Abstract
Objective:
To evaluate the therapeutic efficacy and safety of ultrasound-guided percutaneous bipolar radiofrequency ablation (BRFA) of benign thyroid nodules by comparison with a matched untreated control group.
Methods:
The therapeutic efficacy and safety in 35 patients who were subjected to a single session of ultrasound-guided percutaneous BRFA (Group A) for benign thyroid nodules were compared with those in 35 untreated patients (Group B) with benign nodules. The benign nature of all the nodules was confirmed by ultrasound-guided fine-needle aspiration biopsy (FNAB), and all the patients had normal thyroid functions. BRFA was performed with a bipolar electrode (CelonProSurge 150–T20) with an output power of 20 W. Nodule volume, thyroid function and clinical symptoms of all the patients were compared before treatment and during follow-up.
Results:
In Group A, the BRFA procedures were completed with a mean time of 10.02 ± 3.30 min (range, 5.47–16.03 min) and with a mean total energy deposition of 10.747 ± 3704 J (range, 5510–17.770 J). The procedures were tolerated well in all the patients without causing any major complications. At the 6-month follow-up, all of the nodule volume decreased significantly (from 8.81 ± 8.66 to 1.59 ± 1.55 ml, p < 0.001) in Group A, whereas the nodule volume increased from 6.90 ± 3.77 to 7.87 ± 3.95 ml in Group B (p < 0.001). All (100%) the 35 nodules in Group A had volume reduction ratios (VRRs) of >50%, among which 3 (8.57%) had VRRs >90%. In Group A, the clinical symptoms of the patients who had symptoms before BRFA disappeared, whereas in Group B, the patients had no resolution of clinical symptoms at the 6-month follow-up.
Conclusion:
Ultrasound-guided percutaneous BRFA seems to be an effective and safe method for the treatment of benign thyroid nodules. It may gain a wide use in clinical practice.
Advances in knowledge:
Based on the comparable efficacy and clinical symptoms between the BRFA and untreated groups, the technique of BRFA can be used as an effective and safe method for the treatment of benign thyroid nodules.
INTRODUCTION
Thyroid nodules are increasingly popular with the wide use of high-frequency ultrasound, which can be detected in 20–76% of the adult population.1 Most thyroid nodules are benign, but if nodular growth causes the onset of nodule-related symptoms such as dysphagia, cosmetic problems or a malignant change, the available remedy so far has been mainly surgery.2–4 The curative surgery is not only invasive but also produces pain and cosmetic defects in patients. In addition, the damage to the thyroid function associated with surgery cannot be ignored, and a long-term administration of thyroid hormone therapy is usually needed. In cases of several surgical drawbacks and ineligibility, such as patient refusal and poor medical condition, non-surgical techniques for benign thyroid nodules are needed.5,6 Radiofrequency ablation (RFA) is a minimally invasive modality that has been used to treat benign thyroid nodules. RFA can induce thyroid tissue necrosis by heat, and the nodule will shrink during follow-up, which in turn alleviates or even eliminates the associated symptoms or psychological burden associated with the thyroid nodules.7 Kim et al8 firstly reported the application of RFA to treat benign thyroid nodules of the human thyroid gland in 2006. Since then, numerous studies have been carried out in this regard, and in most of the studies, conventional monopolar RFA (MRFA) has been applied.7–9
Bipolar radiofrequency ablation (BRFA), as a novel technique, has more advantages than MRFA. Usually, a MRFA system requires a grounding pad so that the electrical current is able to flow between the electrode and the grounding pad. As a result, heat retention, perspiration and skin burns at the grounding pad patch site may be caused.10 BRFA has been proven to be an effective technique to treat liver cancers.10–12 The technique has the potential to be suitable for treating thyroid nodules. Although BRFA has been tried for thyroid ablation, only experimental studies on porcine thyroid glands have been performed.13,14 To our knowledge, no studies using BRFA for treatment of thyroid nodule in human beings have been carried out. The purpose of the present study was to evaluate the efficacy and safety of BRFA in the treatment of benign thyroid nodules by comparison with a matched untreated control group.
METHODS AND MATERIALS
Patients
The study was approved by the ethical committee of the Shanghai Tenth People's Hospital, and informed consent was obtained from each patient. From April 2013 to March 2015, 35 consecutive patients with benign thyroid nodules were subjected to ultrasound-guided BRFA in the hospital. For patients with multiple nodules (ten patients had two nodules and four patients had three nodules even more), only the largest nodule were subjected to treatment, and these patients formed the treatment group (Group A). The inclusion criteria for all the patients were as follows: (1) with cystic and solid mixed nodule or predominantly solid (with a fluid component ≤75% of the volume) nodule on ultrasound; (2) with normal thyroid function; and (3) without a history of treatment. All the patients were subjected to one session of BRFA and a benign diagnosis by ultrasound-guided fine-needle aspiration biopsy (FNAB) was obtained in each patient before BRFA. In the same period, 35 patients with 35 nodules who met the above-mentioned inclusion criteria chose to follow-up with periodical ultrasound instead of any medical or surgical treatment, and all the nodules had a benign diagnosis by ultrasound-guided FNAB. Of the 35 patients from Group A, the patient number with clinical symptoms were 13 and the rest of the patients were anxious about possible malignancy; of the 35 patients from Group B, however, the patient number with clinical symptoms were 8 and the rest of the patients had no symptoms (Table 1). Table 1 summarized the data of the baseline characteristics of the patients in the two groups, and no differences were found between them.
Table 1.
Baseline characteristics of the bipolar radiofrequency ablation (BRFA) group and the control group before treatment or at enrollment
| Characteristic | BRFA group (n = 35) | Control group (n = 35) | p-value |
|---|---|---|---|
| Sex (M/F) | 8/27 | 6/29 | 0.55 |
| Age (years) | 42.69 ± 14.94 (17–67) | 47.37 ± 13.77 (20–70) | 0.38 |
| Baseline volume (ml) | 8.81 ± 8.66 (1.27–33.70) | 6.90 ± 3.77 (1.01–14.62) | 0.37 |
| Thyroid-stimulating hormone (mU/l−1) | 1.62 ± 0.87 (0.61–3.23) | 1.54 ± 0.52 (0.66–2.12) | 0.68 |
| Free tri-iodothyronine (pmol l−1) | 4.99 ± 0.48 (4.31–5.97) | 4.90 ± 0.35 (4.15–5.26) | 0.57 |
| Free thyroxine (pmol l−1) | 14.60 ± 1.97 (11.54–17.72) | 13.50 ± 1.28 (12.11–15.25) | 0.13 |
| Number of clinical symptoms | 13 | 8 | |
| Voice change | 2 | 1 | 0.56 |
| Pressure symptom | 6 | 4 | 0.50 |
| Cosmetic problem | 5 | 3 | 0.45 |
F, female; M, male.
Except for the number of males and females and number of clinical symptoms, values are mean ± standard deviation with range.
Equipment
A BRFA system (Celon AG Medical Instruments, Teltow, Germany), as applications of conventional technique in clinical settings, was commercially available. A power control unit works at 470 kHz and provides a maximum output power of 250 W (CelonLabPower; Celon AG). Unlike conventional MRFA system requiring a grounding pad, the BRFA system is designed as a bipolar unit and grounding pad is not necessary. The conducting part of the applicator is of 9 or 20 mm length (type of electrode: CelonProSurge 150-T09, 18 gauge, output power: 3 W; and CelonProSurge 150–T20, 15.5 gauge, output power: 20 W), including both the insulator and the tip. Both electrodes were 15 cm long. The high-frequency current flows between the two exposed portions of the tip of the bipolar electrode and the exposed portions are separated by an insulator at the tip. Then, the tissue surrounding the electrodes is heated up and the heat is conducted to the distant tissues. An internal liquid (e.g. 0.9% normal saline solution) circulation is necessary for the electrode of CelonProSurge 150-T20, which reduces tissue carbonization around the electrode and thus enables increased efficiency of coagulation. The delivery rate of the internal liquid circulation is set at 30 ml min−1 and the internal liquid is at a suitable temperature (temperature: < 25°C). A triple peristaltic pump, as a part of the system, is used to drive liquid flow. Once the resistance increases beyond a limited value power (700 Ω), the energy delivery will stop automatically.
A Logiq E9 ultrasound machine (GE Medical Systems, Milwaukee, WI) with a ML6-15 liner transducer with a centre frequency of 12 MHz (frequency range: 4–15 MHz) was used for ultrasound, ultrasound-guided FNAB and BRFA. Another 9-l linear transducer with a centre frequency of 7 MHz (frequency range: 2–8 MHz) was used for contrast-enhanced ultrasound (CEUS) with an ultrasound contrast agent (SonoVue®, Bracco, Italy). The same ultrasound system was used at each follow-up.
Pre-treatment assessment
Ultrasound examinations, laboratory tests and FNABs were performed before treatment. Laboratory tests included thyroid tests [thyroid-stimulating hormone (normal range, 0.35–5.5 mU l−1), free tri-iodothyronine (normal range, 3.5–6.5 pmol l−1), free thyroxine (normal range, 10.2–31 pmol l−1)], complete blood counts and blood coagulation tests (prothrombin time, activated partial thromboplastin time). Three orthogonal diameters of the nodules (including the largest diameter and the other two perpendicular diameters) were measured by ultrasound. The nodule volume was calculated using the equation: V = π a × b × c/6 (where V is the volume; a is the largest diameter, and b and c are the other two perpendicular diameters). On ultrasound, a nodule was judged to be “predominantly solid” if it was >75% solid and to be “mixed solid and cystic” if it was <75% solid. Free-hand biopsy technique was used for ultrasound-FNAB with a 22-gauge needle. The cytopathological results were obtained according to the World Health Organization criteria.15 SonoVue was used as a contrast agent to evaluate the macro- and microcirculation of the nodules. Before administration, 5 ml of sterile normal saline solution was injected into the vial for preparation of the contrast agent. A total of 2.4-ml SonoVue was then injected via the antecubital vein in a bolus fashion. An optimal puncture path was selected by the scan of ultrasound. On the basis of previous experience, the trans-isthmic approach was usually performed and a careful observation was necessary to avoid damage to the vessels and nerves along the approach route.9
Procedure
The patients in the treatment group (Group A) were subjected to BRFA in an operating room. All procedures of BRFA were performed by one radiologist with 5 years' of experience in MRFA for thyroid nodules. Before the study, the radiologist was trained for BRFA with 10 cases. Patients were placed on an operation bed in a supine position with a mild neck extension and a multiparametric monitor was used to monitor continuous electrocardiogram, breath rate, blood pressure and oxyhaemoglobin saturation. Local anaesthesia with 2% lidocaine was applied to the puncture site on the skin and the puncture route. A 2-mm-long incision was performed with a scalpel. Then, the BRFA electrode was introduced into the target nodule under ultrasound guidance. In the present study, all nodules were managed with bipolar electrodes (CelonProSurge 150–T20) by the moving-shot technique.9 The BRFA was performed in the transverse ultrasound view. The electrode tip was initially positioned in the deepest portion of the nodule and ablation was then begun using a 20-W output power. A hyperechoic cloud would appear in the surrounding tissue around the electrode tip on ultrasound, and then, the electrode was pulled back slowly to ablate the more superficial tissue in the nodule until the front margin of the nodule. Then, the electrode was introduced into the deepest portion of the nodule at a different angle again, from the medial to the lateral portion of the target nodule and along the short axis of the nodule. Finally, the nodule was covered by the hyperechoic cloud completely, and no obvious intranodular blood flow was visible on colour Doppler ultrasound. The procedure of ablation was continuously monitored to make a clear visualization of the relationship between the electrode tip and the thyroid nodule, as well as the surrounding critical structures such as the carotid artery, recurrent laryngeal nerve etc. Afterwards, the electrode was slowly pulled out, and the puncture route was cauterized to avoid bleeding.
For Group A, if patients could not tolerate pain or other minor complications during the procedure, the power would be turned off temporarily. Meanwhile, the patients were asked to make a sound to evaluate whether the voice was changed or not. If severe pain or other major complications happened, the procedure would be given up. 5 min after ablation, CEUS was performed to evaluate whether the treatment was complete or not. A total of 2.4 ml of SonoVue was injected again via the antecubital vein in a bolus fashion. If the nodule showed complete non-enhancement in the nodule during the arterial phase, the ablation was regarded as complete. If not, additional ablation was carried out until there was no obvious enhancement in the nodule during the arterial phase of CEUS. A mild compression of the neck lasting 20–30 min was performed to all the patients in Group A. Afterwards, the patients were sent back to their wards if there were no abnormal findings in the observation period. The day after ablation, ultrasound examinations were performed to scan the thyroid and adjacent structures to evaluate the possible complications. If not, the patients were discharged from the hospital. On the other hand, all the patients in Group B only received observation without any treatment.
Follow-up
The patients in both groups were asked to come back to the hospital 1, 3, and 6 months after treatment or enrollment. In each follow-up, ultrasound examination for the thyroid was performed, and the volume of the treated or targeted nodule was calculated. Volume reduction ratio (VRR) was calculated by the following equation: VRR = [(initial volume − final volume) × 100%]/initial volume, which was calculated at each visit. Thyroid tests (i.e. thyroid-stimulating hormone, free tri-iodothyronine, free thyroxine etc.) were also evaluated at the 1-, 3-, and 6-month follow-up. The clinical symptoms were evaluated at each follow-up visit. Major or minor complications were evaluated at each visit.
Statistical analysis
Data analysis was performed using a software package (SPSS® v. 17.0 for Windows; IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Quantitative data were expressed as mean ± standard deviation (SD). χ2 tests were used to compare sex and the number of clinical symptoms between the two groups at enrollment. Quantitative data between the two groups were compared at enrollment and follow-up by means of the Mann–Whitney U tests. The follow-up nodule volume recorded was compared with baseline volume by means of the Wilcoxon tests. VRRs of “predominantly solid” and “mixed solid and cystic” nodules after BRFA were compared at follow-up by means of the Wilcoxon tests. p < 0.05 was considered as statistical significance.
RESULTS
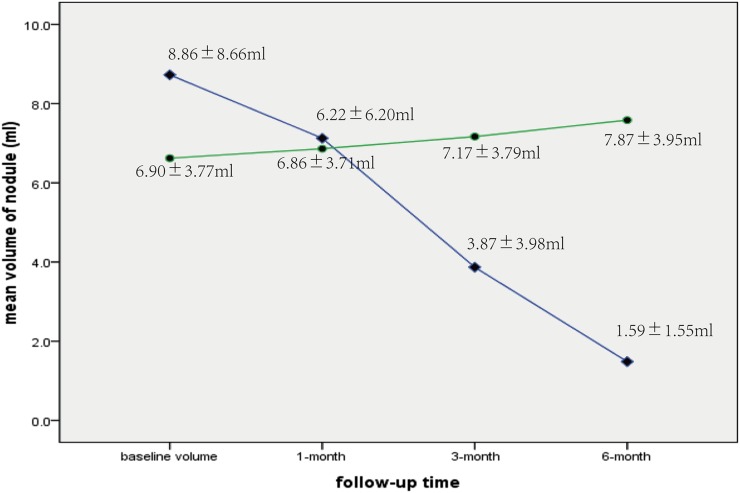
Before treatment, no clinical data, such as sex (p = 0.55), age (p = 0.38) or pre-treatment nodule volume (p = 0.37), were significantly different between Groups A and B (Table 1). The changes in nodule volume and VRR of Groups A and B at follow-up are summarized in Table 2. For Group A, the mean volume of nodules decreased from 8.81 ± 8.66 ml (range, 1.27–33.70 ml) to 1.59 ± 1.55 ml (range, 0.25–7.43 ml) (p < 0.001) at the 6-month follow-up visit (Figure 1). On the other hand, the mean volume of nodules in Group B increased from 6.90 ± 3.77 ml (range, 1.10–14.62 ml) to 7.85 ± 3.95 ml (range, 1.25–15.60 ml) (p < 0.001) (Figure 2).
Table 2.
The change in nodule volume in bipolar radiofrequency ablation (BRFA) group and control group
| Nodule volume | Nodule volume (ml) |
p-value | |
|---|---|---|---|
| BRFA group | Control group | ||
| Baseline | 8.81 ± 8.66 (1.27–33.70) | 6.90 ± 3.77 (1.10–14.62) | 0.37 |
| 1 month | 6.22 ± 6.20 (1.12–27.52) | 6.86 ± 3.71 (1.10–14.79) | 0.20 |
| 3 months | 3.87 ± 3.98a (0.72–15.33) | 7.17 ± 3.79b (1.16–15.00) | <0.001 |
| 6 months | 1.59 ± 1.55c (0.25–7.43) | 7.87 ± 3.95d (1.25–15.60) | <0.001 |
Mean volumes as mean ± standard deviation.
p <0.001, nodule volume at the 3-month follow-up was compared with baseline volume.
p <0.001, nodule volume at the 6-month follow-up was compared with baseline volume.
p <0.001, nodule volume at the 3-month follow-up was compared with baseline volume.
p <0.001, nodule volume at the 6-month follow-up was compared with baseline volume.
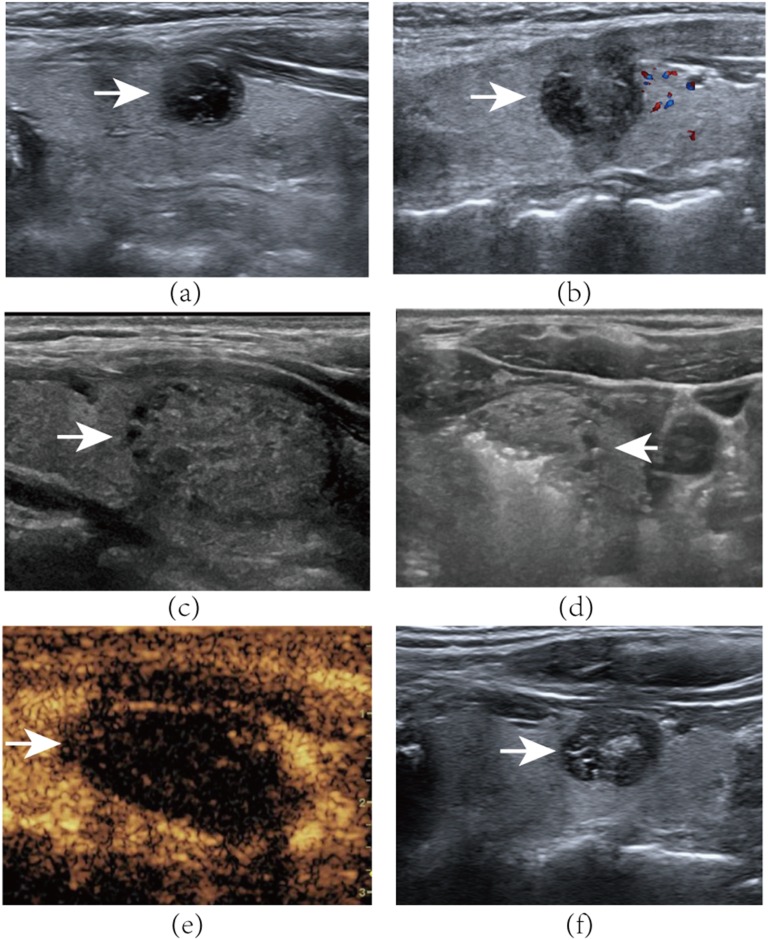
Figure 1.
A 45-year-old female with a mixed nodule in the left of her thyroid gland. (a) Axial ultrasound examination reveals baseline volume of the nodule (arrow) is 2.15 ml. (b) Axial ultrasound examination of the left thyroid nodule (arrow) volume is 2.78 at the 6-month follow-up visit. A 21-year-old female with a predominantly solid nodule in the left of her thyroid gland. (c) Axial ultrasound examination reveals the nodule (arrow) to be 3.52 ml in volume before bipolar radiofrequency ablation (BRFA). (d) Transverse ultrasound image shows moving-shot technique of the bipolar electrode. Tip of the bipolar electrode is positioned in the nodule (arrow). Hyperechoic gas appears during the ablation procedure. (e) Contrast-enhanced ultrasound after BRFA: the nodule (arrow) shows non-enhancement during the arterial phase. (f) Axial ultrasound examination of the left thyroid nodule 6 months after BRFA. The nodule (arrow) volume is 1.25 ml, with a 64% reduction of initial nodule.
Figure 2.
The mean volume of thyroid nodules in the bipolar radiofrequency ablation (BRFA) (♦) and control (●) groups at enrollment (8.81 ± 8.66 vs 6.90 ± 3.77 ml) and at 1 month (6.22 ± 6.20 vs 6.86 ± 3.71 ml), 3 months (3.87 ± 3.98 vs 7.17 ± 3.79 ml) and 6 months (1.59 ± 1.55 vs 7.87 ± 3.95 ml) after treatment. In contrast to the findings of the control group, significant mean volume reduction is achieved in the BRFA group.
In group A, the mean ablation time and mean total energy deposition were 10.02 ± 3.30 min (range, 5.47–16.03 min) and 10747 ± 3704 J (range, 5510–17770 J). After treatment, all nodules in Group A decreased in volume. Mixed cystic and solid nodules showed a significantly better treatment outcome than the predominantly solid nodules at the latest visit (VRR, 84.92 ± 4.96% vs 75.52 ± 5.30%; p < 0.001) (Table 3). In Group A, the VRR was 17.97%, 54.99% and 80.63% at the 1-, 3- and 6-month follow-up, respectively. However, no nodules showed complete disappearance at the 6-month follow-up. All nodules in Group A had VRRs of >50%, among which 3 nodules (8.57%) had VRRs of >90%. On the other hand, in Group B, the volume of 9 (25.71%) nodules were stable throughout the follow-up period (VRR <±5%). The volume of 25 (71.43%) nodules increased >10%, among which 9 (25.71%) nodules increased >20%. What is more, the volume of 1 (2.86%) nodule increased 8.05%.
Table 3.
Difference in mean volume reduction ratio (VRR) of “predominantly solid” and “mixed solid and cystic” nodules after bipolar radiofrequency ablation
| Composition | VRR of 1 month (%) | VRR of 3 months (%) | VRR of 6 months (%) |
|---|---|---|---|
| Predominantly solid (n = 16) | 17.11 ± 3.79 (10.11–22.37) | 55.51 ± 8.20 (33.33–65.31) | 75.52 ± 5.30 (63.34–81.20) |
| Mixed solid and cystic (n = 19) | 19.70 ± 4.46 (11.49–28.00) | 54.56 ± 5.94 (42.54–62.82) | 84.92 ± 4.96 (76.38–94.77) |
| p-value | 0.34 | 0.76 | <0.001 |
Mean VRR as mean±standard deviation.
All patients in Group A tolerated the ablation procedure well. Most patients reported minor complications such as mild local pain and a sensation of heat. When encountered, a short break of energy output was carried out and then was continued after a while. No one asked to give up the procedure. The minor complications were relieved without treatment within 24 h after ablation. The patients with clinical symptoms decreased to zero after BRFA at the 6-month follow-up visit. However, in Group B, the patient number of symptoms increased at the 6-month follow-up visit (Table 4). No procedure-related major complications occurred, such as skin burn, haematoma formation or recurrent laryngeal nerve injury. In Group A, the thyroid-stimulating hormone was 1.75 ± 0.23 mU l (0.61–3.23); free tri-iodothyronine was 4.21 ± 0.52 pmol l−1 (4.02–5.65); and free thyroxine was 12.67 ±1.87 pmol l−1 (10.96–16.35) at the 1-month follow-up. In Group B, the thyroid-stimulating hormone was 1.69 ± 0.66 mU l (0.57–2.79); free tri-iodothyronine was 5.12 ± 0.41 pmol l−1 (4.22–5.97); and free thyroxine was 13.37 ± 1.51 pmol l−1 (11.98–16.57) at the 1-month follow-up. The thyroid tests of all patients were normal at the 1-month follow-up visit and thereafter.
Table 4.
The number of clinical symptoms in the bipolar radiofrequency ablation (BRFA) and control groups at the 6-month follow-up visit
| Characteristic | Number of clinical symptoms |
|---|---|
| BRFA group | |
| Voice change | 0 |
| Pressure symptom | 0 |
| Cosmetic problem | 0 |
| Control group | |
| Voice change | 2 |
| Pressure symptom | 5 |
| Cosmetic problem | 3 |
DISCUSSION
Traditionally, surgery can remove the symptomatic thyroid nodule and reduce the concern for malignancy change. However, surgery usually carries some risks, such as laryngeal nerve injury, hypoparathyroidism or bleeding.16 Therefore, non-surgical treatments such as levothyroxine therapy and percutaneous thermal ablation have been proposed for the treatment of thyroid nodules.3–6 However, the efficacy of levothyroxine is still controversial, and long-term treatment with medication may cause adverse effects such as reduction of bone density or atrial fibrillation.3,4,17 Laser ablation and microwave ablation as thermal ablation therapy have been introduced for treatment of benign thyroid nodules in recent years.5,18–20 Dossing et al21 reported that the efficacy of laser ablation for predominantly solid thyroid nodules were superior to those nodules acting as controls. On the other hand, recently, a study of 477 benign thyroid nodules showed microwave ablation was a safe and effective technique.20 Laser ablation and microwave ablation were used widely in the present stage.5,18–22 RFA is another way to induce necrosis of the thyroid tissue. A recent guideline from Republic of Korea was developed, in which recommendations for the optimal use of RFA in treatment of benign thyroid nodules were included.23 RFA consists of both BRFA and MRFA.24 By comparing the two techniques, BRFA seems to be more convenient, and it has better applicability and fewer side effects than the MRFA system.10–14
To our knowledge, there has been no clinical report of BRFA for treatment of thyroid nodules. In addition, no comparison of the efficacy between the BRFA and untreated groups has been performed. In the present study, the volume and symptom relating to thyroid nodules in the BRFA group tended to decrease as months went by, whereas in the control group of similar patients who were not treated, the nodule volume and clinical symptom increased. The mean VRR of the BRFA group was 80.63% at the 6-month follow-up, which was consistent with the previous studies that the VRR ranged from 75% to 97% using MRFA.25,26 By using laser ablation and microwave ablation, the mean VRR of laser ablation and microwave ablation was 44–62% and 46–65% at the 6-month follow-up.5,20,27,28 In another study, Jeong et al9 reported the clinical practice of MRFA for benign thyroid nodules in 236 patients and the mean VRR was 84.79% at the 6-month follow-up. Our result was at least equivalent to that of Jeong's with a MRFA system and seemed to be better than that achieved by laser ablation and microwave ablation. However, in our study, the advantage of the BRFA system is that electric current is immediately retrieved, which can prevent electric current from flowing to unintended sites and avoid heat retention, perspiration and skin burns at the grounding pad patch site. What is more, in our study all the nodule VRRs in BRFA group were >50%. According to previous studies,23,29 it was meant to be successful treatment if the VRR was >50%. On the other hand, the mean nodule volume in the control group increased significantly at the 6-month follow-up. Dossing et al21 also reported that the control group had an increase in median nodule volume from 7.5 to 9.0 ml at the 6-month follow-up visit. Therefore, through comparison between BRFA group and untreated group, it can be concluded that BRFA rather than spontaneous reduction leads to the volume reduction of the nodules.
With regard to the comparison of treatment efficacy between mixed cystic and solid nodules and predominantly solid nodules, 6 months after BRFA, the mixed nodules decreased in volume more obviously than the predominantly solid nodules (VRR, 84.92 ± 4.96% vs 75.52 ± 5.30%; p < 0.001). Kim et al8 also found that mixed and cystic nodules had a better therapeutic response in comparison with predominantly solid nodules. For mixed nodules, removal of the cystic component was usually performed, which significantly reduced the nodule volume that should be ablated and also the volume of the nodule would decreased significantly. However, for the predominantly solid nodules, the ablated tissues were also solid, which had to be absorbed in a relatively long period.
In the study, the clinical symptoms resolved in the patients who had symptoms before BRFA of Group A. However, the patient number with clinical symptoms in the control group increased. Papini et al27 also reported symptom or cosmetic problem aggravation in 45% of untreated patients at the 12-month follow-up evaluation. This difference suggests that BRFA may be considered an alternative treatment, which can be efficient to resolve symptoms or cosmetic problems of patients.
No procedure-related major complications occurred in the current study. Dupuy et al reported one patient experienced skin burn at the grounding pad patch site using a monopolar system.30 However, no one experienced skin burn in the current study. What is more, previous studies20,23 using a monopolar system described the incidence of nerve injury to be 0–3.3%. The bipolar system used in this study overcomes the disadvantages of possibility of major complications. On the other hand, the bipolar electrode with an internal liquid circulation can generate a larger coagulation volume, which decreases the ablation time and leads to a lower complication rate after treatment. Similarly, in laser ablation and microwave ablation, the incidence of nerve injury was 0–8% and 0–4.3%.20–22,27,31 This difference suggests that more security might be expected if BRFA is used in thyroid nodules. Thyroid function tests of BRFA group were normal after treatment in this study. However, thyroid functions may change after surgery. In a previous study with 1051 patients who were subjected to surgery, postoperative hormone requirement was reported to be up 28%.32 Our results were encouraging, the minimally invasive modality and the moving-shot manipulation only destroys normal thyroid tissue as little as possible.
Our study has some limitations. (1) Only 35 patients were included in both groups thus future studies with more patient numbers is needed. (2) No nodule disappeared after BRFA at the 6-month follow-up in this study. The follow-up was relatively short, and long-term results were not available at the current stage. (3) The technique of BRFA is not compared directly with laser ablation, microwave ablation or surgery. Therefore, to confirm the efficacy and safety of BRFA in the treatment of benign thyroid nodules, a prospective, multicentre study with larger sample size and longer follow-up is needed.
CONCLUSION
Based on the comparable efficacy and clinical symptoms between the BRFA and untreated groups, the thyroid nodule in BRFA group shrinks significantly, and the associated clinical symptoms resolve after BRFA. BRFA is a safe procedure as no procedure-related major complications occur. BRFA can be used as an alternative technique to treat benign thyroid nodules.
FUNDING
This work was supported in part by grant SHDC12014229 from the Shanghai Hospital Development Center, grant 14441900900 from the Science and Technology Commission of Shanghai Municipality, grant 2012045 from the Shanghai Municipal Human Resources and Social Security Bureau, and grant 81401417 from the National Natural Science Foundation of China.
Contributor Information
Xiao-long Li, Email: 15275388623@163.com.
Hui-Xiong Xu, Email: xuhuixiong@126.com.
Feng Lu, Email: uslufeng@163.com.
Wen-wen Yue, Email: yww19870902@163.com.
Li-ping Sun, Email: sunliping_s@126.com.
Xiao-wan Bo, Email: boxiaowan0908@126.com.
Le-hang Guo, Email: gopp1314@hotmail.com.
Jun-mei Xu, Email: junmei_510@126.com.
Bo-ji Liu, Email: jxjj1990@126.com.
Dan-dan Li, Email: candyaugustcarter@163.com.
Shen Qu, Email: 214753456@qq.com.
REFERENCES
- 1.Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 2009; 39: 699–706. doi: 10.1111/j.1365-2362.2009.02162.x [DOI] [PubMed] [Google Scholar]
- 2.Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med 1997; 126: 226–31. doi: 10.7326/0003-4819-126-3-199702010-00009 [DOI] [PubMed] [Google Scholar]
- 3.Lima N, Knobel M, Cavaliere H, Sztejnsznajd C, Tomimori E, Medeiros-Neto G. Levothyroxine suppressive therapy is partially effective in treating patients with benign, solid thyroid nodules and multinodular goiters. Thyroid 1997; 7: 691–7. doi: 10.1089/thy.1997.7.691 [DOI] [PubMed] [Google Scholar]
- 4.Tsai CC, Pei D, Hung YJ, Wang TF, Tsai WC, Yao CY, et al. The effect of thyroxine-suppressive therapy in patients with solitary non-toxic thyroid nodules—a randomised, double-blind, placebo-controlled study. Int J Clin Pract 2006; 60: 23–6. doi: 10.1111/j.1368-5031.2006.00632.x [DOI] [PubMed] [Google Scholar]
- 5.Spiezia S, Vitale G, Di Somma C, Pio Assanti A, Ciccarelli A, Lombardi G, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid 2003; 13: 941–7. doi: 10.1089/105072503322511346 [DOI] [PubMed] [Google Scholar]
- 6.Sung JY, Baek JH, Kim YS, Jeong HJ, Kwak MS, Lee D, et al. One-step ethanol ablation of viscous cystic thyroid nodules. AJR Am J Roentgenol 2008; 191: 1730–3. doi: 10.2214/AJR.08.1113 [DOI] [PubMed] [Google Scholar]
- 7.Kanauchi H, Mimura Y, Kaminishi M. Percutaneous radio-frequency ablation of the thyroid guided by ultrasonography. Eur J Surg 2001; 167: 305–7. doi: 10.1080/110241501300091561 [DOI] [PubMed] [Google Scholar]
- 8.Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 2006; 16: 361–7. doi: 10.1089/thy.2006.16.361 [DOI] [PubMed] [Google Scholar]
- 9.Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 2008; 18: 1244–50. doi: 10.1007/s00330-008-0880-6 [DOI] [PubMed] [Google Scholar]
- 10.Osaki Y, Ikeda K, Izumi N, Yamashita S, Kumada H, Hatta S, et al. Clinical effectiveness of bipolar radiofrequency ablation for small liver cancers. J Gastroenterol 2013; 48: 874–83. doi: 10.1007/s00535-012-0685-x [DOI] [PubMed] [Google Scholar]
- 11.Baldwin K, Katz SC, Rubin A, Somasundar P. Bipolar radiofrequency ablation of liver tumors: technical experience and interval follow-up in 22 patients with 33 ablations. J Surg Oncol 2012; 106: 905–10. doi: 10.1002/jso.23147 [DOI] [PubMed] [Google Scholar]
- 12.Bo XW, Xu HX, Sun LP, Zheng SG, Guo LH, Lu F, et al. Bipolar radiofrequency ablation for liver tumors: comparison of contrast-enhanced ultrasound with contrast-enhanced MRI/CT in the posttreatment imaging evaluation. Int J Clin Exp Pathol 2014; 7: 6108–16. [PMC free article] [PubMed] [Google Scholar]
- 13.Ritz JP, Lehmann KS, Schumann T, Knappe V, Zurbuchen U, Buhr HJ, et al. Effectiveness of various thermal ablation techniques for the treatment of nodular thyroid disease--comparison of laser-inducedthermotherapy and bipolar radiofrequency ablation. Lasers Med Sci 2011; 26: 545–52. doi: 10.1007/s10103-011-0907-0 [DOI] [PubMed] [Google Scholar]
- 14.Holmer C, Lehmann KS, Knappe V, Zurbuchen U, Frericks B, Schumann T, et al. Bipolar radiofrequency ablation for nodular thyroid disease--ex vivo and in vivo evaluation of a dose-response relationship. J Surg Res 2011; 169: 234–40. doi: 10.1016/j.jss.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 15.Maitra A. The endocrine system. In: Robbins SL, Kumar V, Abbas AK, Cotran RS, Fausto N, eds. Robbins and Cotran pathologic basis of disease. 8th edn. Philadelphia, PA: Saunders/Elsevier; 2010. pp. 1097–1163. [Google Scholar]
- 16.Jeannon JP, Orabi AA, Bruch GA, Abdalsalam HA, Simo R. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract 2009; 63: 624–9. doi: 10.1111/j.1742-1241.2008.01875.x [DOI] [PubMed] [Google Scholar]
- 17.Yousef A, Clark J, Doi SA. Thyroxine suppression therapy for benign, non-functioning solitary thyroid nodules: a quality-effects meta-analysis. Clin Med Res 2010; 8: 150–8. doi: 10.3121/cmr.2010.881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dossing H, Bennedbaek FN, Hegedüs L. Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid 2003; 13: 885–8. [DOI] [PubMed] [Google Scholar]
- 19.Amabile G, Rotondi M, De Chiara G, Silvestri A, Di Filippo FB, Bellastella A, et al. Low-energy interstitial laser photocoagulation for treatment of nonfunctioning thyroid nodules: therapeutic outcome in relation to pretreatment and treatment parameters. Thyroid 2006; 16: 749–55. doi: 10.1089/thy.2006.16.749 [DOI] [PubMed] [Google Scholar]
- 20.Yue W, Wang S, Wang B, Xu Q, Yu S, Yonglin Z, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol 2013; 82: e11–6. doi: 10.1016/j.ejrad.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 21.Døssing H, Bennedbaek FN, Hegedüs L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules—a randomised study. Eur J Endocrinol 2005; 152: 341–5. doi: 10.1530/eje.1.01865 [DOI] [PubMed] [Google Scholar]
- 22.Yue W, Chen L, Wang S, Yu S. Locoregional control of recurrent papillary thyroid carcinoma by ultrasound-guided percutaneous microwave ablation: a prospective study. Int J Hyperthermia 2015; 31: 403–8. doi: 10.3109/02656736.2015.1014433 [DOI] [PubMed] [Google Scholar]
- 23.Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. ; Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 2012; 13: 117–25. doi: 10.3348/kjr.2012.13.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. ; Society of Interventional Radiology Technology Assessment Committee. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2005; 16: 765–78. doi: 10.1097/01.RVI.0000170858.46668.65 [DOI] [PubMed] [Google Scholar]
- 25.Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 2009; 33: 1971–7. doi: 10.1007/s00268-009-0130-3 [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Kim YS, Lee D, Choi H, Yoo H, Baek JH. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg 2010; 34: 1488–93. doi: 10.1007/s00268-010-0565-6 [DOI] [PubMed] [Google Scholar]
- 27.Papini E, Guglielmi R, Bizzarri G, Graziano F, Bianchini A, Brufani C, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid 2007; 17: 229–35. doi: 10.1089/thy.2006.0204 [DOI] [PubMed] [Google Scholar]
- 28.Feng B, Liang P, Cheng Z, Yu X, Yu J, Han Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol 2012; 166: 1031–7. doi: 10.1530/EJE-11-0966 [DOI] [PubMed] [Google Scholar]
- 29.Sung JY, Baek JH, Kim KS, Lee D, Yoo H, Kim JK, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 2013; 269: 293–300. doi: 10.1148/radiol.13122134 [DOI] [PubMed] [Google Scholar]
- 30.Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 2001; 130: 971–7. doi: 10.1067/msy.2001.118708 [DOI] [PubMed] [Google Scholar]
- 31.Yue W, Wang S, Yu S, Wang B. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia 2014; 30: 150–7. doi: 10.3109/02656736.2014.885590 [DOI] [PubMed] [Google Scholar]
- 32.Vaiman M, Nagibin A, Hagag P, Kessler A, Gavriel H. Hypothyroidism following partial thyroidectomy. Otolaryngol Head Neck Surg 2008; 138: 98–100. doi: 10.1016/j.otohns.2007.09.015 [DOI] [PubMed] [Google Scholar]