Abstract
The British Thoracic Society has published new comprehensive guidelines for the management of pulmonary nodules. These guidelines are significantly different from those previously published, as they use two malignancy prediction calculators to better characterize the risk of malignancy. There are recommendations for a higher nodule size threshold for follow-up (≥5 mm or ≥80 mm3) and a reduction of the follow-up period to 1 year for solid pulmonary nodules; both of these will reduce the number of follow-up CT scans. PET-CT plays a crucial role in characterization also, with an ordinal scale being recommended for reporting. Radiologists will be the key in implementing these guidelines, and routine use of volumetric image-analysis software will be required to manage patients with pulmonary nodules correctly.
INTRODUCTION
The 2015 British Thoracic Society (BTS) guidelines for the investigation and management of pulmonary nodules have recently been published.1 These guidelines are based on a comprehensive review of the literature on pulmonary nodules and expert opinion. The Guideline Development Group highlight the new evidence, which has led to significant changes in management recommendations from previously published guidelines. These include the use of two prediction calculators to better characterize the risk of malignancy. There are recommendations for a larger nodule size threshold for follow-up (≥5 mm or ≥80 mm3) and a reduction of the follow-up period to 1 year for solid pulmonary nodules; both of these will reduce the number of follow-up CT scans. Volumetry is more accurate than diameter measurements and has been recommended as the preferred measurement method. Acknowledging the good prognosis of subsolid nodules (SSNs), there are recommendations for less aggressive options for their management.
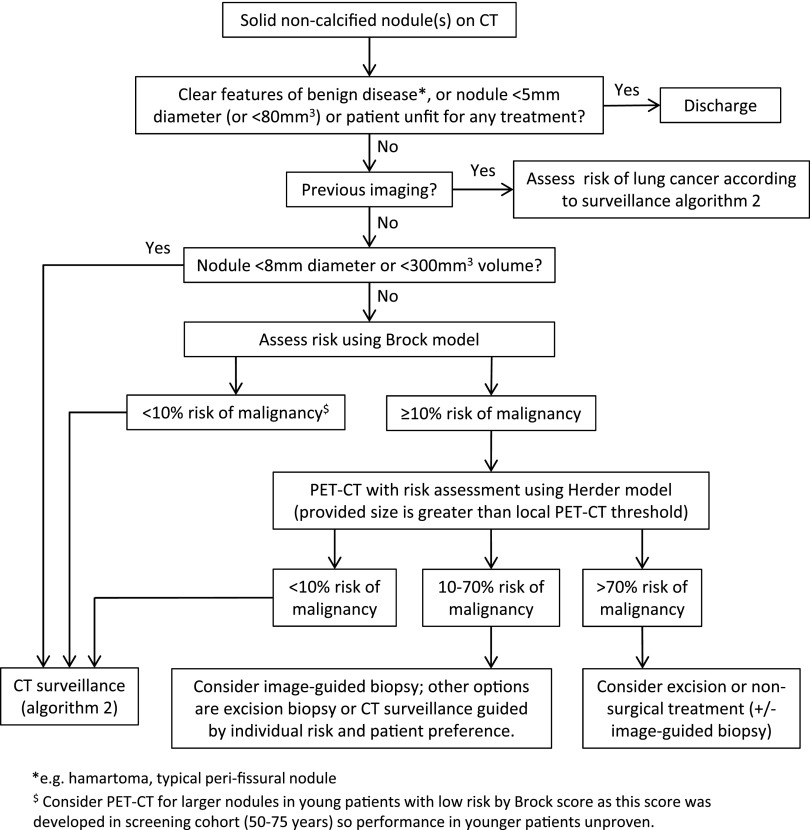
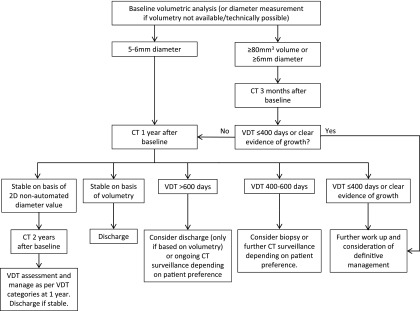
The guidelines provide more clarity in the use of further imaging, with ordinal scale reporting for PET-CT recommended to facilitate incorporation into risk models and more clarity about the place of biopsy. Radiologists will be the key in the successful implementation of these guidelines, as radiological characterization of nodules is crucial for determining management decisions in the algorithms (Figure 1, Figure 2).
Figure 1.
British Thoracic Society guidelines initial approach to solid pulmonary nodules (reproduced from Callister et al1 with permission from BMJ Publishing Group Ltd). PET, positron emission tomography.
Figure 2.
British Thoracic Society guidelines solid pulmonary nodule surveillance algorithm (reproduced from Callister et al1 with permission from BMJ Publishing Group Ltd). 2D, two-dimensional; VDT, volume-doubling time.
The BTS guidelines differ from those of the Fleischer Society.2 In the latter, patients are divided into low risk and high risk on the basis of smoking history and other known risk factors. Once this risk is assigned, advice is given on what management to take with respect to the nodule size. Nodules that are ≤4 mm need no follow-up in the low-risk group but do in the high-risk group. This can be difficult for the radiologist, as often smoking history and risk factors are not in the request information. The BTS guideline allows the radiologist to dismiss nodules <5 mm in diameter (or <80 mm3) without knowing a pre-test probability of cancer, thus making them easier to apply than the Fleischner Society guidelines. Caution is required, however, where there is a previous history of malignancy or patients are under oncology follow-up The Fleischner Society Guidelines also recommend different follow-up intervals for different-sized nodules: >4–6 mm, >6–8 mm and >8 mm. There is also separate guidance for SSNs.3 This is stratified on size and on whether it is a solitary pure ground-glass nodule (pGGN), solitary part-solid nodule (PSN) or there are multiple SSNs.
The guidelines are not prescriptive in how to organize nodule pathways in your institution. Virtual nodule clinics have not been specifically advocated, and it is presumed that the prediction models will be applied during multidisciplinary/post-multidisciplinary team meetings. Overall, services should develop their own preferred approach. An example pathway is provided in appendix 3 of the guideline (Figure 3).
Figure 3.
British Thoracic Society guidelines example of a pulmonary nodule service pathway (reproduced from Callister et al1 with permission from BMJ Publishing Group Ltd). MDT, multidisciplinary team.
NODULE MEASUREMENT ON MULTIDETECTOR CT
To prevent confusion when compared with other guidelines and articles, the authors of the BTS guidelines have used the following nomenclature. A pulmonary nodule is defined as a focal, rounded opacity ≤3 cm diameter, mostly surrounded by aerated lung, including contact with pleura, but without potentially related abnormalities in the thorax. A SSN is a PSN or pGGN. A PSN is a focal opacity that has both solid and ground-glass components of ≤3-cm diameter. A pGGN (synonymous with a non-solid nodule) is a focal ground-glass opacity of ≤3 cm diameter that does not obscure vascular pattern. The solid component of a nodule is the part of a nodule that obscures the underlying bronchovascular structure. Ground glass is opacification that is greater than that of the background, but through which the underlying vascular structure is visible.1
Perifissural nodules (PFNs) detected on CT screening were specifically assessed in the Dutch–Belgian randomized lung cancer screening trial (NELSON) by de Hoop et al4 These are also frequently termed subpleural nodules or subpleural nodes by UK radiologists. They are homogeneous solid nodules, attached to a fissure with a lentiform or triangular shape and may be subpleural (Figure 4). 794 (19.7%) nodules of the 4026 nodules detected at baseline screening were classified as PFNs, and were followed up according to the standard protocol. At first follow-up, 66 PFNs (8.3% of all PFNs) grew with a volume-doubling time (VDT) <400 days. One PFN was resected and was proved to be a lymph node. None of the other PFNs turned out to be malignant after 5 years of follow-up. PFNs are thought to be intrapulmonary lymph nodes, on the basis of their CT features and histological correlates. Four studies examined histologically confirmed intrapulmonary lymph nodes (n = 38, 19, 18 and 11, respectively) and characterized their CT features.5–8 In all these studies and that of de Hoop, the nodules were relatively small (<10 mm). Caution may be required in larger PFNs (>10 mm) in the presence of known non-lung primary cancers, as there is anecdotal evidence of malignancy in these nodules. Subpleural nodules, like PFNs, are assumed to be benign as long as they are within 1 cm of the pleural surface, are homogeneous, smooth, solid with a lentiform or triangular in shape.
Figure 4.
Appearance of perifissural nodules (PFNs) as defined in the studies of de Hoop et al4 (reproduced from de Hoop et al4 with permission from The Rediological Society of North America).
From the NELSON study, Horeweg et al9 demonstrated that nodules <5 mm in diameter or <100 mm3 volume did not require any CT surveillance, as they are not associated with a significantly increased risk of lung cancer. However, two other studies reported variation in absolute volume measurement between volumetric software packages,10,11 suggesting that until there is improved software concordance, it is safer to reduce the threshold to 80 mm3.
A number of studies have shown volumetric measurements of pulmonary nodules to be superior to two-dimensional (2D) calliper measurements in both reproducibility and sensitivity to nodule growth. For example, Revel et al12 assessed variability in 2D CT measurements of 54 subcentimetre pulmonary nodules in 24 patients both between readers and in the same reader's measurements at different times. Both intrareader and interreader agreement for 2D measurements were found to be poor, with a change in size of <1.7 mm only, having a 5% chance of corresponding to an actual change in nodule size. Ko et al13 compared semi-automated three-dimensional volumetric analysis against standard calliper cross-sectional diameter measurement of 123 lung nodules in a retrospective analysis of 59 patients recruited through a CT lung cancer-screening programme. Abnormal growth was detected in nodules subsequently proved to be malignant at a much shorter time interval (183 ± 158 days) by three-dimensional volumetry than by standard radiological diagnosis (344 ± 284 days), suggesting greater sensitivity of the volumetric technique.
VDT has been proven in a number of studies14–17 to be a good method for risk stratification of pulmonary nodules. Unfortunately, extremely long VDTs for some lung cancers, and the observation that a proportion of malignant nodules reduces in size on interval screening, indicates that there is no upper limit of VDT above which nodules can be guaranteed to be benign. Similarly, the observation that some malignant nodules show a long period of radiological stability before growth is identified means that it is not possible to define a period of surveillance during which stability will completely exclude the possibility of malignancy.
The largest series of pulmonary nodules followed up by volumetric analysis comes from the NELSON study. The trial used VDT (calculated by automated volumetric analysis after a 3-month or 12-month interval) to guide management of indeterminate pulmonary nodules (50–500 mm3), so that patients with nodules with a VDT of <400 days were referred to a chest physician for investigation and diagnosis, whereas those with nodules with a VDT >400 days were considered benign and re-entered the screening programme.18 At least a 25% change in volume was required to indicate a significant change.19 It should be noted that where the automated software was unable to calculate volume, VDT was measured by manually measuring maximum diameter in three perpendicular planes. Thus, any conclusions about follow-up periods relying on diameter measurements can apply only when VDT is calculated using this method.
As a result of the evidence supporting volumetric nodule analysis, it will be vital for all radiologists to measure lung nodule volumes in subcentimetre nodules routinely in CT reporting. This will require high-quality nodule analysis software, used as a thin client, on all picture archiving and communication system (PACS) workstations where CT is reported. The radiologist will need to perform volumetric analysis on the baseline CT, and then, the automated package will store these data and nodule-tagging information, which can be used in a semi-automated way to calculate the VDT at follow-up. Screen shots of these volume data will also need to be automatically sent to PACS, to allow review at multidisciplinary teams and clinicoradiological meetings and to future proof against PACS and software vendor change or upgrades.
PET-CT OF NODULES
The utility of PET and PET-CT as an imaging technique in the evaluation of pulmonary nodules has been reported, but data using modern scanners and reconstruction algorithms are poor. Cronin et al20 performed a meta-analysis of cross-sectional imaging modalities for the diagnosis of malignancy in solitary pulmonary nodules (up to 3 cm diameter). A pooled analysis of 1008 nodules from 22 eligible studies reported a sensitivity and specificity of fludeoxyglucose (FDG) PET of 95% and 82%, respectively.
The utility of PET for characterizing nodules <10 mm is not clear, with sparse data available in both meta-analyses. Gould et al21 noted a paucity of data on nodules <1 cm; the eight instances where results were available showed three true-positive, two true-negative and three false-negative observations. A size threshold of 8 mm has been set in the new guidelines, as a nodule of this size should be imaged accurately on modern PET-CT scanners. As PET-CT scanners become more sensitive with hardware and software improvements, this size threshold is likely to fall. Common sense needs to be applied according to the PET-CT scanner in your institution, and a recent article highlights the change in measurements with new technology.21
Gould et al22 in their meta-analysis found that semi-quantitative analysis of FDG uptake provided no additional benefit to the diagnostic accuracy achieved through qualitative visual assessment. Furthermore, they suggested that the best use of FDG PET was in conjunction with an estimation of the pre-test probability of malignancy. Herder et al23 confirmed this in a retrospective study of 106 patients with indeterminate solitary pulmonary nodules evaluated with FDG PET. They validated the Mayo clinical risk-prediction model and reported a high diagnostic accuracy (86%) of FDG PET for malignancy. Importantly, combined information gained from both clinical assessment and FDG PET resulted in the best diagnostic accuracy, with FDG PET significantly increasing the area under the receiver-operating characteristic by 13% from 0.79 to 0.92. More recently, Evangelista et al24 retrospectively reviewed 59 patients with cancer with indeterminate solitary or multiple lung nodules, who underwent FDG PET-CT. They used the Mayo clinic and Veteran Affairs clinical risk prediction models to assign risk categories and assessed the additional role of FDG PET-CT. They found that the use of FDG PET-CT was most efficacious and improved risk stratification in those with a low-to-intermediate pre-test probability of malignancy. In the Herder model, FDG uptake was classified as absent, faint, moderate or intense. The authors did not provide objective measures or definitions, but others have.25,26 The last two studies used a five-point scale that the Guideline Development Group adapted to a four-point scale to facilitate consistency in reporting and use with the Herder model (Table 1).
Table 1.
Four-point ordinal scale for fludeoxyglucose PET-CT reporting of lung nodules
| Grade | Descriptor |
|---|---|
| Absent | Uptake indiscernible from the background lung tissue |
| Faint | Uptake less than or equal to mediastinal blood pool |
| Moderate | Uptake greater than mediastinal blood pool |
| Intense | Uptake markedly greater than mediastinal blood pool |
RISK PREDICTION CALCULATOR
The risk predication calculator for the Brock and Herder models can be found at:
This site also has a VDT calculator. These calculators will not need to be used routinely by radiologists; however, it is useful for radiologists to have an appreciation of the data fields involved and the process involved to generate the risk of malignancy. Radiologists will need to report the following features so that they can be entered into the Brock Model: emphysema (Y/N), largest nodule size, nodule type (pure ground glass, part solid or solid), number of nodules and speculation (Y/N). When the nodules (other than the largest one under assessment) are thought to be inflammatory, it may be prudent to discount the inflammatory nodules as part of the overall nodule count, as it could lead to artificial reduction of the predicted risk. This is especially important in younger patients.
CONCLUSION
These new guidelines are likely to reduce the amount of nodule follow-ups compared with current practice. The fact that the nodules <5 mm or <80 mm3 can be dismissed without knowing the clinical pre-test probability will mean the radiologist can provide a report stating that follow-up is not required. This recommendation may not apply to smaller nodules detected in patients with active cancer, where a more cautious approach may be required, especially if there is to be no repeat CT as part of oncology follow-up. The new cut-off is, however, a major step forward, as the number of incidental nodules being identified is rising owing to the increased use of CT. The use of clinical risk prediction models will allow objective standardized decisions to be made about patient management based on their clinical and imaging data. The implementation of these guidelines is hugely reliant on radiologists learning the algorithms and radiology departments purchasing good automated nodule volumetry software that can be used for routine reporting.
Contributor Information
Richard N J Graham, Email: rnjgraham@gmail.com, richard.graham1@nhs.net.
David R Baldwin, Email: David.Baldwin@nuh.nhs.uk.
Matthew E J Callister, Email: matthew.callister@nhs.net.
Fergus V Gleeson, Email: fgleeson@icloud.com.
REFERENCES
- 1.Callister MJ, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. ; British Thoracic Society Pulmonary Nodule Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015; 70: ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168 [DOI] [PubMed] [Google Scholar]
- 2.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. ; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237: 395–400. doi: 10.1148/radiol.2372041887 [DOI] [PubMed] [Google Scholar]
- 3.Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013; 266: 304–17. doi: 10.1148/radiol.12120628 [DOI] [PubMed] [Google Scholar]
- 4.de Hoop B, van Ginneken B, Gietema H, Prokop M. Pulmonary perifissural nodules on CT scans: rapid growth is not a predictor of malignancy. Radiology 2012; 265: 611–16. doi: 10.1148/radiol.12112351 [DOI] [PubMed] [Google Scholar]
- 5.Wang CW, Teng YH, Huang CC, Wu YC, Chao YK, Wu CT. Intrapulmonary lymph nodes: computed tomography findings with histopathologic correlations. Clin Imaging 2013; 37: 487–92. doi: 10.1016/j.clinimag.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 6.Shaham D, Vazquez M, Bogot NR, Henschke CI, Yankelevitz DF. CT features of intrapulmonary lymph nodes confirmed by cytology. Clin Imaging 2010; 34: 185–90. doi: 10.1016/j.clinimag.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 7.Hyodo T, Kanazawa S, Dendo S, Kobayashi K, Hayashi H, Kouno Y, et al. Intrapulmonary lymph nodes: thin-section CT findings, pathological findings, and CT differential diagnosis from pulmonary metastatic nodules. Acta Med Okayama 2004; 58: 235–40. [DOI] [PubMed] [Google Scholar]
- 8.Oshiro Y, Kusumoto M, Moriyama N, Kaneko M, Suzuki K, Asamura H, et al. Intrapulmonary lymph nodes: thin-section CT features of 19 nodules. J Comput Assist Tomogr 2002; 26: 553–7. doi: 10.1097/00004728-200207000-00014 [DOI] [PubMed] [Google Scholar]
- 9.Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014; 15: 1332–41. doi: 10.1016/S1470-2045(14)70389-4 [DOI] [PubMed] [Google Scholar]
- 10.de Hoop B, Gietema H, van Ginneken B, Zanen P, Groenewegen G, Prokop M. A comparison of six software packages for evaluation of solid lung nodules using semi-automated volumetry: what is the minimum increase in size to detect growth in repeated CT examinations. Eur Radiol 2009; 19: 800–8. doi: 10.1007/s00330-008-1229-x [DOI] [PubMed] [Google Scholar]
- 11.Zhao YR, van Ooijen PM, Dorrius MD, Heuvelmans M, de Bock GH, Vliegenthart R, et al. Comparison of three software systems for semi-automatic volumetry of pulmonary nodules on baseline and follow-up CT examinations. Acta Radiol 2014; 55: 691–8. doi: 10.1177/0284185113508177 [DOI] [PubMed] [Google Scholar]
- 12.Revel MP, Bissery A, Bienvenu M, Aycard L, Lefort C, Frija G. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable? Radiology 2004; 231: 453–8. doi: 10.1148/radiol.2312030167 [DOI] [PubMed] [Google Scholar]
- 13.Ko JP, Berman EJ, Kaur M, Babb JS, Bomsztyk E, Greenberg AK, et al. Pulmonary nodules: growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology 2012; 262: 662–71. doi: 10.1148/radiol.11100878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings SG, Winer-Muram HT, Tann M, Ying J, Dowdeswell I. Distribution of stage I lung cancer growth rates determined with serial volumetric CT measurements. Radiology 2006; 241: 554–63. doi: 10.1148/radiol.2412051185 [DOI] [PubMed] [Google Scholar]
- 15.Winer-Muram HT, Jennings SG, Tarver RD, Aisen AM, Tann M, Conces DJ, et al. Volumetric growth rate of stage I lung cancer prior to treatment: serial CT scanning. Radiology 2002; 223: 798–805. [DOI] [PubMed] [Google Scholar]
- 16.Henschke CI, Yankelevitz DF, Yip R, Reeves AP, Farooqi A, Xu D, Smith JP, et al. ; Writing Committee for the I-ELCAP Investigators. Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology 2012; 263: 578–83. doi: 10.1148/radiol.12102489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson DO, Ryan A, Fuhrman C, Schuchert M, Shapiro S, Siegfried JM, et al. Doubling times and CT screen-detected lung cancers in the Pittsburgh Lung Screening Study. Am J Respir Crit Care Med 2012; 185: 85–9. doi: 10.1164/rccm.201107-1223OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu DM, Gietema H, de Koning H, Vernhout R, Nackaerts K, Prokop M, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006; 54: 177–84. doi: 10.1016/j.lungcan.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 19.Horeweg N, van der Aalst CM, Vliegenthart R, Zhao Y, Xie X, Scholten ET, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J 2013; 42: 1659–67. doi: 10.1183/09031936.00197712 [DOI] [PubMed] [Google Scholar]
- 20.Cronin P, Dwamena BA, Kelly AM, Carlos RC. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 2008; 246: 772–82. doi: 10.1148/radiol.2463062148 [DOI] [PubMed] [Google Scholar]
- 21.Teoh EJ, McGowan DR, Bradley KM, Belcher E, Black E, Gleeson FV. Novel penalised likelihood reconstruction of PET in the assessment of histologically verified small pulmonary nodules. Eur Radiol 2016; 26: 576–84. doi: 10.1007/s00330-015-3832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis (structured abstract). JAMA 2001; 285: 914–24. doi: 10.1001/jama.285.7.914 [DOI] [PubMed] [Google Scholar]
- 23.Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005; 128: 2490–6. doi: 10.1378/chest.128.4.2490 [DOI] [PubMed] [Google Scholar]
- 24.Evangelista L, Panunzio A, Polverosi R, Pomerri F, Rubello D. Indeterminate lung nodules in cancer patients: pretest probability of malignancy and the role of 18F-FDG PET/CT. AJR Am J Roentgenol 2014; 202: 507–14. doi: 10.2214/AJR.13.11728 [DOI] [PubMed] [Google Scholar]
- 25.Fletcher JW, Kymes SM, Gould M, Alazraki N, Coleman RE, Lowe VJ, et al. ; VA SNAP Cooperative Studies Group. A comparison of the diagnostic accuracy of 18F-FDG PET and CT in the characterization of solitary pulmonary nodules. J Nucl Med 2008; 49: 179–85. doi: 10.2967/jnumed.107.044990 [DOI] [PubMed] [Google Scholar]
- 26.Vansteenkiste JF, Stroobants SG, Dupont PJ, De Leyn PR, De Wever WF, Verbeken EK, et al. FDG-PET scan in potentially operable non-small cell lung cancer: do anatometabolic PET-CT fusion images improve the localisation of regional lymph node metastases? The Leuven Lung Cancer Group. Eur J Nucl Med 1998; 25: 1495–501. doi: 10.1007/s002590050327 [DOI] [PubMed] [Google Scholar]