Abstract
Background
Obesity is a risk factor for increased peri-operative morbidity and mortality in surgical patients. There have been limited studies to correlate the morbidity of lung cancer resection with obesity.
Methods
We performed a retrospective study of patients who underwent surgical resection for lung cancer at the Medical College of Wisconsin from 2006 to 2010. Data on patient demographics, weight, pathology findings and hospital course were abstracted after appropriate IRB approval. Peri-operative morbidity was defined as atrial fibrillation, heart failure, respiratory failure, pulmonary embolism or any medical complications arising within 30 days after surgery. Fisher’s exact test was used to test the association between BMI and peri-operative morbidities.
Results
Between 2006 and 2010,320 lung resections were performed for lung cancer. Median age was 67(IQR 59–75) years and 185(57.8%) were females.121 (37.8%) patients had a BMI<25 and 199(62.18%) patients had a BMI≥25. The 30-day mortality rate was 1.8 % (n=6) in the whole group; only 2 of these patients had a BMI ≥ 25. Peri-operative morbidity occurred in 28(23.14%) of normal BMI patients and in 47(23.61%) of BMI ≥ 25 patients (p=0.54). Specific morbidities encountered by patients with normal vs. BMI ≥ 25 were: atrial fibrillation 11(9.09%) vs. 24(12.06%) (p=0.46), Pulmonary embolism 1(0.83%) vs. 3(1.51%) (p=1.0), congestive heart failure 2(1.65%) vs. 2(1.01%) (p=0.63), renal failure 4(3.3%) vs.2 (1.0%)(p=0.29), respiratory failure 12(9.92%) vs. 17(8.54%) (p=0.69) and acute respiratory distress syndrome 2(1.65%) vs. 1(0.50%) (p=0.55). Median hospital stay was 5 days in the lower BMI group and 4 days in the BMI ≥25 groups (p=0.52).
Conclusions
Overweight and normal weight patients do not differ significantly in rates of perioperative morbidities, 30-day mortality and length of stay. Our study indicates that potential curative surgical resections can be offered to even significantly overweight patients.
Keywords: Lung Cancer, Surgery, BMI, Peri-operative morbidities
Introduction
It is estimated that more than 65%of US residents are currently overweight or obese (1). Obesity is associated with many medical co-morbidities including hypertension, diabetes and coronary artery disease (2, 3). Its presence has been associated with an increased risk of cancers including breast, colon, endometrium and kidney (4, 5). There is evidence that obese patients may also be at increased risk of developing lung cancer regardless of smoking status (5).
Obesity is considered a risk factor for poor outcome in many surgical procedures including urologic, gynecologic, cardiac and pancreatic surgical procedures (6–8). However this issue remains controversial, as it has not been found to be consistent with all studies, some studies indicate that in non-cardiac surgery, obesity alone is not a risk factor for peri-operative morbidity and mortality (8–11).
Not much is known about the effects of an elevated BMI on surgical morbidity and mortality after the resection of lung cancers. However it is commonly and widely assumed that obese patients are at higher risk of surgical complications than those who are not obese. Many studies assessing the outcomes have included obesity as one variable with little comment on its prognostic significance (12, 13). Whether or not higher BMI is associated with worse outcomes in patients undergoing lung cancer resections has been poorly studied (14–17).To our knowledge there have been only three published studies on the subject. In a retrospective review of 499 patients who underwent resections for non-small cell lung cancer (NSCLC), a higher BMI was not associated with increased incidence of perioperative complications, mortality or length of stay (18).In contrast two retrospective studies have shown no effect on mortality but higher incidence of intra-thoracic complications (19, 20).
In order to further describe the effects of a higher BMI in patients undergoing resection of lung cancer we performed a retrospective study to assess the impact of obesity on perioperative complications and mortality after resections for lung cancer.
Methods
We performed a retrospective study of patients undergoing resection for lung cancer at The Medical College of Wisconsin. After obtaining appropriate Institutional Review Board approval, data from patients, who underwent lung cancer resection from January 2006 to December 2010, were abstracted. Resections of lung metastasis or benign tumors were excluded.
Patient demographics were collected that included age, sex, height and weight. Patient variables and outcomes were stratified by BMI into two groups BMI<25 and the BMI>=25.Subjects were staged according to the 2010 American Joint Committee on Cancer staging. Outcomes measured included mortality within the 30 days of surgery, length of stay and in-hospital complications. The in-hospital complications were defined as atrial fibrillation, heart failure, respiratory failure, pulmonary embolism or any medical complications arising within 30 days of surgery. Respiratory failure was defined as any condition of respiratory distress from severe COPD, Pneumonia that requires close monitoring including the non-invasive and invasive ventilation.
Patients were admitted on the day of surgery unless they required preoperative management of medical comorbidities. One of three board certified thoracic surgeons performed all the procedures. The prophylaxis for venous thromboembolism and the management of post-operative pain was carried out as per standard guidelines. The duration of hospital stay was calculated from the day of surgery until the patient was discharged. The duration of chest tube was calculated from the day chest tube was placed (on the day of surgery) until the tube was taken out which may happen even after discharge in cases of an air leak. We defined mortality as any death occurring within 30 days after surgery.
Statistical Methods
Association for BMI and hospital days was tested using the Wilcoxon Rank Sum test and Pearson and Spearman correlation. Fisher’s exact test was used to test the dichotomous association between BMI greater than (or equal to) 25 and the presence of perioperative morbidities. Odds ratios are given with 95%exact confidence intervals. The log-rank test was used to compare the overall mortality between the groups.
Multivariate logistic regression of factors predicting perioperative morbidities were considered for all types of morbidity combined, and for specific types where there were more than 25 events. None of these multivariate models indicate more than a single predictive factor for any of the morbidities considered.
The analysis was performed using SAS version 9.2(The SAS Institute, Cary, NC).
Results
Patient characteristics
Between 2006 and 2010, there were 320 lung resections for NSCLC. The patient baseline demographics are shown in Table 1. The median age was 67(IQR 59–75) years and consisted of 135 (44.2%) men and 185(55.7%) women. The frequencies of surgical procedures were wedge resections in 99(32.7%), lobectomies in 207 (62.9%), and pneumonectomies in 14 (4.3%) patients. Adenocarcinoma was the most prevalent histology present in 138(43.9%), followed by squamous cell carcinoma in 107(31.6%), bronchoalveolar carcinoma in 25(7.1%), large cell carcinoma in 19(5.4%) and others in 31(11.7%). The median tumor size was 2.3 cm (IQR 1.5–3.9) and was highest in the pneumonectomy group. Out of 14 pneumonectomies, 8 were left sided and the remaining 6 right sided.
Table 1.
Baseline Characteristics, Surgery type and Pathology
| BMI<25 | BMI>=25 | p value | |
|---|---|---|---|
|
| |||
| Characteristics | |||
| Female sex-no. (%) | 72(59.50) | 113(56.78) | 0.632 |
| Male sex-no. (%) | 49(40.50) | 86(43.22) | |
| Age-yrs. | |||
| Median | 67 | 67 | 0.96 |
| Range | 25–88 | 44–85 | |
| Surgery type | |||
| Lobectomy-no. (%). | 76(62.81) | 131(65.83) | 0.61 |
| Wedge Resection-no. (%). | 38(31.40) | 61(30.65) | |
| Pneumonectomy-no. (%). | 7(5.79) | 7(3.52) | |
| Tumor size- | |||
| Median cm (range) | 2.3(0.5–10) | 2.4(0.6–7) | 0.32 |
| Pathology | |||
| Adenocarcinoma-no (%) | 52(16.25) | 111(34.6) | 0.0817 |
| Squamous cell carcinoma-no (%) | 50(15.62) | 57(17.8) | |
| Large Cell Carcinoma-no (%). | 6(1.8) | 13(4.06) | |
| Mixed type=no (%). | 10(3.1) | 21(6.5) | |
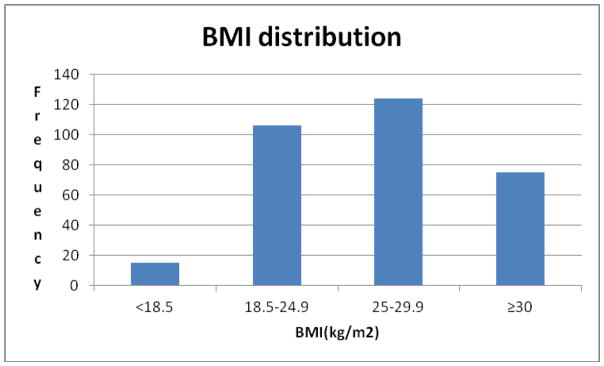
The distribution of patients in different BMI groups is shown in a histogram in figure 2. The majority of patients were overweight 124(38.7%) defined as BMI 25–29.9. Patient demographics, tumor characteristics and types of surgery performed were similar in two groups of BMII<25 and >=25 as shown in table 1.
Figure 2.
Morbidities from surgery
The morbidities encountered peri-operatively are shown in Table 3. A total of 62(19.3%) peri-operative morbidities were encountered in 320 patients, with no significant difference in the rate of overall and specific morbidities (p>0.05 in all cases) in between the two groups. The distributions of these morbidities between the two BMI groups are shown in Table 3.
Table 3.
Morbidities during perioperative period
| BMI<25 | BMI≥25 | p value | |
|---|---|---|---|
|
| |||
| All-no. (%) | 28(8.75) | 47(14.6) | 0.54 |
| Atrial fibrillation-no. (%) | 11(9.09) | 24(12.06) | 0.46 |
| Respiratory Failure-no. (%) | 12(9.92) | 17(8.54) | 0.69 |
| Pulmonary embolism-no. (%) | 1(0.83) | 3(1.51) | 1.00 |
| CHF exacerbation-no. (%) | 2(1.65) | 2(1.01) | 0.63 |
| Acute respiratory distress syndrome-no. (%) | 2(1.65) | 1(0.50) | 0.55 |
| Renal Failure-no. (%) | 4(3.3) | 2(1.0) | 0.29 |
| 30-d mortality-no (%) | 4(1.25) | 2(0.62) | 0.29 |
The median hospital stay in the normal BMI group was 5 days compared to 4 days (p=0.52) in the higher BMI group. The median chest tube duration was same 3 days across both the groups (p=0.052).
Mortality from surgery
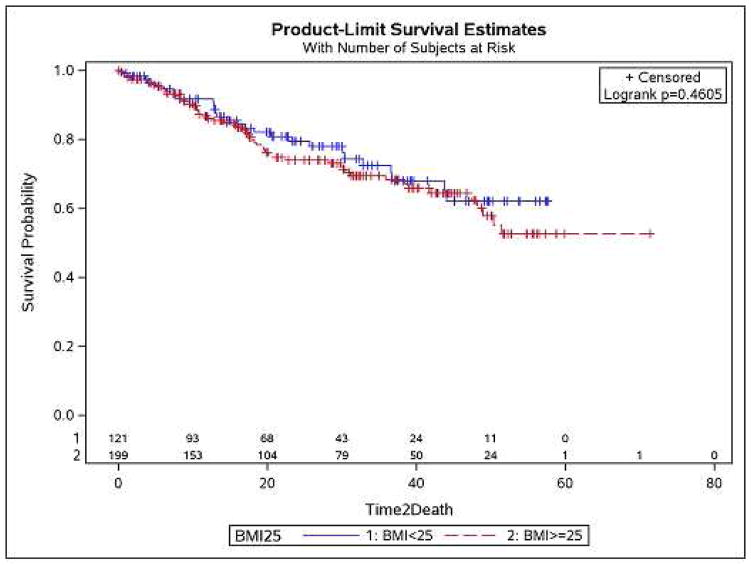
The mortality rate in our population was 1.8% (n=6)(table=4). The causes of death were different for all of them and only 2 of the people that died had a BMI greater than 25.The overall survival was similar between the two groups (p=0.46)(fig.1).
Figure 1.
Kaplan-Meier survival curve showing no significant difference in the survival between the two groups.
Interpretation of Multivariate results(Table 5)
Table 5.
Univariate and Multivariate analysis of all and specific peri-operative morbidities with different predictors.
| Outcome | predictors | OR (95% CI) | univariate p-value | multivariate p-values |
|---|---|---|---|---|
| Any morbidities | COPD (Y v N) | 1.85 (1.09, 3.15) | 0.0253 | 0.2001 |
| CHF (Y v N) | 0.72 (0.34, 1.55) | 0.4614 | 0.1544 | |
| Sex (M v F) | 1.02 (0.63, 1.63) | 1.0000 | 0.9376 | |
| Surgery Procedure | 0.7887 | 0.6734 (df=2) | ||
| BMI (linear | 0.98 (0.95, 1.02) | 0.3095 | 0.3944 | |
| BMI (5 df) | 0.5053 | 0.5687 (df=5) | ||
| BMI25 (25+ v <25) | 0.84 (0.52, 1.35) | 0.5405 | 0.5879 | |
| BMI20 (20+ v <20) | 0.49 (0.24, 1.00) | 0.0513 | 0.0683 | |
| FEV1 (per 1 unit FEV) | 0.98 (0.97, 1.00) | 0.0228 | 0.0228 | |
| FEV1 (per 10 unit FEV) | 0.86 (0.75, 0.98) | 0.0228 | 0.0228 | |
| DLCO (per 1 unit) | 0.99 (0.98, 1.01) | 0.3144 | 0.9945 | |
| age (per year) | 1.01 (0.99, 1.03) | 0.5367 | 0.7376 | |
| Air leak | COPD (Y v N) | 1.45 (0.71, 2.93) | 0.3382 | 0.3969 |
| CHF (Y v N) | 0.52 (0.15, 1.77) | 0.4450 | 0.2800 | |
| Sex (M v F) | 1.05 (0.55, 1.99) | 1.0000 | 0.9461 | |
| Surgery Procedure | 0.0998 | 0.3295 (df=2) | ||
| BMI (linear | 0.97 (0.92, 1.02) | 0.2731 | 0.2731 | |
| BMI (5 df) | 0.0400 | *** | ||
| BMI25 (25+ v <25) | 0.77 (0.40, 1.47) | 0.5035 | already in MV model | |
| BMI20 (20+ v <20) | 0.26 (0.12, 0.58) | 0.0011 | *** | |
| FEV1 (per 1 unit FEV) | 0.99 (0.98, 1.01) | 0.4876 | 0.6211 | |
| DLCO (per 1 unit) | 1.00 (0.98, 1.01) | 0.5914 | 0.7174 | |
| age (per year) | 1.00 (0.97, 1.03) | 0.9867 | 0.9402 | |
| Atrial Fibrillation | COPD (Y v N) | 1.15 (0.51, 2.57) | 0.8325 | |
| CHF (Y v N) | 0.99 (0.33, 2.97) | 1.0000 | ||
| Sex (M v F) | 0.98 (0.65, 1.47) | 1.0000 | ||
| Surgery Procedure | 0.0990 (df=2) | |||
| BMI (linear | 1.01 (0.96, 1.06) | 0.6344 | ||
| BMI (5 df) | 0.6320 | |||
| BMI25 (25+ v <25) | 1.37 (0.65, 2.91) | 0.4639 | ||
| BMI20 (20+ v <20) | 0.88 (0.29, 2.66) | 0.8181 | ||
| FEV1 (per 1 unit FEV) | 0.99 (0.97, 1.01) | 0.2530 | ||
| DLCO (per 1 unit) | 1.00 (0.98, 1.02) | 0.7790 | ||
| age (per year) | 1.02 (0.98, 1.05) | 0.3862 | ||
| Respiratory failure | COPD (Y v N) | 4.13 (1.89, 9.01) | 0.0008 | 0.0008 |
| CHF (Y v N) | 1.25 (0.41, 3.82) | 0.7589 | 0.7104 | |
| Sex (M v F) | 1.31 (0.61, 2.82) | 0.5558 | 0.5659 | |
| Surgery Procedure | 0.7809 | 0.7068 | ||
| BMI (linear | 0.98 (0.93, 1.05) | 0.6122 | 0.8222 | |
| BMI (5 df) | 0.8557 | 0.8630 | ||
| BMI25 (25+ v <25) | 0.85 (0.39, 1.84) | 0.6917 | 0.8794 | |
| FEV1 (per 1 unit FEV) | 0.98 (0.96, 1.00) | 0.1095 | 0.7572 | |
| DLCO (per 1 unit) | 1.00 (0.98, 1.03) | 0.7444 | 0.1751 | |
| age (per year) | 1.01 (0.97, 1.05) | 0.6074 | 0.8353 |
-Multivariate analysis not performed for atrial fibrillation as there are no significant predictors.
only in place of BMI 25.
Any Perioperative Morbidity
FEV1 was the only significant predictor of morbidity considered overall. For each 10 points decrease in FEV1 the odds of any sort of morbidity increased by about 14% (95% CI 2%, 25%), p=0.0228. Although COPD was significant in univariate analysis (p=0.0253), COPD was no longer significant in multivariate analysis controlling for FEV1 (p=0.2001).
Air Leak
Among the factors considered, including a considerable effort to detect non-linear effects of BMI, the only significant predictor was BMI<20 vs BMI 20+, with the BMI 20+ group having considerable lower odds of an air-leak morbidity (OR-0.26, 95% CI 0.12, 0.58, p=0.0011). This effect was not significant when considering BMI<25 vs. BMI 25+ (p=0.5035). We consider the BMI<20 vs. 20+ result to be a post-hoc test. It is reported for completeness, but it does not relate directly to our main result about high BMI and morbidity.
Atrial fibrillation
No significant predictors were found.
Respiratory Failure
COPD was the only significant predictor of Respiratory Failure morbidity on univariate and multivariate analysis (OR=4.1, 95% CI 1.9,9.0, p=0.0008).
DISCUSSION
In order to further describe the surgical risks of overweight patients undergoing resections for lung cancer we carried out a retrospective single-institution review. Our series identified no difference between the morbidities encountered post-surgically nor in mortality between overweight and normal patients. However, there could be small associations, which are present, but too weak to be detected. BMI did not have any effect on the overall and specific peri-operative morbidities whether as a linear (continuous variable) or dichotomous variable with 25 as the cutoff (table 5). Our analysis also divided BMI into four different groups as per WHO classification; with no significant difference in the overall morbidities in these groups except for the air leak which tends to occur more in the lower BMI groups (table 5).
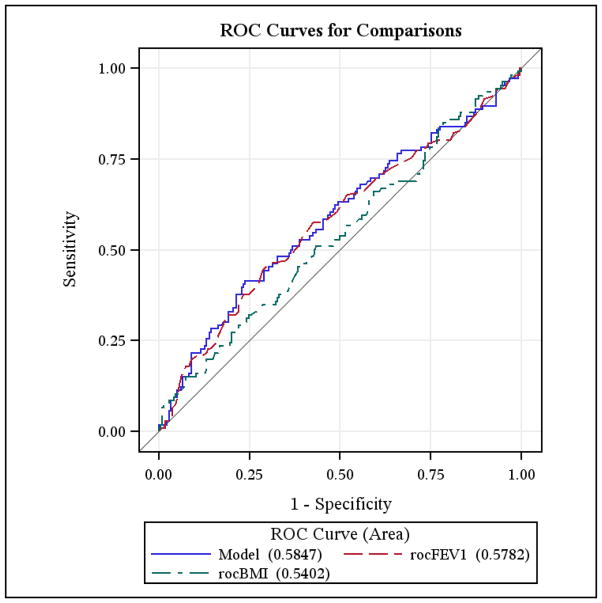
In the logistic regression model considering any perioperative morbidity, after controlling for FEV1 (p=0.0228), continuous BMI is not significant (p=0.3944). ROC plots show no difference all in the model including both FEV1 and BMI, compare to FEV1 alone. There is no indication that BMI at any cut-point would add significant predictive power.
In the logistic regression model considering Air-Leak, continuous BMI is not significant (p=0.2731). The ROC plot for BMI maximizes the sum of Sensitivity and Specificity around 24–25 BMI, but there is no significant predictive power(fig 3).
Figure 3.
The duration of hospital stay is determined by many factors like wound healing, comorbidities and physical deconditioning from surgery. Our study suggests that a higher BMI doesn’t have any effect on the duration of hospital stay, as the median length of hospital stay was same between the two groups. The median chest tube duration was 3 days in both the groups, however due to higher incidence of outliers in the non-obese groups the p value approached the level of significance, this should be interpreted with caution as this number is highly influenced by a few patients with a high value of chest tube days, and is not adjusted for multiple comparisons. Length of stay and even the chest tube duration is influenced by many factors including the surgeon preference, variable discharge criteria and the varying protocols for chest tube management along with other social factors.
The outcome of our study is similar to the study by Smith and colleagues, where no difference in overall morbidity, mortality, and length of stay was found (18). The mortality rate in their study was 1.4%, slightly lower than our 1.8%mortality rate but both were within the accepted standards. In the study by Smith and collaborators, the patients were divided into two groups of BMI<30 and≥30. Our study is different from that, as we divided our patients into two groups with a cut off BMI of 25 as we decided to study the risks of overweight population versus those who have normal BMI. Analysis of our data using the cutoffs used by Smith and collaborators didn’t change our results. In the same study it was shown that obese patients were at higher risk of developing renal failure, (0.3% vs. 3.9%, p=0.001). However this was not confirmed in our series. They also showed a decreased tendency for respiratory complications in obese patients (21.8% vs. 14.2%, p=0.06 on bivariate analysis) due to higher DLCO and low smoking rates in obese patients. Our study showed the opposite trend, as there was no significant difference in respiratory complications when compared to normal BMI patients (9.94 % vs. 8.54%, p= 0.69). FEV1 was the only significant predictor of morbidity considered overall.
In contrast, another study by Petrella and colleagues showed that overweight and obese patients that underwent pneumonectomies for lung cancer had a higher risk of respiratory complications as compared to patients with a normal BMI (21.4 % vs. 4.9 %, p=0.005) (20). The study however did not show any difference in cardiac complcations, 30-d mortality and length of stay between the two groups (20). Our study was not limited to pneumonectomies, only 14 patients with such procedure were included part of our cohort. No differences in outcomes between normal and overweight patients were seen in this subgroup in our series.
Our study is limited by its retrospective nature; it is possible that selection bias is responsible for our findings. The sample size is also small in our study to detect the meaningful differences between the two groups. We also did not take into account the other factors that can affect the morbidity and mortality like diabetes, hypertension, and underlying lung disease among others and were limited to COPD and CHF only. Moreover in our series the sample size is even smaller when we use higher BMI (>35) to see if the differences exist. We also did not look into the baseline nutritional status of our patients like prealbumin/albumin, which might influence the overall outcome. Our study did not look the outcomes with minimal invasive surgery and also the extent of resection. In our multivariate analysis we took into account the three major types of surgery and found no difference in outcomes with the types of surgery. Over 5 year period the changing approach for lung resection would have influenced the peri-operative outcomes and our study was limited to that aspect. Large multi-institutional and prospective studies are needed to fully elucidate the effects of higher BMI due to several limitations of our retrospective study.
Due to the changing lifestyles both the BMI and lung cancer is on the rise. In the future thoracic surgeons are likely to encounter more patients with higher BMI with non-small cell lung cancer. Our study suggests that higher BMI doesn’t carry significant risk for lung cancer surgery. However large, multi-institutional and prospective studies are needed to support this evidence.
Table 2.
Hospital duration, Chest tube duration
| BMI<25 | BMI≥25 | P value | |
|---|---|---|---|
|
| |||
| Hospital duration-median (d) | 5 | 4 | 0.52 |
| Chest tube duration-median (d) | 3 | 3 | 0.052 |
Table 4.
30 d mortality (causes)
| BMI | Surgery types | |
|---|---|---|
|
| ||
| Pulmonary Embolism/CHF | 27.5 | Lobectomy |
| Hypotension/Ischemic bowel | 24.8 | Lobectomy |
| Renal failure | 24.8 | Lobectomy |
| GI bleed | 23.1 | Lobectomy |
| Suicide | 19.7 | Lobectomy |
| Cerebrovascular accident | 25.3 | Wedge resection |
Acknowledgments
Supported, IN PART, by grant 1UL1RR031973 from the Clinical and Translational Science award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002 Oct 9;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.DeMaria EJ, Carmody BJ. Perioperative management of special populations: Obesity. Surg Clin North Am. 2005 Dec;85(6):1283, 9, xii. doi: 10.1016/j.suc.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Raebel MA, Malone DC, Conner DA, Xu S, Porter JA, Lanty FA. Health services use and health care costs of obese and nonobese individuals. Arch Intern Med. 2004 Oct 25;164(19):2135–40. doi: 10.1001/archinte.164.19.2135. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer. 2007 May 1;120(9):1993–8. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008 Feb 16;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993 Oct 1;119(7 Pt 2):655–60. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey AM, Martin RC. Body mass index and outcomes from pancreatic resection: A review and meta-analysis. J Gastrointest Surg. 2011 Sep;15(9):1633–42. doi: 10.1007/s11605-011-1502-1. [DOI] [PubMed] [Google Scholar]
- 8.Wigfield CH, Lindsey JD, Munoz A, Chopra PS, Edwards NM, Love RB. Is extreme obesity a risk factor for cardiac surgery? an analysis of patients with a BMI > or = 40. Eur J Cardiothorac Surg. 2006 Apr;29(4):434–40. doi: 10.1016/j.ejcts.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Klasen J, Junger A, Hartmann B, Jost A, Benson M, Virabjan T, et al. Increased body mass index and peri-operative risk in patients undergoing non-cardiac surgery. Obes Surg. 2004 Feb;14(2):275–81. doi: 10.1381/096089204322857708. [DOI] [PubMed] [Google Scholar]
- 10.Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SF, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol. 2008 Aug;15(8):2164–72. doi: 10.1245/s10434-008-9990-2. [DOI] [PubMed] [Google Scholar]
- 11.Thomas EJ, Goldman L, Mangione CM, Marcantonio ER, Cook EF, Ludwig L, et al. Body mass index as a correlate of postoperative complications and resource utilization. Am J Med. 1997 Mar;102(3):277–83. doi: 10.1016/S0002-9343(96)00451-2. [DOI] [PubMed] [Google Scholar]
- 12.Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest. 1992 May;101(5):1332–7. doi: 10.1378/chest.101.5.1332. [DOI] [PubMed] [Google Scholar]
- 13.Stolz AJ, Schutzner J, Lischke R, Simonek J, Pafko P. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg. 2005 Feb;27(2):334–6. doi: 10.1016/j.ejcts.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Allen MS, Darling GE, Pechet TT, Mitchell JD, Herndon JE, 2nd, Landreneau RJ, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: Initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006 Mar;81(3):1013, 9. doi: 10.1016/j.athoracsur.2005.06.066. discussion 1019–20. [DOI] [PubMed] [Google Scholar]
- 15.Berggren H, Ekroth R, Malmberg R, Naucler J, William-Olsson G. Hospital mortality and long-term survival in relation to preoperative function in elderly patients with bronchogenic carcinoma. Ann Thorac Surg. 1984 Dec;38(6):633–6. doi: 10.1016/s0003-4975(10)62324-7. [DOI] [PubMed] [Google Scholar]
- 16.Damhuis RA, Schutte PR. Resection rates and postoperative mortality in 7,899 patients with lung cancer. Eur Respir J. 1996 Jan;9(1):7–10. doi: 10.1183/09031936.96.09010007. [DOI] [PubMed] [Google Scholar]
- 17.Weiss W. Operative mortality and five year survival rates in patients with bronchogenic carcinoma. Am J Surg. 1974 Dec;128(6):799–804. doi: 10.1016/0002-9610(74)90074-9. [DOI] [PubMed] [Google Scholar]
- 18.Smith PW, Wang H, Gazoni LM, Shen KR, Daniel TM, Jones DR. Obesity does not increase complications after anatomic resection for non-small cell lung cancer. Ann Thorac Surg. 2007 Oct;84(4):1098, 105. doi: 10.1016/j.athoracsur.2007.04.033. discussion 1105–6. [DOI] [PubMed] [Google Scholar]
- 19.Suemitsu R, Sakoguchi T, Morikawa K, Yamaguchi M, Tanaka H, Takeo S. Effect of body mass index on perioperative complications in thoracic surgery. Asian Cardiovasc Thorac Ann. 2008 Dec;16(6):463–7. doi: 10.1177/021849230801600607. [DOI] [PubMed] [Google Scholar]
- 20.Petrella F, Radice D, Borri A, Galetta D, Gasparri R, Solli P, et al. The impact of preoperative body mass index on respiratory complications after pneumonectomy for non-small-cell lung cancer. results from a series of 154 consecutive standard pneumonectomies. Eur J Cardiothorac Surg. 2011 May;39(5):738–44. doi: 10.1016/j.ejcts.2010.09.007. [DOI] [PubMed] [Google Scholar]