Abstract
Objective:
We evaluated whether patients with early-stage non-small-cell lung cancers (NSCLCs) treated with stereotactic body radiation therapy (SBRT) without full prescription dose coverage of the planning target volume (PTV) had inferior outcomes.
Methods:
The SBRT regimen was 54 Gy in three fractions. Dosimetric constraints were as per the Radiation Therapy Oncology Group 0236 guidelines. All patients underwent four-dimensional CT (4D-CT) simulation. The internal target volume (ITV) was defined using 4D-CT, and the PTV was defined as a 6-mm longitudinal and a 3-mm axial expansion from the ITV. If normal tissue constraints were beyond tolerance, ITV-based dosing was employed where priority was made for full ITV coverage at the expense of PTV coverage. Patients with and without full PTV dose coverage were compared, and control rates were estimated using Kaplan–Meier analysis.
Results:
120 NSCLC cases were evaluated with 81% having adequate PTV dose coverage. ITV and PTV were significantly larger in the cohort with inadequate PTV dose coverage (p = 0.0085 and p = 0.0038, respectively), and the mean ITV and PTV doses were higher in patients with adequate PTV dose coverage (p = 0.002 and p < 0.0001, respectively). The 3-year local control rate was 100% for both cohorts. There was no difference in 3-year regional control (p = 0.36), disease-specific survival (p = 0.79) or overall survival (p = 0.73).
Conclusion:
When delivering a highly ablative SBRT regimen for early-stage NSCLC, full-dose coverage of the ITV is sufficient for local control.
Advances in knowledge:
Our data are among the first to evaluate the utility of PTV margins in a highly ablative SBRT regimen and suggest that when dosing constraints cannot be met, full tumouricidal dose coverage of the ITV is sufficient for local control.
INTRODUCTION
Non-small-cell lung cancers (NSCLCs) are a leading cause of mortality worldwide and are the most frequent cause of cancer death in North America. About 20% of patients with NSCLCs present with early-stage disease and are candidates for curative-intent local therapies.1 Although lobectomy is the current gold standard of care, stereotactic body radiation therapy (SBRT) for early-stage NSCLCs is an established and effective treatment option for medically inoperable patients with local control rates comparable to surgical resection.2 Advances in radiation therapy planning and delivery have allowed for SBRT which safely concentrates radiation on a tumour while sparing the surrounding tissues. The precision of SBRT has allowed for dose escalation, which has been shown to be critical for outcomes in the treatment of early-stage NSCLCs. Specifically, dose escalation has been found to provide for superior tumour control and overall survival in the setting of SBRT for early-stage NSCLCs.3–7
As part of SBRT planning, the physician outlines the tumour, and then additional margins in the axial and longitudinal planes are added to create a planning target volume (PTV) to which the dose is prescribed. The PTV margin takes into account variability in treatment setup and is designed to ensure that the prescribed dose is actually delivered to the target tumour. Importantly, margins are applied in three dimensions; therefore, even small margins can significantly increase the irradiated volume. In recent early-stage lung SBRT studies including Radiation Therapy Oncology Group (RTOG) 0618 and RTOG 0236 in which conventional CT simulation was used for target delineation, an additional 0.5-cm margin in the axial plane and 1.0-cm margin in the longitudinal direction were added to create the PTV. Similarly in RTOG 0915, an additional 0.5-cm margin in both the axial and longitudinal directions was used to create the PTV in patients who had four-dimensional CT (4D-CT) simulation that captured the tumour position in all breathing phases. Of note, when conceptualizing margins around the primary target, it is necessary to note that the prescription dose is linked with margin coverage. Therefore, with highly ablative SBRT regimens, a margin with biologically significant dose may be incidentally created around the target tumour secondary to the dose fall-off gradient, without the intentional application of a PTV margin.
With SBRT for early-stage lung cancers, there are many organs at risk which have a limited tolerance to radiation, including the spinal cord, oesophagus, brachial plexus, heart, lungs, ribs and skin. Therefore, prescribing a tumouricidal dose of radiation to the target lesion can compete with sparing critical organs at risk from excess radiation. Thus, situations arise in which compromises in dose prescriptions or target coverage need to be made. We therefore sought to determine if patients with early-stage NSCLC treated with highly ablative SBRT without full prescription dose coverage of the PTV had inferior outcomes.
METHODS AND MATERIALS
Patient selection
Records of consecutive patients treated with SBRT with curative intent at a single institution between February 2009 and December 2013 were retrospectively analysed. The study was approved by the UCLA Medical Center institutional review board, and retrospectively acquired data were deidentified according to the Health Insurance Portability and Accountability Act guideline. Inclusion criteria included patients with early-stage NSCLC who were older than or equal to 18 years of age. Pathological diagnosis was not required for inclusion, although the vast majority, 76% of all cases, were biopsy-proven disease. Patients were excluded if they had small-cell histology, presented with metastatic disease, were not treated with SBRT alone or were treated with palliative intent. Furthermore, in order to have a patient population with homogeneous dose prescriptions that would allow for our analysis, only patients with peripheral tumours defined as outside the 2-cm zone from the trachea and bronchial tree were included in this study. Patients who had <94.5% of their internal target volume (ITV) covered by the prescription dose were also excluded from analysis. After inclusion and exclusion criteria were applied, 110 patients, with 120 primary NSCLCs, were included for analysis.
Treatment
Immobilization for radiation therapy-planning CT simulation and treatment used a whole-body Vac-Lok™ system (CIVCO Medical Solutions Inc., Coralville, IA). No implanted fiducial markers were used for target localization. All patients underwent a 4D-CT simulation scan for radiation planning. Maximum-intensity-projection images from 4D-CT were generated, and the target lesion was then outlined by the treating radiation oncologist using the maximum-intensity-projection images on the lung window and was designated as the ITV. The ITV represented the gross tumour volume and clinical target volume. A PTV was then generated with an expansion margin of 3 mm in the axial direction and of 6 mm in the longitudinal direction from the ITV. Critical structures were outlined and maximum point dose constraints for normal structures were as per RTOG 0236 guidelines.
The prescription dose was 54 Gy in three fractions for peripheral tumours, which corresponds to a biological effective dose (BED) of 151.2 Gy (assuming α/β = 10). Treatment planning used three-dimensional conformal planning, intensity-modulated radiation therapy or dynamic arcs. Initially, a three-dimensional plan was created for each patient given faster treatment delivery, but arcs or intensity-modulated radiation therapy plans were used if a more optimal plan was necessary. Patients were treated with 6-MV photons to the prescription dose. Patients were treated with 9–12 beams with 3–5 non-coplanar beams. The margin from the PTV to the beam edge ranged from 1 to 3 mm, and patients were treated on a designated machine commissioned for small-field SBRT with a 5-mm minimum field aperture. Initially, target coverage at 95% of the PTV was normalized to the prescription dose. However, if normal tissue constraints were beyond tolerance, ITV-based dosing was employed where priority was made for full ITV coverage at the expense of PTV coverage, without compromising on the prescription dose. Organs at risk frequently necessitating the treating physician to employ ITV-based dosing included the chest wall, skin, brachial plexus and great vessels. All dose calculations were performed in iPlan® RT Dose (Brainlab AG, Feldkirchen, Germany) treatment-planning system using the Monte Carlo method.8,9 This dose calculation engine has been demonstrated to be accurate in heterogeneous media10 and specifically in the setting of lung SBRT.11 The dose was calculated with a spatial resolution setting of 2 mm and mean variance of 1%. The result was specified as dose to water. The treatment-planning system used the accuracy-optimized multileaf collimator model for dose calculations which models the actual leaf design including the tongue-and-groove leaf design and leaf leakage.
Treatment was delivered every other day with daily cone beam CT for image guidance to confirm the position of the target. Cone beam CT imaging was evaluated by the treating radiation oncologist before the delivery of each fraction to ensure proper patient setup and target localization. No other manoeuvres including abdominal compression, tumour tracking or active breath-holding techniques were used in the treatment delivery process.
Follow-up
Patients were seen for follow-up at 2 months post-treatment at which point clinical examination and PET/CT were completed as per institutional practice. Patients were subsequently followed up with clinical examination and CT imaging every 3 months for the first 2 years and then every 6 months to assess treatment response and toxicity.
Statistical analysis
Descriptive parameters, including age and T-stage, and dosimetric parameters, including ITV and PTV (cm3); ITV and PTV mean, minimum and maximum doses (Gy); the percentage of both the PTV and ITV receiving at least the prescription dose; and the dose to both 95% of the PTV and ITV (Gy), were determined through chart review.
Kaplan–Meier analysis was used to estimate the 3-year local control, regional control, distant control, disease-specific survival and overall survival rates of our entire study patient population. Local failure utilized the RTOG 0236 definition of primary tumour control and was defined as primary tumour failure within the PTV. Regional failure was defined as in-lobe failure or failure within the regional lymph node stations. Distant failure was failure beyond the regional nodes.
For our analysis, two cohorts were created to evaluate patients with full PTV coverage with the prescription dose and those for whom priority was made for full ITV coverage at the expense of PTV coverage. Specifically, patients with inadequate coverage, defined as those with <90% of their PTV covered by the prescription dose, were compared with patients with adequate coverage, defined as those with ≥90% of their PTV covered by the prescription dose. The descriptive and dosimetric parameters between both cohorts were compared to determine if there were any statistical differences between both patient cohorts using two independent samples t-test for continuous variables with a normal distribution, the Wilcoxon–Mann–Whitney U test for continuous variables that were not normally distributed and the χ2 test for categorical variables. Kaplan–Meier analysis was used to compare local control, regional control, disease-specific survival and overall survival rates between both cohorts using the log-rank test. We had originally planned univariate and multivariate analyses to evaluate the association between the various PTV and ITV dosimetric parameters and local control; however, because of the very high local control rate in our patient population, these analyses were deemed invalid. Statistical analysis was performed using SAS® v. 9.4 (SAS Institute Inc., Cary, NC).
Additionally, an exploratory analysis was performed among patients with inadequate PTV coverage to find the minimum distance from the ITV at which the 42-Gy dose volume would extend. 42 Gy represents a BED of 100.8 Gy (assuming α/β = 10) which is greater than the 100-Gy BED shown to be biologically effective for early-stage lung cancers.3
RESULTS
A total of 141 cases of early-stage NSCLCs were treated with SBRT alone with definitive intent in the study period. 20 of these cases represented non-peripheral tumours and were excluded from this analysis. One other patient was excluded given that there was <94.5% coverage of the ITV with the prescription dose. This left 120 primary NSCLC cases for analysis which represented 110 separate patients with median follow-up of 28.9 months. Among this population of 110 patients, the 3-year overall survival and disease-specific survival rates were 75.46% and 88.89%, respectively. The 3-year regional and distant control rates were 91.27% and 95.11%, respectively. There were no local failures in this population of patients corresponding to a 100% 3-year local control rate. However, when accounting for all peripheral tumours, there was one local failure, but this was the one excluded case secondary to poor ITV coverage with the prescription dose.
Among the patients analysed, 62.4% had T1a disease, 26.5% had T1b disease and the remaining 11.1% had T2a disease. The median ITV and PTV were 6.71 and 19.2 cm3, respectively, and the mean ITV and PTV doses were 61.4 and 58.8 Gy, respectively. 81% of cases (n = 95) had adequate coverage of the PTV with the prescription dose, leaving 19% of cases (n = 22) with inadequate PTV coverage (Table 1). Among these patients with inadequate PTV coverage, actual percentages of PTV coverage with the prescription dose ranged from 43.2% to 89.7% (Table 1).
Table 1.
Dosimetric parameters among all patients and between patients with adequate and inadequate planning target volume (PTV) coverage with the prescription dose
| Variable | All cases | Adequate PTV coverage |
Inadequate PTV coverage |
p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Median | Median | Min. | Max. | Median | Min. | Max. | ||
| Age (years) | 75 | 75 | 41 | 92 | 75 | 52 | 93 | 0.791 |
| ITV (cm3) | 6.71 | 5.86 | 0.37 | 52 | 13 | 2.28 | 36.5 | 0.0085 |
| ITV mean dose (Gy) | 61.4 | 61.9 | 56.2 | 75.3 | 59.2 | 55.2 | 67 | 0.002 |
| ITV min. dose (Gy) | 55.1 | 55.6 | 44.9 | 66 | 51.5 | 45.8 | 57 | <0.0001 |
| ITV max. dose (Gy) | 65.6 | 65.7 | 58 | 94 | 65 | 57.1 | 76.6 | 0.264 |
| 95% ITV dose (Gy) | 59.1 | 59.3 | 54 | 68.4 | 56 | 53.1 | 59.4 | <0.0001 |
| % ITV ≥ Rx dose | 100 | 100 | 95.5 | 100 | 99.4 | 94.5 | 100 | <0.0001 |
| PTV (cm3) | 19.2 | 17.2 | 2.78 | 92.1 | 34.8 | 10.3 | 71.2 | 0.0038 |
| PTV mean dose (Gy) | 58.8 | 59 | 54.5 | 68.9 | 56 | 51.3 | 62.7 | <0.0001 |
| PTV min. dose (Gy) | 45.8 | 46.7 | 28.9 | 59.7 | 44.3 | 17.9 | 47.83 | 0.0043 |
| PTV max. dose (Gy) | 65.6 | 65.7 | 58.2 | 94 | 64 | 57.2 | 74.4 | 0.0138 |
| 95% PTV dose (Gy) | 54 | 54 | 54 | 66.6 | 51.3 | 26.1 | 53.1 | <0.0001 |
| % PTV ≥ Rx dose | 94.9 | 95.3 | 90.8 | 100 | 80.4 | 43.2 | 89.7 | <0.0001 |
ITV, internal target volume; max., maximum; min., minimum; Rx, prescription dose.
The cases with adequate PTV dose coverage had a median ITV of 5.86 cm3 and a median ITV mean dose of 61.9 Gy delivered in three fractions. The median PTV for these cases was 17.2 cm3 and the median PTV mean dose was 59 Gy delivered in three fractions. The cases with inadequate PTV coverage with the prescription dose had a median ITV of 13 cm3 and a median ITV mean dose of 59.2 Gy delivered in three fractions. The median PTV volume for these cases was 34.8 cm3, and the median PTV mean dose was 56 Gy delivered in three fractions. Both ITV and PTV for the patients with inadequate PTV coverage were significantly larger than the volumes of the cases with adequate PTV dose coverage (p = 0.0085 and p = 0.0038, respectively). Additionally, the ITV and PTV mean doses were significantly higher in the patients with adequate coverage of the PTV with the prescription dose (p = 0.002 and p < 0.0001, respectively) (Table 1). There were no statistical differences in the distribution of T-stages between both of these patient cohorts (p = 0.3369).
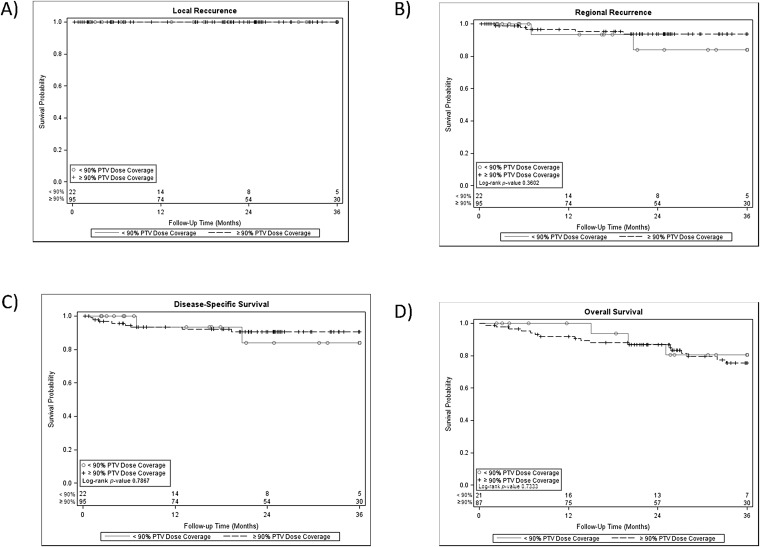
When looking at the main outcome of local control between the cases with adequate and inadequate PTV coverage, there was no difference given the 3-year local control rate of 100% in both cohorts. Additionally, there was no difference in 3-year regional control rates between both cohorts (p = 0.36) and no difference in 3-year disease-specific or overall survival rates (p = 0.79 and p = 0.73, respectively) (Figure 1). Furthermore, our exploratory analysis among the 22 patients with ITV-based dosing found that the 42-Gy volume, the volume of tissue encompassing the 42-Gy isodose line, representing a BED of 100.8 Gy (assuming α/β = 10), extended a median minimum distance of 8.2 mm from the ITV with a range of 3.7–11.4 mm from the ITV.
Figure 1.
Kaplan–Meier analysis was used to determine local control rates (a), regional control rates (b), disease-specific survival (c) and overall survival (d) among patients with early-stage non-small-cell lung cancers treated definitively with stereotactic body radiation therapy who had adequate vs inadequate coverage of the planning target volume (PTV) with the prescription dose.
DISCUSSION
Many studies have evaluated the dose and fractionation needed for optimal outcomes in the setting of SBRT for early-stage NSCLC. However, fewer studies have evaluated the treatment-planning process with regard to PTV margins. We report that in our series of >100 patients who all underwent SBRT with a highly ablative regimen of 54 Gy in three fractions and had adequate coverage of their ITVs with BEDs >150 Gy, there was no increase in local failures among patients without full prescription dose coverage of the PTV. Additionally, we found no increase in regional failures or detriments in disease-specific or overall survival among these patients with poor PTV coverage. Our patients all underwent 4D-CT simulation for radiation planning, but no fiducials were used for target localization and no active motion management strategies were used in treatment delivery.
SBRT has offered medically inoperable patients with early-stage lung cancers the opportunity to obtain treatment results that compare favourably with surgery.2,12 Multiple studies have concluded there to be a dose response with regard to local control and overall survival in the setting of SBRT for early-stage NSCLCs. As reported by Onishi et al,3,4 in a Japanese multi-institutional retrospective review, local control and overall survival rates were inferior in patients receiving a BED <100 Gy. Additionally, a more recent study from the University of Chicago, Chicago, IL, found that among patients with T2 disease, BEDs >150 Gy are associated with a significant survival benefit.7 Therefore, delivering a tumouricidal dose is paramount to the treatment success seen with SBRT in the setting of early-stage NSCLC. However, given the competing needs to spare critical organs from excess radiation, compromises during the treatment-planning process may be required, especially with larger target volumes. Furthermore, if further dose escalation is to be evaluated, especially in patients with T2 disease, meeting current constraints will become even more difficult.
Recent RTOG protocols define the PTV margin as 0.5-cm expansion in all directions if 4D-CT simulation is used or a 1-cm expansion in all directions if a standard CT simulation is used for radiation planning. Similar to these guidelines, we used an institutional standard PTV expansion of 3 mm in the axial and 6 mm in the longitudinal plane from the ITV. Studies of intrafraction variation (IFV) in the setting of SBRT for early-stage NSCLCs have found IFV to vary depending on immobilization devices, treatment delivery time and body weight and have found IFV vectors to range from 1.1 to 5.1 mm.13 These data stress the need for advanced image guidance techniques and margins to ensure that the prescribed radiation dose is actually given to the target. However, in difficult cases where normal tissue dose constraints cannot be met, our data, although limited to a single-institution experience, provide support for prioritizing delivering a tumouricidal dose to the ITV over ensuring full PTV coverage. Furthermore, with highly ablative SBRT regimens, there can be a larger dose fall-off gradient that can produce an incidental margin with the BED. We found that even among our patients with ITV-based dosing, there was an incidentally created minimum margin that ranged from 3.7 to 11.4 mm from the ITV where at least a BED of 100 Gy was delivered.
PTV size reductions have been evaluated in other disease sites given the advances in image-guided radiation therapy. In the setting of head and neck cancers, a modest reduction in PTV expansion from 5 to 3 mm was found to be associated with no detriments in long-term locoregional control and, in fact, was associated with significant reductions in late toxicity.14 In the setting of SBRT for early-stage NSCLC, a larger PTV has been found to be predictive for radiation pneumonitis, and therefore further explorations of PTV reductions in the setting of SBRT for early-stage NSCLC may allow for decreased toxicity in the future.15
Our study was limited by the retrospective nature of our data. Although there were no local failures in the entire analysed patient population, there were imbalances between our two patient cohorts. However, these imbalances were not in favour of the cohort with inadequate PTV coverage as this cohort had significantly larger ITV and PTV and lower mean PTV and ITV doses. Another limitation was that only 19% of all analyzed cases had inadequate PTV coverage. However, only in challenging cases where there is difficulty meeting constraints would inadequate PTV coverage be allowed. Another limitation is that we only studied one dose fractionation scheme which, although provided a very homogeneous study population, limits the translatability of this work. Further studies, with advanced real-time onboard imaging, are planned to potentially allow for tighter PTV margins especially in the context of centrally located tumours and in the setting of reirradiation where the risk of excessive overdosing of organs at risk is even greater.
In conclusion, given that the prescription dose is critically linked to outcomes in the setting of SBRT for early-stage NSCLCs, challenging cases will arise in which normal tissue dose constraints cannot be met without dose reductions or compromises on PTV coverage. We found in this series of patients with early-stage NSCLCs treated with a highly ablative SBRT regimen with optimal ITV dose coverage with BEDs >150 Gy that there was no increase in local failures or detriments in disease-specific survival or overall survival among the patients with inadequate PTV coverage.
Contributor Information
Narek Shaverdian, Email: nshaverdian@mednet.ucla.edu.
Stephen Tenn, Email: stenn@mednet.ucla.edu.
Darlene Veruttipong, Email: DVeruttipong@mednet.ucla.edu.
Jason Wang, Email: JasonW@mednet.ucla.edu.
John Hegde, Email: jhegde@mednet.ucla.edu.
Chul Lee, Email: chullee@mednet.ucla.edu.
Minsong Cao, Email: MinsongCao@mednet.ucla.edu.
Nzhde Agazaryan, Email: NAgazaryan@mednet.ucla.edu.
Michael Steinberg, Email: MSteinberg@mednet.ucla.edu.
Patrick Kupelian, Email: PKupelian@mednet.cual.edu.
Percy Lee, Email: percylee@mednet.ucla.edu.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M. SEER cancer statistics review, 1975–2011. [Updated 17 December 2014.] Available from: http://seer.cancer.gov/csr/1975_2011/
- 2.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–107. doi: 10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onishi H, Araki T, Shirato H. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma. Cancer 2004; 101: 1623–31. doi: 10.1002/cncr.20539 [DOI] [PubMed] [Google Scholar]
- 4.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFx SRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007; 2(7 Suppl. 3): S94–100. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Yang F, Li B, Li H, Huang W, Wang D, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011; 81: e305–16. doi: 10.1016/j.ijrobp.2011.04.034 [DOI] [PubMed] [Google Scholar]
- 6.Kestin L, Grills L, Guckenberger M, Belderbos J, Hope AJ, Werner-Wasik M, et al. Dose–response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother Oncol 2014; 110: 499–504. doi: 10.1016/j.radonc.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Koshy M, Malik R, Weichselbaum RR, Sher DJ. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2015; 91: 344–50. doi: 10.1016/j.ijrobp.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 8.Fippel M. Fast Monte Carlo dose calculation for photon beams based on the VMC electron algorithm. Med Phys 1999; 26: 1466–75. doi: 10.1118/1.598676 [DOI] [PubMed] [Google Scholar]
- 9.Fippel M. Efficient particle transport simulation through beam modulating devices for Monte Carlo treatment planning. Med Phys 2004; 31: 1235–42. doi: 10.1118/1.1710734 [DOI] [PubMed] [Google Scholar]
- 10.Künzler T, Fotina I, Stock M, Georg D. Experimental verification of a commercial Monte Carlo-based dose calculation module for high-energy photon beams. Phys Med Biol 2009; 54: 7363–77. doi: 10.1088/0031-9155/54/24/008 [DOI] [PubMed] [Google Scholar]
- 11.Fragos M, Wen N, Kumar N, Liu D, Ryu S, Movsas B, et al. Dosimetric verification and clinical evaluation of a new commercially available Monte Carlo-based dose algorithm for application in stereotactic body radiation therapy (SBRT) treatment planning. Phys Med Biol 2010; 55: 4445–64. doi: 10.1088/0031-9155/55/16/S02 [DOI] [PubMed] [Google Scholar]
- 12.Crabtree TD, Denlinger CE, Meyers BF, El Naqa I, Zoole J, Krupnick AS, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010; 140: 377–86. doi: 10.1016/j.jtcvs.2009.12.054 [DOI] [PubMed] [Google Scholar]
- 13.Shah C, Kestin LL, Hope AJ, Bissonnette JP, Guckenberger M, Xiao Y, et al. Required target margins for image-guided lung SBRT: assessment of target position intrafraction and correction residuals. Pract Radiat Oncol 2013; 3: 67–73. doi: 10.1016/j.prro.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 14.Chen AM, Yu Y, Daly ME, Farwell DG, Benedict SH, Purdy JA. Long-term experience with reduced planning target volume margins and intensity-modulated radiotherapy with daily image-guidance for head and neck cancer. Head Neck 2014; 36: 1766–72. doi: 10.1002/hed.23532 [DOI] [PubMed] [Google Scholar]
- 15.Baker R, Han G, Sarangkasiri S, DeMarco M, Turke C, Stevens CW, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys 2013; 85: 190–5. doi: 10.1016/j.ijrobp.2012.03.041 [DOI] [PubMed] [Google Scholar]