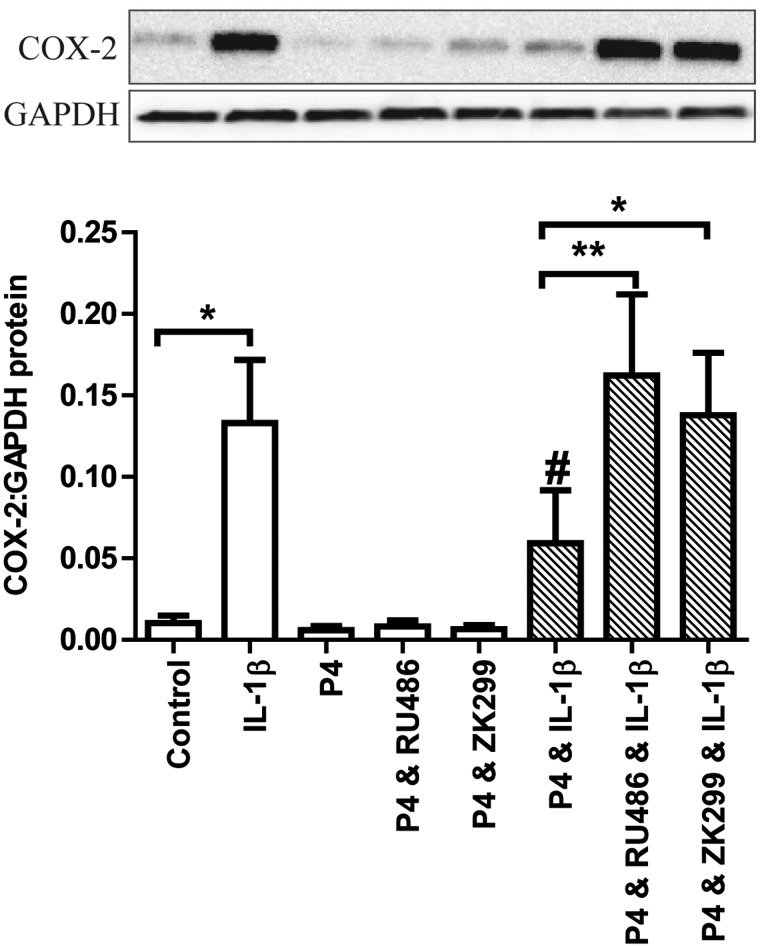
P4 acts via PR in myometrial explants and represses IL-1β-induced activation of p65 and AP-1
The mixed PR/GR antagonist RU486 reversed the repressive effect of 1 µM P4 on IL-1β-induced COX-2 expression in myometrial explants (Fig. 4). The more PR-selective inhibitor ZK299 at the PR-specific dose of 1 µM (Kohmura et al., 2000), also reversed the P4 effect (Fig. 4).
Figure 4.
Mifepristone (RU486) and onapristone (ZK299) reverse the progesterone (P4)-mediated reduction in IL-1β-driven cyclooxygenase-2 (COX-2) in myometrial explants. Myometrial tissue obtained from term non-labouring women was finely dissected into 3 × 3 × 3 mm3 explants. These were immediately pre-treated for 6 h with ethanol and DMSO vehicle, 1 µM P4 ± 1 µM RU486 or 1 µM ZK299 followed by a 24 h treatment with IL-1β (10 ng/ml). Protein was extracted and quantified. Western blotting for cyclooxygenase-2 (COX-2) was performed as described in Methods. A representative western blot is shown at the top of the figure with densitometric analysis below. The data are expressed as mean + SEM. Normality was tested using a Kolmogorov–Smirnov test. Paired t tests were used to compare control versus IL-1β and IL-1β versus P4 & IL-1β (#P < 0.05). The shaded groups were compared with a Friedman test followed by Dunn's Multiple Comparison Test; *P < 0.05; **P < 0.01, n = 6–7.
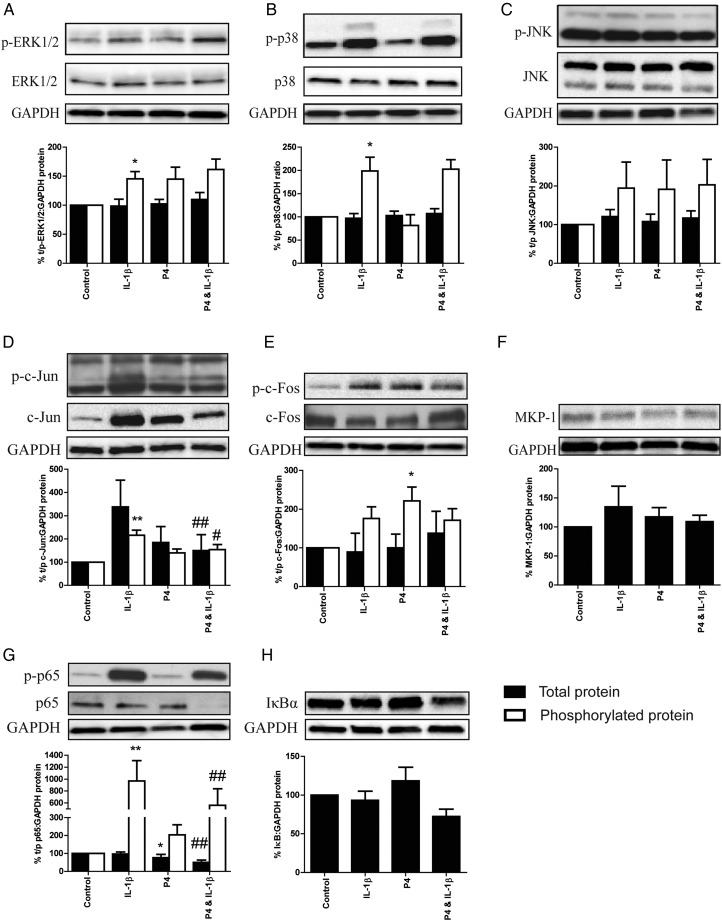
IL-1β increased the phosphorylation of ERK (P = 0.0156) and p38 (P = 0.0313) as well as the transcription factor targets c-Jun (P = 0.0078) and p65 (P = 0.0078; Fig.5) with no alteration to levels of total protein. Although a trend was observed, IL-1β did not significantly increase the phosphorylation of JNK or c-Fos (Fig. 5). Pre-incubation with 1 µM P4 reduced the IL-1β induced increase in p65 and c-Jun phosphorylation in association with a reduction in total protein levels. Interestingly, there was no reduction in MAPK phosphorylation, nor any change in MKP-1 or IκBα levels following P4 treatment alone or in combination with IL-1β (Fig. 5). P4 treatment alone did not drive the MAPKs, c-Jun or p65 phosphorylation, but did lead to a decrease in total p65 levels and an increase in c-Fos phosphorylation (Fig. 5).
Figure 5.
Progesterone (P4) reduces the phosphorylation of pro-inflammatory transcription factors in myometrial explants. Myometrial tissue obtained from term non-labouring women was finely dissected into 3 × 3 × 3 mm3 explants. These were immediately pre-treated for 6 h with ethanol or 1 µM P4 followed by a 30 min treatment with IL-1β (10 ng/ml). Protein was extracted and quantified, and western blotting for total protein ± phosphorylated protein (where applicable) for (A) ERK1/2, (B) p38, (C) JNK, (D) c-Jun, (E) c-Fos, (F) MKP-1, (G) p65 and (H) IκBα was performed as described in Methods. Representative western blots are shown above each densitometric analysis plot. The data are normalized to control and expressed as mean + SEM. Normality was tested using a Kolmogorov–Smirnov for six replicates or a Shapiro–Wilk test for more than six replicates. Wilcoxon-signed rank testing was used to compare between pairs; *P < 0.05 versus control; **P < 0.01 versus control; #P < 0.05 versus IL-1β; ##P < 0.01 versus IL-1β. n = 6–9. Black bars: total (t) protein; clear bars: phosphorylated (p) protein.
Lack of a functional withdrawal of P4 action in myometrial explants
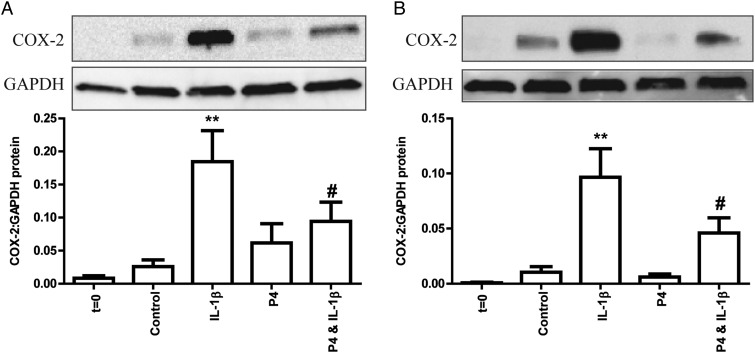
To determine whether there was any evidence of a functional P4 withdrawal with the onset of labour, we obtained myometrium from women before the onset of labour, in early labour (≤3 cm), during which the cervix effaces, begins to dilate and contractions become regular and strong, and in established labour (>3 cm), during which the cervix dilates more rapidly and contractions are regular and strong. The increase in COX-2 levels induced by IL-1β was similar in all 3 groups (Supplementary Fig. S2). Pretreatment with 1 µM P4, reduced the expression of IL-1β-driven COX-2 in explants from patients in both early and established labour (Fig. 6). Treatment with P4 alone was not associated with a significant change in COX-2 levels compared with control (Fig. 6).
Figure 6.
Progesterone (P4) maintains its anti-inflammatory role throughout labour. Myometrial tissue obtained from women in term (A) early labour and (B) established labour was finely dissected into 3 × 3 × 3 mm3 explants after a portion was immediately snap frozen (t = 0). Explants were immediately pre-treated for 6 h with ethanol vehicle or 1 µM P4 followed by a 24 h treatment with IL-1β (10 ng/ml). Protein was extracted and quantified. Western blotting for cyclooxygenase-2 (COX-2) was performed as described in Methods. Representative western blots are shown above each densitometric analysis plot. The data are expressed as mean + SEM. Normality was tested using a Shapiro–Wilk test. A Wilcoxon signed rank test was used for non-normally distributed data and a paired t test for normally distributed data. **P < 0.01 versus control; ***P < 0.001 versus control; #P < 0.05 versus IL-1β. n = 8.
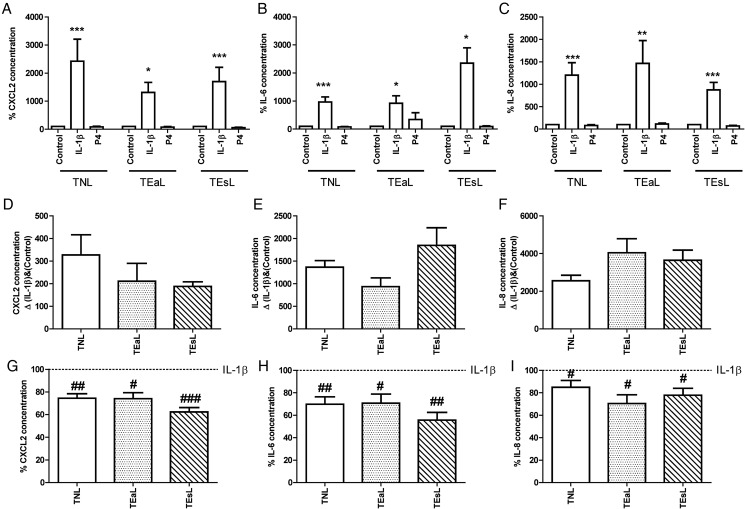
The role of P4 was further studied by quantification of a panel of pro-inflammatory cytokines in the tissue culture media of explants obtained from non-labouring women as well as women in early and established labour. In the first instance, using non-labouring samples, we sought to identify which cytokines were driven by IL-1β in our model and, if so, whether P4 was able to significantly reduce these levels. We identified a list of chemokines/cytokines fulfilling these two criteria comprised of CXCL2, IL-6 and IL-8 (Fig. 7A–C, Supplementary Fig. S3). CCL11 is not shown as it was not within the detection range of the assay. In addition, even though LIF was originally included in the list above, it was not detectable in the labouring culture media.
Figure 7.
Progesterone does not become pro-inflammatory with labour onset and it reduces the expression of proinflammatory cytokines/chemokines irrespective of labour status. The media from explant cultures was used to run human Bio-Plex© Pro™ chemokine/cytokine assays for (A, D, G) C–X–C motif chemokine 2 (CXCL2), (B, E, H) IL-6 and (C, F, I) IL-8 as per the manufacturer's protocol. Data were normalized to tissue weight. The data in (A–C) are standardized to control and expressed as mean + SEM. The data in (D–F) are expressed as mean + SEM of the delta (Δ) between IL-1β and control groups. The data in (E–I) are standardized to IL-1β and expressed as mean + SEM. Normality was tested using a Kolmogorov–Smirnov for six replicates or a Shapiro–Wilk test for more than six replicates. For groups of three, a Kruskal–Wallis test was performed followed by Dunn's Multiple Comparison Test for non-normally distributed data and an ANOVA followed by Bonferroni's Multiple Comparison Test was performed for normally distributed data. Wilcoxon-matched pair testing was used for non-normally distributed paired data and paired t tests for normally distributed data. For comparisons to vehicle control within matched labour status group, statistical significance is indicated as follows: *P < 0.05; **P < 0.01; ***P < 0.001. For comparisons to IL-1β within matched labour status group, statistical significance is indicated as follows: #P < 0.05; ##P < 0.01; ###P < 0.001, n = 6–16. Labour status groups are labelled as TNL (term no labour), TEaL (term early labour) and TEsL (term established labour).
Next we determined whether the effect of IL-1β was altered by labour status by calculating the delta change (Δ) between control and IL-1β treated samples. As with COX-2, we found that the effect of IL-1β was similar in all 3 groups for CXCL2, IL-6 and IL-8 (Fig. 7D–F). We then assessed whether P4 treatment alone altered the release of pro-inflammatory cytokines into the medium in either of the labouring groups and found that P4 had no effect on cytokine levels (Fig. 7A–C). Finally, we confirmed that there was no difference in the ability of P4 to repress the IL-1β-induced increase in CXCL2, IL-6 and IL-8 levels in the labouring samples (Fig. 7G–I).
Discussion
In this study, we sought to establish a model that reflects the in vivo situation more accurately than the current in vitro cell models and to use this model to study P4 signalling in the myometrium.
As expected, we demonstrated that none of the in vitro models overlapped with the in vivo (t = 0) condition in the principal component analysis (PCA) plot; however, the explant gene expression profile was most closely associated with it. The separation between t = 0 and both myometrial cells and the hTERT cell line was much more marked. This was supported by the distinct distribution of top genes shown in the volcano plots of the three models compared with t = 0. Importantly, this pattern was preserved on examining genes relevant to reproductive function and parturition; for example the explant levels of PR (PGR), oxytocin receptor (OXTR) and connexin-43 (GJA1) did not vary significantly compared with t = 0. In contrast, PR RNA levels were significantly lower in both passage 4 myometrial cells and hTERT. Indeed, the same pattern was observed for PR on the protein level with no significant change in explants versus t = 0, but a significant reduction in myometrial cells. In addition, OXTR and GJA1 RNA levels were significantly lower and higher, respectively, in myometrial cells. Although COX-2 (PTGS2) levels were significantly elevated in explants as well as hTERT, upon comparing this up-regulation in relation to COX-2 levels after IL-1β treatment, the effect was negligible with a signal-to-noise ratio of 17.7 (FDA, 2003). Indeed, it was noted that genes associated with inflammation were elevated in all three models as evidenced by gene ontology analysis (Supplementary Table SI). We also determined that the expression pattern of smooth muscle markers such as alpha smooth muscle actin (ACTA2) and myosin light chain kinase (MYLK) remained unaltered in explants whereas both were significantly reduced in hTERT.
We noted with interest that the uniquely up-regulated genes in the explant group versus t = 0 comprised glucose metabolism pathways. We hypothesized that this was the result of the explants being cultured in DMEM without any additional nutrient supplementation and hence utilizing alternative biochemical pathways to produce glucose. Although high lactate levels have been associated with dystocia (Quenby et al., 2004) and hence it is plausible that glucose metabolism may play a role in the success of the parturition, further study is required. In an attempt to mimic the physiological conditions as closely as possible, we did supplement culture media with P4, estradiol and/or the cAMP agonist forskolin, but found that this had no effect on restoring PR levels even closer to t = 0 and hence these conditions were not incorporated into the final experimental model used (data not shown).
To our knowledge, this is the first microarray study directly comparing gene expression in pregnant myometrium at t = 0 with that of myometrial explants, passage 4 myometrial cells and hTERT. One study comparing non-pregnant myometrium at t = 0 to matched primary cell culture reported changes in expression of more than 1000 genes (Zaitseva et al., 2006) supporting our finding of marked changes in gene expression in primary cell culture systems. Some gene expression studies focused on gene expression in myometrial explants (Cordeaux et al., 2010) or cultured myometrial cells (Lei et al., 2015b; Chandran et al., 2016) in the presence or the absence of progestins and hence cannot be compared with our study. Other authors (Rehman et al., 2003; Bollapragada et al., 2009; Mittal et al., 2010; Chan et al., 2014) have sought to compare the gene expression profile of myometrium from non-labouring and labouring patients.
One possible limitation of this gene expression analysis was that it was conducted via microarray and not RNA-Seq, the latter being widely accepted as a superior platform with better concordance with quantitative RT–PCR (Wang et al., 2014). However, microarray and quantitative RT–PCR share high correlation and for the purpose of this study, microarray was deemed appropriate. One notable case of poor concordance between microarray and quantitative RT–PCR data was GR (NR3C1), where the microarray indicated up-regulation in all three models whereas quantitative RT–PCR showed down-regulation. We sought to clarify our findings by undertaking protein level analysis and confirmed that GR levels do not vary significantly in explants or passage 4 myometrial cells compared with t = 0.
On the protein level, we demonstrated that the treatment of explants with 1 µM P4 significantly reduces inflammation, whereas in passage 4 myometrial cells it does not. Intriguingly, this occurs despite the fact that the PR-A:PR-B ratio increases in explant culture versus t = 0. Interestingly, the response to IL-1β is greater in cultured myometrial cells and this could reflect the architectural difference of the explants and cells. For consistency, we opted to treat both myometrial explants and cells with the same dose of IL-1β; however, it could be argued that this stimulus is supraphysiological and that corresponding in vivo events are much subtler. Nevertheless, no similar evidence of increased sensitivity to P4 treatment was observed in cells, suggesting that explants do not just differ to myometrial cells in their cellular organization, but also in their overall sensitivity to different treatments.
Even though other studies have shown that passaging of primary cells does not affect the myometrial phenotype (Mosher et al., 2013), they have crucially not included tissue at t = 0. Our data suggest that there are marked functional differences between explants and passage 4 myometrial cell cultures. Explants contain a heterogeneous collection of cell types, including myometrial smooth muscle, vascular smooth muscle, endothelial cells, stromal fibroblasts and blood cells which may all contribute to the phenotype which, upon comparison to cell culture systems which only contain one cell type, most closely resembles t = 0. Indeed, by virtue of its similarity to the in vivo status, we believe that the study of myometrial function on the explant level, with its variety of cell types, is superior to cell culture systems. Nevertheless, we have observed by immunohistochemistry for alpha smooth muscle actin that smooth muscle cells predominate (data not shown) and additionally that explants contract spontaneously and after treatment with oxytocin (data not shown).
We have shown that both RU486 and ZK299 are capable of reversing the effect of P4. Although neither drug is a pure PR antagonist (Miner et al., 2003), 1 µM ZK299 has previously been shown to reverse the effect of P4 but not that of dexamethasone (Kohmura et al., 2000), suggesting that P4, in contrast to our observations from myometrial cell cultures (Lei et al., 2012), may signal via PR in explants. The reason for this may lie in the difference in nuclear receptor levels in the two models, with the explant model more closely resembling the in vivo state. Trial of other ‘specific’ PR and GR antagonists demonstrated that they have non-specific actions in myometrial explants (data not shown).
Consistent with previous findings (Lei et al., 2012, 2015a; Hardy et al., 2006), we show that P4 is able to repress the IL-1β-induced activation of two major pro-inflammatory transcription factors: NF-κB and AP-1. Both of these transcription factors have previously been shown to be required for IL-1β-mediated up-regulation of COX-2 in gestational tissues (Allport et al., 2000). In support of our previous findings (Lei et al., 2015a) and in contrast to other reports in the literature (Hardy et al., 2006), the mechanism of P4 action does not appear to be via an increase in IκBα. Further, the reduction in c-Jun phosphorylation does not seem to be mediated via an increase in MKP-1 (Lei et al., 2015a). Rather, it appears that total p65 and c-Jun levels are reduced by P4 treatment. These data suggest that the in vivo mechanism of P4 action may differ markedly compared with the in vitro models.
Our data indicate that with the onset of labour, P4 does not become pro-inflammatory as suggested by other groups (Allport et al., 2000; Tan et al., 2012), nor does it lose its anti-inflammatory action in myometrial explants. Furthermore, the explant sensitivity to IL-1β does not alter with labour status, nor does the ability of P4 to down-regulate the IL-1β response suggesting that there is no functional withdrawal of P4 action, at least in terms of the ability of P4 to repress inflammation. It should however be noted that these represent ex vivo observations and may not be reflective of in vivo events. Nevertheless, our data suggest that should a functional P4 withdrawal occur in vivo, then it is unlikely to involve COX-2 and at least the inflammatory chemokine/cytokines examined in the present study. It does remain possible that there is a withdrawal of other P4-mediated functions that lead to the onset of labour.
In conclusion, this study has established the validity of using an explant model to study myometrial P4 signalling. We provide evidence that P4 acts via PR to reduce IL-1β-induced COX-2 synthesis in association with a reduction in NF-κB and AP-1 activation. Furthermore, we show that P4 is able to repress the expression IL-1β-induced genes even after the onset of labour, suggesting that, at least in this regard, there is no functional withdrawal of P4 action.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
E.X.G. designed the study, recruited patients, performed the experiments and wrote the manuscript. K.L., P.F.L., S.R.S and M.R.J contributed to the design of the study and data interpretation. A.Y. contributed to the design of the study. B.R.H. assisted with chemokine/cytokine assay data acquisition. M.C. and S.T.M. carried out microarray data acquisition and analysis. All authors assisted with drafting of the article and approved the final version to be published.
Funding
This work was supported by grants from the Joint Research Committee of the Westminster Medical School Research Trust, Borne (No. 1067412-7; a sub-charity of the Chelsea and Westminster Health Charity) and the Imperial National Institute for Health Research Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the National Health Service or the Department of Health.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
The authors would like to thank all patients who kindly donated biopsies to this study and also clinical staff at the Chelsea & Westminster NHS Foundation Trust who assisted with biopsy collection.
References
- Allport VC, Slater DM, Newton R, Bennett PR. NF-kappaB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH). Mol Hum Reprod 2000;6:561–565. [DOI] [PubMed] [Google Scholar]
- Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod 2011;7:581–586. [DOI] [PubMed] [Google Scholar]
- Bamberger AM, Bamberger CM, Gellersen B, Schulte HM. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc Natl Acad Sci U S A 1996;93:6169–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol 2009;200:104e1–10411. [DOI] [PubMed] [Google Scholar]
- Chan YW, van den Berg HA, Moore JD, Quenby S, Blanks AM. Assessment of myometrial transcriptome changes associated with spontaneous human labour by high-throughput RNA-seq. Exp Physiol 2014;99:510–524. [DOI] [PubMed] [Google Scholar]
- Chandran S, Cairns MT, O'Brien M, O'Connell E, Mashayekhi K, Smith TJ. Effects of combined progesterone and 17beta-estradiol treatment on the transcriptome of cultured human myometrial smooth muscle cells. Physiol Genomics 2016;48:50–61. [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci U S A 2003;100:9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeaux Y, Tattersall M, Charnock-Jones DS, Smith GC. Effects of medroxyprogesterone acetate on gene expression in myometrial explants from pregnant women. J Clin Endocrinol Metab 2010;95:E437–E447. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen MO, Kaihola HL. The effect of luteectomy-induced progesterone-withdrawal on the oxytocin and prostaglandin response of the first trimester pregnant human uterus. Prostaglandins 1973;4:421–429. [DOI] [PubMed] [Google Scholar]
- da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2003;188:419–424. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Sciences, 2003. [Google Scholar]
- Frydman R, Baton C, Lelaidier C, Vial M, Bourget P, Fernandez H. Mifepristone for induction of labour. Lancet 1991;337:488–489. [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol 2006;20:2724–2733. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem 1996;271:6217–6224. [DOI] [PubMed] [Google Scholar]
- Khanjani S, Kandola MK, Lindstrom TM, Sooranna SR, Melchionda M, Lee YS, Terzidou V, Johnson MR, Bennett PR. NF-kappaB regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. J Cell Mol Med 2011;15:809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanjani S, Terzidou V, Johnson MR, Bennett PR. NFkappaB and AP-1 drive human myometrial IL8 expression. Mediators Inflamm 2012;2012:504952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmura Y, Kirikae T, Kirikae F, Nakano M, Sato I. Onapristone (ZK299) blocks the suppressive effect of progesterone, but not that of dexamethasone, on inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. Int J Immunopharmacol 2000;22:765–774. [DOI] [PubMed] [Google Scholar]
- Lee Y, Sooranna SR, Terzidou V, Christian M, Brosens J, Huhtinen K, Poutanen M, Barton G, Johnson MR, Bennett PR. Interactions between inflammatory signals and the progesterone receptor in regulating gene expression in pregnant human uterine myocytes. J Cell Mol Med 2012;16:2487–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Chen L, Georgiou EX, Sooranna SR, Khanjani S, Brosens JJ, Bennett PR, Johnson MR. Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1beta-induced COX-2 expression in human term myometrial cells. PloS One 2012;7:e50167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Georgiou EX, Chen L, Yulia A, Sooranna SR, Brosens JJ, Bennett PR, Johnson MR. Progesterone and the Repression of Myometrial Inflammation: The Roles of MKP-1 and the AP-1 System. Mol Endocrinol 2015a;29:1454–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Sooranna SR, Johnson MR. Expression data from primary culture human myometrial cells. Genomics Data 2015b;6:182–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Lappas M. Differential expression of AP-1 proteins in human myometrium after spontaneous term labour onset. Eur J Obstet Gynecol Reprod Biol 2014;177:100–105. [DOI] [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW et al. . Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379–2385. [DOI] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab 2007;92:1927–1933. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002;87:2924–2930. [DOI] [PubMed] [Google Scholar]
- Miner JN, Tyree C, Hu J, Berger E, Marschke K, Nakane M, Coghlan MJ, Clemm D, Lane B, Rosen J. A nonsteroidal glucocorticoid receptor antagonist. Mol Endocrinol 2003;17:117–127. [DOI] [PubMed] [Google Scholar]
- Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang CL, Chaiworapongsa T, Lye S et al. . Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med 2010;38:617–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher AA, Rainey KJ, Bolstad SS, Lye SJ, Mitchell BF, Olson DM, Wood SL, Slater DM. Development and validation of primary human myometrial cell culture models to study pregnancy and labour. BMC Pregnancy Childbirth 2013;13 (Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000;289:1751–1754. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A 2003;100:9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JE, Marlow N, Messow CM, Shennan A, Bennett PR, Thornton S, Robson SC, McConnachie A, Petrou S, Sebire NJ et al. . Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet 2016;387:2106–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, Soma-Pillay P, Porter K, How H, Schackis R et al. . Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2007;30:687–696. [DOI] [PubMed] [Google Scholar]
- Quenby S, Pierce SJ, Brigham S, Wray S. Dysfunctional labor and myometrial lactic acidosis. Obstet Gynecol 2004;103:718–723. [DOI] [PubMed] [Google Scholar]
- Rehman KS, Yin S, Mayhew BA, Word RA, Rainey WE. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol Hum Reprod 2003;9:681–700. [DOI] [PubMed] [Google Scholar]
- Soloff MS, Cook DL Jr, Jeng YJ, Anderson GD. In situ analysis of interleukin-1-induced transcription of cox-2 and il-8 in cultured human myometrial cells. Endocrinology 2004;145:1248–1254. [DOI] [PubMed] [Google Scholar]
- Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod 2004;10:109–113. [DOI] [PubMed] [Google Scholar]
- Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab 2012;97:E719–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gong B, Bushel PR, Thierry-Mieg J, Thierry-Mieg D, Xu J, Fang H, Hong H, Shen J, Su Z et al. . The concordance between RNA-seq and microarray data depends on chemical treatment and transcript abundance. Nat Biotechnol 2014;32:926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod 2006;12:187–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.