Abstract
Since chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow obstruction, inhaled bronchodilators form the mainstay of treatment. A variety of new inhaled drugs and inhaler devices have recently been licensed and approved for prescribing to patients with COPD; many such drugs have been formulated in devices to deliver two different drugs at the same time. The evidence based review article highlights all of the drugs now licensed, describes some of the evidence surrounding their use and highlights practical steps in helping decide when these drugs should be considered in the context of guidelines.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major global health problem characterized by progressive irreversible airflow obstruction typically caused by cigarette smoking and continued exposure to indoor biomass fuels in developing countries. Its presence generally heralds the onset of chronic respiratory symptoms and exercise limitation, punctuated by exacerbations which are associated with undesirable effects on overall health status in the short, medium and long-term.1–3
Since airflow obstruction is a universal feature of COPD, topically delivered bronchodilators form the cornerstone of pharmacological treatment with inhaled corticosteroids (ICS) and other options reserved for patients with more advanced disease with frequent exacerbations.4,5 Over the past decade, considerable research—requiring vast sums of money and significant input by both the scientific and pharmaceutical communities—has taken place into the development of new strategies and approaches in management. This in turn has generated a myriad of published data and the introduction of a bewildering array of new inhaled treatments and devices available within the armamentarium of clinicians and prescribers.
This evidence based review article is not designed to be a systematic review but aims to highlight to practising clinicians the most important and clinically advanced new inhaled drugs designed for use in COPD and where they most comfortably sit within the existing framework of national and international guidelines. To achieve this, both authors performed an extensive literature search using Pubmed and the Cochrane library up to July 2015 with the following keywords: long acting muscarinic antagonist, long acting β2-agonist, inhaled corticosteroids, chronic obstructive pulmonary disease, exacerbations, pulmonary function, adverse effects and pneumonia.
Management overview
As with most chronic diseases, the overall management of COPD can broadly be classified into non-pharmacological and pharmacological manoevres, with a minority of patients being considered suitable for lung transplantation and other interventional approaches. Since COPD is a heterogeneous condition—with different patients having different specific needs—an individualized treatment approach should ideally be pursued.4,5
When considering which inhaled treatment is most suitable to the needs of a patient with COPD, it is imperative that a comprehensive assessment is firstly made focusing primarily upon factors such as extent of breathless, frequency of exacerbations, exercise limitation and spirometric values [forced expiratory volume in 1 s (FEV1) and forced vital capacity]. The National Institute of Clinical Excellence guidelines on COPD management suggest that inhaled treatment is prescribed in a step-wise manner according to FEV1% predicted, symptoms and exacerbations,4 while the Global initiative for Chronic Obstructive Lung Disease (GOLD) classification of disease severity adopts a similar approach but categorizes patients into groups A, B, C or D as a guide to directing appropriate treatment.5
Short-acting bronchodilators
Short-acting β2-agonists (SABAs)—such as salbutamol and terbutaline—act directly upon bronchial smooth muscle to dilate the airways. Drugs within this class reduce breathlessness, improve lung function and are effective when used on an ‘as required basis’.6 Salbutamol and terbutaline have an onset of action of 5 min, peak effect within 60–90 min with duration of action between 4 and 6 h. Ipratropium is the only available short-acting muscarinic antagonist (SAMA) and offsets high-resting bronchomotor vagally induced tone to dilate the airways. It has been shown in some studies to reduce breathlessness and improve lung function and quality of life.7 Ipratropium has an onset of action within 15 min, peak effect within 30–60 min and duration of action between 3 and 5 h.
All patients with COPD should be prescribed a short-acting inhaled bronchodilator (SABA or SAMA) for as required relief of symptoms; guidelines indicate that there are no major differences in responses to either class, no great benefit from combining both classes or from using them on a regular daily basis.4,5 It is important to note that patients using a long-acting muscarinic antagonist (LAMA) should not be prescribed ipratropium, but use a SABA for acute symptom relief. This is because ipratropium is a non selective muscarinic antagonist which blocks inhibitory pre-junctional M2 receptors which removes ‘the brake’ from post junctional acetylcholine release; this may overcome selective M3 receptor antagonism conferred by a LAMA (such as tiotropium).
In patients with minimal symptoms, preserved lung function (FEV1 > 80% predicted) and few (if any) exacerbations, a SAMA or SABA should be prescribed as monotherapy (without the need for any other inhaled treatment) for as required use.4,5
Long-acting bronchodilators
Inhaled long acting bronchodilators form the mainstay of treatment of COPD. The two classes in widespread use are LAMAs and long-acting β2-agonists (LABAs) (Table 1).8 These drugs promote sustained bronchodilation, improve symptoms and quality of life, and reduce exacerbations; both classes are usually well tolerated although adverse effects are shown in Table 2.
Table 1.
Characteristics of long acting bronchodilator monotherapy inhalers
| Class | Drug | Brand name | Delivery device type | Delivery device name | Dose (µg) | Frequency |
|---|---|---|---|---|---|---|
| LAMA | Tiotropium | Spiriva | DPI | Handihaler | 18 | Once daily |
| LAMA | Tiotropium | Spiriva | FMI | Respimat | 5 | Once daily |
| LAMA | Aclidinium | Eklira | DPI | Genuair | 322 | Twice daily |
| LAMA | Glycopyrronium | Seebri | DPI | Breezhaler | 44 | Once daily |
| LAMA | Umeclidinium | Incruse | DPI | Ellipta | 55 | Once daily |
| LABA | Formoterol | Atimos | pMDI | pMDI | 12 | Twice daily |
| LABA | Formoterol | Oxis | DPI | Turbohaler | 12 | Twice daily |
| LABA | Formoterol | Non-proprietary | DPI | Easyhaler | 12 | Twice daily |
| LABA | Formoterol | Foradil | DPI | Aerolizer | 12 | Twice daily |
| LABA | Salmeterol | Serevent | DPI | Accuhaler | 50 | Twice daily |
| LABA | Salmeterol | Serevent | pMDI | pMDI | 50 | Twice daily |
| LABA | Indacaterol | Onbrez | DPI | Breezhaler | 150/300 | Once daily |
| LABA | Olodaterol | Striverdi | FMI | Respimat | 5 | Once daily |
pMDI, pressurized metered dose inhaler; FMI, fine mist inhaler; DPI, dry powder inhaler.
Table 2.
Adverse effects of long acting bronchodilators
| Long-acting β2-agonists | Long-acting anti-muscarinics |
|---|---|
| Tachycardia | Dry mouth |
| Palpitation | Nausea |
| Myocardial ischaemia | Constipation |
| Peripheral vasodilation | Diarrhoea |
| Fine tremor | Cough |
| Headache | Headache |
| Muscle cramps | Tachycardia |
| Prolongation of the QT interval | Acute angle glaucoma |
| Hypokalaemia | Bladder outflow obstruction |
| Feeling of nervousness | Paradoxical bronchospasm |
| Paradoxical bronchospasm | Blurred vision |
| Sleep disturbance | Dry mouth |
| Tachycardia | Nausea |
Guidelines indicate that a long acting bronchodilator as monotherapy (i.e. LAMA or LABA) should be given initially in symptomatic patients with FEV1 ≥ 50% predicted.4,5 If symptoms persist thereafter, both classes of long-acting bronchodilator may be used in either two separate devices or more conveniently in a combined LAMA/LABA inhaler device.
Long-acting muscarinic antagonists
LAMAs cause a reduction in resting bronchomotor tone, smooth muscle relaxation and prolonged bronchodilation by selectively blocking the effects of acetylcholine on post junctional M3 receptors on airway smooth muscle. However, it is now recognized that acetylcholine is also a paracrine mediator of inflammation on the epithelium and mucosal cells such that LAMAs may also confer anti-inflammatory activity as evidenced by reductions in exacarbations.9 Tiotropium is the most well-established LAMA with recent introduced additions to this class being umeclidinium, glycopyrronium and aclidinium (Table 1). All LAMAs are administered via breath actuated dry powder inhalers (DPI), while tiotropium is also available as a solution-based multi-dose Respimat fine mist solution inhaler (FMI). Data have generally indicated that the new LAMAs confer significant advantages compared with placebo, with effects comparable, on the whole, to tiotropium.10–12
In a Cochrane review of 22 studies,13 tiotropium was associated with a significant improvement in quality of life and reduction in exacerbations compared with placebo. In a further Cochrane review across seven studies,14 tiotropium was similar to LABAs in improving quality of life and lung function, although the former was more effective than LABAs in preventing exacerbations and disease-related hospital admission. The mechanism by which tiotropium reduces exacerbations is probably due to attenuating the pro-inflammatory effects of acetylcholine rather than classical effects of smooth muscle relaxation.9 Despite safety concerns relating to tiotropium delivered via the Respimat, in a randomized controlled trial comparing tiotropium delivered via DPI and FMI of 17 135 patients with COPD over ∼2 years, no differences occurred in risk of death, major adverse cardiovascular events or exacerbations.15 Moreover the lung deposition, measured as its kinetic bioavailability, has been shown to be 24% lower with tiotropium delivered by FMI compared with DPI.16
A Cochrane review evaluated outcomes using the twice daily LAMA aclidinium across 12 studies involving ∼10 000 patients.11 Aclidinium was associated with improved quality of life and reduced hospital admission due to severe exacerbations vs. placebo, although it failed to reduce mortality, serious adverse events or exacerbations requiring oral steroids or antibiotics. In the same study, firm conclusions regarding any benefits of aclidinium vs. either tiotropium or LABAs were not able to be made due to insufficient data. However, in a randomized placebo controlled study of over 400 individuals with mean FEV1 of 56% predicted, aclidinium was as effective as tiotropium over 6 weeks in terms of 24 h bronchodilation, although only the former resulted in a greater (P ≤ 0.05) improvement in symptoms vs. placebo.12 In an early study, the once daily LAMA umeclidinium significantly improved lung function vs. placebo, with effects comparable to tiotropium.10 In the GLISTEN trial, 17 773 patients were randomized to receive the once daily LAMA glycopyrronium, tiotropium or placebo as add-on therapy to a combination of salmeterol (a LABA) and fluticasone propionate (an ICS) for 12 weeks. Add on treatment with glycopyrronium resulted in comparable improvements in lung function to add on tiotropium, whereas the former demonstrated significant improvements in lung function, health status and rescue medication compared with the LABA/ICS combination.
Long-acting β2-agonists
LABAs act directly upon β2-adrenoceptors causing smooth muscle to relax and airways to dilate. The two most widely used LABAs—formoterol and salmeterol—are given on a twice daily basis. A Cochrane review of 26 trials evaluated their effects after treatment of at least 3 months,18 and demonstrated they were associated with improved quality of life and fewer exacerbations compared with placebo, but failed to reduce mortality. In contrast to SABAs, both salmeterol and formoterol are relatively lipophilic (fat soluble) and have prolonged receptor occupancy. Factors such as these—as well as exo-receptor binding with salmeterol - may in part explain their prolonged duration of action. Formoterol is a more potent agonist than salmeterol in terms of smooth muscle relaxation, and has a more rapid onset of action; this property may have beneficial effects in patients perceiving more immediate benefit due to relatively quick bronchodilation.19
New ultra-LABAs have been developed for use on a once daily basis as monotherapy; examples include indacaterol and olodaterol. Another once daily LABA—vilanterol—is currently only available in combination with the LAMA umeclidinium or with the ICS, fluticasone furoate. In the largest meta-analysis of once daily LABAs across 13 studies,20 indacaterol resulted in statistically significant and clinically meaningful improvements in lung function and quality of life compared with placebo. In the same study, lung function improvements were comparable to that achieved with more established twice daily LABAs. In a further multicentre non-inferiority study,21 3444 patients with severe COPD were randomized to receive indacaterol or tiotropium for 1 year. Both treatments conferred clinically relevant improvements in lung function at 12 weeks with indacaterol being non-inferior to tiotropium, while patients receiving tiotropium experienced fewer exacerbations.
Long acting bronchodilator combination inhalers
As well as being given as the individual drug, some long acting bronchodilators have been introduced to deliver both classes of drug (i.e. LAMA and LABA) in a single combination inhaler device, with four such distinct types being available for use in the UK (Table 3). Many studies have demonstrated superiority of the combination product compared with each respective monocomponent in terms of FEV1 and symptoms, and in some cases exacerbations and quality of life.22–26 However, no data demonstrates greater all round efficacy between different combined LAMA/LABA formulations. Inevitably the choice of LABA/LAMA inhaler device will be influenced by considerations including cost and patient preference. Combining drugs in a single inhaler device is an increasingly popular method of delivering different classes of drugs to the lungs; doing so not only minimizes the number of inhaler device patients need to use, but may well enhance adherence to treatment, reduce exacerbations and overall costs.27–29
Table 3.
Characteristics of long acting bronchodilator combination inhalers
| Class | Drug | Brand name | Delivery device type | Delivery device name | Dose (µg) | Frequency |
|---|---|---|---|---|---|---|
| LAMA/LABA | Aclidinium/Formoterol | Duaklir | DPI | Genuair | 340/12 | Twice daily |
| LAMA/LABA | Umeclidium/Vilanterol | Anoro | DPI | Ellipta | 55/22 | Once daily |
| LAMA/LABA | Glycopyrronium/Indacaterol | Ultibro | DPI | Breezhaler | 43/85 | Once daily |
| LAMA/LABA | Tiotropium/Olodaterol | Spiolto | FMI | Respimat | 5/5 | Once daily |
LABA, long acting β2-agonist; LAMA, long acting muscarinic antagonist; DPI, dry powder inhaler; FMI, fine mist inhaler.
In a systematic review of 11 trials involving approximately 10 000 patients lasting >4 weeks,24 the effects of a combination of umeclidinium plus vilanterol were explored; comparisons were against its monocomponents, tiotropium or fluticasone plus salmeterol. The LAMA/LABA combination was superior (P < 0.05) against all comparators in terms of lung function, plus associated with a greater likelihood of demonstrating a minimal clinically important difference on the transition dyspnoea index and reducing exacerbations vs. its monocomponents. In the same study, no significant differences between the LAMA/LABA combination were observed vs. tiotropium in symptoms or risk of COPD exacerbation, with a comparable safety profile between all treatments. In a further study,23 a combination of indacaterol and glycopyrronium was compared with a combination of tiotropium plus formoterol. The former treatment was deemed to be non-inferior to the latter in terms of improvements in quality of life, although it conferred a small benefit (P < 0.001) in lung function. Bateman et al.30 combined outcomes of two studies involving more than 3000 patients receiving aclidinium plus formoterol vs. the individual constituents and placebo. Over 24 weeks, improvements (P < 0.05) in symptoms with the combination inhaler vs. the LABA and LAMA alone and placebo occurred, with moderate or severe exacerbations significantly reduced with the LAMA/LABA vs. placebo (but not monotherapies).
Combined ICS plus long-acting β2-agonist inhalers
Considerable debate has taken place over the years as to the exact clinical effects of ICS in COPD, whether they should be prescribed, and if so, who should receive them, at what dose, whether they are associated with an increased risk of pneumonia and other serious adverse effects, and if any overall benefits outweigh drawbacks associated with chronic use. Given these contentions, multiple large multicentre studies and meta-analysis have attempted to address uncertainties such as these surrounding their use. When used for COPD, ICS are only licensed for use when combined with a LABA in a single inhaler device.
Guidelines and licensing authorities generally indicate that co-administration of ICS should be considered in patients with FEV1 < 60% predicted (in a combination inhaler with a LABA) who experience frequent (≥2 per year) exacerbations.4,5 However, the dose of ICS required to achieve maximal beneficial effect with minimal adverse effect (optimum therapeutic ratio) is not firmly established, and partly as a consequence of this, different combined ICS/LABA inhaler devices contain ICS of different potency and dose.
Four combined ICS/LABA inhalers are available for use in the UK (Table 4), with the combinations of fluticasone furoate plus vilanterol and beclomethasone plus formoterol being the most recent additions to the well-established combinations of budesonide plus formoterol and fluticasone propionate plus salmeterol. All are multidose DPI devices with the exception of the solution extra fine particle beclomethasone/formoterol formulation. Most studies evaluating LABAs and ICS have shown superiority of the combination product over its monocomponent alone. For example, in the largest study evaluating this combination of drugs (TORCH),31 fluticasone plus salmeterol in combination was better than either drug as monotherapy in terms of survival, FEV1, exacerbation frequency and quality of life over a 3-year period. In the same study, the primary end-point of all cause mortality with the combination product was not achieved. In studies evaluating the combination of budesonide with formoterol, the proportion of reductions in exacerbations (24%) were of a similar magnitude to the TORCH study with the combination product vs. placebo.32,33 In a further study (INSPIRE) involving 1323 patients with FEV1 39% predicted,34 salmeterol/fluticasone propionate vs. tiotropium was evaluated. The annual exacerbation rate was similar between randomized treatments: 1.28 vs. 1.32, P = 0.656 for salmeterol/fluticasone and tiotropium, respectively. The quality of life score was lower (P = 0.04) after 2 years with salmeterol/fluticasone vs. tiotropium, although this failed to reach a clinically relevant difference. Although overall numbers were small, mortality was lower (P = 0.03) with salmeterol/fluticasone (21 died) vs. tiotropium (38 died), although the former was associated with a high incidence of pneumonia (P = 0.008 for the difference). In > 3000 patients with COPD,35 the addition of fluticasone furoate to vilanterol resulted in a reduction in moderate and severe exacerbations compared with the vilanterol only group, but was linked to an increased risk of pneumonia.
Table 4.
Characteristics of inhaled corticosteroid and long acting beta-agonist combination inhalers
| Class | Drug | Brand name | Delivery device | Delivery device name | Dose (µg) | Frequency |
|---|---|---|---|---|---|---|
| ICS/LABA | Fluticasone Propionate/Salmeterol | Seretide | DPI | Accuhaler | 500/50 | Twice daily |
| ICS/LABA | Budesonide/Formoterol | Symbicort | DPI | Turbohaler | 400/12 | Twice daily |
| ICS/LABA | Budesonide/Formoterol | DuoResp Spiromax | DPI | 400/12 | Twice daily | |
| ICS/LABA | Fluticasone Furoate/Vilanterol | Relvar | DPI | Ellipta | 92/22 | Once daily |
| ICS/LABA | Beclometasone/Formoterol | Fostair | pMDI | pMDI | 200/12 | Twice daily |
pMDI, pressurized metered dose inhaler; ICS, inhaled corticosteroid; LABA, long acting beta-agonist; DPI, dry powder inhaler.
In a Cochrane review8 of 19 studies of varying length (mean 42 weeks) different combined LABA/ICS devices were assessed. Compared with placebo, the combination product resulted in fewer exacerbations, and an overall reduction in mortality was observed, although this finding was skewed by data from the largest study (TORCH).31 Similarly, combined ICS/LABA resulted in significant improvement in health status and lung function, while in the same study, an increase in the risk of pneumonia was observed with combined inhalers vs. placebo. The SUMMIT trial 36 reported on 16 485 patients with COPD who also had an increased risk of cardiovascular disease and compared fluticasone furoate with vilanterol vs. each monocomponent or placebo (all delivered by the Ellipta DPI device over 15–44 months). Preliminary data37 demonstrated that the primary end point of risk of death with fluticasone furoate with vilanterol was 12% lower compared with placebo but non-significant (P = 0.14). Moreover, the secondary end point of composite cardiovascular events was 7.4% lower with the combination product vs. placebo, although this was also not significant (P = 0.48).
Adverse effects of ICS
ICS cause both local and systemic adverse effects. Common local adverse sequelae include oropharyngeal candidiasis and dysphonia.38 Previous studies have shown that skin bruising occurs more commonly in patients using ICS, while variable dose-related effects have been observed in terms of reduction of bone mineral density (osteoporosis) and cortisol (adrenal insufficiency).38 In the TORCH study31 and in another study35 evaluating fluticasone furoate, an increased risk of pneumonia was observed in patients receiving ICS alone and when used in conjunction with a LABA. Other data have shown the propensity for pneumonia to be greater with fluticasone propionate compared with budesonide.39–41 In a further study, it was suggested that the association with pneumonia and ICS s may not be a class effect, as across 7 trials (including 3801 patients), no increased incidence of pneumonia was observed with budesonide.42 Possible explanations behind the observed association between fluticasone and pneumonia have been postulated such as its higher lipophilicity resulting in more prolonged local immune suppression in the lung, altering the lung microbiome in patients with COPD who have impaired mucociliary clearance.43 In a Cochrane review across 43 studies,44 it was concluded that budesonide and fluticasone (delivered alone or in combination with a LABA) were associated with increased risk of pneumonia, but neither significantly affected mortality vs. controls. Despite the apparent ‘signal’ in terms of pneumonia and ICS in COPD, it is important to note that no large well conducted clinical trial has been specifically designed to explore the presence or absence of this association. In some of the studies highlighted above, ‘pneumonia’ did not have to be confirmed radiologically, and it is pertinent to emphasize that increased pneumonia events have not been directly linked to an increase in mortality.45
Triple therapy
In advanced symptomatic COPD, many patients are prescribed triple therapy with a combination of a LAMA, LABA and ICS. Currently, no inhaled device contains all three drug classes, and patients require to typically use a monocomponent LAMA inhaler plus combined ICS/LABA device. However, combination triple inhaler devices are currently in clinical development; examples include combinations of fluticasone/vilanterol/umeclidinium and beclomethasone/formoterol/glycopyrronium.
In studies by Tashkin46 and Welte,47 the addition of tiotropium to fluticasone plus salmeterol resulted in a reduction in exacerbations in one study, but not the other. The GLISTEN study demonstrated significant improvements in FEV1 and quality of life plus reduction in rescue medication use when either tiotropium or glycopyrronium were added to fluticasone/salmeterol over 3 months.17 In a retrospective cohort study,48 2853 patients with moderate-to-severe COPD were followed up for between 4 and 5 years of whom 996 were receiving ICS/LABA and 1857 receiving ICS/LABA/LAMA. Comparing outcomes using triple vs. dual therapy, there was a 15% reduction for hospital admissions, 29% reduction for oral corticosteroid bursts and 26% reduction for all-cause mortality. A further study (analysing overall outcomes of two separate studies across a total of > 1000 patients) involving the combination of umeclidinium plus fluticasone furoate/vilanterol,49 also supports the use of triple therapy in improving lung function with some improvement in symptom reduction. Whether adding LAMA to ICS/LABA in a triple inhaler enables the dose of ICS to be lowered—hence mitigating any possible pneumonia risk—remains to be seen.
Practical considerations
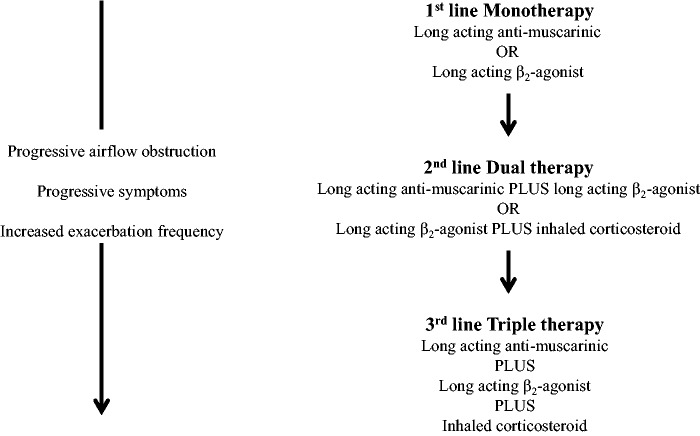
Reduced to the most simplest of forms, regular inhaled treatment in the management of patients with COPD has three basic pharmacological steps based around parameters influencing disease severity (Figure 1). While this appears at first glance to represent an easy, practical and pragmatic guide by which to escalate treatment, in ‘real-life’ this is unfortunately not always the case. Difficulties and complications arise since:
Monocomponent corticosteroids inhalers are not licensed for use in COPD.
No single inhaler device is designed to deliver all different classes of drug to facilitate prevention and relief of acute symptoms across all severities of disease.
Escalating treatment—to include a different class of drug—may involve patients switching inhaler device.
Patients will invariably need to correctly recall how (and when) to use different inhaler devices and dosing regimens (i.e. once or twice daily administration).
Many primary and secondary care prescribing clinicians and nurses are unlikely to have in-depth knowledge of the array of new inhaled drugs and devices currently available.
Different inhaler devices are of a single dose, and others of a multi-dose, type.
Figure 1.
Simplified step-wise management of inhaled treatment for COPD showing first, second and third line treatment options.
Given all of this, which inhaler device is prescribed should usually be based upon which class (or classes) of drug individuals are likely to benefit from, patient preference and ability to use specific inhaler types, cost and availability on local formularies. It is also intuitive that if a patient requires switched from LAMA alone to LAMA/LABA combination, the same device should be used; similarly—wherever possible—when an ICS is required, a combination inhaler device containing ICS plus LABA in addition to separate LAMA (using the same device) is firstly considered.
It is therefore reasonable to consider that a pragmatic starting point is therefore for local health boards and formulary groups to perhaps contain a series of preferred options in terms of drug and delivery devices for patients requiring first (i.e. LAMA), second (i.e. LAMA plus LABA combination) and third (LAMA alone plus LABA/ICS) line treatment. This is most likely to be based around factors such as minimizing different types of inhaler device, cost, simple dosing regimens (i.e. all treatment either once or twice daily) and general ease of use.
Since airflow obstruction is the universal feature of clinically significant COPD, bronchodilators play an integral role in all stages of disease, while ICS should be reserved for patients with more advanced airflow obstruction who experience frequent exacerbations. A multitude of new drugs and inhaler devices have been recently introduced to be used as both monotherapy and combined with different classes. In addition to tiotropium, new LAMAs include umcledinium, aclidinium and glycopyrronium; as well as being formulated individually, all of these LAMAs have been formulated to be delivered in a single combination inhaler also containing a LABA. In addition to the well-established LABA/ICS inhalers containing formoterol/budesonide and salmeterol/fluticasone, newer combination inhalers containing fluticasone furoate plus vilanterol and beclomethasone/formoterol have more recently been introduced.
Guidelines suggest that regular inhaled drugs (alone or in combination) should be given depending on a composite risk assessment based on airflow obstruction (FEV1% predicted), exacerbation frequency and symptoms. If FEV1 is ≥50% predicted, options include a LAMA or LABA alone, or preferably in a combination LAMA/LABA single inhaler if symptoms and exacerbations persist. If FEV1 is <50% predicted - and if symptoms and exacerbations persist - options include dual therapy with a LAMA/LABA combination inhaler, ICS/LABA combination inhaler or triple therapy with ICS/LABA combination plus LAMA (as two separate inhalers). In such circumstances, patients should be made aware of the potential risk of developing side effects including pneumonia and osteoporosis with high ICS doses. Given the introduction of new inhaler devices and drugs, and potential complexities which may arise due to the large number of choices and configurations possible, health boards and formulary groups should devise and disseminate a series of preferred options to help primary and secondary health care professionals initiate and escalate treatment in a coherent and pragmatic manner. Failure to do so, will inevitably create a confusing, difficult and haphazard transition into this new unchartered era of COPD management.
Acknowledgments
Conflicts of interest: B.J.L. has received personal payments for consulting, being on advisory boards or speaker bureau from Boehringer Ingelheim, Cheisi, Teva, Dr Reddys, Cipla, Meda and Sandoz. He has also received financial support to attend national and international meetings from Boerhinger Ingelheim, Cheisi and Teva. The Scottish Centre for Respiratory Research has received unrestricted grant support from Cheisi, Teva and Almirral and also multi-centre grant support from Astra Zeneca, Janssen, Roche and Pearl. G.P.C. has received honoraria from AZ, Chiesi, Pfizer and GlaxoSmithKline (GSK) for giving postgraduate educational talks.
References
- 1.Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA 1995; 274:1852–7. [PubMed] [Google Scholar]
- 2.Piquet J, Chavaillon JM, David P, Martin F, Blanchon F, Roche N. High-risk patients following hospitalisation for an acute exacerbation of COPD. Eur Respir J 2013; 42:946–55. [DOI] [PubMed] [Google Scholar]
- 3.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161:1608–13. [DOI] [PubMed] [Google Scholar]
- 4.NICE Guideline Chronic Obstructive Pulmonary Disease CG101: Chronic Obstructive Pulmonary Disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.
- 5.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–65. [DOI] [PubMed] [Google Scholar]
- 6.Sestini P, Renzoni E, Robinson S, Poole P, Ram FS. Short-acting beta 2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; CD001495. [DOI] [PubMed] [Google Scholar]
- 7.Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, et al. Ipratropium bromide versus short acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; CD001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev 2014; 3:CD010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kistemaker LE, Oenema TA, Meurs H, Gosens R. Regulation of airway inflammation and remodeling by muscarinic receptors: perspectives on anticholinergic therapy in asthma and COPD. Life Sci 2012; 91:1126–33. [DOI] [PubMed] [Google Scholar]
- 10.Donohue JF, Anzueto A, Brooks J, Mehta R, Kalberg C, Crater G. A randomized, double-blind dose-ranging study of the novel LAMA GSK573719 in patients with COPD. Respir Med 2012; 106:970–9. [DOI] [PubMed] [Google Scholar]
- 11.Ni H, Soe Z, Moe S. Aclidinium bromide for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 9:CD010509.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beier J, Kirsten AM, Mroz R, Segarra R, Chuecos F, Caracta C, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled Phase IIIb study. COPD 2013; 10:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 7:CD009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 9:CD009157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013; 369:1491–501. [DOI] [PubMed] [Google Scholar]
- 16.Hohlfeld JM, Sharma A, van Noord JA, Cornelissen PJ, Derom E, Towse L, et al. Pharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary disease. J Clin Pharmacol 2014; 54:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frith PA, Thompson PJ, Ratnavadivel R, Chang CL, Bremner P, Day P, et al. Glycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trial. Thorax 2015; 70:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kew KM, Mavergames C, Walters JA. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 10:CD010177.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partridge MR, Schuermann W, Beckman O, Persson T, Polanowski T. Effect on lung function and morning activities of budesonide/formoterol versus salmeterol/fluticasone in patients with COPD. Ther Adv Respir Dis 2009; 3:1–11. [DOI] [PubMed] [Google Scholar]
- 20.Geake JB, Dabscheck EJ, Wood-Baker R, Cates CJ. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta(2)-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 1:CD010139.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 2013; 1:524–33. [DOI] [PubMed] [Google Scholar]
- 22.Moitra S, Bhome AB, Brashier BB. Aclidinium bromide/formoterol fixed-dose combination therapy for COPD: the evidence to date. Drug Des Devel Ther 2015; 9:1989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buhl R, Gessner C, Schuermann W, Foerster K, Sieder C, Hiltl S, et al. Efficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to-severe COPD (QUANTIFY): a randomised, non-inferiority study. Thorax 2015; 70:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigo GJ, Neffen H. A Systematic Review of the Efficacy and Safety of a Fixed-Dose Combination of Umeclidinium and Vilanterol for the Treatment of COPD. Chest 2015; 148:397–407. [DOI] [PubMed] [Google Scholar]
- 25.Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med 2013; 1:199–209. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigo GJ, Plaza V. Efficacy and safety of a fixed-dose combination of indacaterol and Glycopyrronium for the treatment of COPD: a systematic review. Chest 2014; 146:309–17. [DOI] [PubMed] [Google Scholar]
- 27.Toy EL, Beaulieu NU, McHale JM, Welland TR, Plauschinat CA, Swensen A, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med 2011; 105:435–41. [DOI] [PubMed] [Google Scholar]
- 28.Tashkin DP. Multiple dose regimens. Impact on compliance. Chest 1995; 107:176S–82S. [DOI] [PubMed] [Google Scholar]
- 29.Yu AP, Guerin A, de Leon DP, Ramakrishnan K, Wu EQ, Mocarski M, et al. Clinical and economic outcomes of multiple versus single long-acting inhalers in COPD. Respir Med 2011; 105:1861–71. [DOI] [PubMed] [Google Scholar]
- 30.Bateman ED, Chapman KR, Singh D, D'Urzo AD, Molins E, Leselbaum A, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res 2015; 16:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–89. [DOI] [PubMed] [Google Scholar]
- 32.Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003; 21:74–81. [DOI] [PubMed] [Google Scholar]
- 33.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J 2003; 22:912–9. [DOI] [PubMed] [Google Scholar]
- 34.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008; 177:19–26. [DOI] [PubMed] [Google Scholar]
- 35.Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med 2013; 1:210–23. [DOI] [PubMed] [Google Scholar]
- 36.Vestbo J, Anderson J, Brook RD, Calverley PM, Celli BR, Crim C, et al. The Study to Understand Mortality and Morbidity in COPD (SUMMIT) study protocol. Eur Respir J 2013; 41:1017–22. [DOI] [PubMed] [Google Scholar]
- 37.J V. GSK and Theravance announce results from the SUMMIT COPD CV Survival Stud. https://wwwgskcom/en-gb/media/press-releases/2015/gsk-and-theravance-announce-results-from-the-summit-copd-cv-survival-study/. 2015.
- 38.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 7:CD002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013; 68:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janson C, Larsson K, Lisspers KH, Stallberg B, Stratelis G, Goike H, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS). BMJ 2013; 346:f3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halpin DM, Gray J, Edwards SJ, Morais J, Singh D. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract 2011; 65:764–74. [DOI] [PubMed] [Google Scholar]
- 42.Sin DD, Tashkin D, Zhang X, Radner F, Sjobring U, Thoren A, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet 2009; 374:712–9. [DOI] [PubMed] [Google Scholar]
- 43.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185:1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 3:CD010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzoratti L, Iannella HA, Waterer GW. Inhaled corticosteroids and the increased risk of pneumonia. Ther Adv Respir Dis 2013; 7:225–34. [DOI] [PubMed] [Google Scholar]
- 46.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–54. [DOI] [PubMed] [Google Scholar]
- 47.Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180:741–50. [DOI] [PubMed] [Google Scholar]
- 48.Short PM, Williamson PA, Elder DH, Lipworth SI, Schembri S, Lipworth BJ. The impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting beta-agonist therapy in COPD. Chest 2012; 141:81–6. [DOI] [PubMed] [Google Scholar]
- 49.Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: Results of two randomized studies. Respir Med 2015; 109:1155–63. [DOI] [PubMed] [Google Scholar]