Abstract
Objective:
To evaluate spatial displacement of breast lesions from prone MR to supine ultrasound positions, and to determine whether the degree of displacement may be associated with breast density and lesion histotype.
Methods:
380 patients underwent breast MR and second-look ultrasound. The MR and ultrasound lesion location within the breast gland, distances from anatomical landmarks (nipple, skin and pectoral muscle), spatial displacement (distance differences from the landmarks within the same breast region) and region displacement (breast region change) were prospectively evaluated. Differences between MR and ultrasound measurements, association between the degree of spatial displacement and both breast density and lesion histotypes were calculated.
Results:
In 290/380 (76%) patients, 300 MR lesions were detected. 285/300 (95%) lesions were recognized on ultrasound. By comparing MR and ultrasound, spatial displacement occurred in 183/285 (64.3%) cases while region displacement in 102/285 (35.7%) cases with a circumferential movement along an arc centred on the nipple, having supine ultrasound as the reference standard. A significant association between the degree of lesion displacement and breast density was found (p < 0.00001) with a significant higher displacement in case of fatty breasts. No significant association between the degree of displacement and lesion histotype was found (p = 0.1).
Conclusion:
Lesion spatial displacement from MRI to ultrasound may occur especially in adipose breasts. Lesion–nipple distance and circumferential displacement from the nipple need to be considered for ultrasound lesion detection.
Advances in knowledge:
Second-look ultrasound breast lesion detection could be improved by calculating the lesion–nipple distance and considering that spatial displacement from MRI occurs with a circumferential movement along an arc centred on the nipple.
INTRODUCTION
The role of dynamic contrast-enhanced MRI of the breast has been established in numerous research studies with MRI being able to improve the detection and characterization of primary and recurrent breast cancers and evaluate the response to therapy.1–10
Suspicious lesions at MRI of the breast require second-look ultrasound or MRI-guided biopsy. Second-look ultrasound with ultrasound-guided core biopsy represents an effective and widespread approach, offering some advantages compared with MRI-guided one.11,12 In fact, ultrasound-guided biopsy is less expensive, more readily available and more comfortable for the patient. Moreover, ultrasound-guided biopsy does not require intravenous contrast material injection and allows sampling of lesions located near the chest wall and nipple. However, MRI-guided biopsy remains mandatory when MRI lesions and second-look ultrasound findings do not match perfectly.12–14
For this reason, the main limitation of second-look ultrasound is that MRI information has to be translated into ultrasound examination. In fact, often, it is difficult to identify MRI lesions on second look ultrasound because lesion spatial or region displacement may occur changing from the prone to the supine patient positioning.15 Lesion spatial displacement occurs within the same breast region while region displacement refers to a lesion movement towards an adjacent region. Both the lesion location in the breast and the breast volume could be associated with spatial or region displacement.
The aim of this study was to evaluate the spatial displacement of breast lesions from prone MR to supine ultrasound patient positions, and to determine whether the degree of spatial displacement may be associated with breast density and lesion histotype.
METHODS AND MATERIALS
Patients
Between September 2011 and January 2014, a total of 380 patients [age range, 26–79 years; mean age ± standard deviation, 52.3 ± 9.2 years] with family history of breast cancer and dense glandular structure (120 out of 380 patients) or with suspected breast lesions detected by mammography (parenchymal distortion; diffuse and polymorphous microcalcifications; and ill-defined opacity) and ultrasound (inhomogeneous hypo-echoic areas; shadowing areas with irregular margins) (260 out of 380 patients) underwent MRI before histological examination. Written informed consent was obtained from all patients according to the Declaration of Helsinki principles. MR examination was performed in the second week of menstrual cycle in case of pre-menopausal females. In case of MRI positivity, ultrasound examination was performed within 7–10 days with meticulous exploration of all breast regions, and the detected breast lesions underwent ultrasound-guided cytological or histological examination. In case of MRI negativity, patients were invited to resume periodic mammography and ultrasound after 6 months.
MR protocol
MR examinations were performed in prone position with arms up on a 1.5 T MR device (Achieva®; Philips Medical Systems, Best, Netherlands) by using a four-channel breast coil. The protocol consisted of:
transverse short-inversion-time (TI) inversion recovery turbo-spin-echo sequence [repetition time (TR)/echo time (TE)/TI = 3.800/60/165 ms, field of view (FOV) = 250 × 450 mm [antero-posterior (AP) × right–left (RL)], matrix 168 × 300 pixels, 50 slices with 3-mm slice thickness and without gaps, 3 averages and turbo factor 23, resulting in a voxel size of 1.5 × 1.5 × 3.0 mm3];
transverse T2 weighted turbo spin echo (TR/TE = 6.300/130 ms, FOV = 250 × 450 mm (AP × RL), matrix 336 × 600 pixels, 50 slices with 3-mm slice thickness and without gaps, 3 averages, turbo factor 59, and sensitive encoding (SENSE) factor 1.7, resulting in a voxel size of 0.75 × 0.75 × 3.0 mm3);
three-dimensional dynamic contrast-enhanced T1 weighted high-resolution isotropic volume sequences (TR/TE = 4.4/2.0 ms, FOV = 250 × 450 × 150 mm (AP × RL × FH), matrix 168 × 300 pixels, 100 slices with 1.5-mm slice thickness, turbo factor 50, SENSE factor 1.6 and 6 dynamic acquisitions, resulting in 1.5-mm3 isotropic voxels a dynamic data acquisition time of 1 min 30 s and total sequence duration of 9 min).
Gadobenate dimeglumine (Multihance®; Bracco, Milan, Italy) was intravenously injected at a dose of 0.1 mmol kg−1 of body weight and flow rate of 1.5 ml s−1, followed by 20 ml of saline solution, the most accurate contrast medium used in this field, as already reported in other experiences.16
Image and statistical analysis
All MRI data were transferred to and analysed on a diagnostic workstation equipped with dedicated software for MRI examination (View-Forum R5.1 V1L1 2006). Two radiologists with 5 years' experience in the field of breast imaging prospectively evaluated MRI data searching for mass-like breast lesions. In order to evaluate lesion displacement from MR to ultrasound examination, all foci or non-mass-like lesions which can be difficult to identify on ultrasound were excluded.
For each detected lesion, the position and the following distances from such anatomical landmarks as the nipple, skin and pectoral muscle were assessed on MR images (Figures 1–3), as similarly and already reported by Carbonaro et al,17 as follows:
distance from the lesion margin to the horizontal plane passing through the nipple on coronal images;
distance from the lesion margin to the vertical plane passing through the nipple on coronal images;
distance from the lesion margin to the skin on axial images;
distance from the lesion margin to the pectoral muscle on axial images;
distance from the lesion margin to the nipple on sagittal images.
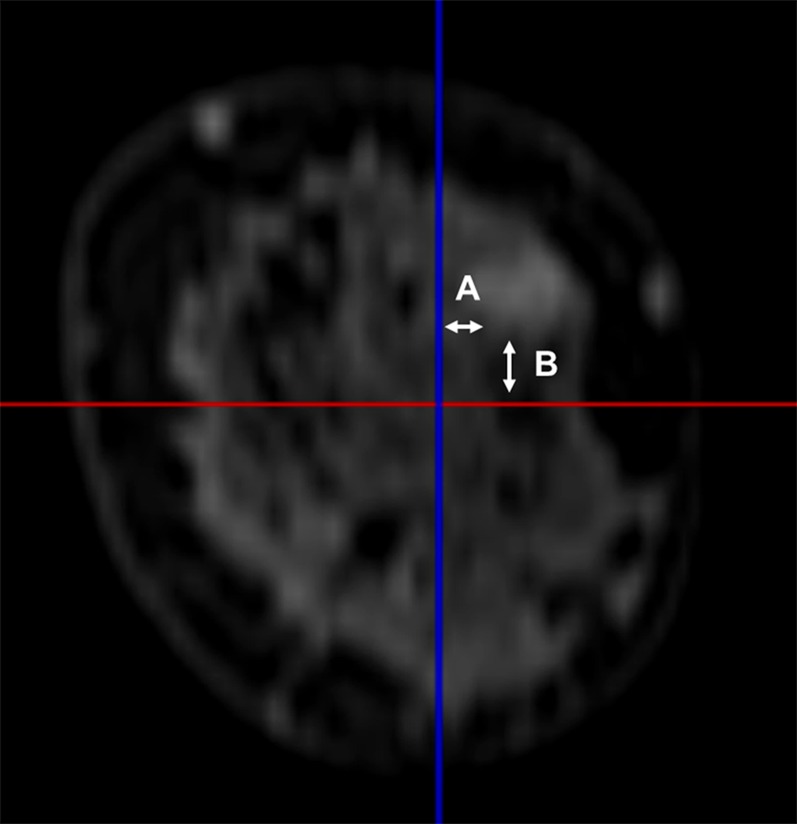
Figure 1.
MR coronal image showing the distance from the lesion margin to the vertical (A) and horizontal planes (B) passing through the nipple.
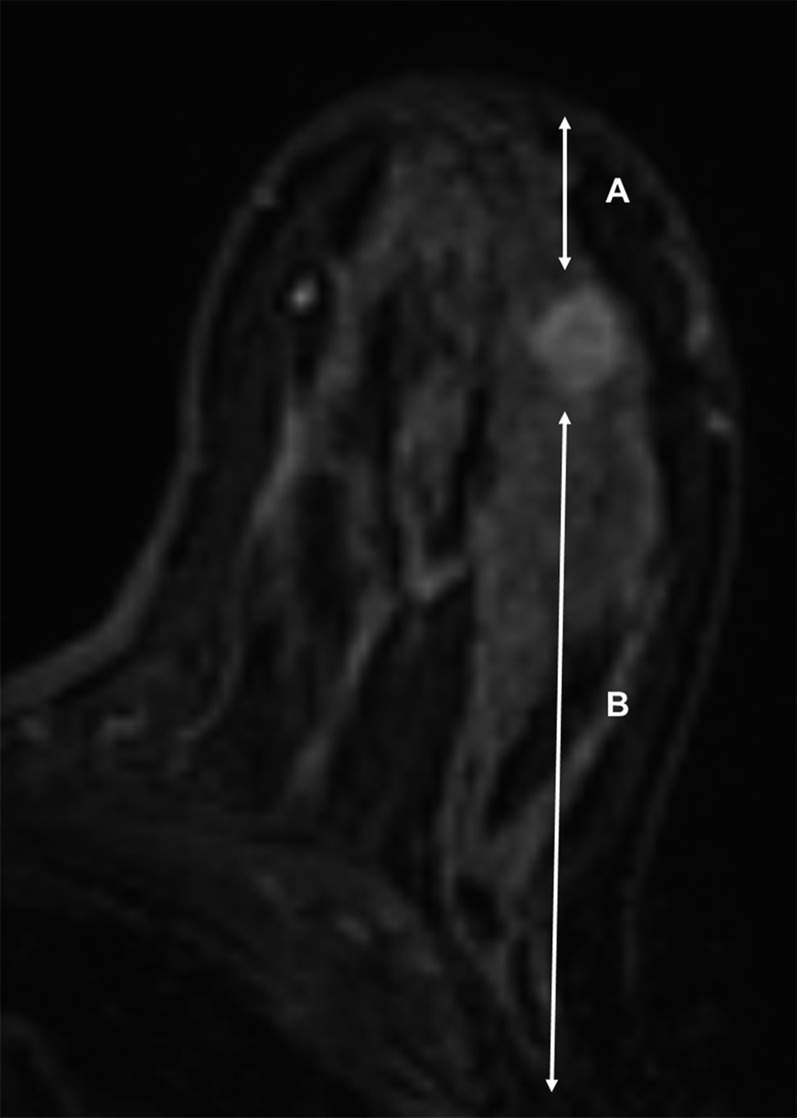
Figure 3.
MR sagittal image showing the distance (double headed arrow) from the lesion margin to the nipple.
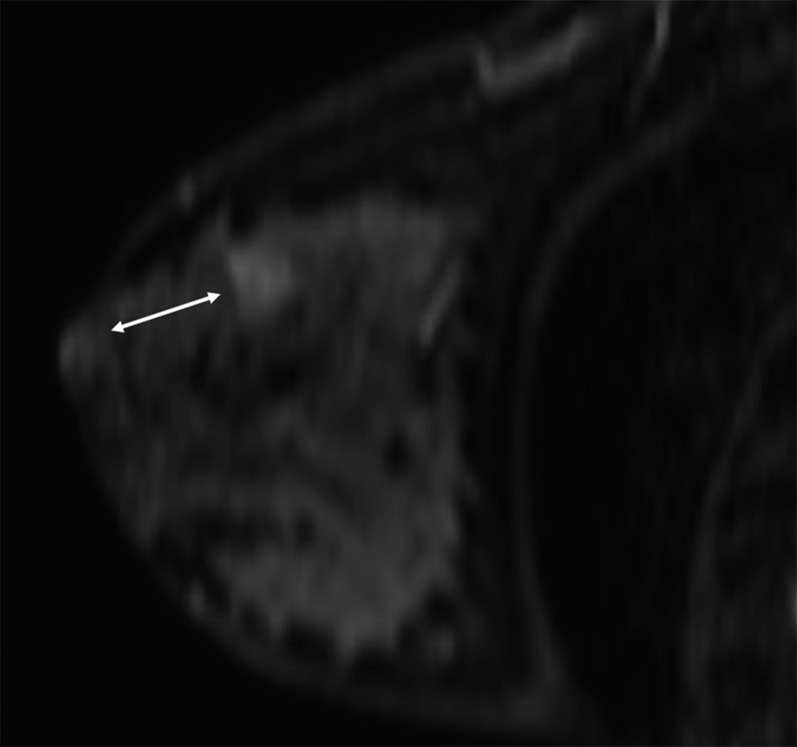
Figure 2.
MR axial image showing the distance from the lesion margin to the skin (A) and the pectoral muscle (B).
In order to assess the interobserver agreement between the two readers and the reliability of MR measurements, Cohen's kappa (κ) test was applied. A value of κ >0.81 was considered representative of almost perfect agreement while values of 0.61–0.80 and 0.41–0.60 were considered representative of substantial and moderate agreement, respectively.
Ultrasound examination was performed by one of the two radiologists within 7–10 days in case of MRI positivity, evaluating both the already known breast lesions and any incidental findings at MRI. Ultrasound examinations were performed with patients in the supine positions and arms up and in the supine oblique position for examining the outer breast regions. A radial technique was used in all cases, with the ultrasound probe held vertically. For each ultrasound-detected lesion, the position and the following distances from such anatomical landmarks as the nipple, skin and pectoral muscle were assessed (Figures 4–6):
distance from the lesion margin to the horizontal line passing through the nipple
distance from the lesion margin to the vertical line passing through the nipple
distance from the lesion margin to the skin
distance from the lesion margin to the pectoral muscle
distance from the lesion margin to the nipple.
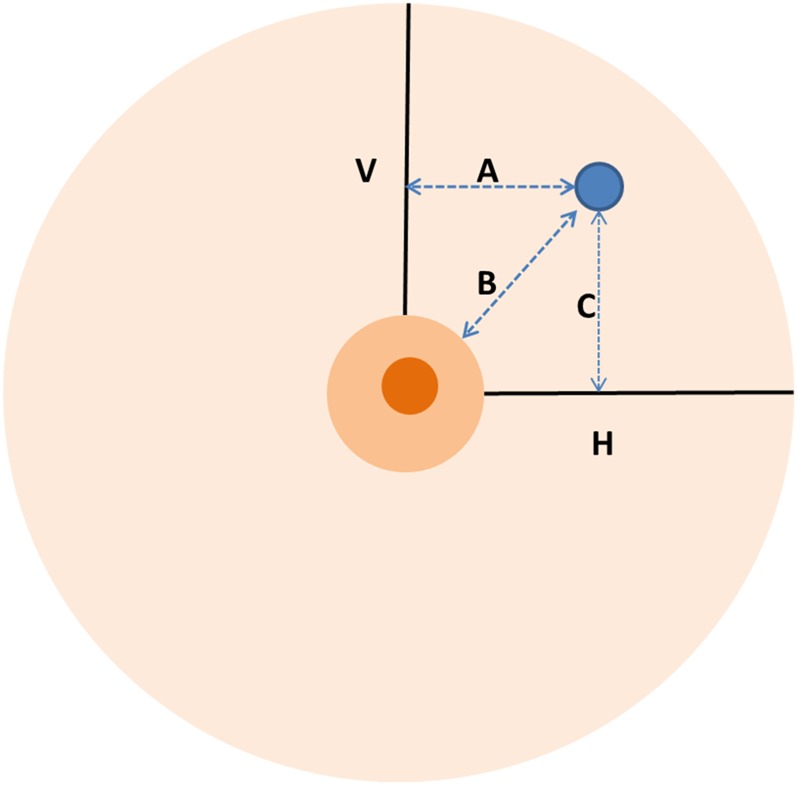
Figure 4.
Ultrasound technique for measuring the distance from the lesion margin to the vertical (A) and horizontal lines (C) passing through the nipple and to the nipple (B). H, horizontal line; V, vertical line.
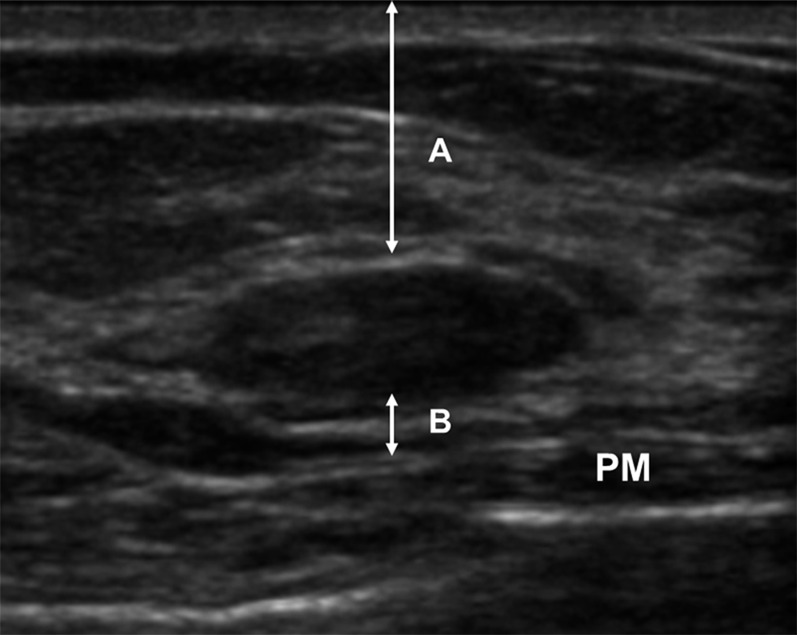
Figure 6.
Ultrasound measurement of the distance from the lesion margin to the skin (A) and the pectoral muscle (B). PM, pectoral muscle.
Figure 5.
Ultrasound technique for measuring the distance from the lesion margin to the skin (A) and the pectoral muscle (B). PM, pectoral muscle.
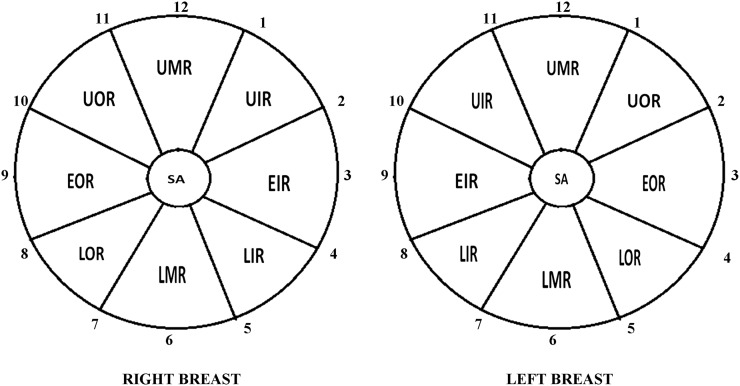
The spatial displacement was evaluated by considering the region in which breast lesions were assigned by the radiologists on MRI and ultrasound examinations. Breast glands were divided into nine regions based on the clock positions (eight 45 ° segments plus a central subareolar region) in order to identify even minimum lesion displacement (Figure 7). In particular:
upper outer region (UOR) (centred between 1 and 2 o'clock in the left breast and between 10 and 11 o'clock in the right breast)
equatorial outer region (EOR) (centred on 3 o'clock in the left breast and on 9 o'clock in the right breast)
lower outer region (LOR) (centred between 4 and 5 o'clock in the left breast and between 7 and 8 o'clock in the right breast)
lower median region (LMR) (centred on 6 o'clock for both breasts)
lower inner region (LIR) (centred between 7 and 8 o'clock in the left breast and between 4 and 5 o'clock in the right breast)
equatorial inner region (EIR) (centred on 9 o'clock in the left breast and on 3 o'clock in the right breast)
upper inner region (UIR) (centred between 10 and 11 o'clock in the left breast and between 1 and 2 o'clock in the right breast)
upper median region (UMR) (centred on 12 o'clock for both breasts)
subareolar (SA) region (the glandular tissue located in the central part of the breast extending from the nipple to the pectoral muscle).
Figure 7.
Graphical representation of breast division into nine regions (eight 45° segments plus a central subareolar region) based on the clock positions. EIR, equatorial inner region; EOR, equatorial outer region; LIR, lower inner region; LMR, lower median region; LOR, lower outer region; SA, subareolar; UIR, upper inner region; UMR, upper median region; UOR, upper outer region.
By considering the lesion distances from the above-mentioned anatomical landmarks (nipple, skin and pectoral muscle), any difference between MRI and ultrasound measurements was calculated.
χ2 test was used to evaluate the association between the degree of spatial displacement and the breast density according to Breast Imaging Reporting and Data System classification18 and the association between the degree of spatial displacement and the benign or malignant nature of breast lesions, having the histological examination as the reference standard.
RESULTS
In 290 out of 380 (76%) patients, MR sequences indicated the presence of 300 mass-like breast lesions including five multicentric lesions. 285 out of 300 (95%) lesions were recognized on ultrasound. 260 out of 300 (86%) lesions corresponded to the suspected breast lesions as detected by mammography and ultrasound; 40 out of 300 (14%) represented incidental findings on MR examination. 25 out of them (63%) were recognized on second look ultrasound. In the remaining 15 (37%) patients, breast MR and ultrasound examinations repeated after 6 months confirmed the absence of breast lesions. Ultrasound-guided histological examination revealed 100 malignant lesions (invasive ductal carcinoma n = 85; invasive lobular carcinoma n = 15) and 185 benign lesions (cysts n = 39; fibrotic areas n = 19; fibroadenolipoma n = 32; fibroadenomas n = 95). The breast lesion size ranged between 0.7 cm and 2.5 cm (mean diameter ± standard deviation, 1.6 ± 9 cm).
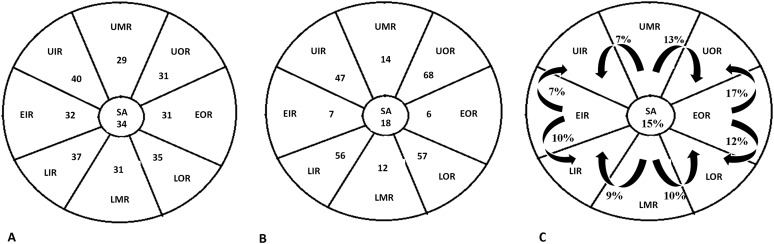
On MR examination, 31 breast lesions were located in UOR (10.3%), 31 in EOR (10.3%), 35 in LOR (11.6%), 31 in LMR (10.3%), 37 in LIR (12.3%), 32 in EIR (10.6%), 40 in UIR (13.3%), 29 in UMR (9.6%) and 35 in SA position (11.3%).
On ultrasound examination, 68 breast lesions were located in UOR (23.8%), 6 in EOR (2.1%), 57 in LOR (20%), 12 in LMR (4.2%), 56 in LIR (19.6%), 7 in EIR (2.4%), 47 in UIR (16.4%), 14 in UMR (4.9%) and 18 in SA (6.3%).
By comparing lesion position on MRI and ultrasound, region displacement occurred in 102 out 285 (35.7%) cases (Figures 8, 9; Table 1).
Figure 8.
Breast lesion spatial distribution on MRI (a) and on second-look ultrasound (b). (c) Graphical representation of region displacement from MRI to second-look ultrasound showing a circumferential position changing of lesions along an arc centred on the nipple. EIR, equatorial inner region; EOR, equatorial outer region; LIR, lower inner region; LMR, lower median region; LOR, lower outer region; SA, subareolar; UIR, upper inner region; UMR, upper median region; UOR, upper outer region.
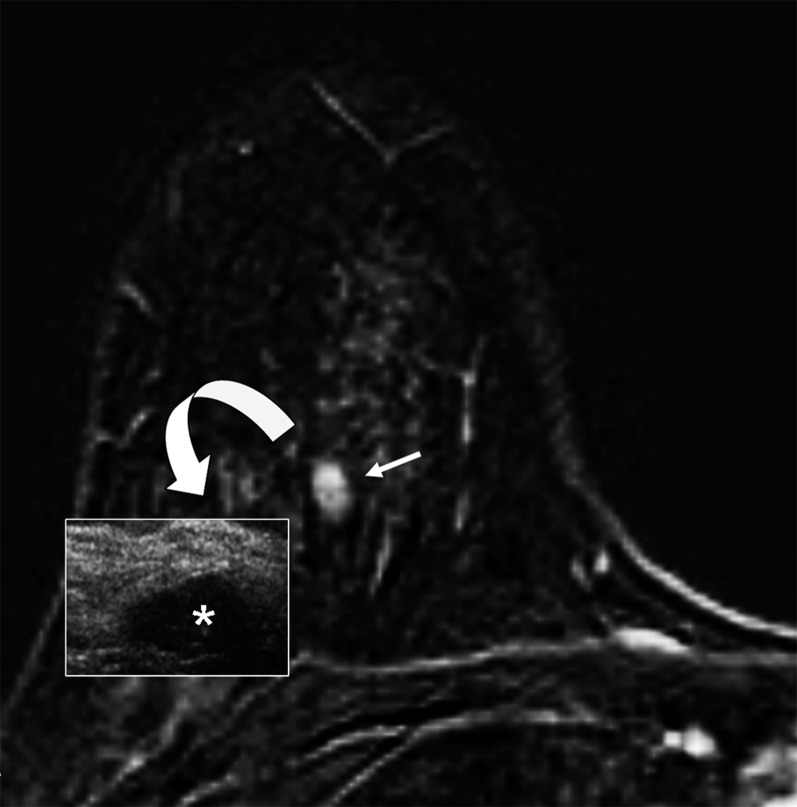
Figure 9.
Example of spatial displacement (curved arrow) from the MR lower median region (arrow) to the lower external region at second-look ultrasound (asterisk) in a case of incidental fibroadenoma of the right breast.
Table 1.
Percentages of breast lesions which showed spatial or region displacement from MRI to ultrasound examination
| Ultrasound-detected lesions | 285 |
|---|---|
| Spatial displacement from MRI | 183 (64.3%) |
| Region displacement from MRI | 102 (35.7%) |
In the remaining 183 out 285 (64.3%) cases, lesion spatial displacement occurred within the same region (Table 1). The mean difference between the measurements obtained on MR examination and on ultrasound resulted of 5 ± 3.2 cm for the distance from lesion margin to the horizontal plane passing through the nipple; 5 ± 2.5 cm for distance from lesion margin to vertical plane passing through the nipple; 0.8 ± 0.5 cm for distance from lesion margin to the skin; 2.5 ± 1.3 cm for the distance from lesion margin to the pectoral muscle; 0.7 ± 0.2 cm for the distance from lesion margin to the nipple (Table 2).
Table 2.
Mean values of difference between MR and ultrasound measurements of distances from the lesion margins to the anatomical landmarks
| MR/ultrasound distances between lesion and anatomical landmarks | MR/ultrasound measurement difference, mean value ± standard deviation |
|---|---|
| Lesion—horizontal plane/line through the nipple | 5 cm ± 3.2 cm |
| Lesion—vertical plane/line through the nipple | 5 cm ± 2.5 cm |
| Lesion—skin | 0.8 cm ± 0.5 cm |
| Lesion—pectoral muscle | 2.5 cm ± 1.3 cm |
| Lesion—nipple | 0.7 cm ± 0.2 cm |
In the group of breast lesions which showed region displacement, 50 were almost entirely fatty with <25% glandular tissue, 20 contained scattered fibro-glandular densities with 25–50% glandular tissue, 15 were heterogeneously dense with 51–75% glandular tissue and 17 extremely dense with >75% glandular. In the group of breast lesions which did not show region displacement, 20 were almost entirely fatty with <25% glandular tissue, 28 contained scattered fibro-glandular densities with 25–50% glandular tissue, 70 were heterogeneously dense with 51–75% glandular tissue and 65 extremely dense with >75% glandular.
χ2 test revealed a statistically significant association between the degree of spatial displacement of lesions and breast density (p-value < 0.00001).
In the group of breast lesions which showed region displacement, 60 out of 102 (58%) lesions resulted as benign and 42 out of 102 (42%) lesions as malignant. In the group of breast lesions which did not show region displacement, 125 out of 183 (68%) lesions resulted as be benign and 58 out of 183 (32%) malignant. χ2 test revealed no statistically significant association between the degree of spatial displacement and the nature of breast lesions (p-value = 0.1).
Almost perfect agreement between the two readers for MR measurements was found (k = 0.86).
DISCUSSION
Second-look ultrasound is widely used in clinical practice and allows finding a correlation to additional MR findings in 64% of cases (range 23–89% cases).15,19–26 Ultrasound lesion detection is influenced by lesion echo texture compared with breast background tissue and by lesion MRI appearance. A higher correlation of second-look ultrasound for mass lesions rather than non-mass ones has been reported.15 In our series, all the included lesions were mass-like at MRI. 40 out of 300 (14%) MRI-detected lesions represented incidental findings. 25 (63%) lesions were recognized on second-look ultrasound examination, underwent histological examination and resulted as being benign in all cases.
The identification of breast lesions at second-look ultrasound is crucial for ultrasound-guided breast biopsies and pre-surgical wire-localization procedures. However, it could be limited by patient positioning differences between ultrasound and MR examinations. Comstock and Tartar27 suggest not being rigid about ultrasound localization of breast MRI findings. In fact, positioning differences cause considerable variability in the apparent position of the corresponding lesions. They further suggest to examine with ultrasound at least a quadrant's worth of tissue on either side of the clock position of any concerning lesion identified on MRI. The distance from the nipple is considered the most reliable measurement.28
In our series, the distance from the lesion margin to the nipple represented the most reliable measurement with a variation of 0.7 ± 0.2 cm between MRI and ultrasound. For this reason, it could be considered as a reference distance for locating breast lesions on ultrasound. On the other side, distances from the lesion margin to the horizontal and vertical planes passing through the nipple, skin and pectoral muscle showed considerable variations with patient positioning. Comstock and Tartar also suggested that ultrasound performed by the same radiologist who interpreted MRI could increase its accuracy.15,27–29 For this purpose, in our study, both MR and ultrasound examinations were performed by the same radiologist as often occurs in clinical practice. We calculated interobserver agreement for MR measurements because MR reliability ensures correct establishment of the lesion position and prediction of how the lesion will move at ultrasound. Almost perfect agreement has been demonstrated in our series, showing the reliability of the used MR measurements.
Moreover, Trop et al suggested an ultrasound lateral patient position in order to improve lesion identification. In our series, we used only the oblique supine position for examining the outer breast regions.30
Fausto et al proposed an ultrasound/MRI co-registration with volume navigation, combining the two different techniques.15,31 However, the image quality of supine breast MRI could be compromised by respiratory artefacts and by the lack of a dedicated coil, as reported by Tozaki et al32
Our study aimed to assess lesion spatial displacement with patient positioning. In fact, few studies are reported in the literature about the degree of lesion spatial displacement and the possible existence of spatial displacement vectors.20,27
All lesions showed even minimal displacement between prone MRI and supine ultrasound, probably owing to the change of patient positioning and breast density. In 64.3% of cases, lesion spatial displacement occurred within the same region. In the remaining 35.7% of cases, region displacement occurred with a statistically significant association with almost entirely fatty or fibroglandular breasts. The greater amount of adipose tissue could cause considerable variability in the lesion apparent position and also a more difficult lesion detection.
However, fatty breasts tend to be larger than dense ones, and lesion displacement may depend on the breast size rather than on density in these cases.
The absence of a statistically significant association between the degree of spatial displacement and lesion histotype could suggest that displacement is more influenced by breast density as compared with lesion histotype.
In case of region displacement, our data suggest that lesions located in the median and equatorial regions tend to move more frequently and typically to the inner and outer ones. This circumferential movement along an arc centred on the nipple is probably related to the breast shape which is elongated during MR examination and which becomes flat and lies on the chest wall during ultrasound. Therefore, the distance from the lesion margin to the nipple provided by MRI and the knowledge of the most frequent vectors of lesion spatial displacement could represent useful tools for detecting breast lesions on second-look ultrasound. In fact, our results suggest that it is possible to predict lesion movement from MRI to ultrasound.
Our study presents some limitations mainly represented by the relatively small number of incidental MR findings undergoing second-look ultrasound; the potential selection bias due to the presence of already-known breast lesions among MR findings; the lack of a correlation between lesion displacement and size; the potential lower reliability of ultrasound measurements being an operator-dependent technique; the absence of MR-guided biopsies and of non-mass-like MRI lesions; and the influence of ultrasound probe compression on measurements causing potential differences with MRI.
CONCLUSION
Patient positioning differences existing between MR and ultrasound could cause lesion spatial displacement which is more frequent in the adipose breasts. Ultrasound breast lesion detection could be improved by calculating the distance between the lesion and the nipple, which is quite similar in the two diagnostic techniques, and by considering that spatial displacement occurs in a circumferential way along an arc centred on the nipple. Second-look ultrasound should be extended to the quadrants in which MRI lesions are expected to move in order to improve their detection which is crucial for ultrasound-guided biopsies and wire-localization procedures.
Contributor Information
Michele Telegrafo, Email: mikitele@hotmail.it.
Leonarda Rella, Email: lea.rella@gmail.com.
Amato A Stabile Ianora, Email: mtelegrafo02@gmail.com.
REFERENCES
- 1.Heywang-Köbrunner SH. Contrast-enhanced magnetic resonance imaging of the breast. Invest Radiol 1994; 29: 94–104. [DOI] [PubMed] [Google Scholar]
- 2.Orel SG, Schnall MD, Livolsi VA, Troupin RH. Suspicious breast lesions: MR imaging with radiologic-pathologic correlation. Radiology 1994; 190: 485–93. doi: 10.1148/radiology.190.2.8284404 [DOI] [PubMed] [Google Scholar]
- 3.Huang W, Fisher PR, Dulaimy K, Tudorica LA, O'Hea B, Button TM. Detection of breast malignancy: diagnostic MR protocol for improved specificity. Radiology 2004; 232: 585–91. doi: 10.1148/radiol.2322030547 [DOI] [PubMed] [Google Scholar]
- 4.Kuhl CK. MRI of breast tumors. Eur Radiol 2000; 10: 46–58. doi: 10.1007/s003300050006 [DOI] [PubMed] [Google Scholar]
- 5.Baum F, Fischer U, Vosshenrich R, Grabbe E. Classification of hypervascularized lesions in CE MR imaging of the breast. Eur Radiol 2002; 12: 1087–92. doi: 10.1007/s00330-001-1213-1 [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann U, Brix G, Knopp MV, Hess T, Lorenz WJ. Pharmacokinetic mapping of the breast: a new method for dynamic MR mammography. Magn Reson Med 1995; 33: 506–14. doi: 10.1002/mrm.1910330408 [DOI] [PubMed] [Google Scholar]
- 7.Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 1999; 211: 101–10. doi: 10.1148/radiology.211.1.r99ap38101 [DOI] [PubMed] [Google Scholar]
- 8.Orel SG. High-resolution MR imaging for the detection, diagnosis, and staging of breast cancer. Radiographics 1998; 18: 903–12. doi: 10.1148/radiographics.18.4.9672975 [DOI] [PubMed] [Google Scholar]
- 9.Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 2008; 246: 116–24. doi: 10.1148/radiol.2461061298 [DOI] [PubMed] [Google Scholar]
- 10.Moschetta M, Telegrafo M, Rella L, Capolongo A, Stabile Ianora AA, Angelelli G. MR evaluation of breast lesions obtained by diffusion-weighted imaging with background body signal suppression (DWIBS) and correlations with histological findings. Magn Reson Imaging 2014; 32: 605–9. doi: 10.1016/j.mri.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 11.Demartini WB, Eby PR, Peacock S, Lehman CD. Utility of targeted sonography for breast lesions that were suspicious on MRI. AJR Am J Roentgenol 2009; 192: 1128–34. doi: 10.2214/AJR.07.3987 [DOI] [PubMed] [Google Scholar]
- 12.Meissnitzer M, Dershaw DD, Lee CH, Morris EA. Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended. AJR Am J Roentgenol 2009; 193: 1025–9. doi: 10.2214/AJR.09.2480 [DOI] [PubMed] [Google Scholar]
- 13.Abe H, Schmidt RA, Shah RN, Shimauchi A, Kulkarni K, Sennett CA, et al. MR-detected (“Second-Look”) ultrasound examination for breast lesions detected initially on MRI: MR and sonographic findings. AJR Am J Roentgenol 2010; 194: 370–7. doi: 10.2214/AJR.09.2707 [DOI] [PubMed] [Google Scholar]
- 14.Leung JW. Second-look ultrasound: only for biopsy or more? Eur J Radiol 2012; 81(Suppl. 1): S87–9. doi: 10.1016/S0720-048X(12)70035-9 [DOI] [PubMed] [Google Scholar]
- 15.Fausto A, Casella D, Mantovani L, Giacalone G, Volterrani L. Clinical value of second-look ultrasound: is there a way to make it objective? Eur J Radiol 2012; 81(Suppl. 1): S36–40. doi: 10.1016/S0720-048X(12)70015-3 [DOI] [PubMed] [Google Scholar]
- 16.Pediconi F, Catalano C, Occhiato R, Venditti F, Fraioli F, Napoli A, et al. Breast lesion detection and characterization at contrast-enhanced MR mammography: gadobenate dimeglumine versus gadopentat dimeglumine. Radiology 2005; 237: 45–56. doi: 10.1148/radiol.2371041369 [DOI] [PubMed] [Google Scholar]
- 17.Carbonaro LA, Tannaphai P, Trimboli RM, Verardi N, Fedeli MP, Sardanelli F. Contrast enhanced breast MRI: spatial displacement from prone to supine patient's position. Preliminary results. Eur J Radiol 2012; 81: e771–4. doi: 10.1016/j.ejrad.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 18.Boyer B, Canale S, Arfi-Rouche J, Monzani Q, Khaled W, Balleyguier C. Variability and errors when applying the BIRADS mammography classification. Eur J Radiol 2013; 82: 388–97. doi: 10.1016/j.ejrad.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 19.LaTrenta LR, Menell JH, Morris EA, Abramson AF, Dershaw DD, Liberman L. Breast lesions detected with MR imaging: utility and histopathologic importance of identification with US. Radiology 2003; 227: 856–61. doi: 10.1148/radiol.2273012210 [DOI] [PubMed] [Google Scholar]
- 20.Sim LS, Hendriks JH, Bult P, Fook-Chong SM. US correlation for MRI-detected breast lesions in women with familial risk of breast cancer. Clin Radiol 2005; 60: 801–6. doi: 10.1016/j.crad.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 21.Beran L, Liang W, Nims T, Paquelet J, Sickle-Santanello B. Correlation of targeted ultrasound with magnetic resonance imaging abnormalities of the breast. Am J Surg 2005; 190: 592–4. doi: 10.1016/j.amjsurg.2005.06.019 [DOI] [PubMed] [Google Scholar]
- 22.Shin JH, Han BK, Choe YH, Ko K, Choi N. Targeted ultrasound for MR-detected lesions in breast cancer patients. Korean J Radiol 2007; 8: 475–83. doi: 10.3348/kjr.2007.8.6.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Candelaria R, Fornage BD. Second-look US examination of MR-detected breast lesions. J Clin Ultrasound 2011; 39: 115–21. doi: 10.1002/jcu.20784 [DOI] [PubMed] [Google Scholar]
- 24.Luciani ML, Pediconi F, Telesca M, Vasselli F, Casali V, Miglio E, et al. Incidental enhancing lesions found on preoperative breast MRI: management and role of second-look ultrasound. [In Italian.] Radiol Med 2011; 116: 886–904. doi: 10.1007/s11547-011-0630-8 [DOI] [PubMed] [Google Scholar]
- 25.Laguna AD, Arranz SJ, Checa VQ, Roca SA, Jiménez DE, Oliver-Goldaracena J. Sonographic findings of additional malignant lesions in breast carcinoma seen by second look ultrasound. J Clin Imaging Sci 2011; 1: 34. doi: 10.4103/2156-7514.82338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spick C, Baltzer PA. Diagnostic utility of second-look US for breast lesions identified at MR imaging: systematic review and meta-analysis. Radiology 2014; 273: 401–9. doi: 10.1148/radiol.14140474 [DOI] [PubMed] [Google Scholar]
- 27.Comstock CC, Tartar M. Detection of breast cancer: screening of asymptomatic patients. Case 12. In: Tartar M, Comstock CC, Kipper MS, eds. Breast cancer imaging, 1st edn. New York: Elsevier Health Sciences–Mosby; 2008. pp. 26–8. [Google Scholar]
- 28.Erguvan-Dogan B, Whitman GJ. Breast ultrasound MR imaging correlation. Ultrasound Clin 2007; 1: 593–601. doi: 10.1016/j.cult.2006.10.001 [DOI] [Google Scholar]
- 29.McMahon K, Medoro L, Kennedy D. Breast magnetic resonance imaging: an essential role in malignant axillary lymphadenopathy of unknown origin. Australas Radiol 2005; 49: 382–9. doi: 10.1111/j.1440-1673.2005.01499.x [DOI] [PubMed] [Google Scholar]
- 30.Trop I, Labelle M, David J, Mayrand MH, Lalonde L. Second-look targeted studies after breast magnetic resonance imaging: practical tips to improve lesion identification. Curr Probl Diagn Radiol 2010; 39: 200–11. doi: 10.1067/j.cpradiol.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 31.Fausto A, Rizzatto G, Preziosa A, Gaburro L, Washburn MJ, Rubello D, et al. A new method to combine contrast-enhanced magnetic resonance imaging during live ultrasound of the breast using volume navigation technique: a study for evaluating feasibility, accuracy and reproducibility in healthy volunteers. Eur J Radiol 2012; 81: e332–7. doi: 10.1016/j.ejrad.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 32.Tozaki M, Fukuda K. Supine MR mammography using VIBE with parallel acquisition technique for the planning of breast-conserving surgery: clinical feasibility. Breast 2006; 15: 137–40. doi: 10.1016/j.breast.2005.03.003 [DOI] [PubMed] [Google Scholar]