Abstract
Increasing occurrences of harmful algal blooms (HABs) in the ocean are a major concern for countries around the globe, and with strong links between HABs and climate change and eutrophication, the occurrences are only set to increase. Of particular concern with regard to HABs is the presence of toxin-producing algae. Six major marine biotoxin groups are associated with HABs. Ingestion of such toxins via contaminated shellfish, fish, or other potential vectors, can lead to intoxication syndromes with moderate to severe symptoms, including death in extreme cases. There are also major economic implications associated with the diverse effects of marine biotoxins and HABs. Thus, effective monitoring programmes are required to manage and mitigate their detrimental global effect. However, currently legislated detection methods are labour-intensive, expensive and relatively slow. The growing field of biosensor diagnostic devices is an exciting area that has the potential to produce robust, easy-to-use, cost-effective, rapid and accurate detection methods for marine biotoxins and HABs. This review discusses recently developed biosensor assays that target marine biotoxins and their microbial producers, both in harvested fish/shellfish samples and in the open ocean. The effective deployment of such biosensor platforms could address the pressing need for improved monitoring of HABs and marine biotoxins, and could help to reduce their global economic impact.
Keywords: marine biotoxins, shellfish poisoning, harmful algal blooms, marine monitoring, biosensors
Introduction–harmful algal blooms: a global issue
Harmful algal blooms (HABs) are global phenomena throughout the world's oceans that have led to increasing concerns in terms of human health, environmental preservation and economic challenges. These concerns stem from the increasing frequency and geographic distribution of a number of toxin-producing algal species that cause HABs in locations widespread around the globe. The associated marine biotoxins produced by certain HAB species become a major concern to consumers and fishery industries when filter-feeding shellfish, such as mussels, oysters, clams and cockles, ingest toxic algae and accumulate their biotoxins to dangerously high levels. In the case of ciguatera fish poisoning (CFP), the causative ciguatoxins of algal (dinoflagellate) origin are transferred and accumulated through multiple trophic levels ranging from small herbivores to larger predatory fish species that are generally the most toxic due to biomagnification of ciguatoxins. Bacterially produced pufferfish poisoning (PFP) toxin has been detected in a number of pufferfish species as well as various marine invertebrates (e.g. gastropods, crabs and octopus) and is considered to reflect the acquisition of both toxigenic bacteria and exogenous PFP toxin via trophic interactions [1,2]. Consumption of contaminated fish/shellfish can lead to acute food poisoning syndromes. These food poisoning syndromes vary depending on the toxin group and the exposure level. The six major marine biotoxin syndromes are summarized in Table 1.
Table 1. Summary of the six major marine biotoxin syndromes.
| Syndrome | Toxin group | Algae/bacteria producer | Cellular target | Symptoms |
|---|---|---|---|---|
| Amnesic shellfish poisoning (ASP) | Domoic acid (DA) | Pseudo-nitzschia spp., Nitzschia navis-varingica, Chondria armata | Glutamate receptors | Short-term memory loss, confusion, disorientation, vomiting, diarrhoea, death |
| Diarrhetic shellfish poisoning (DSP) | Okadaic acid (OA) | Dinophysis spp., Prorocentrum spp. | Protein phosphatases | Nausea, vomiting, diarrhoea, stomach cramps |
| Azaspiracid shellfish poisoning (AZP) | Azaspiracid (AZA) | Azadinium spp., Amphidoma languida | Nausea, vomiting, diarrhoea, stomach cramps | |
| Paralytic shellfish poisoning (PSP) | Saxitoxin (STX) | Alexandrium spp., Gymnodinium catenatum, Pyrodinium bahamense | Sodium channels | Gastrointestinal symptoms, numbness/tingling in mouth and extremities, dizziness, headache, fever, ataxia, respiratory distress, death |
| Neurotoxic shellfish poisoning (NSP) | Brevetoxin (BTX) | Karenia spp. | Sodium channels | Gastrointestinal symptoms, numbness, tingling sensation, hypotension, paralysis, seizures, coma |
| Ciguatera fish poisoning (CFP) | Ciguatoxin (CTX) | Gambierdiscus spp. | Sodium channels | Gastrointestinal symptoms, numbness/tingling in mouth and extremities, temporary blindness, bradycardia, ataxia, paralysis |
Taking into consideration the health hazards posed by marine biotoxins, the European Commission has developed regulatory limits for each toxin. However, fearing that current regulations do not sufficiently protect human health, the European Food Safety Authority (EFSA) has made recommendations to change the current European Union (EU) regulations to impose stricter limits on marine biotoxins (Table 2). These limits were developed based on the criteria that, if consuming a large portion (400 g) of shellfish, one would not exceed the acute reference dose (ARfD) for the associated toxin. For example, consumption of a 400 g portion of shellfish contaminated with the EU limit of 160 μg of okadaic acid (OA) equivalent/kg of shellfish would lead to a dietary exposure approximately three times higher than the ARfD [3].
Table 2. European Commission (EC) regulatory limits for marine biotoxins compared with the EFSA recommended guidelines.
| Toxin and equivalents | EC regulatory limit (μg of toxin eq./kg of shellfish/fish) | EFSA recommended limit (μg of toxin eq./kg of shellfish/fish) |
|---|---|---|
| Domoic acid (DA) | 20 000 | 4500 |
| Okadaic acid (OA) | 160 | 45 |
| Azaspiracid (AZA) | 160 | 30 |
| Saxitoxin (STX) | 800 | 75 |
| Brevetoxin (BTX) | 800* | NA |
| Ciguatoxin (CTX) | No EU limit in place. Products containing CTX must not be placed on the market | 0.01 |
*No EU limit in place for BTX. Value equals US Food and Drug Administration (FDA) action limit
The economic implications of HABs and marine biotoxins are associated with the closure of coastal fisheries, losses of valuable shellfish goods, job losses and a reduction in tourism and recreational activities. Few studies are available that can accurately estimate the associated economic losses. One study from Washington State, USA, estimated the annual losses from two counties due to coastal fishery closures were in the tens of millions of US dollars [4]. Another study estimated the global economic impact of marine biotoxins was approximately US$ 4 billion per year [5]. HABs have been linked with climate change [6,7] and eutrophication [8]; therefore, as HABs and, by extension, marine biotoxins are likely to occur more frequently and over a larger area due to these factors, it is imperative that sufficient monitoring programmes are in place to reduce the risk to consumer health and global economies.
In this review, the monitoring of HABs and their associated marine biotoxins will be discussed in the context of two applications: first, the methods currently used to monitor HABs will be considered (e.g. light microscopy (LM)) and compared with emerging autonomous platforms that can monitor these algae in situ and that can, in certain cases, also detect and quantify levels of algal toxins in seawater; secondly, methods for the detection of marine biotoxins in shellfish samples will be outlined, comparing the current regulatory-approved methods with emerging biosensors.
Case study–inside the most recent marine biotoxin discovery: azaspiracid
In 1995, a human food poisoning event occurred in The Netherlands following the consumption of blue mussels (Mytilus edulis) originating from Ireland. A number of people became ill, displaying symptoms characteristic of diarrhetic shellfish poisoning (DSP). However, subsequent analyses did not find any DSP toxins present. It was not for another two years that, in 1997, using nuclear magnetic resonance (NMR) and mass spectrometry (MS) analysis, the causative toxin, a novel biotoxin designated azaspiracid (AZA) was discovered. To date, over 20 AZA analogues have been identified in plankton and shellfish (reviewed in [9]). Until 2007, the plankton species Protoperidinium crassipes was deemed to be the primary producer of AZA. However, in pure culture, P. crassipes could not produce the toxin. It was subsequently discovered that P. crassipes was engaged in a predator–prey relationship with the true AZA producer, Azadinium spinosum. Tillmann et al. [10] identified the small thecate photosynthetic dinoflagellate as a new species. To date, AZA has been detected largely in Europe, but also on the western coast of North America, both coasts of South America and in East Asia.
Monitoring of HABs: from humans to robots
Monitoring of HAB species is currently heavily reliant on the use of traditional methods, such as LM. This technique is highly time-consuming and labour-intensive, and requires trained personnel. Accurate identification of certain species using LM is also very difficult, often requiring the use of electron microscopy for confirmation, and there is a demand for frequent sample acquisition. Other advanced methods of HAB monitoring make use of satellite imaging to remotely sense ocean colour that serves as a proxy for ‘in-water’ molecules, such as chlorophyll, which can be used to estimate levels of and changes in algal biomass. However, this method is limited by factors such as poor sensitivity at low cell concentrations, detection being restricted to the water surface, and interference from clouds and coloured dissolved organic matter (CDOM) in the water. Additionally, HAB detection methods such as LM and satellite imaging cannot determine toxin levels associated with a given bloom population and thus the likelihood of adverse effects, such as the contamination of fishery resources.
Molecular methods, namely quantitative polymerase chain reaction (qPCR), hold great potential to address the specificity and sensitivity limitations associated with the above techniques. Such methods involve the amplification of specific genomic deoxyribonucleic acid (DNA) sequences in the target species using gene-specific primers. These primers incorporate fluorescent probes that can be quantified and correlated with the relative abundance of the target gene. One such assay involves the amplification of genes associated with saxitoxin (STX) production in Alexandrium species and could allow the side-by-side identification and enumeration of potentially toxic algal species [11]. Note, however, that simply the presence of toxin genes does not necessarily translate into the production of toxin, only the potential to synthesize these metabolites under the appropriate environmental conditions. Such assays are amenable for point-of-need (PON) use, but currently require trained personnel to carry out the procedure. However, one can envisage such systems being automated in the near future, thereby allowing the fully autonomous monitoring of toxigenic algal species in situ (for further reading, see [12]).
Autonomous and in situ monitoring of algae and their associated marine biotoxins is a concept that is emerging rapidly [13]. Such systems, which are now becoming commercially available, allow for real-time or near-real-time monitoring of algae and their toxins at the site of an ongoing bloom or early in the process of bloom initiation and the relaying of this information to the relevant authorities. They can be deployed on stationary moorings or potentially on autonomous underwater vehicles (AUVs). One example is the Imaging Flow Cytobot (IFCB; McLane Research Laboratories, Inc., East Falmouth, MA, USA), which combines high-resolution video imagery with flow cytometry to allow the autonomous in situ classification of marine algae to species level [14]. Another example is the Environmental Sample Processor (ESP) developed by the Monterey Bay Aquarium Research Institute (MBARI) and collaborators at the National Oceanic and Atmospheric Administration (NOAA), which has been deployed in Monterey Bay, CA, USA, for the detection of both Pseudo-nitzschia species and the neurotoxin domoic acid (DA) produced by some of these organisms. The robotic ESP system, also available from McLane Research Laboratories, Inc., allows the autonomous sub-surface detection of the toxin and algae using enzyme-linked immunosorbent assay (ELISA) and DNA probe arrays, respectively [15,16]. Other ESPs have been deployed in the Gulf of Maine to monitor levels of Alexandrium species and paralytic shellfish poisoning (PSP) toxin levels. This project will determine the feasibility and the economic advantage of deploying remote, autonomous sensors for harmful algal species (see the NOAA website at http://oceanservice.noaa.gov/news/weeklynews/jun13/esp-robot.html and the NCCOS website at http://www.coastalscience.noaa.gov/projects/detail?key=137). Another technology platform that could revolutionize HAB monitoring is that of AUVs. These unmanned systems currently have the ability to autonomously provide spatial and temporal information on algal pigment distributions that can be useful for HAB monitoring, but provide no data on the species or toxin present. In order to address this shortcoming, scientists are currently developing a redesigned ESP that will fit within the payload of an AUV (C. Scholin, personal communication) and thus enable measurements of algal and toxin concentrations on this mobile platform, which is something as yet unavailable to marine scientists and coastal managers. This could allow an advanced understanding of HAB occurrences, their frequency and their causes, and may potentially allow the enhanced prediction and protection of public health as well as economic interests in the future.
Detection of marine biotoxins in shellfish
To date, the detection of marine biotoxins, as opposed to the HAB toxin-producers, has focused mainly on detection in shellfish tissue more so than in seawater. This is largely due to the regulation of toxin levels in seafood and the absence of regulation of toxins in seawater. The most widely employed method for the screening of shellfish tissue was the mouse bioassay (MBA). This method involves the intraperitoneal (IP) injection of suspect shellfish tissue extract into a mouse and the monitoring of symptoms over time until death. Aside from the obvious ethical issues, this method is also limited in terms of its poor sensitivity. It also does not provide information as to the exact toxins present in the sample. For these reasons, as of 1 January 2015, MBA has been prohibited in the EU for use in detecting the presence of marine biotoxins in shellfish material [17]. The approved reference methods for the detection of marine biotoxins in shellfish samples are now established as high-performance liquid chromatography (HPLC) (for DA, OA and STX) and liquid chromatography http://www.coastalscience.noaa.gov/projects/detail?key 137 tandem mass spectrometry (MS) (LC http://www.coastalscience.noaa.gov/projects/detail?key 137 MS/MS) (for brevetoxin (BTX) and AZA). However, although the HPLC methods have greater sensitivity than MBA at toxin concentrations reflecting the current regulatory levels, these sensitivities would need to be improved for certain methods should the regulatory limits be lowered. This issue may be addressed through use of LC http://www.coastalscience.noaa.gov/projects/detail?key 137 MS/MS, as it possesses excellent sensitivity and is also amenable for high-throughput analysis. Nonetheless, LC http://www.coastalscience.noaa.gov/projects/detail?key 137 MS/MS has not yet been validated in inter-laboratory studies for all toxin classes [18] and both of these methods are labour-intensive and require highly trained personnel and expensive equipment.
Thus, there is an open niche for an ‘easy-to-use’, rapid, inexpensive and accurate PON monitoring device for marine biotoxins in shellfish tissue. An accepted and proven approach that fits these criteria is the use of biosensors, which have been discussed extensively in this volume and other review articles (see [19]). Assays conducted on such devices incorporate a biorecognition element coupled to a chemical/physical measurement system that allow the detection of a target analyte(s). They also have excellent potential to serve as an in situ screening tool for marine biotoxins in shellfish samples and also in seawater. Many of these assays utilize the naturally high binding affinity of marine biotoxins to their cellular target, e.g. voltage-gated sodium channel receptors for PSP, BTX, ciguatoxin (CTX) and tetrodotoxin (TTX), glutamate receptor for DA or protein phosphatase for OA (see Table 1 and Figure 1). Receptor-based assays (RBAs), although currently restricted to the laboratory bench, have excellent sensitivity and working range for their respective toxins, provide an estimate of integrated toxic potency for a sample and have a very rapid turnaround time (TAT). However, RBAs may have limited applicability for PON testing as their biorecognition elements have a limited stability at temperatures greater than 4°C [20–25].
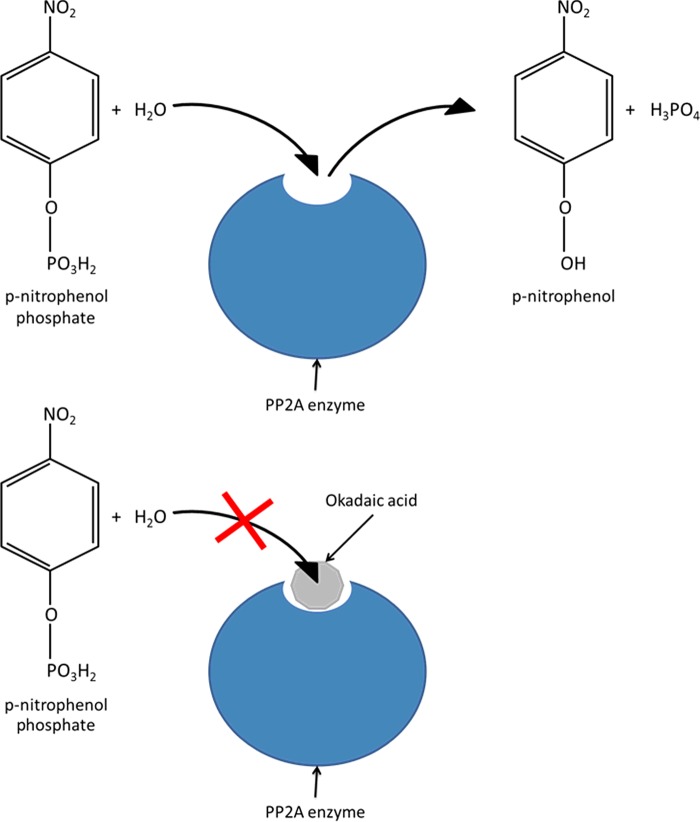
Figure 1. Illustration of protein phosphatase 2A (PP2A) inhibition assay concept.
PP2A catalyses the conversion of p-nitrophenyl phosphate (p-NPP) into p-nitrophenol (p-NP). The coloured product can be measured spectrophotometrically. DSP group toxins, such as OA, block the binding site of the PP2A enzyme, preventing the catalysis of p-NPP.
Many biosensor assays for small haptenic molecules such as marine biotoxins use antibodies as their biorecognition elements, and are based on a competitive immunoassay format, utilizing the highly specific interaction between an antibody and its target antigen (Figure 2). Plate-based ELISA is amenable for high-throughput analysis and has excellent performance characteristics. However, this assay is not truly rapid (i.e. TAT of less than 1 h) and requires numerous reagent additions and wash steps, incubations, trained personnel and dedicated equipment for the measurement of results [26–29]. Other systems for toxin detection use the same competitive format principle, but have improved read-out or incorporate high-density spotting in a microarray format [30,31]. Surface plasmon resonance (SPR) biosensors allow automated and multiplexed analysis as well as the regeneration and reusability of assay surfaces [27,32]. Luminex microsphere, liquid-based array assays, as used by Fraga et al. [33,34], use flow cytometry principles coupled with the competitive format to allow the high-sensitivity multiplexed detection of toxins. However, both of these systems generally require dedicated expensive equipment and trained personnel, limiting their use at the PON. In the case of SPR, the potential for PON applications is starting to be addressed through the development of field-portable instrumentation. The field-portable SPIRIT device (Seattle Sensor Systems, Inc.) has been demonstrated for the detection of the amnesic shellfish poisoning (ASP) toxin DA [35], although extensive validation of this technology is lacking. The MBio waveguide-based system, in development at MBio Diagnostics, Inc., is a highly flexible detection device, [36–39], has a 15 min test time, is highly sensitive and allows multiplexed detection of ASP, DSP and PSP or additional toxins. Moreover, the MBio single-use waveguide cartridges along with a dedicated reader represent a very economical approach to testing for multiple toxins in a single sample and require minimal training of end-users. Direct label-free immunoassays are also beginning to emerge such as that developed by Hayat et al. [40]. This sensor, which utilizes anti-OA monoclonal antibodies bound to an impedimetric electrochemical surface, was capable of high-sensitivity detection with a short TAT. Also, being relatively simple to perform and inexpensive to produce means that such an assay shows promise for PON testing.
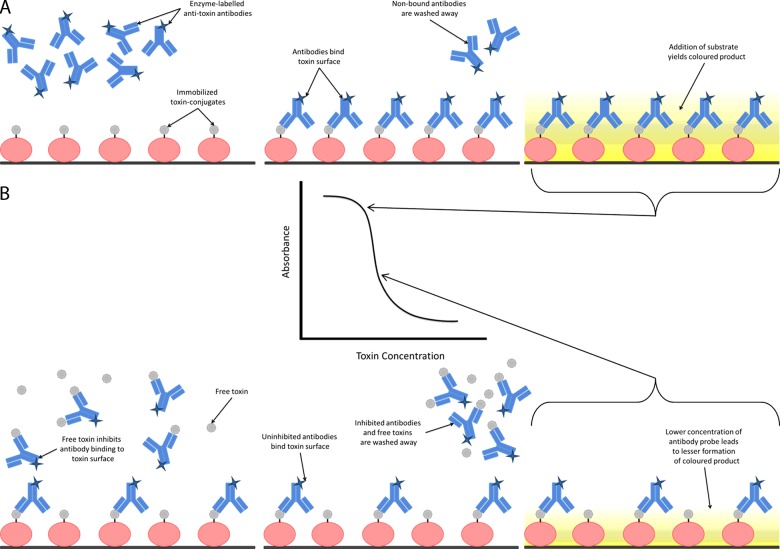
Figure 2. Illustration of a competitive ELISA format.
Enzyme-labelled anti-toxin antibodies are added to a microwell plate coated with toxins conjugated to a carrier protein. (A) In the absence of free toxin in solution, the antibodies are uninhibited and bind to the coated surface at a high concentration. Addition of a substrate (e.g. 3,3′,5,5′-tetramethylbenzidine (TMB) in the case of horseradish peroxidase (HRP)-labelled antibodies) produces a coloured product of high intensity. (B) In the presence of free toxin in solution, the free toxin competes with the immobilized toxin-conjugate for binding to the antibodies. The uninhibited antibodies bind to the coated surface but in smaller amounts. Addition of a substrate produces a coloured product of lower intensity.
Many, if not all, of the above assays are likely to be laboratory-based alternatives to high-performance analytical methods such as HPLC and LC–MS/MS. None of these assays, perhaps with the exceptions of the MBio system and direct electrochemical assay, are currently amenable to PON testing. A final assay format, lateral flow immunoassay (LFIA), may present a suitable alternative for this. These tests are cheap to manufacture, easy to use, rapid, have good performance characteristics and, with the incorporation of simple reader systems, are easy to interpret. These benefits, coupled with excellent shelf life and temperature stability, infer that LFIAs have great potential to be used as dockside screening assays of recently landed shellfish for the presence of marine biotoxins. However, due to the qualitative or semi-quantitative output of these assays, further confirmatory laboratory-based analysis would probably be required for regulatory clearance of a commercial product [41–44].
A potential problem with antibody-based toxin sensors is the issue of cross-reactivity (CR) between toxin analogues of the same group. For example, the PSP toxin group has more than 20 analogues that may differ by only a single side group, each with differing toxicities. Therefore, an assay incorporating an antibody that targets STX and shows strong CR for only a small number of STX analogues would probably underestimate the overall PSP toxicity of a sample, depending on the sample's toxin composition. A potential solution to this would be to incorporate a cocktail of different antibodies, each with a different CR profile for the PSP toxins. However, it would be highly challenging to characterize the CR of such a large number of antibodies for numerous targets. Chemical conversion of low into high CR analogues is also a potential means of addressing this issue.
A growing field of bioanalytical molecules is that of aptamers. These short oligo-DNA or -RNA molecules can bind to a wide range of targets with high affinity and specificity, including environmental pollutants (the reader is directed to [45] for a review). Without the need for an animal host, they are relatively easy to generate to a range of targets. Their small size may also allow them to distinguish subtle differences between toxin analogues. To date, aptamers have been developed against STX, OA and BTX-2 [46–48]. Aptamers represent an expanding area in the field of diagnostics and may have potential for marine biotoxin detection due their relatively high stability.
In many cases, there will be a need to pre-concentrate and extract the specific targeted toxin or its producer from marine or freshwater. In this case, the use of the aforementioned biorecognition ligands in the form of a solid-phase extraction system may also be important prior to determination of actual toxin levels by biosensors.
Conclusion
HABs and their associated marine biotoxins present a serious concern for global economies, consumer health and the environment. These toxins are associated with acute short-term symptoms as well as long-term risks such as cancer. Currently, international regulatory-approved detection methods for toxins require expensive equipment, trained personnel, long TAT, and laborious sample acquisition and processing. Thus, there is a need for inexpensive, easy-to-use, rapid and sensitive tests that can take toxin monitoring out of the laboratory and into the field (or water). Biosensors have the potential to address this need, in particular those with antibody- and aptamer-based recognition elements, as their excellent sensitivity, specificity and stability lend themselves extremely well to PON testing. However, few automated systems are currently available to allow completely hands-off remote monitoring, which is highly relevant to in-sea monitoring of HABs and toxins. Sample processing to concentrate algal target cells and their toxins is a significant challenge, yet a critical component for such automated systems in order to achieve appropriate limits of detection. The area of microfluidics has proven very promising for allowing automated lab-on-a-disc devices to carry out immunoassays remotely and could have potential to address this need. Additionally, further work must go into developing higher specificity antibodies for various toxin analogues, as currently there is the possibility of underestimation of sample toxicity.
This review discussed two applications of toxin/algae monitoring: first, front-line sensors for in-sea detection may help to mitigate the harvesting of contaminated shellfish in areas prone to blooms; secondly, sensors for the shipboard and dockside detection of toxins serve as a second line of defence and may help to avoid the processing of already harvested contaminated shellfish and prevent their consumption by consumers. These sensors will hopefully help to mitigate some of the adverse effects of the growing problem of HABs and marine biotoxins in the coming years, allowing the harvesting of safe food and reduction of global economic losses.
Summary
HABs and marine biotoxins are a major global health, economic and environmental issue.
The six major marine intoxication syndromes each have their own unique mode of action and associated symptoms, which range from moderate to severe.
Current monitoring programmes for HABs and marine biotoxins are expensive, labour-intensive and have a relatively long turnaround time.
Biosensors present a favourable alternative to the laboratory-based analytical methods currently used in monitoring as they boast excellent performance characteristics, ease-of-use, cost-effectiveness and short turnaround time. A number of recently developed biosensors for marine biotoxins were discussed. In particular, immunoassay-based methods have great promise as their robustness and stability mean that they are flexible for analysis in complex matrices such as seawater and shellfish extract.
Currently, the main drawback of immunoassays for toxins is their underestimation of sample toxicity due to the poor cross-reactivity of antibodies to certain toxin analogues. This could be addressed in the future via the development of higher-specificity antibodies or aptamers for the various toxin analogues.
Abbreviations
- ARfD
acute reference dose
- ASP
amnesic shellfish poisoning
- AUV
autonomous underwater vehicle
- AZA
azaspiracid
- BTX
brevetoxin
- CR
cross-reactivity
- DA
domoic acid
- DSP
diarrhetic shellfish poisoning
- ESP
Environmental Sample Processor
- EFSA
European Food Safety Authority
- EU
European Union
- HAB
harmful algal bloom
- LFIA
lateral flow immunoassay
- MBA
mouse bioassay
- NOAA
National Oceanic and Atmospheric Administration
- OA
okadaic acid
- PFP
pufferfish poisoning
- PON
point-of-need
- PSP
paralytic shellfish poisoning
- RBA
receptor-based assay
- SPR
surface plasmon resonance
- STX
saxitoxin
- TAT
turnaround time
Funding
We acknowledge support from Science Foundation Ireland [grant number 10/CE/B1821] and the Irish Research Council. We also acknowledge support from the National Science Foundation [grant numbers OCE-0982032 and OCE-314222]. This material is based upon work supported by the National Science Foundation [grant numbers OCE-1440299 and pOCE-1440198] (EAGER Collaborative).
Competing Interests
We have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Disclaimer: this publication does not constitute an endorsement of any commercial product or intend to be an opinion beyond scientific or other results obtained by the National Oceanic and Atmospheric Administration (NOAA). No reference shall be made to NOAA, or this publication furnished by NOAA, to any advertising or sales promotion which would indicate or imply that NOAA recommends or endorses any proprietary product mentioned herein, or which has as its purpose an interest to cause the advertised product to be used or purchased because of this publication.
References
- 1.Noguchi T., Onuki K., Arakawa O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. ISRN Toxicol. 2011;2011:276939. doi: 10.5402/2011/276939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bane V., Lehane M., Dikshit M., O'Riordan A., Furey A. Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins. 2014;6:693–755. doi: 10.3390/toxins6020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander J., Auðunsson G.A., Benford D., Cockburn A., Cravedi J., Dogliotti E., et al. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the European Commission on marine biotoxins in shellfish–okadaic acid and analogues. EFSA J. 2008;589:1–62. [Google Scholar]
- 4.Dyson K., Huppert D.D. Regional economic impacts of razor clam beach closures due to harmful algal blooms (HABs) on the pacific coast of Washington. Harmful Algae. 2010;9:264–271. doi: 10.1016/j.hal.2009.11.003. [DOI] [Google Scholar]
- 5.Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP) Protecting the oceans from land-based activities. GESAMP Rep. Stud. 2001;71:1–168. [Google Scholar]
- 6.Anderson D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs) Ocean Coast Manag. 2009;52:342–347. doi: 10.1016/j.ocecoaman.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore S.K., Trainer V.L., Mantua N.J., Parker M.S., Laws E.A., Backer L.C., et al. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health. 2008;7:S4. doi: 10.1186/1476-069X-7-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson D., Glibert P., Burkholder J. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries. 2002;25:704–726. doi: 10.1007/BF02804901. [DOI] [Google Scholar]
- 9.Twiner M.J., Hess P., Doucette G.J. Azaspiracids: toxicology, pharmacology, and risk assessment. In: Botana L.M., editor. Seafood and Freshwater Toxins. Pharmacology, Physiology, and Detection. 3rd edn. Oxford: Taylor & Francis; 2014. pp. 823–855. [DOI] [Google Scholar]
- 10.Tillmann U., Elbrächter M., Krock B., John U., Cembella A. Azadinium spinosum gen. et sp. nov. (Dinophyceae) identified as a primary producer of azaspiracid toxins. Eur. J. Phycol. 2009;44:63–79. doi: 10.1080/09670260802578534. [DOI] [Google Scholar]
- 11.Gao Y., Yu R., Murray S.A., Chen J., Kang Z., Zhang Q., et al. High specificity of a quantitative PCR assay targeting a saxitoxin gene for monitoring toxic algae associated with paralytic shellfish toxins in the yellow sea. Appl. Environ. Microbiol. 2015;81:6973–6981. doi: 10.1128/AEM.00417-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonella P., Luca G. The quantitative real-time PCR applications in the monitoring of marine harmful algal bloom (HAB) species. Environ. Sci. Pollut. Res. Int. 2013;20:6851–6862. doi: 10.1007/s11356-012-1377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seltenrich N. Keeping tabs on HABs: new tools for detecting, monitoring, and preventing harmful algal blooms. Environ. Health Perspect. 2014;122:A206–A213. doi: 10.1289/ehp.122-A206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell L., Olson R.J., Sosik H.M., Abraham A., Henrichs D.W., Hyatt C.l.J., et al. First harmful Dinophysis (Dinophyceae, Dinophysiales) bloom in the U.S. is revealed by automated imaging flow cytometry. J. Phycol. 2010;46:66–75. doi: 10.1111/j.1529-8817.2009.00791.x. [DOI] [Google Scholar]
- 15.Doucette G.J., Mikulski C.M., Jones K.L., King K.L., Greenfield D.I., Marin R., III, et al. Remote, subsurface detection of the algal toxin domoic acid onboard the environmental sample processor: assay development and field trials. Harmful Algae. 2009;8:880–888. doi: 10.1016/j.hal.2009.04.006. [DOI] [Google Scholar]
- 16.Greenfield D.I., Marin R., Doucette G.J., Mikulski C., Jones K., Jensen S., et al. Field applications of the second-generation environmental sample processor (ESP) for remote detection of harmful algae: 2006–2007. Limnol. Oceanogr. Methods. 2008;6:667–679. doi: 10.4319/lom.2008.6.667. [DOI] [Google Scholar]
- 17.European Commission. 2011. Commission regulation (EU) No 15/2011. OJ L6/3.
- 18.van den Top H.J., Gerssen A., McCarron P., van Egmond H.P. Quantitative determination of marine lipophilic toxins in mussels, oysters and cockles using liquid chromatography-mass spectrometry: Inter-laboratory validation study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011;28:1745–1757. doi: 10.1080/19440049.2011.608382. [DOI] [PubMed] [Google Scholar]
- 19.McPartlin D.A., O'Kennedy R.J. Point-of-care diagnostics, a major opportunity for change in traditional diagnostic approaches: Potential and limitations. Expert Rev. Mol. Diagn. 2014;14:979–998. doi: 10.1586/14737159.2014.960516. [DOI] [PubMed] [Google Scholar]
- 20.Doucette G.J., Powell C.L., Do E.U., Byon C.Y., Cleves F., McClain S.G. Evaluation of 11-[3H]-tetrodotoxin use in a heterologous receptor binding assay for PSP toxins. Toxicon. 2000;38:1465–1474. doi: 10.1016/S0041-0101(99)00240-8. [DOI] [PubMed] [Google Scholar]
- 21.Van Dolah F.M., Leighfield T.A., Haynes B.L., Hampson D.R., Ramsdell J.S. A microplate receptor assay for the amnesic shellfish poisoning toxin, domoic acid, utilizing a cloned glutamate receptor. Anal. Biochem. 1997;245:102–105. doi: 10.1006/abio.1996.9889. [DOI] [PubMed] [Google Scholar]
- 22.Van Dolah F.M., Leighfield T.A., Doucette G.J. Single-laboratory validation of the microplate receptor binding assay for paralytic shellfish toxins in shellfish. J. AOAC Int. 2009;92:1705–1713. [PubMed] [Google Scholar]
- 23.Van Dolah F.M., Fire S.E., Leighfield T.A., Mikulski C.M., Doucette G.J. Determination of paralytic shellfish toxins in shellfish by receptor binding assay: collaborative study. J. AOAC Int. 2012;95:795–812. doi: 10.5740/jaoacint.CS2011_27. [DOI] [PubMed] [Google Scholar]
- 24.Volpe G., Cotroneo E., Moscone D., Croci L., Cozzi L., Ciccaglioni G., et al. A bienzyme electrochemical probe for flow injection analysis of okadaic acid based on protein phosphatase-2A inhibition: an optimization study. Anal. Biochem. 2009;385:50–56. doi: 10.1016/j.ab.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Garibo D., Devic E., Marty J., Diogène J., Unzueta I., Blázquez M., et al. Conjugation of genetically engineered protein phosphatases to magnetic particles for okadaic acid detection. J. Biotechnol. 2012;157:89–95. doi: 10.1016/j.jbiotec.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Vdovenko M.M., Hung C., Sakharov I.Y., Yu F. Determination of okadaic acid in shellfish by using a novel chemiluminescent enzyme-linked immunosorbent assay method. Talanta. 2013;116:343–346. doi: 10.1016/j.talanta.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 27.McNamee S., Elliott C., Delahaut P., Campbell K. Multiplex biotoxin surface plasmon resonance method for marine biotoxins in algal and seawater samples. Environ. Sci. Pollut. Res. Int. 2013;20:6794–6807. doi: 10.1007/s11356-012-1329-7. [DOI] [PubMed] [Google Scholar]
- 28.Liu R., Xu D.F., Dong Y.F., Liang Y. An indirect competitive ELISA to detect domoic acid in seawater and shellfish. Wei Sheng Yan Jiu. 2009;38:622–624. [PubMed] [Google Scholar]
- 29.Garet E., González-Fernández Á., Lago J., Vieites J.M., Cabado A.G. Comparative evaluation of enzyme-linked immunoassay and reference methods for the detection of shellfish hydrophilic toxins in several presentations of seafood. J. Agric. Food Chem. 2010;58:1410–1415. doi: 10.1021/jf904448z. [DOI] [PubMed] [Google Scholar]
- 30.Szkola A., Campbell K., Elliott C.T., Niessner R., Seidel M. Automated, high performance, flow-through chemiluminescence microarray for the multiplexed detection of phycotoxins. Anal. Chim. Acta. 2013;787:211–218. doi: 10.1016/j.aca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Desmet C., Blum L.J., Marquette C.A. High-throughput multiplexed competitive immunoassay for pollutants sensing in water. Anal. Chem. 2012;84:10267–10276. doi: 10.1021/ac302133u. [DOI] [PubMed] [Google Scholar]
- 32.Prieto-Simón B., Miyachi H., Karube I., Saiki H. High-sensitive flow-based kinetic exclusion assay for okadaic acid assessment in shellfish samples. Biosens. Bioelectron. 2010;25:1395–1401. doi: 10.1016/j.bios.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 33.Fraga M., Vilariño N., Louzao M.C., Campbell K., Elliott C.T., Kawatsu K., et al. Detection of paralytic shellfish toxins by a solid-phase inhibition immunoassay using a microsphere-flow cytometry system. Anal. Chem. 2012;84:4350–4356. doi: 10.1021/ac203449f. [DOI] [PubMed] [Google Scholar]
- 34.Fraga M., Vilariño N., Louzao M.C., Rodríguez P., Campbell K., Elliott C.T., et al. Multidetection of paralytic, diarrheic, and amnesic shellfish toxins by an inhibition immunoassay using a microsphere-flow cytometry system. Anal. Chem. 2013;85:7794–7802. doi: 10.1021/ac401146m. [DOI] [PubMed] [Google Scholar]
- 35.Stevens R.C., Soelberg S.D., Eberhart B.L., Spencer S., Wekell J.C., Chinowsky T.M., et al. Detection of the toxin domoic acid from clam extracts using a portable surface plasmon resonance biosensor. Harmful Algae. 2007;6:166–174. doi: 10.1016/j.hal.2006.08.001. [DOI] [Google Scholar]
- 36.McNamee S.E., Elliott C.T., Greer B., Lochhead M., Campbell K. Development of a planar waveguide microarray for the monitoring and early detection of five harmful algal toxins in water and cultures. Environ. Sci. Technol. 2014;48:13340–13349. doi: 10.1021/es504172j. [DOI] [PubMed] [Google Scholar]
- 37.Murphy C., Stack E., Krivelo S., McPartlin D.A., Byrne B., Greef C., et al. Detection of the cyanobacterial toxin, microcystin-LR, using a novel recombinant antibody-based optical-planar waveguide platform. Biosens. Bioelectron. 2015;67:708–714. doi: 10.1016/j.bios.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 38.Devlin S., Meneely J.P., Greer B., Greef C., Lochhead M.J., Elliott C.T. Next generation planar waveguide detection of microcystins in freshwater and cyanobacterial extracts, utilising a novel lysis method for portable sample preparation and analysis. Anal. Chim. Acta. 2013;769:108–113. doi: 10.1016/j.aca.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Meneely J.P., Campbell K., Greef C., Lochhead M.J., Elliott C.T. Development and validation of an ultrasensitive fluorescence planar waveguide biosensor for the detection of paralytic shellfish toxins in marine algae. Biosens. Bioelectron. 2013;41:691–697. doi: 10.1016/j.bios.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 40.Hayat A., Barthelmebs L., Marty J. Electrochemical impedimetric immunosensor for the detection of okadaic acid in mussel sample. Sens. Actuators B Chem. 2012;171–172:810–815. doi: 10.1016/j.snb.2012.05.075. [DOI] [Google Scholar]
- 41.Gao L.L., Cheng J.P., Liu Y.Y., Wang Q., Wang W.H. Development of colloidal gold immunochromatographic strip for rapid detection of domoic acid. Huan Jing Ke Xue. 2011;32:2492–2496. [PubMed] [Google Scholar]
- 42.Jawaid W., Meneely J., Campbell K., Hooper M., Melville K., Holmes S., et al. Development and validation of the first high performance-lateral flow immunoassay (HP-LFIA) for the rapid screening of domoic acid from shellfish extracts. Talanta. 2013;116:663–669. doi: 10.1016/j.talanta.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 43.Jawaid W., Campbell K., Melville K., Holmes S.J., Rice J., Elliott C.T. Development and validation of a novel lateral flow immunoassay (LFIA) for the rapid screening of paralytic shellfish toxins (PSTs) from shellfish extracts. Anal. Chem. 2015;87:5324–5332. doi: 10.1021/acs.analchem.5b00608. [DOI] [PubMed] [Google Scholar]
- 44.Lu S., Lin C., Li Y., Zhou Y., Meng X., Yu S., et al. A screening lateral flow immunochromatographic assay for on-site detection of okadaic acid in shellfish products. Anal. Biochem. 2012;422:59–65. doi: 10.1016/j.ab.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 45.Hayat A., Marty J.L. Aptamer based electrochemical sensors for emerging environmental pollutants. Front. Chem. 2014;2:41. doi: 10.3389/fchem.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Handy S.M., Yakes B.J., DeGrasse J.A., Campbell K., Elliott C.T., Kanyuck K.M., et al. First report of the use of a saxitoxin–protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon. 2013;61:30–37. doi: 10.1016/j.toxicon.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Eissa S., Ng A., Siaj M., Tavares A.C., Zourob M. Selection and identification of DNA aptamers against okadaic acid for biosensing application. Anal. Chem. 2013;85:11794–11801. doi: 10.1021/ac402220k. [DOI] [PubMed] [Google Scholar]
- 48.Eissa S., Siaj M., Zourob M. Aptamer-based competitive electrochemical biosensor for brevetoxin-2. Biosens. Bioelectron. 2015;69:148–154. doi: 10.1016/j.bios.2015.01.055. [DOI] [PubMed] [Google Scholar]