Abstract
Objective:
To measure paddle motion during the clamping phase of a breast phantom for a range of machine/paddle combinations.
Methods:
A deformable breast phantom was used to simulate a female breast. 12 mammography machines from three manufacturers with 22 flexible and 20 fixed paddles were evaluated. Vertical motion at the paddle was measured using two calibrated linear potentiometers. For each paddle, the motion in millimetres was recorded every 0.5 s for 40 s, while the phantom was compressed with 80 N. Independent t-tests were used to determine differences in paddle motion between flexible and fixed, small and large, GE Senographe Essential (General Electric Medical Systems, Milwaukee, WI) and Hologic Selenia Dimensions paddles (Hologic, Bedford, MA). Paddle tilt in the medial–lateral plane for each machine/paddle combination was calculated.
Results:
All machine/paddle combinations demonstrate highest levels of motion during the first 10 s of the clamping phase. The least motion is 0.17 ± 0.05 mm/10 s (n = 20) and the most motion is 0.51 ± 0.15 mm/10 s (n = 80). There is a statistical difference in paddle motion between fixed and flexible (p < 0.001), GE Senographe Essential and Hologic Selenia Dimensions paddles (p < 0.001). Paddle tilt in the medial–lateral plane is independent of time and varied from 0.04 ° to 0.69 °.
Conclusion:
All machine/paddle combinations exhibited motion and tilting, and the extent varied with machine and paddle sizes and types.
Advances in knowledge:
This research suggests that image blurring will likely be clinically insignificant 4 s or more after the clamping phase commences.
INTRODUCTION
Breast cancer is the most common cancer among females and the second most common cause of death from cancer in the UK.1 Mammographic screening is the key to early detection of breast cancer. In a randomized control trial of 282,777 females in Sweden, there was a 24% reduction of breast cancer mortality compared with females without screening.2 Screening can identify ductal carcinoma in situ, which may never cause symptoms or death in a female's lifetime. A study by Bleyer and Gilbert3 estimated that 31% of breast cancers detected by screening in the USA are considered to be over diagnosis, and according to the study by Biesheuvel et al,4 the over diagnosis rate can be as high as 54% for females aged between 50 and 59 years. Although over diagnosis might occur, the benefit of screening is generally considered to outweigh the harm of over diagnosis. An independent review carried out by Marmot et al5 estimated that for 10,000 females aged 50 years who are invited to screening in the next 20 years, 129 females would have been over diagnosed, while 43 deaths from breast cancer would have been prevented. This suggests that one death from breast cancer is prevented for every three over diagnosed cases.
Early detection of breast cancer relies on good image quality, but factors such as image blurring, inadequate compression, incorrect exposure and skin folds can degrade image quality.6 Repeat imaging for technical reasons such as these will increase radiation dose and possibly increase client anxiety.7
Research studies to specifically evaluate image-blurring rates within mammography services are limited. Within the UK screening service, the overall technical recall and repeat rates for each service should be below 3% with a target of 2%.8 One study reviewed a unit’s recall and repeat rates and reported that 0.86% of females were recalled owing to image blur, constituting almost one-third (29%) of the 3% maximum permissible rate for repeats.9 A second study within the same unit reported that over half of all their total clients were recalled owing to blurring with 1/20th repeated owing to blurring.10 A study within another unit reported that over 90% of their total technical recalls were due to blurred images.11 Despite much anecdote within the UK National Health Breast Screening Programme, and others, about image blurring and the need for repeat imaging because of blurring, this technical problem continues to be under-reported within the literature.
Groot et al12 suggested that breast compression consists of a deformation phase for flattening and a clamping phase for immobilization. During the deformation phase, the breast is gradually flattened by the compression paddle, by increasing the compression force. The clamping phase starts when the maximum compression force is reached. The deformation and clamping phases last approximately 7.5 and 12.8 s, respectively.12 Groot et al in their study, which involved 117 females, observed that during the clamping phase, the compression force continues to change for a short period and it decreases substantially in the first few seconds after the clamping phase commences. This suggests that paddle movement is likely to be occurring during mammography because of this change in compression force.
Ma et al13 proposed that paddle motion could be one source of image blurring. They found that the extent of paddle motion during a mammography exposure could be as much as 1.5 mm in the vertical plane. One of the limitations of the study by Ma et al is that they assessed mammography machines from only one manufacturer; so, their finding may be limited to the Hologic Selenia Dimensions. Our present study extends the work of Ma et al13 to examine paddle motion during the clamping phase of a deformable breast phantom for a wider range of machine/paddle combinations.
METHODS AND MATERIALS
The present study used the same approach as that described by Ma et al.13 A deformable breast phantom, made of silicone (medium 360 cm3, Bodicool Triangle, Trulife, Sheffield, UK) was mounted on a wooden board to simulate the chest wall. A line was marked on the centre of the phantom to ensure it was aligned to the centre of the paddle prior to applying compression. For each combination of full-field digital mammography (FFDM) machines and paddles, the phantom was compressed to 80 N. In a previous work,14 we found that the phantom integrity would be preserved only if the compression force does not exceed 100 N. 80 N was selected to preserve phantom integrity, and it is within the range of compression forces used by mammography practitioners.15–17
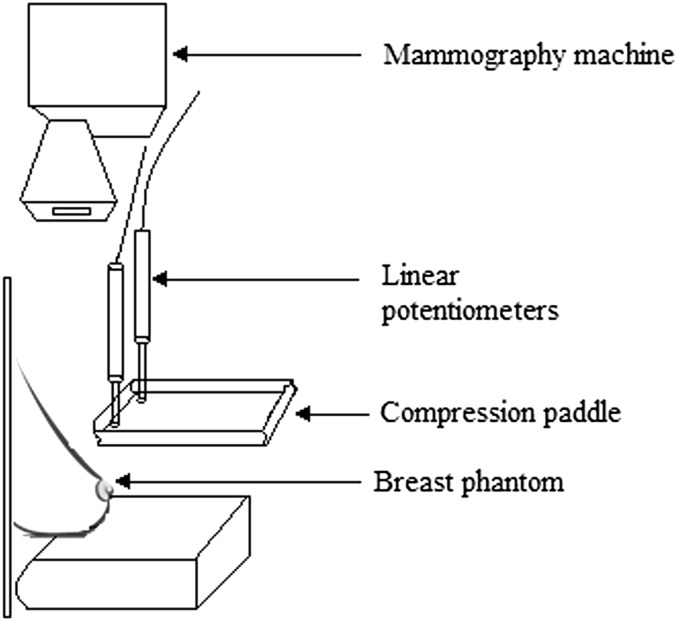
Motion at the paddle in the vertical plane was measured mechanically by two calibrated linear potentiometers (CLS1321) (Indianapolis, Indiana), placed at the corners of the compression paddle near the phantom chest wall (Figures 1 and 2). For each paddle, the measurement was repeated three times and averaged to minimize random error; the same team performed the experiment on all paddle/machine combinations to ensure consistency in setup and measurements. Previous research into paddle motion13 demonstrated that the time required for the paddle motion to stabilize was approximately 30 s; therefore, data were recorded for a period of 40 s at 0.5-s intervals.
Figure 1.
Two calibrated linear potentiometers (arrows) were located near the phantom chest wall.
Figure 2.
Schematic diagram showing the location of linear potentiometers.
Vertical paddle motion for 10 s time periods after the clamping phase commenced was calculated. The first 10 s after the clamping phase commenced was chosen for comparing machines and paddles. The rationale of choosing this time period is that the average exposure time and clamping phases last 1 and 12.8 s, respectively;12 therefore, 11.8 s after the clamp started is the average time window during which blurring is likely to occur. Vertical paddle motion at 2, 4, 8, 16 and 32 s after commencement of the clamping phase was also calculated to demonstrate how instantaneous paddle motion (the tangent slope to the potentiometer recordings) varies with time.
Paddle tilt across the medial–lateral plane for each combination of FFDM machines and paddles was calculated using trigonometric function, by considering the difference between the two potentiometer readings (tilt level) and the paddle width.
12 FFDM machines from three manufactures (Hologic, Bedford, MA; General Electric Medical Systems, Milwaukee, WI; and Siemens, Erlangen, Germany) which met quality assurance (QA) testing specifications18 were used, and a range of paddle sizes were used: 18 × 24 cm, 24 × 29 cm and 24 × 30 cm. This resulted in 42 FFDM machine/paddle combinations, with 22 flexible and 20 fixed paddles (Table 1). Since the 24 × 29-cm and 24 × 30-cm paddles are very similar in size, for practical purposes, the 24 × 29-cm and 24 × 30-cm paddles are combined into a “large” paddle group, while the 18 × 24-cm paddles are combined into a “small” paddle group. Three independent t-tests were conducted to determine whether there is a significant difference in paddle motion between fixed and flexible paddles, small and large paddles, GE Senographe Essential and Hologic Selenia Dimensions paddles. The reason Hologic Lorad Selenia and Siemens Mammomat Inspiration paddles were not included in the t-test is because the sample size for the Hologic Lorad Selenia and Siemens Mammomat Inspiration paddles are too small compared with that of GE Senographe Essential and Hologic Selenia Dimensions paddles (Table 1). The statistical comparison was performed in the first 10 s of the clamping phase rather than on the entire data set (0–40 s) because the first 10 s is the time period of interest where the probability of blurring is highest.
Table 1.
Mammography machines and paddles used in this study
| Mammography machine | Flexible paddle (small) | Fixed paddle (small) | Flexible paddle (large) | Fixed paddle (large) | Total |
|---|---|---|---|---|---|
| GE Senographe Essential | 6 | 6 | 4 | 5 | 21 |
| Hologic Selenia Dimensions | 4 | 4 | 4 | 4 | 16 |
| Hologic Lorad Selenia | 1 | 0 | 1 | 0 | 2 |
| Siemens mammomat inspiration | 1 | 1 | 1 | 0 | 3 |
| Total | 12 | 11 | 10 | 9 | 42 |
Hologic Lorad Selenia, Hologic Selenia is obtained from Hologic, Bedford, MA; GE Senographe Essential is obtained from General Electric Medical Systems, Milwaukee, WI; and Siemens Mammomat Inspiration is obtained from Siemens, Erlangen, Germany.
RESULTS
Vertical paddle motion for 18 × 24 cm (small), 24 × 29 cm and 24 × 30 cm (large) paddles during the first, second, third and fourth 10 s time periods is shown in Tables 2 and 3, respectively. As can be seen, all machine/paddle combinations have the greatest motion in the first 10 s of clamping phase commencement with a trend of decreasing motion towards 40 s. Vertical paddle motion for 18 × 24 cm (small), 24 × 29 cm and 24 × 30 cm (large) paddles at 2, 4, 8, 16 and 32 s after clamping commencement is shown in Tables 4 and 5. For small and large paddles, the vertical paddle motion has the highest value in the first 2 s of clamping and it decreases gradually 4 s after clamping phase commencement.
Table 2.
Vertical paddle motion for small paddles (18 × 24 cm) during the first, second, third and fourth section of 10-s time periods after clamping commencement
| Time period (s) |
0–10 |
10–20 |
20–30 |
30–40 |
0–40 |
|---|---|---|---|---|---|
| Paddle type | Average paddle motion (x̅ ± SD, n) (mm/10 s) | ||||
| GE Senographe Essential (flexible) | 0.21 ± 0.06, 120 | 0.08 ± 0.03, 120 | 0.04 ± 0.01, 120 | 0.03 ± 0.01, 120 | 0.36 ± 0.09, 480 |
| Hologic Lorad Selenia (flexible) | 0.26 ± 0.07, 20 | 0.05 ± 0.01, 20 | 0.03 ± 0.01, 20 | 0.03 ± 0.01, 20 | 0.37 ± 0.08, 80 |
| GE Senographe Essential (fixed) | 0.26 ± 0.07, 120 | 0.06 ± 0.02, 120 | 0.05 ± 0.01, 120 | 0.02 ± 0.01, 120 | 0.39 ± 0.09, 480 |
| Siemens Mammomat Inspiration (fixed) | 0.28 ± 0.08, 20 | 0.13 ± 0.04, 20 | 0.08 ± 0.02, 20 | 0.05 ± 0.02, 20 | 0.54 ± 0.14, 80 |
| Siemens Mammomat Inspiration (flexible) | 0.35 ± 0.11, 20 | 0.13 ± 0.03, 20 | 0.10 ± 0.02, 20 | 0.05 ± 0.01, 20 | 0.63 ± 0.16, 80 |
| Hologic Selenia Dimensions (flexible) | 0.39 ± 0.12,80 | 0.18 ± 0.05,80 | 0.12 ± 0.04,80 | 0.10 ± 0.03,80 | 0.79 ± 0.22,320 |
| Hologic Selenia Dimensions (fixed) | 0.51 ± 0.15, 80 | 0.18 ± 0.05, 80 | 0.11 ± 0.03, 80 | 0.07 ± 0.02, 80 | 0.87 ± 0.22, 320 |
x̅ is the mean; SD, standard deviation; n, number of observations.
Flexible paddles are in italicized text.
Hologic Lorad Selenia, Hologic Selenia is obtained from Hologic, Bedford, MA; GE Senographe Essential is obtained from General Electric Medical Systems, Milwaukee, WI; and Siemens Mammomat Inspiration is obtained from Siemens, Erlangen, Germany.
Table 3.
Vertical paddle motion for large paddles (24 × 29 cm and 24 × 30 cm) during the first, second, third and fourth 10 s time periods after clamping commencement
| Time period (s) |
0–10 |
10–20 |
20–30 |
30–40 |
0–40 |
|---|---|---|---|---|---|
| Paddle type | Average paddle motion (x̅ ± SD, n) (mm/10 s) | ||||
| Hologic Lorad Selenia (flexible) | 0.17 ± 0.05, 20 | 0.06 ± 0.02, 20 | 0.03 ± 0.01, 20 | 0.01 ± 0.01, 20 | 0.27 ± 0.07, 80 |
| GE Senographe Essential (flexible) | 0.30 ± 0.09, 80 | 0.06 ± 0.02, 80 | 0.05 ± 0.02, 80 | 0.04 ± 0.01, 80 | 0.45 ± 0.10, 320 |
| GE Senographe Essential (fixed) | 0.31 ± 0.09, 100 | 0.08 ± 0.02, 100 | 0.04 ± 0.01, 100 | 0.03 ± 0.01, 100 | 0.46 ± 0.10, 400 |
| Siemens Mammomat Inspiration (flexible) | 0.33 ± 0.10, 20 | 0.12 ± 0.04, 20 | 0.09 ± 0.03, 20 | 0.04 ± 0.01, 20 | 0.58 ± 0.15, 80 |
| Hologic Selenia Dimensions (flexible) | 0.35 ± 0.11, 80 | 0.15 ± 0.04, 80 | 0.10 ± 0.03, 80 | 0.05 ± 0.02, 80 | 0.65 ± 0.17, 320 |
| Hologic Selenia Dimensions (fixed) | 0.42 ± 0.13, 80 | 0.13 ± 0.04, 80 | 0.07 ± 0.02, 80 | 0.06 ± 0.02, 80 | 0.68 ± 0.16, 320 |
x̅ is the mean; SD, standard deviation; n, number of observations.
Flexible paddles are in italicized text.
Hologic Lorad Selenia, Hologic Selenia is obtained from Hologic, Bedford, MA; GE Senographe Essential is obtained from General Electric Medical Systems, Milwaukee, WI; and Siemens Mammomat Inspiration is obtained from Siemens, Erlangen, Germany.
Table 4.
Vertical paddle motion for small paddles (18 × 24 cm) at 2, 4, 8, 16 and 32 s after clamping commencement
| Seconds after clamping |
2 |
4 |
8 |
16 |
32 |
|---|---|---|---|---|---|
| Paddle type | Paddle motion (mm s−1) | ||||
| GE Senographe Essential (flexible) | 0.15 | 0.06 | 0.02 | 0.01 | <0.01 |
| Hologic Lorad Selenia (flexible) | 0.12 | 0.04 | 0.02 | 0.004 | <0.01 |
| GE Senographe Essential (fixed) | 0.14 | 0.05 | 0.02 | <0.01 | <0.01 |
| Siemens Mammomat Inspiration (fixed) | 0.22 | 0.09 | 0.04 | 0.01 | <0.01 |
| Siemens Mammomat Inspiration (flexible) | 0.25 | 0.11 | 0.04 | 0.01 | <0.01 |
| Hologic Selenia Dimensions (flexible) | 0.35 | 0.15 | 0.06 | 0.02 | <0.01 |
| Hologic Selenia Dimensions (fixed) | 0.34 | 0.14 | 0.05 | 0.01 | <0.01 |
Flexible paddles are in italicized text.
Hologic Lorad Selenia, Hologic Selenia is obtained from Hologic, Bedford, MA; GE Senographe Essential is obtained from General Electric Medical Systems, Milwaukee, WI; and Siemens Mammomat Inspiration is obtained from Siemens, Erlangen, Germany.
Table 5.
Vertical paddle motion for large paddles (24 × 29 cm and 24 × 30 cm) at 2, 4, 8, 16 and 32 s after clamping commencement
| Seconds after clamping |
2 |
4 |
8 |
16 |
32 |
|---|---|---|---|---|---|
| Paddle type | Paddle motion (mm s−1) | ||||
| Hologic Lorad Selenia (flexible) | 0.09 | 0.04 | 0.01 | <0.01 | <0.01 |
| GE Senographe Essential (flexible) | 0.16 | 0.06 | 0.02 | 0.01 | <0.01 |
| GE Senographe Essential (fixed) | 0.16 | 0.06 | 0.02 | 0.01 | <0.01 |
| Siemens Mammomat Inspiration (flexible) | 0.23 | 0.10 | 0.03 | 0.01 | <0.01 |
| Hologic Selenia Dimensions (flexible) | 0.28 | 0.12 | 0.04 | 0.01 | <0.01 |
| Hologic Selenia Dimensions (fixed) | 0.26 | 0.10 | 0.04 | 0.01 | <0.01 |
Flexible paddles are in italicized text.
Hologic Lorad Selenia, Hologic Selenia is obtained from Hologic, Bedford, MA; GE Senographe Essential is obtained from General Electric Medical Systems, Milwaukee, WI; and Siemens Mammomat Inspiration is obtained from Siemens, Erlangen, Germany.
For small paddles, the GE Senographe Essential flexible paddle has the lowest mean motion (0.21 ± 0.06 mm/10 s, n = 120) in the first 10 s after clamping commencement, while the Hologic Selenia Dimensions fixed paddle has the largest mean motion (0.51 ± 0.15 mm/10 s, n = 80) (Table 2). For large paddles, the Hologic Lorad Selenia flexible paddle has the lowest mean motion (0.17 ± 0.05 mm/10 s, n = 20) in the first 10 s after clamping commencement, while the Hologic Selenia Dimensions fixed paddle has the largest mean motion (0.42 ± 0.13 mm/10 s, n = 80) (Table 3).
There is a statistical difference in paddle motion between fixed [x̅ = 0.24, standard deviation (SD) = 0.15, n = 400] and flexible paddles (x̅ = 0.20, SD = 0.10, n = 440), t(838) = 5.11, p < 0.001, GE Senographe Essential (x̅ = 0.19, SD = 0.11, n = 420) and Hologic Selenia Dimensions paddles (x̅ = 0.26, SD = 0.15, n = 320), t (738) = 8.15, p < 0.001. However, there is no statistical difference in paddle motion between small (x̅ = 0.21, SD = 0.14, n = 460) and large paddles (x̅ = 0.22, SD = 0.12, n = 380); t (838) = 0.865, p = 0.387.
The mean paddle tilt in the medial–lateral plane for small (18 × 24 cm) and large (24 × 29 cm and 24 × 30 cm) paddles is shown in Figures 3 and 4. As can be seen, all machine/paddle combinations demonstrating tilt are independent of time. The 18 × 24-cm Hologic Lorad Selenia flexible paddle has the smallest tilt (0.04 °) (Figure 3), while the 24 × 30 cm Siemens Mammomat Inspiration flexible paddle has the largest tilt (0.69 °) (Figure 4).
Figure 3.
Paddle tilt against time for small paddles (18 × 24 cm).
Figure 4.
Paddle tilt against time for large paddles (24 × 29 cm and 24 × 30 cm).
DISCUSSION
Research into the perception of motion in FFDM images, using computer-based simulation to mimic blurring, demonstrated that simulated motion as low as 0.4 mm in the horizontal plane can be detected visually.19 Further work is needed to determine what relationship exists between vertical motion and reactionary horizontal displacement in the female breast tissue. Studies show that harmonious breast height (H) to width (W) ratio (H/W) should be between 0.7 and 1.3.20 Given that the female breast deforms rather than squashes when compressed, the vertical thickness reduction will result in horizontal breast tissue displacement, and the ratio could therefore vary between 0.7 and 1.3.
All paddles demonstrated motion. Most of this motion occurred in the first 10 s of clamping. According to the study by Groot et al,12 the average exposure time and clamping phases last 1 and 12.8 s, respectively. If the exposure is made when the paddle is moving, then image blurring could occur. Although paddle motion decreases with time, it would be impractical to wait tens of seconds before making the exposure, for reasons such as patient movement and discomfort.21,22
Our research suggests that the Hologic Selenia Dimensions with 18 × 24-cm fixed paddle (0.51 ± 0.15 mm/10 s, n = 80) has the highest potential to create blurring during imaging, while the Hologic Lorad Selenia with 24 × 29 cm flexible paddle (0.17 ± 0.05 mm/10 s, n = 20) has the lowest potential.
One of the practical solutions to minimize the probability of image blurring is to use the fixed paddle with caution, as our findings show that there is a significant difference (p < 0.001) in motion for fixed and flexible paddles. Fixed paddles have slightly higher motion (x̅ = 0.24, SD = 0.15, n = 400) than flexible paddles (x̅ = 0.20, SD = 0.10, n = 440), suggesting that the fixed paddles might incur more motion artefacts. Extra caution could therefore be exercised by radiographers when positioning patients using fixed paddles because of this. An additional preventative measure could include waiting an additional few seconds prior to making an exposure, thereby allowing any paddle motion to have ceased by the time the exposure commences. Tables 4 and 5 suggest that motion will be clinically insignificant or not visually apparent 4 s or more after the clamping phase commences, as all motion values are likely to be below 0.4 mm for typical exposure times.19 However, caution should be exercised as this prediction is based upon data generated from a phantom breast and motion in the vertical plane from Ma et al19's work. Further research is therefore needed using a human female breast alongside measures of horizontal displacement.
The presence of tilting in the medial–lateral plane among paddles suggests that the compression force applied on the paddle may not be evenly distributed, which could mean that one side of the breast may be compressed more than the other side. A limitation of this study is that the breast phantom used cannot fully represent the compression characteristics of the female breast. Our silicone breast phantom exhibits a purely elastic compression characteristic, whereas the female breast exhibits a visco-elastic compression characteristic.23 If the compression speed is too fast for the viscous effect to occur during the deformation phase, the paddle motion measured in the clamping phase would be influenced by the female breast's viscosity. Consequently, the female breast is likely to continue to flatten during the clamping phase, while the purely elastic phantom may not. Therefore, phantom measurements would give an underestimation of paddle and therefore breast motion if the compression speed is fast.
In this study, we sampled only two points on the paddle surface to measure the paddle motion, as at the time of conducting the study, limited affordable technology existed to map the entire surface. This has now changed—for example, technologies like Kinect (Microsoft, Washington) would allow monitoring of the whole paddle surface over time, which would allow for the assessment of regional differences in motion across the paddle surface.24
The clinical impact of mammography image blurring needs further investigation. For instance, an analysis of lesion-detection performance using free response-operating characteristic with blurred and non-blurred images would give an indication as to whether cancer/non-cancer localization and observer confidence in decision-making would be impaired during blurred image conditions.
Presently, compression paddle QA guidelines (e.g. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis25) indicate only a compression force test and compression plate alignment. There is no manufacturer guidance or QA standards regarding assessment of paddle motion, particularly using a deformable object/phantom in an attempt to mimic clinical demands. Our work suggests that new QA tests/guidelines be developed to assess paddle motion using a suitable deformable object prior to a paddle being used in practice.
CONCLUSION
All machine/paddle combinations exhibited motion and tilt, and the extent varies with machine, paddle sizes and paddle types. Most motion occurred within the first 10 s of clamping, and after 4 s, paddle motion will likely be clinically insignificant. Paddle tilt in the medial–lateral plane is independent of time under compression. Our findings may have implications for practice, including the need for a new QA motion test and the need for radiographers to possibly take additional precautions when using fixed paddles in order to minimize the potential of paddle motion and image blurring.
Acknowledgment
The authors would like to thank Mass Measuring Ltd (Manchester, UK) for developing the system for measuring movement using the potentiometers and for the financial support from the Trustees of Symposium Mammographicum.
Funding
This study is supported by the funding from the Trustees of Symposium Mammographicum.
Contributor Information
Wang KEi Ma, Email: carnby2000@gmail.com.
Mark F McEntee, Email: mark.mcentee@sydney.edu.au.
Claire Mercer, Email: C.E.Mercer@salford.ac.uk.
Judith Kelly, Email: judith.kelly2@nhs.net.uk.
Sara Millington, Email: saramillington@nhs.net.uk.
Peter Hogg, Email: P.Hogg@salford.ac.uk.
REFERENCES
- 1.Cancer statistics registrations, England (series 18 MB1) no. 43. London, UK: Office for National Statistics; 2012. [Google Scholar]
- 2.Nyström L, Rutqvist LE, Wall S, Lindgren A, Lindqvist M, Rydén S, et al. Breast cancer screening with mammography: overview of Swedish randomised trials. Lancet 1993; 341: 973–8. doi: 10.1016/0140-6736(93)91067-V [DOI] [PubMed] [Google Scholar]
- 3.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012; 367: 1998–2005. doi: 10.1056/NEJMoa1206809 [DOI] [PubMed] [Google Scholar]
- 4.Biesheuvel C, Barratt A, Howard K, Houssami N, Irwig L. Effects of study methods and biases on estimates of invasive breast cancer overdetection with mammography screening: a systematic review. Lancet Oncol 2007; 8: 1129–38. doi: 10.1016/S1470-2045(07)70380-7 [DOI] [PubMed] [Google Scholar]
- 5.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M; Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380: 1778–86. doi: 10.1016/S0140-6736(12)61611-0 [DOI] [PubMed] [Google Scholar]
- 6.Guidelines for quality assurance in mammography screening. 3rd edn. Dublin, Ireland: The National Cancer Screening Service Board; 2008. [Google Scholar]
- 7.Hogg P, Kelly J, Claire E, eds. Digital mammography: a holistic approach. 1st edn. London, UK: Springer; 2015. [Google Scholar]
- 8.NHS Cancer Screening Programmes. Consolidated guidance on standards for the NHS Breast Screening Programme. NHSBSP Publication No 60 (version 2). UK: Sheffield; 2005. [Google Scholar]
- 9.Julie R, Claire E, Laura S. Programme evaluation: technical recall and image blur within a breast screening service. Symposium mammographicum 2014. UK: Bournemouth; 2014. [Google Scholar]
- 10.Kinnear L, Mercer C. The detection of visual blurring in 1MP and 5MP monitors within mammography clinical practice. Imaging Ther Practice. In press.
- 11.Seddon D, Schofield KA, Waite CA. Investigation into possible causes of blurring in mammograms. Breast Cancer Res 2000; 2: A64. doi: 10.1186/bcr253 [DOI] [Google Scholar]
- 12.de Groot JE, Broeders MJ, Grimbergen CA, den Heeten GJ. Pain-preventing strategies in mammography: an observational study of simultaneously recorded pain and breast mechanics throughout the entire breast compression cycle. BMC Womens Health 2015; 15: 26. doi: 10.1186/s12905-015-0185-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma WK, Brettle D, Howard D, Kelly J, Millington S, Hogg P. Extra patient movement during mammographic imaging: an experimental study. Br J Radiol 2014; 87: 20140241. doi: 10.1259/bjr.20140241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauge IH, Hogg P, Szczepura K, Connolly P, McGill G, Mercer C. The readout thickness versus the measured thickness for a range of screen film mammography and full-field digital mammography units. Med Phys 2012; 39: 263–71. doi: 10.1118/1.3663579 [DOI] [PubMed] [Google Scholar]
- 15.Mercer C, Szczepura K, Kelly J, Millington S, Denton ERE, Borgen R, et al. A 6-year study of mammographic compression force: practitioner variation within and between screening sites. Radiography 2014; 21: 68–73. doi: 10.1016/j.radi.2014.07.004 [DOI] [Google Scholar]
- 16.Hogg P, Taylor M, Szczepura K, Mercer C, Denton E. Pressure and breast thickness in mammography—an exploratory calibration study. Br J Radiol 2013; 86: 20120222. doi: 10.1259/bjr.20120222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercer CE, Hogg P, Lawson R, Diffey J, Denton ER. Practitioner compression force variability in mammography: a preliminary study. Br J Radiol 2013; 86: 20110596. doi: 10.1259/bjr.20110596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore AC, Dance DR, Evans DS, Lawinski CP, Pitcher EM, Rust A, et al. The commissioning and routine testing of mammographic X-ray systems: a technical quality control protocol. report no. 89. York, UK: IPEM; 2005. [Google Scholar]
- 19.Ma WK, Aspin R, Kelly J, Millington S, Hogg P. What is the minimum amount of simulated breast movement required for visual detection of blurring? an exploratory investigation. Br J Radiol 2015; 88: 20150126. doi: 10.1259/bjr.20150126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiffman A, ed. Breast augmentation: principles and practice. 1st edn. London, UK: Springer; 2009. [Google Scholar]
- 21.Poulos A, Rickard M. Compression in mammography and the perception of discomfort. Australas Radiol 1997; 41: 247–52. [DOI] [PubMed] [Google Scholar]
- 22.Sapir R, Patlas M, Strano SD, Hadas-Halpern I, Cherny NI. Does mammography hurt? J Pain Symptom Manage 2003; 25: 53–63. doi: 10.1016/S0885-3924(02)00598-5 [DOI] [PubMed] [Google Scholar]
- 23.Geerligs M, Peters GW, Ackermans PA, Oomens CW, Baaijens FP. Does subcutaneous adipose tissue behave as an (anti-)thixotropic material? J Biomech 2010; 43: 1153–9. doi: 10.1016/j.jbiomech.2009.11.037 [DOI] [PubMed] [Google Scholar]
- 24.Pohlmann STL, Hewes J, Williamson A I, Sergeant JC, Hufton A, Gandhi A. et al. Breast volume measurement using a games console input device, breast imaging: lecture notes in computer science 8539: international workshop on breast imaging. Gifu, Jpn Switzerland: Springer International; 2014. pp. 666–73. [Google Scholar]
- 25.Perry N, Broeders M, Wolf C, Törnberg S, Holland R, Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. 4th edn. Luxembourg: European Communities; 2006. [DOI] [PubMed] [Google Scholar]