Abstract
Objective:
To investigate the prognostic value of fluorine-18 fludeoxyglucose (FDG) positron emission tomography (PET) parameters for small-cell lung cancer (SCLC), according to the primary tumour location, adjusted by conventional prognostic factors.
Methods:
From 2008 to 2013, we enrolled consecutive patients with histologically proven SCLC, who had undergone FDG-PET/CT prior to initial therapy. The primary tumour location was categorized into central or peripheral types. PET parameters and clinical variables were evaluated using univariate and multivariate analysis.
Results:
A total of 69 patients were enrolled in this study; 28 of these patients were categorized as having the central type and 41 patients as having the peripheral type. In univariate analysis, stage, serum neuron-specific enolase, whole-body metabolic tumour volume (WB-MTV) and whole-body total lesion glycolysis (WB-TLG) were found to be significant in both types of patients. In multivariate analysis, the independent prognostic factor was found to be stage in the central type, but WB-MTV and WB-TLG in the peripheral type. Kaplan–Meier analysis demonstrated that patients with peripheral type with limited disease and low WB-MTV or WB-TLG showed significantly better overall survival than all of the other groups (p < 0.0083).
Conclusion:
The FDG-PET volumetric parameters were demonstrated to be significant and independent prognostic factors in patients with peripheral type of SCLC, while stage was the only independent prognostic factor in patients with central type of SCLC.
Advances in knowledge:
FDG-PET is a non-invasive method that could potentially be used to estimate the prognosis of patients, especially those with peripheral-type SCLC.
INTRODUCTION
Small-cell lung cancer (SCLC) is one of the most aggressive cancers with frequent recurrence, even after an initial favourable response to cytotoxic treatments. In the USA, despite the development of radical therapies, 5-year survival remains at 20–25% for limited disease (LD) and 5% for extensive disease (ED).1 Several prognostic factors for SCLC have previously been reported including stage, age, sex, performance status (PS) and serum lactate dehydrogenase (LDH).2–5 Among them, clinical stage is regarded as the main prognostic factor in clinical practice. No other factor has been well investigated, which could be a potential trigger for subdividing SCLC and existing therapies, although other clinical indices such as age and PS are often referred to in deciding the therapy regimen.
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT-based imaging parameters such as maximum standardized uptake value (SUVmax), metabolic tumour volume (MTV) and total lesion glycolysis (TLG) have been suggested as prognostic factors for various neoplasms.6–9 As for SCLC, only a small number of studies have investigated the relationship between FDG-PET/CT parameters and prognosis.10–12 They regarded whole-body metabolic tumour volume (WB-MTV) as one of the important prognostic factors; however, this has not been well established.
The standard therapy regimens for SCLC have not changed in a long time. This may be because the pathological diagnosis of SCLC is usually sufficient with electroscopic observation. Special immunohistochemical staining is sometimes required but not always necessary, whereas many driver mutations have been detected and surveyed routinely for non-small-cell cancer, especially for adenocarcinoma; this has helped improve prognosis dramatically in some cases. The possible biological difference between centrally and peripherally located SCLC has been discussed in a few articles.13–15 For example, it has been pointed out that hilar nodes in SCLC tend to be involved early and massively, when the primary lesion is located in the peripheral lung parenchyma.13 However, differences in prognosis according to tumour location remain controversial.14,16,17 Although pathological differences have not yet been well established, FDG-PET/CT can supposedly be a helpful tool in observing tumour characteristics and prognosis non-invasively, especially SCLC, where surgery is usually not indicated and direct observation of the entire tumour is unachievable.
The aim of the present study was to investigate the prognostic value of FDG-PET parameters such as SUVmax, WB-MTV and whole-body total lesion glycolysis (WB-TLG) for SCLC, according to the primary tumour location, adjusted by those of conventional prognostic factors in patients with SCLC.
METHODS AND MATERIALS
Patients
From January 2008 to December 2013, a total of 112 consecutive patients who were pathologically diagnosed with SCLC at Kyoto University Hospital were enrolled in the study. We included the patients who had undergone pre-treatment FDG-PET/CT, followed by treatment with at least one course of standard chemotherapy at our institution. The exclusion criteria were combined SCLC and coexisting malignancy. 43 patients were excluded based on the criteria as follows: 39 patients had no PET/CT prior to initial therapy; 3 patients were diagnosed as having combined SCLC; and the remaining 1 patient had coexisting oesophageal cancer. In total, 69 patients (male : female ratio, 57 : 12; median age, 75 years; range, 56–86 years) were eligible for the study.
This retrospective study was approved by the institutional review board, and written informed consent was obtained from each patient regarding access of their data.
Positron emission tomography/CT image acquisition
PET/CT scanning was performed using a combined PET/CT scanner [Discovery ST Elite, GE Healthcare, Waukesha, WI (n = 11); Biograph™ 16 TruePoint™, Siemens Healthcare, Erlangen, Germany (n = 28); Biograph™ 64 TruePoint™, Siemens Healthcare (n = 30)]. We confirmed that each scanner underwent routine maintenance using a consistent cross-calibration factor with an error of <5%. All patients fasted for ≥4 h before the administration of FDG. The plasma glucose level at the time of FDG injection was confirmed to be <200 mg dl−1 in all patients. PET emission data acquisition commenced at 71.8 ± 14.8 (mean ± SD) min after injection of a dose of 3.7–4.5 MBq kg−1 of FDG; a whole-body scan was conducted starting at the level of the thigh and extending to the head. CT scans were acquired for the same coverage with a range of 20–120 mAs of current–time product. The PET images obtained were reconstructed with CT-derived attenuation correction using the iterative reconstruction algorithm.
Measurements of positron emission tomography/CT parameters
The PET images were displayed on a workstation (Advantage Workstation, v. 4.4: GE Healthcare, Waukesha, WI). The outcome of the patients was not disclosed to the observers. Each tumour was examined with a sphere-shaped volume of interest (VOI) that included the entire lesion in the axial, sagittal and coronal planes. The brain was excluded from the calculation because reliability would be decreased, as a result of the high physiological uptake in the background tissue. With the use of CT images, FDG uptake in the normal organs such as the cardiac muscle, bowel, stomach, liver and urinary tract was carefully excluded from the VOI. The maximum standardized uptake value of the primary tumour (P-SUVmax), the highest value of the maximum standardized uptake value among the primary and metastatic disease (H-SUVmax), WB-MTV and WB-TLG were measured for each case. The SUVmax of the VOI was calculated as follows: (decay-corrected activity/tissue volume)/(injected dose/body weight). WB-MTV was defined as total whole-body tumour volume with a cut-off of ≥40% of the H-SUVmax. The cut-off value was determined by referring to data reported in previous studies.18–23 Each VOI for calculating MTV and TLG was first placed manually, paying careful attention not to include high physiological uptake, and then MTV was automatically extracted on a workstation. The summed MTV was produced as the WB-MTV. The WB-TLG was calculated as the sum of the product of mean standardized uptake value and the MTV of each lesion.
Primary tumour location
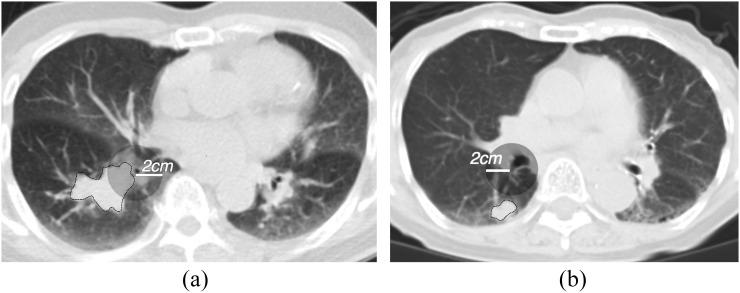
The primary tumour locations were categorized into two types: the central type and peripheral type. Because in SCLC the border of these two types has not been fully established using CT, the tumour location was defined according to one of the criteria used for non-small-cell lung cancer (NSCLC).24 If the tumour margin was <2 cm from the regional segmental bronchi, the tumour was defined as a central type (Figure 1).
Figure 1.
Representative cases of the central type (a) and peripheral type (b) on CT images. The primary tumour is delineated with a thin black line. The transparent grey circle demonstrates a region 2 cm from the origin of the nearest segmental bronchus. If the margin of the primary tumour is within the circle, it is classified as a central type.
The location of the primary tumour was judged using the pre-treatment CT images. If a conventional chest CT scan was unavailable, CT images from the PET/CT scan were evaluated. All images were anonymized and blindly evaluated by two board-certified radiologists (TN and SK). The interreader variability in the location of the primary tumour assessed by the two readers was evaluated using κ statistics. κ values of >0.8, 0.6–0.8, 0.4–0.6 and <0.4 were regarded as excellent, good, moderate and poor, respectively. The discordant cases were consensually determined by discussion between both readers and a third reader, who was a chest radiology expert with 20 years’ experience.
Clinical indices
For all patients, staging was performed using the initial PET/CT and contrast-enhanced brain MRI, with the exception of one patient who did not undergo brain MRI because he had already been diagnosed with ED with PET/CT. The Veterans Administration staging system was applied for disease staging;25 namely LD was defined as disease confined to the ipsilateral hemithorax, ipsilateral hilum, bilateral mediastinum and subclavian areas. The disease beyond the ipsilateral hemithorax, including malignant pleural or pericardial effusion and haematogenous metastasis, was categorized as ED. Other clinical indices including age, sex, smoking index (multiplication of the number of consumed cigarettes per day by the number of years of smoking) and laboratory data regarding blood tests [serum LDH, neuron-specific enolase (NSE), pro-gastrin-releasing peptide (ProGRP), cytokeratin 19 fragment (CYFRA) and carcinoembryonic antigen (CEA)] were obtained from the most recent records prior to PET/CT.
Statistical analysis
For both the central and peripheral types, the patient population was subdivided by the median values of the PET/CT parameters (P-SUVmax, H-SUVmax, WB-MTV and WB-TLG), together with the following clinical characteristics that have been demonstrated to be prognostic factors: age; sex; smoking index; Eastern Cooperative Oncology Group PS; clinical stage; type of therapy; and serum LDH. Tumour markers, namely NSE, ProGRP, cytokeratin 19 fragment and CEA, were also included in the analyses. Progression-free survival (PFS) was calculated as the interval from the initial PET/CT study to any recurrence or last follow-up. Overall survival (OS) was assessed from the initial PET/CT date to death or last follow-up. The association between patient outcome and the factors listed above was examined using the univariate Cox proportional hazards model. Multivariate analysis was performed by means of a Cox proportional hazard model using PET/CT parameters and clinical variables with p-values <0.02 in the univariate analysis. PFS and OS curves were plotted using Kaplan–Meier methods, and survival differences between the groups were assessed using the log-rank test. A two-sided p-value of <0.05 was considered to indicate statistical significance. Bonferroni correction was adopted, if multiple statistical tests were performed simultaneously on a single data set. All statistical analyses were performed using JMP® pro 11 software (SAS® Institute Inc., Cary, NC).
RESULTS
Patients
The characteristics of the patients are listed in Table 1. Of the 69 patients, 27 patients had undergone chemoradiotherapy, while 42 patients had undergone chemotherapy only. The first-line chemotherapy regimens were as follows: cisplatin plus etoposide (n = 29); cisplatin plus irinotecan (n = 4); carboplatin plus etoposide (n = 33); and carboplatin plus irinotecan (n = 3). 4 out of 38 patients with LD (including 1 patient with central type and 3 patients with peripheral type) and 7 out of 31 patients with ED (including 4 patients with central type and 3 patients with peripheral type) were unable to complete fist-line chemotherapy because of toxicity. Radiation therapy was administered concurrently at a total dose of 44–60 Gy to the chest area, when the disease was limited to the tolerable radiation field. Six patients with LD underwent total excision of the tumour without pre-operative pathological diagnosis. After diagnosis as SCLC using tumour specimens, patients received adjuvant therapy (definitive chemoradiotherapy and chemotherapy was performed for two and four patients, respectively). 10 patients (including 7 patients with central type and 3 patients with peripheral type) had brain metastases before the initial treatment. The median follow-up period was 21.8 months (range, 2.3–68.5 months). There were 16 survivors during the study period.
Table 1.
Patient characteristics
| Variables | Total (n = 69) | Central (n = 28) | Peripheral (n = 41) | p-value |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 73.9 ± 8.2 | 76.0 ± 8.3 | 72.4 ± 7.9 | 0.036a |
| Median | 75 | 78 | 72 | |
| Gender | ||||
| Male | 57 | 23 | 34 | 1.00 |
| Female | 12 | 5 | 7 | |
| Smoking index | ||||
| Mean ± SD | 1297 ± 1803 | 1675 ± 2735 | 1039 ± 558 | 0.076 |
| Median | 960 | 1010 | 960 | |
| ECOG PS | ||||
| 0 or 1 | 62 | 25 | 37 | 1.00 |
| 2 or 3 | 7 | 3 | 4 | |
| LDH [normal: 129–241 (IU/l)] | ||||
| Mean ± SD | 247.6 ± 95.8 | 273 ± 131.3 | 230.3 ± 56.4 | 0.034a |
| Median | 223 | 250.5 | 214 | |
| Tumour markers | ||||
| NSE [normal <12 (ng ml−1)] | ||||
| Mean ± SD | 50.4 ± 74.1 | 74.9 ± 100.8 | 33.3 ± 40.9 | 0.11 |
| Median | 22.4 | 31.7 | 20.9 | |
| ProGRP [normal <70 pg ml−1)] | ||||
| Mean ± SD | 996 ± 3118 | 1630 ± 4554 | 562 ± 435 | 0.082 |
| Median | 135 | 407 | 122 | |
| CYFRA [normal <2.2 ng ml−1)] | ||||
| Mean ± SD | 3.59 ± 2.55 | 3.06 ± 1.50 | 3.94 ± 3.02 | 0.92 |
| Median | 3 | 2.7 | 3.05 | |
| CEA [normal <5 (ng ml−1)] | ||||
| Mean ± SD | 21.7 ± 70.3 | 30.6 ± 94.8 | 15.7 ± 47.4 | 0.2 |
| Median | 4.5 | 5.4 | 4.5 | |
| Stage | ||||
| Limited disease | 38 | 14 | 24 | 0.62 |
| Extensive disease | 31 | 14 | 17 | |
| Therapy | ||||
| Chemoradiotherapy | 27 | 14 | 13 | 0.14 |
| Chemotherapy | 42 | 14 | 28 | |
| P-SUVmax [g ml−1] | ||||
| Mean ± SD | 9.86 ± 5.46 | 11.74 ± 5.77 | 8.61 ± 4.93 | 0.0098a |
| Median | 9.75 | 10.6 | 9.3 | |
| H-SUVmax [g ml−1] | ||||
| Mean ± SD | 11.69 ± 4.63 | 12.21 ± 5.52 | 11.32 ± 3.95 | 0.22 |
| Median | 11.6 | 11.65 | 11.5 | |
| WB-MTV [cm3] | ||||
| Mean ± SD | 72.6 ± 82.1 | 100.6 ± 93.6 | 53.4 ± 68.1 | 0.018a |
| Median | 43.9 | 74.0 | 38.3 | |
| WB-TLG [g] | ||||
| Mean ± SD | 568.6 ± 771.2 | 823.6 ± 915.6 | 394.5 ± 607.2 | 0.022a |
| Median | 336.1 | 607.6 | 226.3 | |
CEA, carcinoembryonic antigen; CYFRA, cytokeratin 19 fragment; ECOG, Eastern Cooperative Oncology Group; H-SUVmax, maximum standardized uptake value of the highest lesion of the disease; IU, international unit; LDH, lactate dehydrogenase; NSE, neuron-specific enolase; ProGRP, pro-gastrin-releasing peptide; PS, performance status; P-SUVmax, maximum standardized uptake value of the primary tumour lesion; SD, standard deviation; WB-MTV, whole-body metabolic tumour volume; WB-TLG, whole-body total lesion glycolysis.
The paired t-test was used for the evaluation of age, smoking index, LDH, tumour markers, maximum of standardized uptake value, MTV and TLG. Fisher's exact test was used for the evaluation of sex, performance status, stage and therapy.
p < 0.05.
Subgroups of the central and peripheral types
The reproducibility in identifying the primary tumour location was good (as κ = 0.67). After discussion between the two CT readers and the chest radiology expert, 28 patients were categorized as having the central type and 41 patients as having the peripheral type. Age (p = 0.036), serum LDH (p = 0.034), P-SUVmax (p = 0.0098), WB-MTV (p = 0.018) and WB-TLG (p = 0.022) of the central type were significantly higher than those of the peripheral type (Table 1).
Univariate survival analysis
Univariate analysis for PFS and OS was performed according to the median values for the following clinicolaboratory characteristics: age; Eastern Cooperative Oncology Group PS; LDH level; stage; and tumour markers (Table 2). Among them, ED, high NSE level, high WB-MTV and WB-TLG led to significantly worse prognosis regarding PFS and OS for both the central and peripheral types. Old age was a significant prognostic factor only in the central type. High LDH and CEA level led to a significantly worse prognosis only regarding OS in the central type. All of the other parameters showed no statistical significance in terms of prognosis.
Table 2.
Univariate Cox proportional hazard models for overall survival (OS) and progression-free survival (PFS)
| Variables | PFS |
OS |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Central type (n = 28) | ||||
| Age | 2.67 (1.08–6.38) | 0.034a | 3.19 (1.11–10.49) | 0.031a |
| Gender (M : F) | 1.37 (0.51–4.75) | 0.56 | 4.04 (0.80–73.50) | 0.10 |
| Smoking index | 0.85 (0.36–2.00) | 0.71 | 0.74 (0.27–2.03) | 0.55 |
| ECOG PS | 1.83 (0.42–5.80) | 0.38 | 0.98 (0.053–5.29) | 0.99 |
| Serum LDH | 1.93 (0.83–4.73) | 0.13 | 3.29 (1.15–10.69) | 0.026 |
| Tumour markers | ||||
| NSE | 3.25 (1.36–8.18) | 0.0084b | 3.86 (1.35–12.59) | 0.012b |
| ProGRP | 1.67 (0.70–4.17) | 0.25 | 1.53 (0.53–4.70) | 0.43 |
| CYFRA | 0.84 (0.35–2.01) | 0.69 | 0.55 (0.17–1.55) | 0.26 |
| CEA | 2.04 (0.84–5.03) | 0.12 | 4.51 (1.48–15.41) | 0.0082 |
| Stage | 5.37 (2.01–16.06) | 0.0007b | 30.01 (5.62–557.55) | <0.0001b |
| Therapy | 4.94 (1.84–14.80) | 0.0013b | 15.73 (4.02–104.42) | <0.0001b |
| P-SUVmax | 1.00 (0.41–2.39) | 1 | 0.98 (0.36–2.69) | 0.97 |
| H-SUVmax | 1.04 (0.43–2.48) | 0.930 | 1.15 (0.42–3.15) | 0.78 |
| WB-MTV | 3.93 (1.62–10.21) | 0.0024b | 5.31 (1.78–19.44) | 0.0024b |
| WB-TLG | 3.11 (1.29–7.85) | 0.011b | 5.18 (1.73–19.00) | 0.0028b |
| Peripheral type (n = 41) | ||||
| Age | 1.74 (0.86–3.53) | 0.12 | 1.54 (0.68–3.51) | 0.2 |
| Gender (M : F) | 2.29 (0.87–7.92) | 0.097 | 2.89 (0.96–12.38) | 0.061 |
| Smoking index | 1.10 (0.54–2.22) | 0.79 | 1.43 (0.62–3.31) | 0.4 |
| ECOG PS | 1.77 (0.52–4.60) | 0.32 | 1.44 (0.34–4.25) | 0.57 |
| Serum LDH | 1.24 (0.62–2.49) | 0.55 | 1.62 (0.72–3.88) | 0.25 |
| Tumour markers | ||||
| NSE | 2.65 (1.26–5.78) | 0.01b | 3.98 (1.57–11.48) | 0.0031b |
| ProGRP | 1.28 (0.64–2.59) | 0.49 | 1.12 (0.50–2.60) | 0.79 |
| CYFRA | 1.15 (0.58–2.30) | 0.68 | 0.86 (0.37–1.94) | 0.72 |
| CEA | 1.16 (0.57–2.37) | 0.68 | 1.37 (0.61–3.21) | 0.45 |
| Stage | 3.54 (1.66–7.72) | 0.0012b | 6.27 (2.49–17.95) | <0.0001b |
| Therapy | 1.99 (0.95–4.56) | 0.069 | 1.85 (0.76–5.16) | 0.18 |
| P-SUVmax | 1.65 (0.82–3.36) | 0.16 | 1.86 (0.82–4.39) | 0.14 |
| H-SUVmax | 1.82 (0.91–3.73) | 0.090 | 2.27 (0.99–5.65) | 0.052 |
| WB-MTV | 5.21 (2.35–12.51) | <0.0001b | 8.16 (2.95–28.98) | <0.0001b |
| WB-TLG | 4.98 (2.23–11.98) | <0.0001b | 8.02 (2.89–28.53) | <0.0001b |
CEA, carcinoembryonic antigen; CI, confidence interval; CYFRA, cytokeratin 19 fragment; ECOG, Eastern Cooperative Oncology Group; F, female; H-SUVmax, maximum standardized uptake value of the highest lesion of the disease; LDH, lactate dehydrogenase; M, male; NSE, neuron-specific enolase; ProGRP, pro-gastrin-releasing peptide; PS, performance status; P-SUVmax, maximum standardized uptake value of the primary tumour lesion; WB-MTV, whole-body metabolic tumour volume; WB-TLG, whole-body total lesion glycolysis.
p < 0.05.
p < 0.02.
Multivariate survival analysis
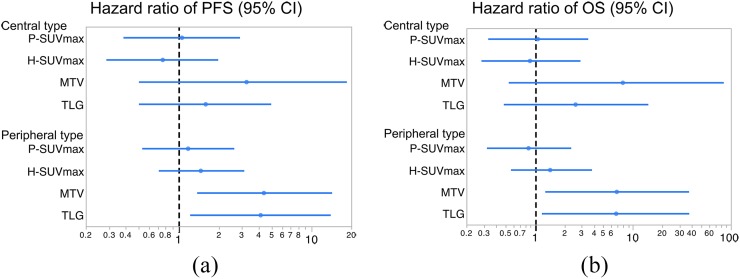
To assess the possible independent effects of the PET/CT parameters, multivariate analyses were performed regarding PFS and OS adjusted for stage and serum NSE, both of which showed p-values of <0.02 in the univariate analyses (Table 3). In the peripheral type, WB-MTV and WB-TLG were revealed to be independent prognostic factors regarding PFS and OS. However, in the central type, stage was the only independent prognostic factor in all of the models, and none of the PET/CT parameters were significant as shown in the forest plot analysis (Figure 2).
Table 3.
Multivariate Cox proportional hazard models for overall survival (OS) and progression-free survival (PFS)
| Variables | PFS |
OS |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Central type (n = 28) | ||||
| Stage | 4.43 (1.42–15.00) | 0.01a | 26.05 (4.28–509.30) | <0.0001a |
| NSE | 2.30 (0.86–6.55) | 0.10 | 2.03 (0.59–7.97) | 0.27 |
| P-SUVmax | 1.06 (0.39–2.86) | 0.900 | 1.07 (0.34–3.43) | 0.90 |
| Stage | 4.14 (1.47–12.97) | 0.0068a | 24.86 (4.34–475.1) | <0.0001a |
| NSE | 2.60 (0.95–7.38) | 0.062 | 2.19 (0.63–8.45) | 0.22 |
| H-SUVmax | 0.76 (0.29–1.96) | 0.56 | 0.89 (0.29–2.86) | 0.85 |
| Stage | 4.04 (1.41–12.80) | 0.0091a | 23.44 (3.97–453.6) | <0.0001a |
| NSE | 0.87 (0.19–5.57) | 0.88 | 0.36 (0.047–4.56) | 0.39 |
| WB-MTV | 3.25 (0.51–18.25) | 0.21 | 7.90 (0.55–83.08) | 0.12 |
| Stage | 3.91 (1.33–12.58) | 0.013a | 23.35 (3.88–453.54) | <0.0001a |
| NSE | 1.92 (0.64–5.73) | 0.24 | 1.14 (0.25–5.90) | 0.87 |
| WB-TLG | 1.60 (0.51–4.91) | 0.42 | 2.60 (0.49–14.10) | 0.26 |
| Peripheral type (n = 41) | ||||
| Stage | 2.39 (0.76–8.65) | 0.14 | 5.99 (1.37–40.16) | 0.015a |
| NSE | 1.48 (0.45–4.36) | 0.51 | 1.13 (0.19–5.45) | 0.89 |
| P-SUVmax | 1.18 (0.54–2.59) | 0.68 | 0.86 (0.33–2.30) | 0.76 |
| Stage | 2.33 (0.82–7.80) | 0.12 | 5.09 (1.25–29.99) | 0.021a |
| NSE | 1.48 (0.46–4.25) | 0.50 | 1.09 (0.20–5.02) | 0.92 |
| H-SUVmax | 1.47 (0.72–3.08) | 0.29 | 1.43 (0.58–3.75) | 0.44 |
| Stage | 1.31 (0.45–4.44) | 0.64 | 2.85 (0.77–14.43) | 0.12 |
| NSE | 0.94 (0.30–2.85) | 0.92 | 0.49 (0.10–2.52) | 0.49 |
| WB-MTV | 4.39 (1.40–14.13) | 0.011a | 6.85 (1.30–36.71) | 0.023a |
| Stage | 1.30 (0.44–4.53) | 0.66 | 2.84 (0.77–14.70) | 0.13 |
| NSE | 0.96 (0.30–2.91) | 0.94 | 0.49 (0.10–2.56) | 0.39 |
| WB-TLG | 4.15 (1.24–13.79) | 0.021a | 6.75 (1.20–36.86) | 0.031a |
CI, confidence interval; H-SUVmax, maximum standardized uptake value of the highest lesion of the disease; NSE, neuron-specific enolase; P-SUVmax, maximum standardized uptake value of the primary tumour lesion; WB-MTV, whole-body metabolic tumour volume; WB-TLG, whole-body total lesion glycolysis.
p < 0.05.
Figure 2.
Hazard ratio of progression-free survival (PFS) (a) and overall survival (OS) (b) using positron emission tomography (PET) parameters. Forest plots describe the hazard ratio regarding the central and peripheral types. PET/CT volumetric parameters such as whole-body metabolic tumour volume and whole-body total lesion glycolysis are independent prognostic factors only in the peripheral type. CI, confidence interval; H-SUVmax, maximum standardized uptake value of the highest lesion of the disease; MTV, metabolic tumour volume; P-SUVmax, maximum standardized uptake value of the primary tumour lesion; TLG, total lesion glycolysis.
Subgroup analysis
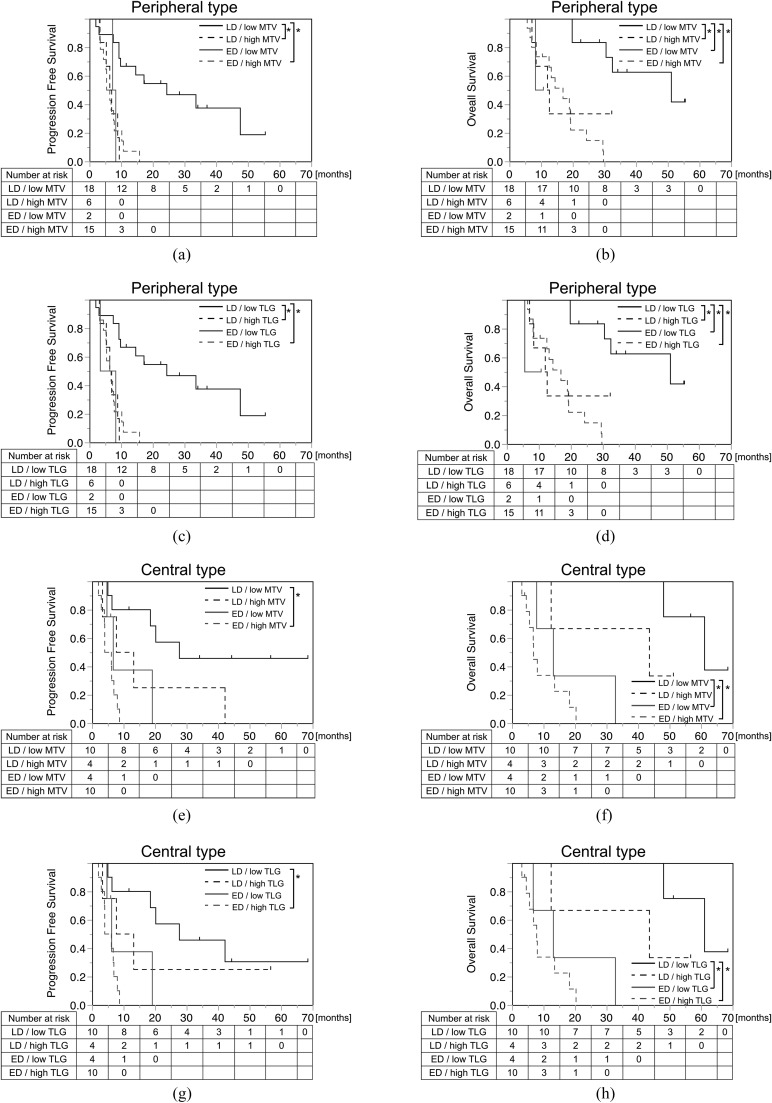
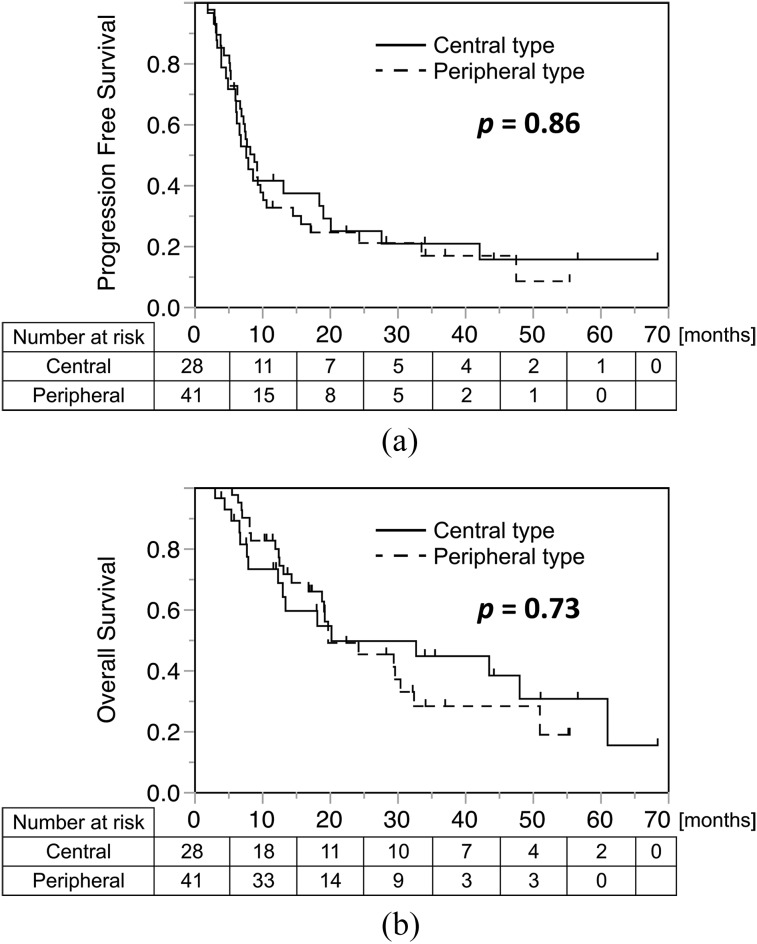
The number of patients in the different subgroups is detailed in Table 4. The number of patients grouped by stage and WB-TLG matched exactly with that for stage and WB-MTV. Since MTV and TLG are interchangeable, the results are demonstrated together in the table. No significant bias was seen regarding the distribution of patient number in each subgroup. Kaplan–Meier curves were produced for each tumour location, divided into four groups involving stage and WB-MTV/TLG (Figure 4). No statistical difference was found concerning patient outcome between the central and peripheral types, both in terms of PFS (p = 0.85) and OS (p = 0.73) (Figure 3). In the peripheral type, patients with LD with low WB-MTV/TLG showed significantly better prognosis than all of the other groups, with the exception of patients with ED with low WB-MTV/TLG, after adjustment using the Bonferroni correction. If WB-MTV/TLG was high, the prognosis was very poor even in patients with LD and was equivalent to the prognosis for patients with ED (Figure 4c, d, g and h and Table 5). In the central type, patients with ED with low WB-MTV/TLG had a worse prognosis than was the case for patients with LD with high WB-MTV/TLG regarding OS (Figure 4b and f); this finding supported the results of the multivariate analysis.
Table 4.
Number of patients assigned to subgroups on the basis of stage and metabolic tumour volume (MTV) [total lesion glycolysis (TLG)]
| Groups | Limited disease | Extensive disease | Total |
|---|---|---|---|
| Central type | |||
| Low WB-MTV(TLG) | 10 | 4 | 14 |
| High WB-MTV(TLG) | 4 | 10 | 14 |
| Total | 14 | 14 | 28 |
| Peripheral type | |||
| Low WB-MTV(TLG) | 18 | 2 | 20 |
| High WB-MTV(TLG) | 6 | 15 | 21 |
| Total | 24 | 17 | 41 |
WB-MTV, whole-body metabolic tumour volume; WB-TLG, whole-body total lesion glycolysis.
Figure 4.
Kaplan–Meier analyses of overall survival and progression-free survival subgrouped by stage and whole-body metabolic tumour volume (WB-MTV) (a, b, e and f) and whole-body total lesion glycolysis (WB-TLG) (c, d, g and h). In the peripheral type (a–d), patients with limited disease with low WB-MTV/TLG showed significantly better prognosis than the other groups, with the exception of extensive disease with low WB-MTV/TLG. In the central type (e–h), WB-MTV and WB-TLG did not contribute much to prognosis as compared with the peripheral type. ED, extensive disease; LD, limited disease; MTV, metabolic tumour volume; TLG, total lesion glycolysis. *, p < 0.0083 (adjusted by Bonferroni correction).
Figure 3.
Kaplan–Meier analyses of progression-free survival (a) and overall survival (b) according to tumour location. Patients who were censored are indicated as tick marks. No significant difference in prognosis was observed between patients with the central and peripheral types.
Table 5.
p-values for Kaplan–Meier analysis according to each subtype using the log-rank test
| Groups | Central |
Peripheral |
||||
|---|---|---|---|---|---|---|
| PFS | OS | PFS | OS | |||
| WB-MTV |
||||||
| LD/low MTV vs LD/high MTV | 0.093 | 0.10 |
0.0005a | 0.0022a |
||
| LD/low MTV vs ED/low MTV | 0.056 | 0.0003a |
0.013 | 0.0036a |
||
| LD/low MTV vs ED/high MTV | 0.0002a | <0.0001a |
<0.0001a | <0.0001a |
||
| LD/high MTV vs ED/low MTV | 0.71 | 0.20 |
0.93 | 0.68 |
||
| LD/high MTV vs ED/high MTV | 0.078 | 0.030 |
0.82 | 0.54 |
||
| ED/low MTV vs ED/high MTV | 0.17 | 0.22 |
0.74 | 0.65 |
||
| WB-TLG |
||||||
| LD/low TLG vs LD/high TLG | 0.39 |
0.098 | 0.0005a |
0.0022a | ||
| LD/low TLG vs ED/low TLG | 0.036 |
0.0003a | 0.013 |
0.0036a | ||
| LD/low TLG vs ED/high TLG | 0.0002a |
<0.0001a | <0.0001a |
<0.0001a | ||
| LD/high TLG vs ED/low TLG | 0.43 |
0.17 | 0.62 |
0.51 | ||
| LD/high TLG vs ED/high TLG | 0.078 |
0.033 | 0.70 |
0.56 | ||
| ED/low TLG vs ED/high TLG | 0.21 | 0.28 | 0.83 | 0.30 | ||
ED, extensive disease; LD, limited disease; MTV, metabolic tumour volume; OS, overall survival; PFS, progression-free survival; TLG, total lesion glycolysis; WB-MTV, whole-body metabolic tumour volume; WB-TLG, whole-body total lesion glycolysis.
p < 0.0083 (adjusted using the Bonferroni correction).
DISCUSSION
In the present study, we categorized patients with SCLC into two types based on tumour location, peripheral and central, and evaluated several parameters measured using pre-treatment FDG-PET/CT scans for the prediction of prognosis. Our results demonstrated that the independent prognostic factor was stage in the central type, but volumetric parameters such as WB-MTV/TLG in the peripheral type. Neither P-SUVmax nor H-SUVmax had much influence on prognosis in both types. Kaplan–Meier subgroup analysis was performed to support the multivariate analysis results. It showed that patients with peripheral type with LD and low WB-MTV/TLG had a better prognosis than those with the other groups, and patients with LD with high WB-MTV/TLG had a poor prognosis equivalent to patients with ED; this was not observed in the central type. When adjusted using Bonferroni correction, the p-value for the comparison between LD with low WB-MTV/TLG and ED with low WB-MTV/TLG in the peripheral type was not statistically significant; this is probably the result of the small number of patients evaluated and does not necessarily mean that stage is an impractical prognostic factor in patients with peripheral type. To summarize, in the peripheral type, PET volumetric parameters could help in extracting patients with better prognosis, especially in patients with LD. However, in the central type, stage had more influence on prognosis than PET volumetric parameters. To our knowledge, the present study is the first to investigate the difference in prognostic power in terms of primary tumour location using FDG-PET/CT.
Subgroup analysis in relation to tumour location in SCLC has not been fully investigated. Only a few reports have discussed the relationship between tumour location regarding pre-treatment images and prognosis. Lally et al14 reported that the location of the main bronchus was one of the mortality risks, together with older age, male sex and African-American ethnicity in multivariate analysis. Bandoh et al13 reported that CEA levels in the peripheral type were significantly higher than those in the central type, but no significant difference in prognosis was found between the peripheral and central types. In surgical series, no significant prognostic difference has been reported between central-type and peripheral-type tumours,16,17 which was also demonstrated in our study. Therefore, differences in prognosis according to tumour location remain controversial. However, as a new insight, we have revealed a possible prognostic difference in terms of glucose metabolic consumption, depending on whether the primary tumour was located in the centre or periphery of CT scans. One possible reason for this is that patients with central type had a significantly larger WB-MTV/TLG than patients with peripheral type in our population. This means that patients with central type with a “low” WB-MTV/TLG had a tumour volume that was large enough to result in a poor clinical course. The central-type tumour is often very difficult to detect at an early stage and is found late when it is already large with lymph-node metastases. Then, WB-MTV/TLG would not contribute significantly to the prediction of the prognosis. The other reason is that there could be some biological differences between the peripheral and central types of SCLC. According to Nomori et al,26 undifferentiated carcinoma of the small-cell type was located more frequently at the periphery of the lung than classical neuroendocrine types, such as oat-cell carcinoma and small-cell carcinoma of the intermediate cell type in surgical series. Most of the SCLC cases are diagnosed using a punch biopsy and are not often surgically resected; this makes it difficult to survey the entire range of tumour characteristics before treatment. There might also be a possible heterogeneity in SCLC, which is undetectable by mere biopsy. It would be meaningful to determine the difference in prognostic factors using non-invasive methods such as PET/CT according to subgroups (e.g. tumour location).
Several studies have demonstrated the prognostic value of FDG-PET parameters. However, the prognostic value of SUVmax is still controversial in SCLC. Pandit et al reported that a high SUVmax was significantly associated with poor survival,27 while the difference in OS according to the SUVmax of the primary tumour was not found to be statistically significant in two independent studies.28,29 SUVmax may have a complex relationship with prognosis in SCLC, unlike NSCLC, in which a favourable relationship with prognosis has already been reported in several studies.29–33 This may reflect the characteristics of SUVmax, which shows the location where FDG uptake is highest within VOI. Conversely, FDG-PET volumetric parameters such as MTV and TLG are generally reported to be favourable prognostic factors. Furthermore, most of the previous studies have focused on WB-MTV in SCLC, unlike other malignancies, in which only the MTV of the primary tumour is usually investigated. For example, Zhu et al12 reported WB-MTV as one of the important prognostic factors in patients with SCLC. Oh et al10 reported that the sum of the extrathoracic MTVs is an independent prognostic biomarker for patients with ED with SCLC. Their results are reasonable, considering the biological nature of SCLC. Because the clinical course of SCLC is generally very rapid, the prognosis would correlate well with WB-MTV, which provides information concerning the total tumour burden and glucose metabolism in the entire target lesion. On the other hand, the MTV of the primary tumour would not directly reflect prognosis; the primary tumour in patients with SCLC can be very small, even if it is accompanied by severe lymphatic and metastatic spread. Overall, FDG-PET whole-body volumetric parameters can be said to be significant prognostic factors in SCLC. However, in our study, they were only found to be independent in the peripheral type, when focusing on the primary tumour location. Especially in the case of LD, patients with high WB-MTV/TLG had a poor prognosis similar to the patients with ED. This means that more careful observation for recurrence would be required, even if these patients once showed a good response to radical therapies.
In the present study using univariate analysis, NSE was one of the prognostic factors in patients with SCLC in both the central and peripheral types. The prognostic role for tumour markers in SCLC has not been fully established to date; however, some studies have investigated the relationship between prognosis and tumour markers.34–36 Shibayama et al36 reported on the complementary role of NSE and ProGRP; NSE was found to be superior to ProGRP as a prognostic factor, while ProGRP was more sensitive than NSE regarding the diagnosis of SCLC. Similar to the previous study, the pre-treatment serum NSE level showed a much better correlation with prognosis than ProGRP in our population; however, it was not found to be an independent prognostic factor, when adjusted for stage and PET volumetric parameters.
Our study had several limitations. First, it was non-randomized and retrospective in design and involved a small number of patients. Especially when divided into subgroups, the numbers of patients with ED with low MTV/TLG and patients with LD with high MTV/TLG were extremely small for analysis. Regimens equivalent to standard chemotherapy were used for the treatment of all of the patients; however, they were not unified. Some patients could not complete chemotherapy because of severe toxicity. These factors possibly confounded our study results, although no prominent bias was seen in our population. Second, brain metastasis, which is one of the major prognostic factors, was not considered in the evaluation of PET parameters because of high physiological FDG uptake in the normal tissue. In our population, approximately one-third of patients with ED had brain metastasis. A different prevalence rate between central type (25.0%) and peripheral type (7.3%) might have affected the results. Third, because three different PET/CT scanners were used, absolute quantitative values might be unreliable. However, we confirmed the consistency of the cross-calibration factor with an error of <5% for each scanner, which would support the reproducibility of the data.37 Moreover, the definition of tumour location used in the present study was based on the Timmerman criteria for NSCLC involving radiation therapy and has not been previously applied for SCLC. There have been no recent studies entailing precise evaluation of primary tumour location using CT; the tumour border was determined using chest X-rays.13,14,26 Because no clear definition concerning central-type or peripheral-type tumours has been established, we applied the Timmerman criteria as a preliminary approach. Potentially, a more feasible border exists elsewhere in terms of biological features. To overcome these issues, further prospective studies involving more patients will be necessary to elucidate the prognostic value of volumetric PET/CT parameters.
Acknowledgments
ACKNOWLEDGMENTS
We thank Dr Eiji Tadamura, Dr Kohei Hayashida, Dr Yasuyo Hamanaka, Dr Masahiro Tomoi and all the associated technologists for preparing images and related information.
Contributor Information
Tomomi Nobashi, Email: nobaco@kuhp.kyoto-u.ac.jp.
Sho Koyasu, Email: sho@kuhp.kyoto-u.ac.jp.
Yuji Nakamoto, Email: ynakamo1@kuhp.kyoto-u.ac.jp.
Takeshi Kubo, Email: tkubo@kuhp.kyoto-u.ac.jp.
Takayoshi Ishimori, Email: ishimori@kuhp.kyoto-u.ac.jp.
Young H Kim, Email: ekim@kuhp.kyoto-u.ac.jp.
Akihiko Yoshizawa, Email: akyoshi@kuhp.kyoto-u.ac.jp.
Kaori Togashi, Email: ktogashi@kuhp.kyoto-u.ac.jp.
CONCLUSION
The FDG-PET volumetric parameters were demonstrated to be significant and independent prognostic factors in the peripheral type of SCLC, whereas PET-based volumetric parameters were not shown to be independent prognostic factors in the central type. It may be necessary to take into account the location of the primary tumours, when considering the prognostic value of FDG-PET/CT in patients with SCLC.
REFERENCES
- 1.Simon GR, Turrisi A; American College of Chest Physicians. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132(Suppl. 3): 324S–39S. doi: 10.1378/chest.07-1385 [DOI] [PubMed] [Google Scholar]
- 2.Planchard D, Le Péchoux C. Small cell lung cancer: new clinical recommendations and current status of biomarker assessment. Eur J Cancer 2011; 47(Suppl. 3): S272–83. doi: 10.1016/S0959-8049(11)70173-3 [DOI] [PubMed] [Google Scholar]
- 3.Lee YJ, Cho A, Cho BC, Yun M, Kim SK, Chang J, et al. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin Cancer Res 2009; 15: 2426–32. doi: 10.1158/1078-0432.CCR-08-2258 [DOI] [PubMed] [Google Scholar]
- 4.Foster NR, Mandrekar SJ, Schild SE, Nelson GD, Rowland KM, Jr, Deming RL, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer 2009; 115: 2721–31. doi: 10.1002/cncr.24314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quoix E, Purohit A, Faller-Beau M, Moreau L, Oster JP, Pauli G. Comparative prognostic value of lactate dehydrogenase and neuron-specific enolase in small-cell lung cancer patients treated with platinum-based chemotherapy. Lung cancer 2000; 30: 127–34. doi: 10.1016/S0169-5002(00)00131-8 [DOI] [PubMed] [Google Scholar]
- 6.Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 2014; 55: 884–90. doi: 10.2967/jnumed.113.133801 [DOI] [PubMed] [Google Scholar]
- 7.Guy MS, Jacob C, McDonald SD, Dowdy YG, Kardan A. 18F-FDG PET/CT metabolic variability in functioning oncocytic parathyroid adenoma with brown tumors. Clin Nucl Med 2014; 39: 393–5. doi: 10.1097/RLU.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 8.Xu HX, Chen T, Wang WQ, Wu CT, Liu C, Long J, et al. Metabolic tumour burden assessed by 18F-FDG PET/CT associated with serum CA19-9 predicts pancreatic cancer outcome after resection. Eur J Nucl Med Mol Imaging 2014; 41: 1093–102. doi: 10.1007/s00259-014-2688-8 [DOI] [PubMed] [Google Scholar]
- 9.Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2012; 39: 27–38. doi: 10.1007/s00259-011-1934-6 [DOI] [PubMed] [Google Scholar]
- 10.Oh JR, Seo JH, Hong CM, Jeong SY, Lee SW, Lee J, et al. Extra-thoracic tumor burden but not thoracic tumor burden on (18)F-FDG PET/CT is an independent prognostic biomarker for extensive-disease small cell lung cancer. Lung cancer 2013; 81: 218–25. doi: 10.1016/j.lungcan.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 11.Oh JR, Seo JH, Chong A, Min JJ, Song HC, Kim YC, et al. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging 2012; 39: 925–35. doi: 10.1007/s00259-011-2059-7 [DOI] [PubMed] [Google Scholar]
- 12.Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung cancer 2011; 73: 332–7. doi: 10.1016/j.lungcan.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 13.Bandoh S, Fujita J, Ueda Y, Fukunaga Y, Dohmoto K, Hojo S, et al. Expression of carcinoembryonic antigen in peripheral- or central-located small cell lung cancer: its clinical significance. Jpn J Clin Oncol 2001; 31: 305–10. doi: 10.1093/jjco/hye067 [DOI] [PubMed] [Google Scholar]
- 14.Lally BE, Geiger AM, Urbanic JJ, Butler JM, Wentworth S, Perry MC, et al. Trends in the outcomes for patients with limited stage small cell lung cancer: an analysis of the surveillance, epidemiology, and end results database. Lung cancer 2009; 64: 226–31. doi: 10.1016/j.lungcan.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 15.Hamanaka W, Motoi N, Ishikawa S, Ushijima M, Inamura K, Hatano S, et al. A subset of small cell lung cancer with low neuroendocrine expression and good prognosis: a comparison study of surgical and inoperable cases with biopsy. Hum Pathol 2014; 45: 1045–56. doi: 10.1016/j.humpath.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 16.Ohkubo T, Ito K, Sugiura H, Ohno K, Morikawa T, Okushiba S, et al. Surgical analysis for small cell lung cancer of the lung. [In Japanese.] Kyobu Geka 1999; 52: 1061–6; discussion 6–8. [PubMed] [Google Scholar]
- 17.Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, Sheppard MN, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002; 26: 1184–97. doi: 10.1097/00000478-200209000-00009 [DOI] [PubMed] [Google Scholar]
- 18.Kruse V, Mees G, Maes A, D'Asseler Y, Borms M, Cocquyt V, et al. Reproducibility of FDG PET based metabolic tumor volume measurements and of their fdg distribution within. Q J Nucl Med Mol Imaging 2015; 59: 462–8. [PubMed] [Google Scholar]
- 19.Kitajima K, Suenaga Y, Ueno Y, Maeda T, Ebina Y, Yamada H, et al. Preoperative risk stratification using metabolic parameters of (18)F-FDG PET/CT in patients with endometrial cancer. Eur J Nucl Med Mol Imaging 2015; 42: 1268–75. doi: 10.1007/s00259-015-3037-2 [DOI] [PubMed] [Google Scholar]
- 20.Maffione AM, Ferretti A, Grassetto G, Bellan E, Capirci C, Chondrogiannis S, et al. Fifteen different 18F-FDG PET/CT qualitative and quantitative parameters investigated as pathological response predictors of locally advanced rectal cancer treated by neoadjuvant chemoradiation therapy. Eur J Nucl Med Mol Imaging 2013; 40: 853–64. doi: 10.1007/s00259-013-2357-3 [DOI] [PubMed] [Google Scholar]
- 21.Chen SW, Chen WT, Wu YC, Yen KY, Hsieh TC, Lin TY, et al. Which FDG/PET parameters of the primary tumors in colon or sigmoid cancer provide the best correlation with the pathological findings? Eur J Radiol 2013; 82: e405–10. doi: 10.1016/j.ejrad.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 22.Fonti R, Larobina M, Del Vecchio S, De Luca S, Fabbricini R, Catalano L, et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med 2012; 53: 1829–35. doi: 10.2967/jnumed.112.106500 [DOI] [PubMed] [Google Scholar]
- 23.Erdi YE, Mawlawi O, Larson SM, Imbriaco M, Yeung H, Finn R, et al. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer 1997; 80(Suppl. 12): 2505–9. doi: [DOI] [PubMed] [Google Scholar]
- 24.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006; 24: 4833–9. doi: 10.1200/JCO.2006.07.5937 [DOI] [PubMed] [Google Scholar]
- 25.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Reports Part 3 1973; 4: 31–42. [PubMed] [Google Scholar]
- 26.Nomori H, Shimosato Y, Kodama T, Morinaga S, Nakajima T, Watanabe S. Subtypes of small cell carcinoma of the lung: morphometric, ultrastructural, and immunohistochemical analyses. Hum Pathol 1986; 17: 604–13. doi: 10.1016/S0046-8177(86)80133-2 [DOI] [PubMed] [Google Scholar]
- 27.Pandit N, Gonen M, Krug L, Larson SM. Prognostic value of [18F]FDG-PET imaging in small cell lung cancer. Eur J Nucl Med Mol Imaging 2003; 30: 78–84. doi: 10.1007/s00259-002-0937-8 [DOI] [PubMed] [Google Scholar]
- 28.Jhun BW, Lee KJ, Jeon K, Suh GY, Chung MP, Kim H, et al. Clinical applicability of staging small cell lung cancer according to the seventh edition of the TNM staging system. Lung cancer 2013; 81: 65–70. doi: 10.1016/j.lungcan.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 29.van der Leest C, Smit EF, Baas J, Versteijlen RJ, van Walree N, Hoogsteden HC, et al. SUVmax during 18FDG-PET scanning in small cell lung cancer: similar information as in non-small cell lung cancer? Lung cancer 2012; 76: 67–71. doi: 10.1016/j.lungcan.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 30.Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol 2004; 22: 3255–60. doi: 10.1200/JCO.2004.11.109 [DOI] [PubMed] [Google Scholar]
- 31.Pottgen C, Levegrün S, Theegarten D, Marnitz S, Grehl S, Pink R, et al. Value of 18F-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in non-small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res 2006; 12: 97–106. doi: 10.1158/1078-0432.CCR-05-0510 [DOI] [PubMed] [Google Scholar]
- 32.van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer 2007; 43: 1392–8. doi: 10.1016/j.ejca.2007.03.027 [DOI] [PubMed] [Google Scholar]
- 33.Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. ; European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008; 3: 6–12. doi: 10.1097/JTO.0b013e31815e6d6b [DOI] [PubMed] [Google Scholar]
- 34.Zhao WX, Luo JF. Serum neuron-specific enolase levels were associated with the prognosis of small cell lung cancer: a meta-analysis. Tumour Biol 2013; 34: 3245–8. doi: 10.1007/s13277-013-0896-7 [DOI] [PubMed] [Google Scholar]
- 35.Ono A, Naito T, Ito I, Watanabe R, Shukuya T, Kenmotsu H, et al. Correlations between serial pro-gastrin-releasing peptide and neuron-specific enolase levels, and the radiological response to treatment and survival of patients with small-cell lung cancer. Lung cancer 2012; 76: 439–44. doi: 10.1016/j.lungcan.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 36.Shibayama T, Ueoka H, Nishii K, Kiura K, Tabata M, Miyatake K, et al. Complementary roles of pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in diagnosis and prognosis of small-cell lung cancer (SCLC). Lung cancer 2001; 32: 61–9. doi: 10.1016/S0169-5002(00)00205-1 [DOI] [PubMed] [Google Scholar]
- 37.Geworski L, Knoop BO, de Wit M, Ivancevic V, Bares R, Munz DL. Multicenter comparison of calibration and cross calibration of PET scanners. J Nucl Med. 2002;43:635–9. [PubMed] [Google Scholar]