Abstract
The rapid diagnosis of many diseases and timely initiation of appropriate treatment are critical determinants that promote optimal clinical outcomes and general public health. Biosensors are now being applied for rapid diagnostics due to their capacity for point-of-care use with minimum need for operator input. Antibody-based biosensors or immunosensors have revolutionized diagnostics for the detection of a plethora of analytes such as disease markers, food and environmental contaminants, biological warfare agents and illicit drugs. Antibodies are ideal biorecognition elements that provide sensors with high specificity and sensitivity. This review describes monoclonal and recombinant antibodies and different immobilization approaches crucial for antibody utilization in biosensors. Examples of applications of a variety of antibody-based sensor formats are also described.
Keywords: antibodies, biosensor, monoclonal antibodies, recombinant antibodies
Introduction
Traditional in vitro diagnostics are time-consuming and require centralized laboratories, experienced personnel and bulky equipment. Recent advances in biosensor technologies have the potential to deliver point-of-care diagnostics that match or surpass conventional standards with respect to time, accuracy and cost. Antibodies (Abs), which are amongst the most exquisitely designed and engineered molecules in Nature, play a vital role in a number of sensor devices due to their exquisite target specificity and affinity.
Introduction to antibodies
The immune system
The immune system functions to protect the body against infectious organisms, which are potentially harmful to the host. It is divided into two main sub-systems; non-adaptive (innate) and acquired (adaptive) immunity. Innate immunity refers to non-specific defence mechanisms that come into play immediately or within hours of an antigen's appearance in the body [1]. Innate immune responses depend on physical barriers such as the skin in addition to groups of proteins and phagocytic cells, such as neutrophils, monocytes, macrophages, mast cells and dendritic cells, which recognize specific features of foreign molecules and become quickly activated to remove/destroy the invaders. In contrast, the acquired immune system is highly specific to a particular pathogen. Acquired immune responses are more complex than innate responses. The antigen must first be processed and recognized. Once an antigen is recognized, the acquired immune system creates an army of immune cells specifically designed to attack that antigen. Acquired immunity is controlled by lymphocytes, which are responsible for the secretion of immunoglobulins (Igs). Acquired immunity creates immunological memory after an initial response to a specific pathogen, which leads to an enhanced response to subsequent encounters with that pathogen.
Antibody structure
Abs or Igs are highly soluble serum glycoproteins involved in the defence mechanisms of the immune system. They can be divided into five classes depending on their heavy chain constant region sequences, i.e. IgM, IgD, IgG, IgE and IgA [2]. The basic structure of an Ab is outlined in Figure 1 and it can be subdivided into two distinct building blocks; the antigen-binding fragment (Fab) and the constant fragment (Fc) [2]. An Ab has four polypeptide chains, i.e. two heavy chains and two light chains (either κ (kappa) or λ (lambda)), which are joined together by disulphide bonds [1]. The heavy chain is composed of one variable region (variable heavy or VH) and three constant regions (CH1, CH2 and CH3). The light chain has one variable region (variable light or VL) and one constant region (CL). The Fab component of the Ab contains the fragment variable (Fv) region, where the complementarity-determining regions (CDRs) can be located [2]. The CDRs form the antigen-binding sites of the Ab and confer antigen specificity. The Fc region is essential for mediating effector functions such as Ab-dependent cell-mediated cytotoxicity (ADCC), Ab-dependent cellular phagocytosis, antigen presentation to the immune system, degranulation, complement-mediated lysis, and regulation of cell activation and proliferation.
Figure 1. Structure of an IgG antibody (Ab).
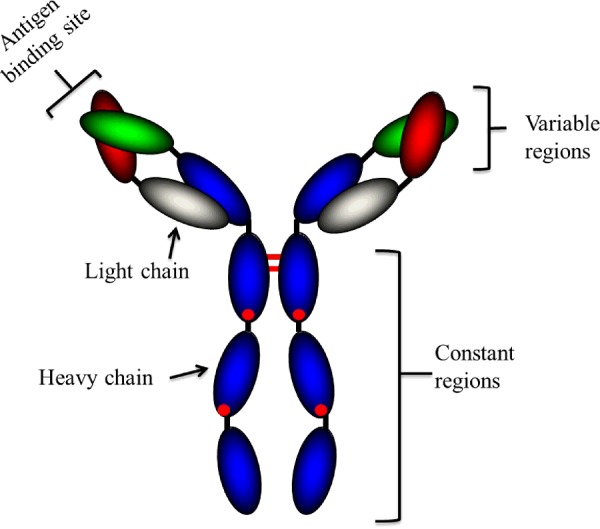
A typical Ab is a large molecule of about 150 kDa made up of four peptide chains. It contains two identical class γ heavy chains of about 50 kDa and two identical light chains of about 25 kDa, and thus a tetrameric quaternary structure. The two heavy chains are linked to each other and to a light chain by disulphide bonds. The resulting tetramer has two identical halves, which together form the Y-like shape. Each end of the fork contains an identical antigen-binding site. (Adapted from [1]).
Monoclonal antibodies
Abs have been used extensively since their initial discovery as diagnostic tools in many different formats due to their exquisite specificity for their cognate antigen. Ab-based immunoassays are the most commonly used diagnostic assays and remain one of the fastest growing technologies for the analysis of biomolecules [3]. Conventional techniques for the preparation of Ab in antiserum against a specific target consistently result in the production of non-homogeneous Abs with different specificity and affinity, referred to as polyclonal Ab (pAbs) [4]. Monoclonal Ab (mAb) technology or hybridoma technology has revolutionized the use of Ab as tools for research for the prevention, detection and treatment of diseases. In hybridoma technology, a myeloma cell is rendered drug-sensitive through mutation of a growth-essential gene for hypoxanthine guanine phosphoribosyl-transferase (HGPRT). It is then fused with immune cells from a host immunized with the target antigen of interest and the resulting cells are grown in a medium containing a selective drug. Since the immune cells have a short lifespan in tissue culture and the myeloma cells are drug-sensitive, the only cells that will survive are those myeloma cells which obtained a normal HGPRT-encoding gene from the immune cells. Such cells also have a high chance of carrying the immune cell's Ab gene, resulting in the generation of a hybridoma that can grow continuously in vitro and secrete a single mAb [5]. Since its discovery, it has provided a number of advantages over the original art of pAb generation: (i) the hybridoma cell line is immortal and hence there is an unlimited source of homogeneous mAb; (ii) Ab with selective properties for specific targets can be generated; and (iii) the use of an impure antigen is acceptable considering that the detection of the target mAb is predominantly based on the selection strategy used. Over many years, mAbs generated using hybridoma technology have provided the means for developing a number of highly specific and reproducible immunological assays for the rapid and accurate diagnosis of an extensive list of diseases. Although traditional IgG mAbs have a future in the diagnosis/therapy of diseases, the introduction of recombinant Ab (rAb) technology and a deeper understanding of the action of Abs have paved the way for new and greatly improved Ab generation strategies for both therapies and diagnostics. Recombinant Ab technology allows the generation of Ab fragments that retain their stability and specificity [1]. In comparison with the parental Ab, these minimized Abs have several advantages in, for example, clinical practice, including better tumour penetration, more rapid blood clearance, lower retention times in non-target tissue and reduced immunogenicity.
Recombinant antibody technology
In the past, Ab fragments could only be generated by proteolytic cleavage which leads to the production of F(ab’)2 and Fab Ab fragment [1]. Two decades ago, Plückthun and Skerra [6] described the use of vectors in bacterial systems that could generate fully functional correctly folded Fv and Fab Ab fragments. These vectors could offer soluble Ab secretion directly into the periplasm space by means of its oxidizing environment, which contributes to the correct formation of disulphide bonds between the Ab domains [7]. Since then, Ab fragments have been produced in a variety of systems, such as mammalian [8], insect [9], yeast [8], plant [10] and ‘cell-free’ [10].
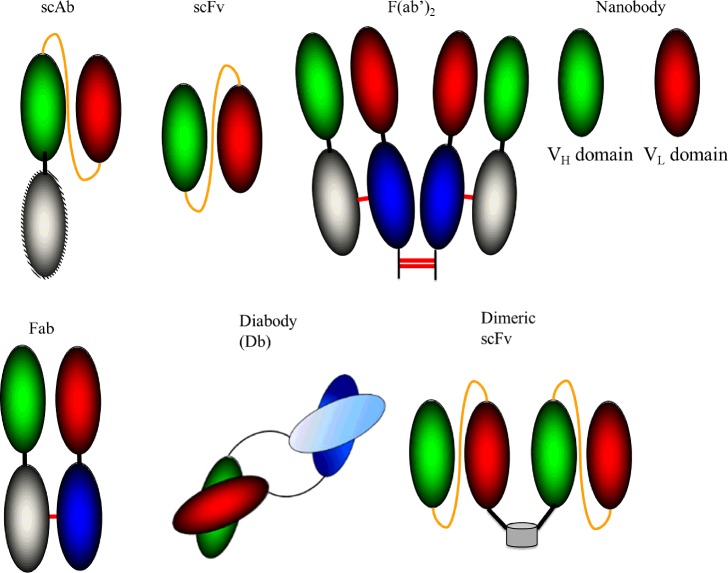
Fv fragments, composed of the VH and VL domains linked via a disulphide bond, are the smallest Ab fragments which function in antigen-binding activities. Stability issues associated with Fv Abs were overcome by introducing a flexible (Gly4Ser)3 peptide linker into the Fv fragment resulting in the generation of a single-chain Fv or scFv. By incorporating a human constant κ light chain into the terminal of the VL region of an scFv, a single-chain Ab (scAb) fragment can be produced which can improve the stability and expression of scFv Abs. An additional extension to the scFv family is the Diabody (see Figure 2 for more detail). Here, the variable domains from two scFv Abs are expressed as two polypeptide chains with the domains connected by a short polypeptide linker, forcing heterodimerization of the two chains. Dimeric scFvs are composed of two scFvs linked via a naturally dimeric protein [1]. A diagnostically valuable variant to the dimeric construct, which facilitate direct detection, is a bifunctional scFv consisting of alkaline phosphatase (AP)-labelled scFvs. Single-domain antigen-binding fragments, known as nanobodies, are currently highly valued proteins for multiple applications, including fundamental research, diagnostics and therapeutics. Once an Ab fragment is generated, an appropriate selection or screening method, such as phage display [11], is used to isolate high-affinity Abs from a vast library [1].
Figure 2. Recombinant antibody formats.
A scFv consists of the variable heavy (VH) and variable light (VL) regions of an Ab joined via a peptide linker. The Fab fragment is composed of VH and VL domains within both constant heavy and light chains. F(ab’)2 involves linking two Fab Abs by disulphide bonds. A Diabody is generated when the variable domains from two scFv Abs are expressed as two polypeptide chains with the domains connected by a short polypeptide linker. A dimeric scFv is generated by the fusion of two scFv Ab via a naturally occurring dimeric protein. A nanobody is an Ab fragment consisting of a single monomeric variable Ab domain. (Adapted from [1].)
Abs are excellent biorecognition components due to their exceptional specificity and affinity for their cognate antigen. Thus, they are ideal recognition elements for incorporation into sensors. A huge number of Ab-based biosensors are applied clinically for the detection of a variety of analytes. The following sections describe different approaches applied in the fabrication of sensors with particular emphasis on Abs and the development of immobilization methods for their incorporation to ensure optimal performance.
Antibody immobilization approaches
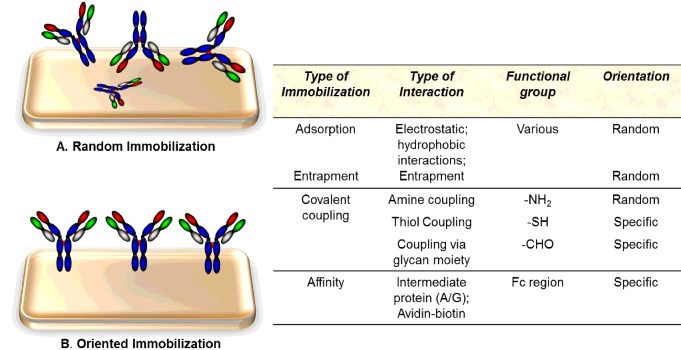
The performance of a bio/immuno-sensor depends on three critical factors: (i) its ability to immobilize recognition elements (biological molecules) while maintaining their natural activity; (ii) the accessibility of the recognition element to the relevant analyte in solution; and (iii) low non-specific adsorption to the solid support. The physico-chemical properties of the interface or sensor surface play a significant role in accomplishing optimal immobilization of the Abs and limiting non-specific adsorption [12]. As mentioned above, immobilization of Abs onto a sensor surface without altering their specificity and immunological activity is one of the most crucial steps in the fabrication of an immunosensor. The immobilization step affects the detection limit, sensitivity and overall performance of the immunosensor [13]. The asymmetric macromolecular Ab can attach to the solid surface in many different orientations. If immobilization is through the Fc region and the binding site of the Ab is fully available for interaction with the cognate antigen then it can function very well, thus maximizing sensor performance. Immobilization of the Ab on to the sensor surface through the antigen-binding sites, encompassing the VH and CH regions, results in either decreased or entirely eliminated binding activity of the Ab [1]. Figure 3 outlines an overview of different functional groups used for random and oriented Ab immobilization on to solid surfaces. In addition, there are a number of intermediate states of immobilization that may either lead to reduced access of the cognate antigen to the Ab-binding site or link the Ab close to this site with an associated reduction in binding and thus sensor-related performance. The extent of linkage between the Ab and the surface may also need to be carefully controlled. This can be successfully achieved if the Ab is engineered to have very well defined points of attachment, e.g. in the use of Ab fragments such as scFv, scAb or Fabs with tags such as polyhistidine, with biotin or engineered thiol points for linkage. However, this is harder to control where chemical crosslinking is utilized, e.g. to -COOH or NH2, as multiple crosslinks can reduce the overall Ab-binding capacity, e.g. by hindering access to the Ab-binding site.
Figure 3. Different types of immobilization methods and functional groups used for random and oriented Ab immobilization on to surfaces.
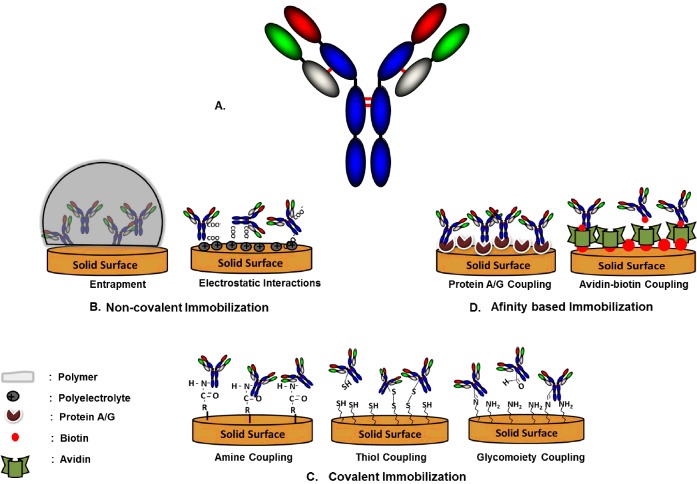
Favourable orientation of Abs and minimum structural modification while immobilized on the solid surface can directly contribute to optimal immunosensor performance, with improvement performance factors as high as 200-fold compared with disoriented immobilization [13]. The orientation of Abs on sensor surfaces can be controlled by the interaction between specific reactive groups on the surface and on the Ab. Various approaches including functionalizing of the surface with specific groups, such as glutaraldehyde, carbodiimide, succinimide ester, maleinimide or periodate, or decorating it with nanostructures, have been reported in the literature to make the sensor surface more compatible for Ab immobilization with specific orientation [14]. The two main approaches that can be used for immunosensor fabrication are non-covalent and covalent immobilization. Figure 4 is a schematic representation of various immobilization methods used to immobilize Abs on to a sensor surface.
Figure 4. Schematic representation of various immobilization methods.
(A). Typical Ab structure. (B) Non-covalent immobilization of an Ab on to a solid sensor surface represented by entrapment and electrostatic interactions. (C) Covalent binding of an Ab to functionalized solid surface via amine, thiol and aldehyde groups. (D). Coupling of a biomolecule by affinity interactions with the solid surface represented by the Protein A/G and the biotin–avidin affinity-based systems.
Non-covalent immobilization
Abs can be adsorbed on to the sensor surface via simple non-covalent forces, including electrostatic or ionic bonds, hydrophobic interactions and van der Waals forces. Adsorption is an attractive method due to its simplicity and can be classified as either physisorption or chemisorption. Physisorption involves mainly weak van der Waals forces and hydrophobic interactions whereas chemisorption is mainly based on electrostatic interactions between the Abs and the surface. Um et al. [15] showed the improved immunological activity of electrochemically immobilized Abs on to a poly-(2-cyano-ethylpyrrole)-coated gold electrode in comparison with physisorbed Abs. This improved activity was attributed to orientation-controlled immobilization. The overall performance of physically adsorbed Ab-based reusable immunosensors is usually low because of leaching and lack of functional orientation of Abs. Ab entrapment into conducting polymer films is another non-covalent approach, reported by John et al. in 1991 [16], where Abs for human serum albumin were entrapped on a galvanostatically polymerized pyrrole on to a platinum wire substrate. Polyacetylene, polythiophene, polyaniline, polyindole and polypyrrole are some of the common conducting polymers that have been extensively used for immunosensor fabrication due to their exceptional biocompatibility in neutral aqueous solutions. Polyquinone is another class of smart materials for biomolecule immobilization. In addition to excellent biocompatibility, it also provides coupling and transduction abilities, thus avoiding the need for an additional redox label attached to the Ab or in solution. The ‘label-free’ immunosensor developed by Sun et al. [17] for carbofuran detection based on a silica sol-gel-entrapped Ab was shown to have a detection limit of 0.33 ng/ml. Silica sol-gel possesses a silicate network and provides a biocompatible microenvironment around the Ab [17]. In spite of the enhanced attachment, the sensitivity of an immunosensor based on entrapment can be compromised due to the burying of the active sites, resulting in poor immunological activity or recognition efficiency. Even though these untreated coupling schemes are favoured due to their simplicity, they allow only limited control over the orientation of the Abs and their practical use is constrained by mass transfer limitations through membranes or gels. This might restrict the correct and desirable immobilization via the Fc region, resulting in poor accessibility for the antigen-binding sites.
Covalent immobilization
Covalent immobilization of Abs on to various solid surfaces is the most intensely studied immobilization approach as it facilitates the long-term storage and reusability of immunosensors. Covalent binding involves surface modification in order to achieve reactive groups such as hydroxy, thiol, carboxy or amino groups on the surface for the subsequent Ab immobilization. Various surface modification techniques including chemical modification, photochemical grafting, plasma gas discharge and ionizing radiation graft co-polymerization have been developed. Coupling Abs to the sensor surface by targeting amine groups present in the lysine amino acid side chains of Ab is an extensively used form of covalent immobilization, due to the relative ease of access to these groups. However, such approaches could also react with amino groups in or close to the binding site, thus reducing Ab activity. Coupling of the amine groups of Abs with terminal carboxylated gold surfaces has also been widely used. The primary amine groups on Abs bind with reactive succinimide esters (formed by the reaction between carboxylic acid and 1-ethyl-3-(3-dimethylaminopropyl)-carbodi-imide (EDC) and N-hydroxysuccinimide (NHS)) to form a covalent linkage as shown in Figure 4. Feyssa et al. [18] showed enhancement in the assay signal by immobilization of an anti-C-reactive protein (CRP) Ab in a microfluidic platform via amine covalent linkage, in comparison with passive binding. The reproducibility of an amine-coupled biochip was found to be comparable with a human-CRP enzyme-linked immunosorbent assay (ELISA) detection kit. Rahman et al. [19] developed an impedimetric immunosensor using a quartz crystal microbalance (QCM) for the label-free detection of bisphenol A (BPA) by covalently immobilizing a pAb on to a carboxylic acid group-functionalized nanoparticle. Under optimized conditions, the linear dynamic range of the developed BPA immunosensor was achieved between 1 and 100 ng/ml with a detection limit of 0.3 ± 0.07 ng/ml [19].
Amine-coupling chemistry usually results in random orientation and less homogeneous binding due to the presence of excessive lysine groups in the Ab. Thiol groups can also be used for Ab immobilization; usually they give more homogeneous immobilization or may allow defined orientation of Abs in comparison with amine coupling, particularly where specific thiol groups have been added to Abs by either chemical or molecular biological approaches. Immobilization of Abs on to a sensor surface using a thiol group is typically based on thiol–disulphide exchange between thiol groups on the Ab and active disulphides introduced on to the surface. Ab fragments immobilized in defined orientation have been shown to achieve a 20-fold enhanced antigen-binding ability, compared with the randomly immobilized Abs, using amine groups [13]. The thiol-coupling approach was proved to be successful with Ab fragments. A sensitive and selective impedimetric immunosensor for the detection of peptides derived from avian influenza haemagglutinin H5 using a Fab Ab fragment immobilized on a gold electrode surface via thiol coupling was reported by Jarocka et al. [20].
Another covalent approach involves the generation of active aldehyde groups by oxidizing hydroxy (-OH) groups of carbohydrates present in the Fc region of Abs using sodium (NaIO4) or potassium (KIO4) periodates. The resulting activated diol groups can be linked efficiently on amine-functionalized surfaces, resulting in partially oriented covalent Ab coupling on the sensor surface. Lin et al. [21] designed a simple and effective boronic acid-assisted strategy for covalent and site-specific Ab conjugation on magnetic nanoparticles (MNPs) through boronate formation at the carbohydrate moiety of the Fc domain. The immobilization method via a glyco moiety demonstrated significant enhancement in immunoaffinity extraction compared with the Ab immobilized through Protein G. However, the usage of highly reactive chemicals for carbohydrate moiety oxidation might occasionally result in the involuntary oxidation of amino acids such as methionine, tryptophan or histidine at different positions on the Ab with the potential to reduce binding capacity. Other associated limitations as mentioned by Shriver-Lake et al. [22] with the use of the carbohydrate-based immobilization procedure involve time delays due to more steps in the procedure, increased loss of Ab and also loss of activity of some of the mAbs after the periodate treatment. They observed 50% losses in the Ab prior to immobilization due to the number of steps in the procedure.
Affinity-based immobilization
Despite the robustness of covalent immobilization it is still not ideal, owing to drawbacks such as partial denaturation and the lack of definite orientation associated with the method. Thus, other non-physical immobilization strategies such as the use of biotin–avidin or immobilizing intermediate binding proteins, such as Protein A or G, first on to the sensor surface followed by the subsequent capture of Abs were introduced. These proteins have a high affinity and binding specificity towards the Fc region of a wide range of Abs, thus encouraging Ab immobilization via the Fc region on the sensor surface. de Juan-Franco et al. [23] introduced a new, simple and fast oriented Ab immobilization approach, using a fusion protein, the Protein A–gold-binding domain (PAG). The human growth hormone-specific immunosensor fabricated using the PAG immobilization approach showed better sensitivity with a limit of detection of 90 ng/ml and inter-chip variability lower than 7% compared with conventional methods [23]. The biotin-tagged Abs can easily be captured on avidin or streptavidin, or genetically derived combinations of both proteins, which are conjugated with functionalized polymeric and metallic surfaces. Barton et al. [24] achieved much higher sensitivities with an avidin–biotin interaction-based immunosensor in comparison with an entrapment-based immunosensor. Ionescu et al. [25] reported a highly sensitive label-free impedimetric immunosensor for the detection of atrazine, a herbicide. They immobilized the anti-atrazine Ab fragments via affinity binding on to a polypyrrole film N-substituted by nitrilotriacetic acid (NTA) electrogenerated on a gold electrode. The subsequent charge transfer resistance analysis measured as low as 10 pg/ml of atrazine with a working range of 10 pg/ml to 1 μg/ml [25]. Trilling et al. [26] showed the in vivo biotinylation of variable domains of llama heavy-chain antibodies (VHH) at the lysine position of the Avi-tag. Although the in vivo biotinylation was time-consuming, uniform orientation improved analyte binding more than 200-fold with up to 227-fold sensitivity enhancement [26].
Engineering antibodies for immobilization
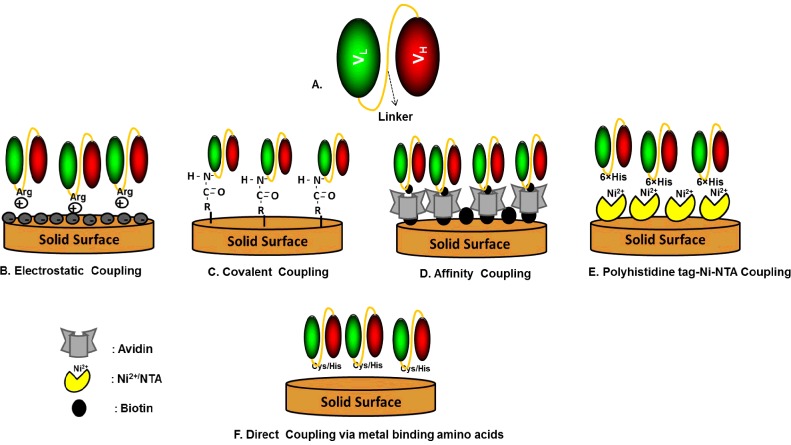
Recombinant Abs have several desirable characteristics including low molecular mass, increased flexibility, high physico-chemical stability and easy access to the antigen which makes them a potential substitute for naturally generated Abs. The directed immobilization approaches developed for intact Abs based on the Fc domain or carbohydrate moiety cannot be used with rAbs or Ab fragments such as scAbs or scFv, due to the absence of the glycosylated Fc domain. Various approaches have been developed to engineer scFv or scAb during their generation so as to easily immobilize them on to the solid surface without denaturation. Ab fragments can be engineered to have positively charged amino acids (e.g. arginine or Arg) in the peptide linker or a 6-histidine amino acid sequence on the C-terminus for immobilization via electrostatic and non-covalent interactions, respectively. Bioaffinity methodology can also be used to immobilize scFv on to streptavidin-coated surfaces by conjugating to biotin via free amines on the scFv. Metal-binding cysteine (Cys) or histidine (His) amino acids in the peptide linker in engineered scFv allow correct self-assembly on to metal surfaces. Figure 5 is a schematic representation of different types of immobilization approaches used for immobilizing rAbs on to sensor surfaces.
Figure 5. Schematic representation of various types of immobilization approaches used for immobilizing recombinant Abs on to sensor surfaces.
A wide variety of sensing transducers have been developed by using rAbs to substitute for intact Abs to develop immunosensors. Table 1 outlines some examples of rAb-based immunosensors. The high specificity of rAbs allows the use of a single rAb to detect an antigen, thus eliminating the need for a second antigen-specific Ab. Additionally, the small size of rAbs permits immobilization on to an immunosensor surface at high density, thus resulting in enhanced assay avidity, sensitivity and stability.
Table 1. Recombinant antibody-based immunosensors.
| Application | Analyte | Antibody type | Transducer | Reference |
|---|---|---|---|---|
| Nitroaromatic explosives detection | 2,4,6-trinitrotoluene (TNT) | scAb | Chemoresistive | [27] |
| Deep vein thrombosis (DVT) disorders | D-dimer | scAb | Electrochemical | [28] |
| Human immunodeficiency virus (HIV) | HIV-1 virion infectivity factor | scFv | Piezoelectric | [29] |
| Toxic metabolite detection | Aflatoxin B1 | scFv | SPR | [30] |
| Disease diagnosis | Fc receptors | scFv | Piezoelectric | [31] |
| Listeriosis diagnosis | Listeria monocytogenes | scFv | Electrochemical | [32] |
| Pesticide concentration detection | Atrazine | scAb | Electrochemical | [33] |
| Encephalomyelitis diagnosis | Venezuelan equine encephalitis virus | scFv | Resonant mirror | [34] |
| Entamoeba histolytica diagnosis | Entamoeba histolytica antigens | scFv | Amperometric | [35] |
| Detection of doping with hormone by | Somatotropin | Half-sized Ab fragment | Surface plasmon resonance (SPR) | [36] |
| Detection of inflammation | C-reactive protein (CRP) | Engineered Ab fragment | SPR | 1 |
Conclusion
The performance of any immunosensor is largely dependent on the type of Ab and the associated Ab-immobilization approach used to fabricate the sensor. Abs play a crucial role in determining the sensitivity and specificity of an immunosensor. A crucial factor in sensor development is the immobilization strategy used with the Ab or biorecognition ligand. Several studies have demonstrated the enhancement in antigen-binding activity by oriented Ab immobilization on a sensor surface. Different types of Abs with a wide variety of immobilization strategies have been discussed in this review. However, the limitations associated with all the described strategies illustrates that a single optimum method is not yet available. Recombinant Ab fragments are valuable and robust tools for the fabrication of immunosensors. Unlike conventional Abs, rAb fragments are of small size, highly stable and can be easily genetically manipulated to have highly oriented immobilization on the sensor surface. In spite of these benefits of rAbs, their applications in the field of immunosensors are still not well exploited. Recombinant Abs are expected to show significant additional promise for the generation of Ab-based sensors with many novel applications in biomedical diagnostics.
Summary
Antibodies are large Y-shaped proteins produced by plasma cells that are utilized by the immune system to identify and target pathogens such as bacteria and viruses.
Their small size, high stability and easy genetic manipulation make recombinant antibody fragments valuable and robust tools for the fabrication of immunosensors.
Antibody-based biosensors have revolutionized diagnostics for the detection of a plethora of analytes such as food and environmental contaminants, biological warfare agents, illicit drugs and disease markers.
Immobilization of antibodies on to a sensor surface without altering their specificity and immunological activity is one of the most crucial steps in the fabrication of a successful immunosensor.
The immobilization step affects the detection limit, sensitivity and overall performance of the immunosensor.
Orientation of antibodies on sensor surfaces can be controlled by the interaction between specific reactive groups on the surface and on the antibody.
Abbreviations
- Ab
antibody
- BPA
bisphenol A
- CDR
complementarity-determining region
- CH
constant heavy
- CL
constant light
- CRP
C-reactive protein
- HGPRT
hypoxanthine guanine phosphoribosyltransferase
- Ig
immunoglobulin
- mAb
monoclonal antibody
- pAb
polyclonal antibody
- PAG
Protein A-gold-binding domain
- rAb
recombinant antibody
- scAb
single-chain Ab
- scFv
single-chain Fv
- VH
variable heavy
- VHH
variable domains of llama heavy-chain antibodies
- VL
variable light.
Funding
The financial support of the Programme for Research in Third Level Institutions through the Bioanalysis and Therapeutics (BioAT) postgraduate support scheme, the European Commission FP7 Programme through the Marie Curie Initial Training Network PROSENSE [grant number 317420, 2012–2016] and Science Foundation Ireland [grant number 10/CE/B1821] are gratefully acknowledged.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Conroy P.J., Hearty S., Leonard P., O'Kennedy R.J. Antibody production, design and use for biosensor-based applications. Semin. Cell Dev. Biol. 2009;20:10–26. doi: 10.1016/j.semcdb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Weiner L.M., Surana R., Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrebaeck C.A. Antibodies in diagnostics–from immunoassays to protein chips. Immunol. Today. 2000;21:379–382. doi: 10.1016/S0167-5699(00)01683-2. [DOI] [PubMed] [Google Scholar]
- 4.Morgan G., Levinsky R.J. Monoclonal antibodies in diagnosis and treatment. Arch. Dis. Child. 1985;60:96–98. doi: 10.1136/adc.60.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988;240:1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- 6.Plückthun A., Skerra A. Expression of functional antibody Fv and Fab fragments in Escherichia coli. Methods Enzymol. 1989;178:497–515. doi: 10.1016/0076-6879(89)78036-8. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad Z.A., Yeap S.K., Ali A.M., Ho W.Y., Alitheen N.B., Hamid M. ScFv antibody: principles and clinical application. Clin. Dev. Immunol. 2012;2012:980250. doi: 10.1155/2012/980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho M., Nagata S., Pastan I. Isolation of anti-CD22 Fv with high affinity by Fv display on human cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9637–9642. doi: 10.1073/pnas.0603653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choo A.B.H., Dunn R.D., Broady K.W., Raison R.L. Soluble expression of a functional recombinant cytolytic immunotoxin in insect cells. Protein Expr. Purif. 2002;24:338–347. doi: 10.1006/prep.2001.1589. [DOI] [PubMed] [Google Scholar]
- 10.Galeffi P., Lombardi A., Pietraforte I. Functional expression of a single-chain antibody to ErbB-2 in plants and cell-free systems. J. Transl. Med. 2006;4:39. doi: 10.1186/1479-5876-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbas C.F., III . Phage Display: a Laboratory Manual. 1st edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 12.Kim D., Herr A.E. Protein immobilization techniques for microfluidic assays. Biomicrofluidics. 2013;7:041501. doi: 10.1063/1.4816934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trilling A.K., Beekwilder J., Zuilhof H. Antibody orientation on biosensor surfaces: a minireview. Analyst. 2013;138:1619–1627. doi: 10.1039/c2an36787d. [DOI] [PubMed] [Google Scholar]
- 14.Kierny M.R., Cunningham T.D., Kay B.K. Detection of biomarkers using recombinant antibodies coupled to nanostructured platforms. Nano Rev. 2012;3:17240. doi: 10.3402/nano.v3i0.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Um H.J., Kim M., Lee S.H., Min J., Kim H., Choi Y.W., et al. Electrochemically oriented immobilization of antibody on poly-(2-cyano-ethylpyrrole)-coated gold electrode using a cyclic voltammetry. Talanta. 2011;84:330–334. doi: 10.1016/j.talanta.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 16.John R., Spencer M., Wallace G.G., Smyth M.R. Development of a polypyrrole-based human serum albumin sensor. Anal. Chim. Acta. 1991;249:381–385. doi: 10.1016/S0003-2670(00)83010-X. [DOI] [Google Scholar]
- 17.Sun X., Du S., Wang X., Zhao W., Li Q. A label-free electrochemical immunosensor for carbofuran detection based on a sol-gel entrapped antibody. Sensors. 2011;11:9520–9531. doi: 10.3390/s111009520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feyssa B., Liedert C., Kivimaki L., Johansson L.S., Jantunen H., Hakalahti L. Patterned immobilization of antibodies within roll-to-roll hot embossed polymeric microfluidic channels. PLoS One. 2013;8:e68918. doi: 10.1371/journal.pone.0068918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman M.A., Shiddiky M.J., Park J.S., Shim Y.B. An impedimetric immunosensor for the label-free detection of bisphenol A. Biosens. Bioelectron. 2007;22:2464–2470. doi: 10.1016/j.bios.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Jarocka U., Sawicka R., Góra-Sochacka A., Sirko A., Zagórski-Ostoja W., Radecki J., et al. An immunosensor based on antibody binding fragments attached to gold nanoparticles for the detection of peptides derived from Avian Influenza Hemagglutinin H5. Sensors. 2014;14:15714–15728. doi: 10.3390/s140915714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin P.C., Chen S.H., Wang K.Y., Chen M.L., Adak A.K., Hwu J.R.R., et al. Fabrication of oriented antibody-conjugated magnetic nanoprobes and their immunoaffinity application. Anal. Chem. 2009;81:8774–8782. doi: 10.1021/ac9012122. [DOI] [PubMed] [Google Scholar]
- 22.Shriver-Lake L.C., Donner B., Edelstein R., Breslin K., Bhatia S.K., Ligler F.S. Antibody immobilization using heterobifunctional crosslinkers. Biosens. Bioelectron. 1997;12:1101–1106. doi: 10.1016/S0956-5663(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 23.de Juan-Franco E., Caruz A., Pedrajas J.R., Lechuga L.M. Site-directed antibody immobilization using a protein A–gold binding domain fusion protein for enhanced SPR immunosensing. Analyst. 2013;138:2023–2031. doi: 10.1039/c3an36498d. [DOI] [PubMed] [Google Scholar]
- 24.Barton A.C., Collyer S.D., Davis F., Garifallou G.Z., Tsekenis G., Tully E., et al. Labeless AC impedimetric antibody-based sensors with pgml−1 sensitivities for point-of-care biomedical applications. Biosens. Bioelectron. 2009;24:1090–1095. doi: 10.1016/j.bios.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Ionescu R.E., Gondran C., Bouffier L., Jaffrezic-Renault N., Martelet C., Cosnier S. Label-free impedimetric immunosensor for sensitive detection of atrazine. Electrochim. Acta. 2010;55:6228–6232. doi: 10.1016/j.electacta.2009.11.029. [DOI] [Google Scholar]
- 26.Trilling A.K., Harmsen M.M., Ruigrok V.J., Zuilhof H., Beekwilder J. The effect of uniform capture molecule orientation on biosensor sensitivity: dependence on analyte properties. Biosens. Bioelectron. 2013;40:219–226. doi: 10.1016/j.bios.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Park M., Cella L.N., Chen W., Myung N.V., Mulchandani A. Carbon nanotubes-based chemiresistive immunosensor for small molecules: detection of nitroaromatic explosives. Biosens. Bioelectron. 2010;26:1297–1301. doi: 10.1016/j.bios.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chebil S., Hafaiedh I., Sauriat-Dorizon H., Jaffrezic-Renault N., Errachid A., Ali Z., et al. Electrochemical detection of d-dimer as deep vein thrombosis marker using single-chain d-dimer antibody immobilized on functionalized polypyrrole. Biosens. Bioelectron. 2010;26:736–742. doi: 10.1016/j.bios.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 29.Encarnação J.M., Rosa L., Rodrigues R., Pedro L., da Silva F.A., Gonçalves J., et al. Piezoelectric biosensors for biorecognition analysis: Application to the kinetic study of HIV-1 Vif protein binding to recombinant antibodies. J. Biotechnol. 2007;132:142–148. doi: 10.1016/j.jbiotec.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Dunne L., Daly S., Baxter A., Haughey S., O'Kennedy R. Surface plasmon resonance-based immunoassay for the detection of Aflatoxin B1 using single-chain antibody fragments. Spectrosc. Lett. 2005;38:229–245. doi: 10.1081/SL-200058689. [DOI] [Google Scholar]
- 31.Yan H., Shen Z., Mernaugh R., Zeng X. Single chain fragment variable recombinant antibody as a template for Fc sensors. Anal. Chem. 2011;83:625–630. doi: 10.1021/ac102087w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benhar I., Eshkenazi I., Neufeld T., Opatowsky J., Shaky S., Rishpon J. Recombinant single chain antibodies in bioelectrochemical sensors. Talanta. 2001;55:899–907. doi: 10.1016/S0039-9140(01)00497-0. [DOI] [PubMed] [Google Scholar]
- 33.Grennan K., Strachan G., Porter A.J., Killard A.J., Smyth M.R. Atrazine analysis using an amperometric immunosensor based on single-chain antibody fragments and regeneration-free multi-calibrant measurement. Anal. Chim. Acta. 2003;500:287–298. doi: 10.1016/S0003-2670(03)00942-5. [DOI] [Google Scholar]
- 34.Love T.E., Redmond C., Mayers C.N. Real time detection of anthrax spores using highly specific anti-EA1 recombinant antibodies produced by competitive panning. J. Immunol. Methods. 2008;334:1–10. doi: 10.1016/j.jim.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Grewal Y.S., Shiddiky M.J., Gray S.A., Weigel K.M., Cangelosi G.A., Trau M. Label-free electrochemical detection of an Entamoeba histolytica antigen using cell-free yeast-scFv probes. Chem. Commun. (Camb.) 2013;49:1551–1553. doi: 10.1039/c2cc38882k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kausaite-Minkstimiene A., Ramanavicius A., Ruksnaite J., Ramanaviciene A. A surface plasmon resonance immunosensor for human growth hormone based on fragmented antibodies. Anal. Methods. 2013;5:4757–4763. doi: 10.1039/c3ay40614h. [DOI] [Google Scholar]