Abstract
Resonant and acoustic wave devices have been researched for several decades for application in the gravimetric sensing of a variety of biological and chemical analytes. These devices operate by coupling the measurand (e.g. analyte adsorption) as a modulation in the physical properties of the acoustic wave (e.g. resonant frequency, acoustic velocity, dissipation) that can then be correlated with the amount of adsorbed analyte. These devices can also be miniaturized with advantages in terms of cost, size and scalability, as well as potential additional features including integration with microfluidics and electronics, scaled sensitivities associated with smaller dimensions and higher operational frequencies, the ability to multiplex detection across arrays of hundreds of devices embedded in a single chip, increased throughput and the ability to interrogate a wider range of modes including within the same device. Additionally, device fabrication is often compatible with semiconductor volume batch manufacturing techniques enabling cost scalability and a high degree of precision and reproducibility in the manufacturing process. Integration with microfluidics handling also enables suitable sample pre-processing/separation/purification/amplification steps that could improve selectivity and the overall signal-to-noise ratio. Three device types are reviewed here: (i) bulk acoustic wave sensors, (ii) surface acoustic wave sensors, and (iii) micro/nano-electromechanical system (MEMS/NEMS) sensors.
Keywords: acoustic biosensors, bulk acoustic waves, microelectromechanical system (MEMS), piezoelectricity, quartz crystal microbalance, surface acoustic waves
Introduction
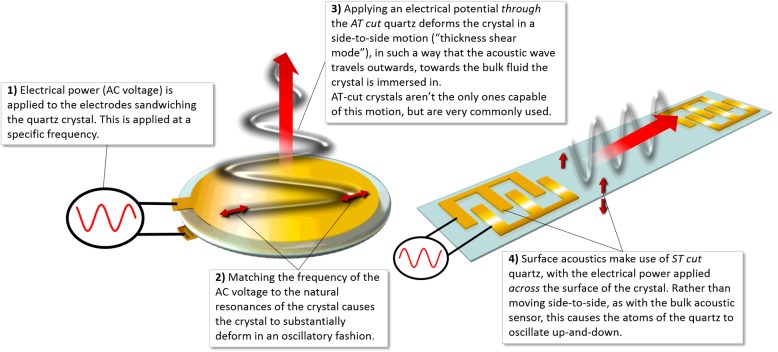
Acoustic wave sensors operate by monitoring the change in the physical properties of an acoustic wave in response to the measurand. Piezoelectric materials (crystalline solids lacking a centre of inversion symmetry and representing strong coupling between mechanical strain and electrical polarization) are commonly employed in acoustic sensors to generate acoustic waves in solid materials using appropriately tailored electric fields and to detect the acoustic waves by the charge generated due to the induced mechanical deformation. Quartz is commonly used as it is abundant, amenable to low-cost volume manufacturing and is associated with excellent mechanical properties and good chemical stability. Depending on the cut of the quartz crystal, different properties can be realized. There are two traditional ‘cuts’ for the quartz crystal used in acoustic sensors: the AT cut and the ST cut. These differences cause different piezoelectric deformations, which allow acoustic waves to efficiently propagate in different directions: either along the surface of the sensor, or away from the sensor surface towards the bulk substrate (Figure 1). This distinction is quite important and is the basis of the classification of acoustic sensors into either surface acoustic wave (SAW) or bulk acoustic wave (BAW) sensors.
Figure 1. Graphic depicting in general terms the processes for the generation of surface and bulk acoustic waves.
Capable of detecting multiple physical parameters (mass, pressure, temperature, etc.), acoustic wave devices are inherently sensitive. Here, we discuss the operating principles of BAW, SAW and microelectromechanical systems (MEMS)/nanoelectromechanical system (NEMS) sensors and share examples of how they are being used to develop solutions for biosensing.
Bulk acoustic wave sensors
QCM technologies
The well-established quartz crystal microbalance (QCM) is a BAW sensor comprising an AT-cut quartz crystal sandwiched between two circular electrodes, such as gold. The piezoelectric nature of the quartz means that the application of an alternating current (AC) voltage between the electrodes causes the crystal to deform in an oscillatory manner, preferentially operated in the fundamental thickness shear mode resonance or associated resonance overtones–optimal amplitudes of displacement of the motion being reached at certain acoustic frequencies (resonant frequencies), which can be reached by altering the voltage input frequencies. This resonant frequency of oscillation of the crystal is sensitive to added mass at the crystal surface as described by Sauerbrey [1], allowing for quantitative measurements of changes at the crystal surface:
| 1 |
Where: Δf is the change in resonance frequency due to the change in sensor mass (typically a few hertz to a few hundred hertz) fr0 is the resonant frequency of the crystal, A is the active surface area, pq is the density of the quartz, and Gq is the shear modulus of the quartz [1] (represented in its current form by Vogt et al. [2]).
Since the resonating frequencies (fr0) are very large (≫1 × 106 Hz), and the change in the mass of the quartz crystal (Δmq in eqn. 1) scales with the square of this, small changes in the mass of a quartz crystal result in significant changes in the resonance frequency.
Sauerbrey's early investigations centred on the resonating properties of very thin and rigid films attached to sensors operating in vacuums [1], and the equations uniting changes in sensor mass to changes in resonance frequencies translated very well to sensors operating in air. Further refinements, by Kanazawa and Gordon [3], described the frequency shift caused by the operation of quartz sensors with the sensing surface exposed to liquid, which opened the way for biosensing to take place on this platform. However, biomolecules are viscoelastic: parts of them tend to resist distortion when a force is applied to them (viscosity) and parts of them tend to deform when a force is applied to them (elasticity) [4,5]. When attaching a film of such molecules to the sensor surface, for example when constructing a biosensor, the motion of the outer areas of the film will not couple to the quartz crystal's oscillating motion and some of the oscillation energy is lost as the molecules themselves dissipate the energy as kinetic motion [5]. This ‘looseness’ of the film results in further dampening of the frequency of oscillation rather than that resulting only from the bound mass, overestimating the bound mass determinable by the Sauerbrey equation [2,6].
QCM-D
Accounting for the viscoelasticity common to biological molecules was important for the development of accurate biosensing utilizing QCM principles. The introduction of the measurement of an additional factor during the oscillation of the crystal, dissipation [3,4,6,7], paved the way for QCM with a dissipation measurement: QCM-D.
The introduction of QCM-D has enabled monitoring of the damping, or dissipation, factor which gives information on the structure of the film adhered to the sensor. The dissipation factor of a quartz oscillator is easily measured by shutting off the electrical power to an oscillating crystal and recording the rate at which oscillation decays. Similarly, dissipative information can also be obtained passively using vector network analysers to monitor the frequency-dependent impedance of the crystal and deriving the dissipation from the position and width (bandwidth) of the resonant frequency peaks [8].
Quantification of dissipative losses can now be measured together with changes in resonant frequencies at a variety of overtones. Combined, this information can then be used to accurately model viscoelastic representations of the film and correctly estimate the viscoelastic mass of the film [2,8,9].
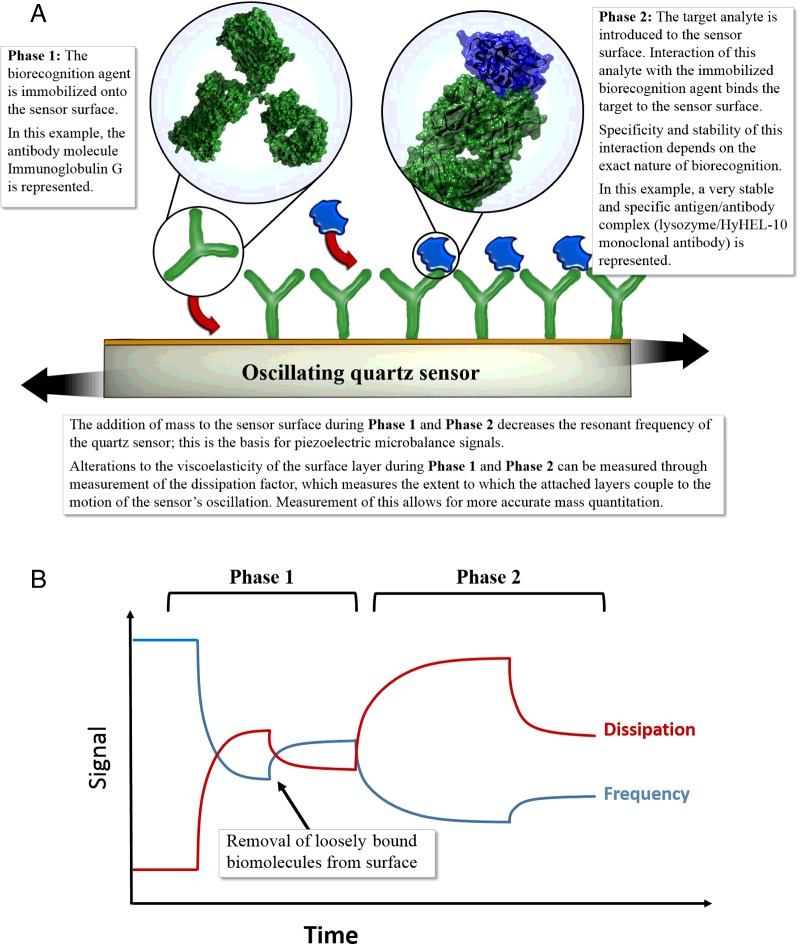
The viscoelastic mass-frequency relationship has allowed the development of quantitative and qualitative biosensors. Figure 2 describes a typical scenario. The resonant frequency of a freely oscillating crystal decreases when mass in the form of an antibody (biorecognition agent) is bound to the gold electrodes in Phase 1. In Phase 2, when a solution containing the analyte molecule of interest is flowed over the electrode, a change in frequency indicates that the target analyte has bound to the antibody (and has hence been detected in the solution). The change in frequency could be used to determine the amount of mass bound to the surface of the gold electrodes.
Figure 2. (A) Schematic depiction of antibody immobilization (Phase 1) on to the surface of a quartz sensor followed by the introduction of the target analyte (Phase 2). (B) Depiction of frequency against dissipation plots may be observed in QCM-D experiments.
In Phase 1, frequency decreases due to loading of the antibody on to the surface, while increased dissipation is recorded, due to the viscoelastic nature of the antibody. After a period, loosely bound antibody is removed, resulting in a slight increase in frequency as mass is lost or as coupled water is lost. In the second phase, the target analyte is introduced, resulting in a frequency decrease and dissipation increase as it binds to the antibody immobilized during Phase 1. PDB accession codes in (A): 1IGT [10] for the antibody and 3HFM [11] for the analyte.
The nature of the QCM-D technique, however, extends its scope beyond traditional biosensor approaches as explored below.
Biosensors and biomolecular interactions
Functioning as a traditional or direct quantitative biosensor, QCM and QCM-D have been successfully used to develop sensitive and specific biosensors for a wide range of targets, e.g. cholera [12] via biorecognition with antibodies, and also via aptamers as biorecognition agents, e.g. influenza [13] and ATP [14], following the approach described in Figure 2.
QCM-D in particular holds promise as a biosensor for sensing interactions between different biological moieties [15]. At the heart of unravelling biochemical pathways is the interaction between biological molecules. Several techniques exist to monitor protein–protein interactions, or the interactions between proteins and different drugs, for example. QCM-D serves to ascertain whether such interactions do occur; moreover, the method provides information on whether the materials added to the electrodes are rigid or viscoelastic, as well as information on any structural changes that may occur during the interaction. Several examples are available in the literature exploring this application of QCM-D, from monitoring of a mussel adhesion protein to different surface functional groups [16], the biochemistry of heat-shock proteins via relevant protein–protein interactions [17] and recently its application in understanding interactions of the enzymes of the thymidylate synthesis cycle [18].
Developing biosensors
Successfully assembling a biosensor is largely reliant on the manner in which the biorecognition agent is immobilized on to the transducer surface. Maintenance of the conformation of the biorecognition agent may be used as an indicator of an immobilization system that successfully maintains the enzyme in as native a state as possible. This can be estimated from the height, state of hydration and viscoelastic properties of the immobilized biomolecule [6,19]. This application of QCM [20] and QCM-D, tracking the formation and the changing structure of functional thin films was initially founded upon fundamental studies [3,4,6], but was rapidly adopted to examine and optimize the various factors that influence immobilizing biorecognition agents and remains one of the most commonly reported functions of this technology. Here, the fundamental nature of the QCM signal provides a distinct advantage: the ability of the QCM-D to provide information as to the mass and rigidity of attached films gives valuable biochemical and engineering information when making decisions regarding immobilization, while its gravimetric nature makes this technique label-less and generalizable to nearly all biomolecules that can be attached to solid surfaces.
In the above manner, QCM-D has been successfully used to select a successful immobilization strategy to attach a monolayer of laccase enzymes on to electrode surfaces to create electrochemical thin-film biosensors [21,22]. In those studies, both the attached masses and viscoelastic properties of the sensing layers were found to be important predictors of the measurable catalytic activity of the immobilized enzymes. Similar applications of QCM-D include aiding the design of biosensor assemblies in hybrid systems combining both biological and synthetic moieties [23], improvement in the attachment of streptavidin to the surface of sensor crystals, to allow improved and specific immobilization of biotin-tagged biomolecules [24], and in modelling studies for the best loading of a biorecognition agent for prostate-specific antigen biosensors [25].
Surface acoustic wave resonators
A SAW device relies on the excitation of a particular acoustic mode where the acoustic energy is confined very near the planar surface of a solid medium [26]. The utility of such a device in biosensing relies on the fact that the energy is confined near the surface and hence is highly sensitive to surface adsorption and the mode is also excited and detected by using thin-film electrodes patterned on the surface of a piezoelectric layer. A SAW device thus typically consists of interdigitated metal electrodes patterned on a piezoelectric substrate or on a piezoelectric thin film deposited on a silicon-based substrate to excite the acoustic wave and detect the resulting response as shown in Figure 1. Surface waves are generated most efficiently when the SAW wavelength equals the spacing between the transducer fingers. These devices have the advantages of high resonant frequencies extending well up to several gigahertz, enabling high mass sensitivity. The device can be operated in either a resonator configuration (with the traditional outputs of a resonant frequency and dissipation shift similar to that discussed for QCM) or a delay line configuration, where the velocity of the SAW is modulated by the surface adsorbates. In the delay line configuration, the time taken for the acoustic wave to travel between the two sets of transducers is the measured output. In a resonator configuration, SAW devices are usually designed to include acoustic reflectors to confine the acoustic energy within the resonant cavity to minimize energy loss. Liquid damping also affects the energy dissipation, although this is usually less than the case for flexural mode resonators. Furthermore, by designing SH-SAW (shear horizontal SAW) resonators, less acoustic energy is radiated into the fluid as the particle displacements are parallel to the surface rather than normal to the surface [27]. A guiding layer such as a thin film of SiO2 is patterned between the substrate and the piezoelectric thin film in order to confine the acoustic energy to the sensing surface. Such shear horizontal waves are also known as Love waves [28,29]. Microfluidic integration of SAW devices has also been demonstrated [30].
SAW devices have been demonstrated in a variety of biosensing applications [31]. A variety of molecular recognition elements including proteins and nucleotides have been used [32]. Harding et al. [33] demonstrated one of the first immunosensing applications of a Love wave device in liquid, which exhibited a sensitivity of ∼1 ng/ml anti-sheep IgG in buffer [33]. SAW devices operated in a contactless mode where radio frequency (RF) energy was inductively coupled to the sensor were used for operation in liquid environments for IgG detection (against anti-IgG using Protein A to immobilize on the sensor surface) in a delay line configuration [34]. Gruhl and Länge [35] demonstrated the detection of penicillin G in milk using a binding inhibition assay by monitoring the frequency difference between the functionalized SAW resonator sensor and a reference device. Yatsuda et al. [36] demonstrated a 250 MHz delay-line SH-SAW biosensor on a quartz substrate with application to human serum albumin detection. Gruhl and Länge [37] also demonstrated the detection of cancer biomarkers (human epidermal growth factor receptor-2 (HER-2) and tissue inhibitor of metalloproteinases-1 (TIMP-1)) at sensitivities sufficient for prognosis and therapeutic response monitoring of breast cancer patients. Barie and Rapp [38] demonstrated the photo-immobilization of a dextran layer to the sensing surface and covalent immobilization of recognition molecules within the dextran matrix for immunosensing with the detection of mouse Ig antibodies. Deoxyribonucleic acid (DNA) detection was demonstrated by Sakong et al. [39] using complementary binding between a probe and a target on a gold-coated SH-SAW device fabricated on a LiTaO3 substrate. More recently, SH-SAW devices have been used to monitor airborne allergens in a semi-continuous manner over a period of 4 days with a detection limit of 6.1 ng/ml established for a house dust mite allergen Der f 1 [40].
Micro/nano-electromechanical systems
The use of micro- and nano-electromechanical resonators for biosensing, particularly cantilever structures, has been widely explored. Such devices are constructed using planar fabrication techniques, typically on silicon or silicon-based substrates, common in semiconductor manufacturing [41]. Following completion of device processing to enable mechanical functionality, the devices are suitably packaged to provide electrical and fluidic access. In the context of a biosensing application, the surface of the device is specifically chemically functionalized by antibodies, DNA, aptamers, etc. to provide specific functional recognition to an analyte of interest. These molecules may be attached using ink-jet printing, micro-contact printing, microfluidic interfacing or other more bespoke techniques [41]. Reference structures are usually designed to account for background/thermal effects or the effects of non-specific binding. Furthermore, as with other surface-modified transducers, the effects of non-specific binding can be reduced by suitable chemical passivation techniques [42].
Due to their dimensional scale, micro- and nano-electromechanical resonant sensors have demonstrated mass sensitivities of attograms or less [43]. MEMS devices have been configured as affinity-based biosensors (relying on the specific chemical recognition provided by surface functionalization), fingerprint sensors where analytes are interrogated against a panel of sensors with imperfect chemical specificity and as detectors in separation-based assays where a separation technique, e.g. electrophoresis is utilized to enhance specificity prior to detection [43]. Even though MEMS devices display extraordinary sensitivity, issues such as non-specific binding currently limit the accuracy of measurement [43].
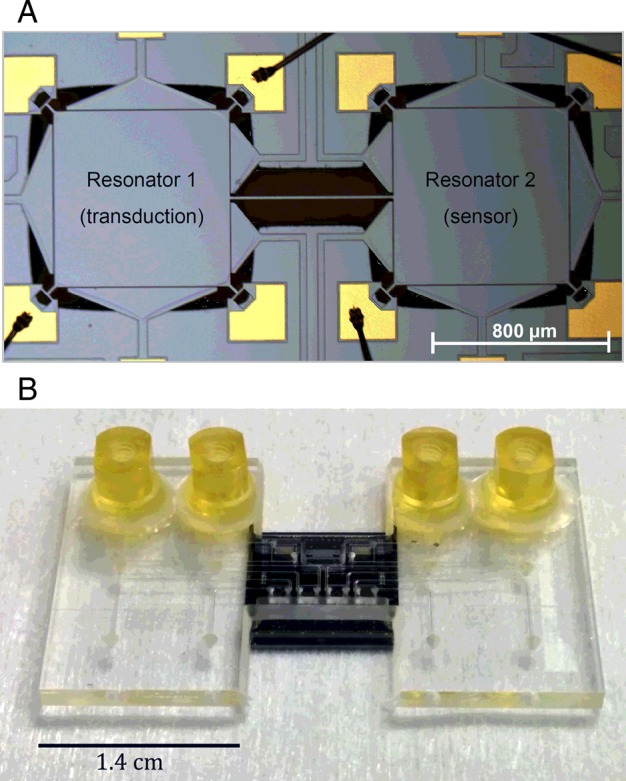
Common limitations of MEMS sensors operating in fluidic environments include fluidic damping limiting the mass resolution and the limitations associated with electrical interfacing in fluidic environments. A number of solutions have been developed to address some of these limitations including: (i) sealed-cavity resonators where the fluidic interfacing is carried out on one side of the device while the other side of the device contains the electrodes [44]; (ii) coupled resonators where fluidic isolation is achieved between a sensor resonator and the transducer resonator [45] as shown in Figure 3; (iii) fluidic channels embedded within the resonator, enabling decoupling of fluidic and electrical interconnect and interfacing to the resonators [46,47]; and (iv) filled-gap or dielectrically transduced resonators [48].
Figure 3. (A) Optical micrograph of a coupled silicon MEMS resonator platform where the spatial separation between the sensor and transducer resonators is utilized to achieve electrical isolation and fluidic interfacing to sensing layer [45]; (B) image of the above device co-integrated with a microfluidic interface.
Measurements may be conducted under atmospheric pressure or in vacuum by desiccating the devices and simply measuring the dried mass [41]. However, such approaches are often limited by surface contaminants that may limit the accuracy of the measured results [43] and do not provide continuous monitoring capability or real-time measurements. Devices may be measured in a fluidic ambient state that enables real-time monitoring; however, such devices have to be designed to deal with issues such as fluidic damping. Dissipation in liquid media can be addressed using embedded micro-channel resonators [49], localized fluid contact using microfluidics or by interrogating higher-order resonant modes such a lateral bulk acoustic modes [50] that are less affected by fluidic damping.
Resonant MEMS biosensors have been used to detect Escherichia coli microcultures in humid environments [51] and the detection and characterization of virus particles using mechanical resonators [52]. Suspended microchannel resonators have been used to weigh single bacterial cells [49] and work by Braun et al. [53] showed the detection of T5 virus particles in fluid via membrane-based protein–ligand interactions under near physiological conditions [53]. Suspended microchannel resonators have been employed to detect activated leukocyte cell adhesion molecules (ALCAMs) in undiluted serum with a detection limit of 10 ng/ml demonstrated [54]. A great deal of work is currently being carried out in this area to enable real-time measurements of physiologically relevant concentrations of biological analytes in buffer and serum.
Future directions for acoustic biosensors
QCM technologies have the potential to form an important section of the biosensor market, due in part to their low operating cost, sensor compactness, real-time data responses and the very high sensitivity of this technique [55]. The gravimetric nature of the signal will ensure that this will remain a routine method of characterizing layer–layer interactions in a label-free fashion, which lends itself very well to investigation of biorecognition immobilization, particularly for the design of other biosensors. In addition, the expanded ability of these sensors to measure and model surface-immobilized nano- and micro-structures as laterally heterogenous thin films [56] can enable real-time and data-rich measurements of complex biological systems providing physical insight into dynamic processes at the micro- and nano-scale. Applications holding promise include, amongst others, the study of protein interactions on biomaterials as a predictor of how tissues may interact with introduced materials, such as biomedical implants. As reviewed in [57], plots of ΔD against Δf aid in deriving information about the protein adlayer, state of hydration and viscoelasticity that will elucidate the biocompatibility of introduced implants. SAW sensors are a promising extension of the technology with relevance to biosensing due to the surface-sensitive nature of the acoustic mode and high operating frequencies enabling significantly scaled mass sensitivities. Further miniaturization of the acoustic wave sensors has been demonstrated using semiconductor processing techniques enabling batch manufacturing and a high degree of precision and reproducibility in the manufacturing process. Integration with microfluidic handling is also possible enabling suitable sample pre-processing/separation/purification/amplification steps that could result in further improvements in selectivity and overall signal-to-noise ratio.
Summary
Acoustic sensors use piezoelectric materials, typically quartz crystals, in order to generate acoustic waves. The direction that the acoustic wave propagates in dictates the nature of the sensor (BAW or SAW) and what type of information can be derived from the sensor.
Quartz crystal microbalances are BAW devices that are configured as sensitive mass sensors by tracking alterations in resonant frequencies of a thickness shear mode caused by changes to the sensor mass. Further improvements (monitoring dissipation via QCM-D) increase the accuracy of measuring viscoelastic films in liquids.
As biosensors, QCM-D can be used to obtain information regarding the masses and structures of attaching layers, both for biosensor purposes and for fundamental research centred on analysing biomolecule interactions.
SAW sensors operate by detecting perturbations in a mode of propagation where the acoustic energy is confined to the surface. Such modes are highly sensitive to surface perturbations and shear-horizontal SAW resonators demonstrate relatively low damping in liquid environments.
SAW sensors have been successfully demonstrated as platforms to detect airborne pathogens, for immunosensing applications in liquid environments and have been successfully integrated with microfluidic platforms and interrogated wirelessly.
Miniaturization of acoustic wave sensors is possible using semiconductor processing techniques enabling integration with microfluidic handling and multiplexed detection using sensor arrays. Free-standing mechanical structure (MEMS/NEMS) devices can be interrogated into a wide variety of resonant modes and are being actively researched for a variety of biosensing applications.
Abbreviations
- BAW
bulk acoustic wave
- MEMS
microelectromechanical system
- NEMS
nanoelectromechanical system
- QCM
quartz crystal microbalance
- QCM-D
QCM with dissipation monitoring
- SAW
surface acoustic wave
- SH-SAW
shear horizontal SAW
Funding
R.F. acknowledges the South Africa Medical Research Council (SAMRC) Career Development Award. J.L. acknowledges DST/NRF SARCHI Chair programme and the SAMRC.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Physik. 1959;155:206–222. doi: 10.1007/BF01337937. [DOI] [Google Scholar]
- 2.Vogt B.D., Lin E.K., Wu W-L., White C.C. Effect of film thickness on the validity of the Sauerbrey equation for hydrated polyelectrolyte films. J. Phys. Chem. B. 2004;108:12685–12690. doi: 10.1021/jp0481005. [DOI] [Google Scholar]
- 3.Kanazawa K.K., Gordon J.G. The oscillation frequency of a quartz resonator in contact with a liquid. Anal. Chim. Acta. 1985;175:99–105. doi: 10.1016/S0003-2670(00)82721-X. [DOI] [Google Scholar]
- 4.Höök F., Kasemo B. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: a quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 2001;73:5796–5804. doi: 10.1021/ac0106501. [DOI] [PubMed] [Google Scholar]
- 5.Reimhult E., Larsson C., Kasemo B., Höök F. Simultaneous surface plasmon resonance and quartz crystal microbalance with dissipation monitoring measurements of biomolecular adsorption events involving structural transformations and variations in coupled water. Anal. Chem. 2004;76:7211–7220. doi: 10.1021/ac0492970. [DOI] [PubMed] [Google Scholar]
- 6.Du B., Johannsmann D. Operation of the quartz crystal microbalance in liquids: derivation of the elastic compliance of a film from the ratio of bandwidth shift and frequency shift. Langmuir. 2004;20:2809–2812. doi: 10.1021/la035965l. [DOI] [PubMed] [Google Scholar]
- 7.Höök F., Rodahl M., Brzezinski P., Kasemo B. Energy dissipation kinetics for protein and antibody−antigen adsorption under shear oscillation on a quartz crystal microbalance. Langmuir. 1998;14:729–734. doi: 10.1021/la970815u. [DOI] [Google Scholar]
- 8.Johannsmann D. Viscoelastic, mechanical, and dielectric measurements on complex samples with the quartz crystal microbalance. Phys. Chem. Chem. Phys. 2008;10:4516–4534. doi: 10.1039/b803960g. [DOI] [PubMed] [Google Scholar]
- 9.Voinova M.V., Jonson M., Kaseom B. ‘Missing mass’ effect in biosensor's QCM applications. Biosens. Bioelectron. 2002;17:835–841. doi: 10.1016/S0956-5663(02)00050-7. [DOI] [PubMed] [Google Scholar]
- 10.Harris L.J., Larson S.B., Hasel K.W., McPherson A. Refined structure of an intact IgG2a monoclonal antibody. Biochemistry. 1997;36:1581–1597. doi: 10.1021/bi962514+. [DOI] [PubMed] [Google Scholar]
- 11.Padlan E.A., Silverton E.W., Sheriff S., Cohen G.H., Smith-Gill S.J., Davies D.R. Structure of an antibody–antigen complex: crystal structure of the HyHEL-10 Fab-lysozyme complex. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5938–5942. doi: 10.1073/pnas.86.15.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson C., Bramfeldt H., Wingren C., Borrebaeck C., Höök F. Gravimetric antigen detection utilizing antibody-modified lipid bilayer. Anal. Biochem. 2005;345:72–80. doi: 10.1016/j.ab.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Wang R., Zhao J., Jiang T., Kwon Y.M., Lu H., Jiao P., et al. Selection and characterization of DNA aptamers for use in detection of avian influenza virus H5N1. J. Virol. Methods. 2013;189:362–369. doi: 10.1016/j.jviromet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Özalp V.C. Acoustic quantification of ATP using a quartz crystal microbalance with dissipation. Analyst. 2011;136:5046–5050. doi: 10.1039/c1an15762k. [DOI] [PubMed] [Google Scholar]
- 15.Heller G.T., Mercer-Smith A.R., Johal M.S. Quartz microbalance technology for probing biomolecular interactions. Methods Mol. Biol. 2015;1278:153–164. doi: 10.1007/978-1-4939-2425-7. [DOI] [PubMed] [Google Scholar]
- 16.Fant C., Sott K., Elwing H., Höök F. Adsorption behavior and enzymatically or chemically induced cross-linking of a mussel adhesive protein. Biofouling. 2000;16:119–132. doi: 10.1080/08927010009378437. [DOI] [Google Scholar]
- 17.Limson J., Odunuga O.O., Green H., Höök F., Blatch G.L. The use of a quartz crystal microbalance with dissipation for the measurement of protein–protein interactions: a qualitative and quantitative analysis of the interactions between molecular chaperones. S. Afr. J. Sci. 2004;100:678–682. [Google Scholar]
- 18.Antosiewicz A., Senkara E., Cieśla J. Quartz crystal microbalance with dissipation and microscale thermophoresis as tools for investigation of protein complex formation between thymidylate synthesis cycle enzymes. Biosens. Bioelectron. 2015;64:36–42. doi: 10.1016/j.bios.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Ozeki T., Morita M., Yoshimine H., Furusaway H., Okahata Y. Hydration and energy dissipation measurements of biomolecules on a piezoelectric quartz oscillator by admittance analyses. Anal. Chem. 2007;79:79–88. doi: 10.1021/ac060873x. [DOI] [PubMed] [Google Scholar]
- 20.Davis F., Higson S.P.J. Structured thin films as functional components within biosensors. Biosens. Bioelectron. 2005;21:1–20. doi: 10.1016/j.bios.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Fogel R., Limson J. Probing fundamental film parameters of immobilized enzymes–towards enhanced biosensor performance. Part I–QCM-D mass and rheological measurements. Enzyme Microb. Technol. 2011;49:146–152. doi: 10.1016/j.enzmictec.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Fogel R., Limson J. Probing fundamental film parameters of immobilized enzymes–towards enhanced biosensor performance. Part II–electroanalytical estimation of immobilized enzyme performance. Enzyme Microb. Technol. 2011;49:153–159. doi: 10.1016/j.enzmictec.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Fogel R., Mashazi P., Nyokong T., Limson J. Critical assessment of the quartz crystal microbalance with dissipation as an analytical tool for biosensor development and fundamental studies: metallophthalocyanine–glucose oxidase biocomposite sensors. Biosens. Bioelectron. 2007;23:95–101. doi: 10.1016/j.bios.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Nilebäck E., Feuz L., Uddenberg H., Valiokas R., Svedhem S. Characterization and application of a surface modification designed for QCM-D studies of biotinylated biomolecules. Biosens. Bioelectron. 2011;28:407–413. doi: 10.1016/j.bios.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 25.Formisano N., Jolly P., Bhalla N., Cromhout M., Flanagan S.P., Fogel R., et al. Optimisation of an electrochemical impedance spectroscopy aptasensor by exploiting quartz crystal microbalance with dissipation signals. Sens. Actuators B. 2015;220:369–375. doi: 10.1016/j.snb.2015.05.049. [DOI] [Google Scholar]
- 26.Ballantine D.S., White R.M., Martin S.J., Ricco A.J., Zellers E.T., Frye G.C., et al. Acoustic Wave Sensors: Theory, Design and Physico-Chemical Applications. San Diego: Academic Press; 1997. [Google Scholar]
- 27.Lange K., Voigt A., Rapp M. Surface acoustic wave biosensors for biomolecular interaction analysis. Sensors 2003 Proc. IEEE. 2003;2:1174–1178. [Google Scholar]
- 28.Rocha-Gaso M-I., March-Iborra C., Montoya-Baides A., Arnau-Vives A. Surface generated acoustic wave biosensors for the detection of pathogens–a review. Sensors. 2009;9:5740–5769. doi: 10.3390/s90705740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gizeli E., Liley M., Lowe C. R., Vogel H. Antibody binding to a functionalized supported lipid layer: a direct acoustic immunosensor. Anal. Chem. 1997;69:4808–4813. doi: 10.1021/ac970519m. [DOI] [PubMed] [Google Scholar]
- 30.Lange K., Blaess G., Voigt A., Gotzen R., Rapp M. Integration of a surface acoustic wave biosensor in a microfluidic polymer chip. Biosens. Bioelectron. 2006;22:227–232. doi: 10.1016/j.bios.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Lange K., Rapp B.E., Rapp M. Surface acoustic wave biosensors: a review. Anal. Bioanal. Chem. 2008;391:1509–1519. doi: 10.1007/s00216-008-1911-5. [DOI] [PubMed] [Google Scholar]
- 32.Gronewald T.M.A. Surface acoustic wave sensors in the bioanalytical field: Recent trends and challenges. Anal. Chim. Acta. 2007;603:119–128. doi: 10.1016/j.aca.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 33.Harding G.L., Du J., Dencher P.R., Barnett D., Howe E. Love wave acoustic immunosensor operating in liquid. Sens. Actuators A. 1997;61:279–286. doi: 10.1016/S0924-4247(97)80275-0. [DOI] [Google Scholar]
- 34.Freudenberg J., Schelle S., Beck K., von Schickfus M., Hunklinger S. A contactless surface acoustic wave biosensor. Biosens. Bioelectron. 1999;14:423–425. doi: 10.1016/S0956-5663(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 35.Gruhl F.J., Länge K. Surface acoustic wave (SAW) biosensor for rapid and label-free detection of Penicillin G in milk. Food Anal. Methods. 2014;7:430–437. doi: 10.1007/s12161-013-9642-4. [DOI] [Google Scholar]
- 36.Yatsuda H., Kogai T., Goto M., Yoshimura N. Shear-horizontal surface acoustic wave biosensors for POCT; 2014 IEEE International Frequency Control Symposium (FCS); Taipei, Taiwan: 2014. pp. 1–4. 19–22 May 2014. [Google Scholar]
- 37.Gruhl F.J., Länge K. Surface modification of an acoustic biosensor allowing the detection of low concentrations of cancer markers. Anal. Biochem. 2012;420:188–190. doi: 10.1016/j.ab.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Barie N., Rapp M. Covalent bound sensing layers on surface acoustic wave (SAW) biosensors. Biosens. Bioelectron. 2001;16:979–987. doi: 10.1016/S0956-5663(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 39.Sakong J., Roh H., Roh Y. Surface acoustic wave DNA sensor with micro-fluidic channels. Jpn. J. Appl. Phys. 2007;46:4729–4733. doi: 10.1143/JJAP.46.4729. [DOI] [Google Scholar]
- 40.Toma K., Miki D., Kishikawa C., Yoshimura N., Miyajima K., Arakawa T., et al. Repetitive immunoassay with a surface acoustic wave device and a highly stable protein monolayer for on-site monitoring of airborne dust mite allergens. Anal. Chem. 2015;87:10470–10474. doi: 10.1021/acs.analchem.5b02594. [DOI] [PubMed] [Google Scholar]
- 41.Waggoner P.S., Craighead H.G. Micro- and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip. 2007;7:1238–1255. doi: 10.1039/b707401h. [DOI] [PubMed] [Google Scholar]
- 42.Kasemo B. Biological surface science. Surf. Sci. 2002;500:656–677. doi: 10.1016/S0039-6028(01)01809-X. [DOI] [Google Scholar]
- 43.Artlett J., Myers E.B., Roukes M. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011;6:203–215. doi: 10.1038/nnano.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White R.M. Acoustic sensors for physical, chemical and biochemical applications. Frequency Control 1998. Proceedings of the 1998 IEEE International, Pasadena, CA, pp. 1998:587–594. [Google Scholar]
- 45.Lin T-H.A., Yan J., Seshia A.A. Electrically addressed dual resonator sensing platform for biochemical detection. IEEE J. Microelectromech. Syst. 2012;21:34–43. doi: 10.1109/JMEMS.2011.2174420. [DOI] [Google Scholar]
- 46.Burg T.P., Manalis S.R. Suspended microchannel resonators for biomolecular detection. Appl. Phys. Lett. 2003;83:2698. doi: 10.1063/1.1611625. [DOI] [Google Scholar]
- 47.Agache V., Blanco-Gomez G., Baleras F., Caillat P. An embedded microchannel in a MEMS plate resonator for ultrasensitive mass sensing in liquid. Lab Chip. 2011;11:2598–2603. doi: 10.1039/c1lc20011a. [DOI] [PubMed] [Google Scholar]
- 48.Heidari A., Yoon Y-J., Park M.K., Park W-T., Tsai J.M-L. High sensitive dielectric filled Lamé mode mass sensor. Sens. Actuators A. 2012;188:82–88. doi: 10.1016/j.sna.2012.03.040. [DOI] [Google Scholar]
- 49.Burg T.P., Godin M., Knudsen S.M., Shen W., Carlson G., Foster J.S., et al. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature. 2007;446:1066–1069. doi: 10.1038/nature05741. [DOI] [PubMed] [Google Scholar]
- 50.Prasad A., Lin A.T-H., Rao V.R., Seshia A.A. Monitoring sessile droplet evaporation on a micromechanical device. Analyst. 2014;139:5538–5546. doi: 10.1039/C4AN01389A. [DOI] [PubMed] [Google Scholar]
- 51.Gfeller K.Y., Nugaeva N., Hegner M. Micromechanical oscillators as rapid biosensor for the detection of active growth of Escherichia coli. Biosens. Bioelectron. 2005;21:528–533. doi: 10.1016/j.bios.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Johnson L., Gupta A.K., Ghafoor A., Akin D., Bashir R. Characterization of vaccinia virus particles using microscale silicon cantilever resonators and atomic force microscopy. Sens. Actuators B. 2006;115:189–197. doi: 10.1016/j.snb.2005.08.047. [DOI] [Google Scholar]
- 53.Braun T., Ghatkesar M.K., Backmann N., Grange W., Boulanger P., Letellier L., et al. Quantitative time-resolved measurement of membrane protein–ligand interactions using microcantilever array sensors. Nat. Nanotechnol. 2009;4:179–185. doi: 10.1038/nnano.2008.398. [DOI] [PubMed] [Google Scholar]
- 54.von Muhlen M.G., Brault N.D., Knudsen S.M., Jiang S., Manalis S.R. Label-free biomarker sensing in undiluted serum with suspended microchannel resonators. Anal. Chem. 2010;82:1905–1910. doi: 10.1021/ac9027356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vashist S.K., Vashist P. Recent advances in quartz crystal microbalance-based sensors. J. Sens. 2011;2011:571405. doi: 10.1155/2011/571405. [DOI] [Google Scholar]
- 56.Reviakine I., Johannsmann D., Richter R.P. Hearing what you cannot see and visualizing what you hear: interpreting quartz crystal microbalance data from solvated interfaces. Anal. Chem. 2011;83:8838–8848. doi: 10.1021/ac201778h. [DOI] [PubMed] [Google Scholar]
- 57.Tagaya M. In situ QCM-D study of nano-bio interfaces with enhanced biocompatibility. Polymer J. 2015;47:599–608. doi: 10.1038/pj.2015.43. [DOI] [Google Scholar]