Abstract
Diffusion tensor imaging (DTI) provides a measure of the directional diffusion of water molecules in tissues. The measurement of DTI indices within the spinal cord provides a quantitative assessment of neural damage in various spinal cord pathologies. DTI studies in animal models of spinal cord injury indicate that DTI is a reliable imaging technique with important histological and functional correlates. These studies demonstrate that DTI is a non-invasive marker of microstructural change within the spinal cord. In human studies, spinal cord DTI shows definite changes in subjects with acute and chronic spinal cord injury, as well as cervical spondylotic myelopathy. Interestingly, changes in DTI indices are visualized in regions of the cord, which appear normal on conventional MRI and are remote from the site of cord compression. Spinal cord DTI provides data that can help us understand underlying microstructural changes within the cord, and assist in prognostication and planning of therapies. In this article, we review the use of DTI to investigate spinal cord pathology in animals and humans, and describe advances in this technique that establish DTI as a promising biomarker for spinal cord disorders.
Keywords: diffusion tensor imaging, spinal cord, spinal cord injury, fractional anisotropy
Introduction
Diffusion tensor imaging (DTI) is a magnetic resonance technique capable of measuring the magnitude and direction of diffusion of water molecules in various tissues. DTI developed from a technique known as diffusion weighted imaging, which measures the attenuation of MR signals due to diffusion, and was initially used for brain imaging.1 DTI was formally introduced by Basser et al2, and subsequent improvements in this technique have led to the development of DTI as a tool to delineate white matter tracts in the brain.
DTI of the spinal cord in humans was initially inadequate due to the small area of the cord, susceptibility artifacts, as well as cardiac and respiratory motion artifacts.3, 4 Improvements in scanning protocols have allowed for useable diffusion images of the spinal cord. Spinal cord DTI, initially performed in animals, is now used to evaluate spinal cord disorders in humans. Investigators have shown that DTI is able to detect cord damage in regions of the cord that appear normal on T2W images.5, 6 Spinal cord DTI, therefore, represents an important advancement in the field of neuroimaging, and its use is being expanded both for prognostication as well as for guiding therapy.
In this paper, we review the literature on spinal cord DTI in both animal models and humans. We provide a summary for the clinical use of spinal cord DTI in a few neurosurgical conditions. We hope that by providing a review on the current status of spinal cord DTI, we may be able to better direct future efforts in this field.
Principles of Diffusion Tensor Imaging
Diffusion MRI provides a measure of the displacement of water molecules in tissues. Displaced water molecules produce an attenuated signal during diffusion MR scanning. By its nature, the axonal architecture in the white matter of the central nervous system promotes diffusion of water molecules in a direction predominantly parallel, rather than perpendicular, to axon fibers.2, 7, 8 Diffusion perpendicular to the fibers seems to be limited by cell membranes more than myelin sheaths.9, 10 This direction-dependent diffusion, described as ‘anisotropy’, is used by DTI to infer the orientation of surrounding axonal fibers and to delineate anatomical boundaries. DTI uses a tensor framework to characterize molecular motion in multiple directions in a three-dimensional space. The diffusivities along the three principle axes are used to calculate DTI indices. The commonly used indices for spinal cord DTI include fractional anisotropy (FA), apparent diffusion co-efficient (ADC), longitudinal apparent diffusion co-efficient (lADC), and transverse apparent diffusion co-efficient (tADC). Investigators determine specific regions of interest on axial or sagittal diffusion images, and DTI indices for these regions are calculated from individual vectors using dedicated software tools. FA, which ranges from 0 to 1, defines the degree of anisotropy, and tissues with high anisotropy, such as white matter tracts, have a value closer to 1. Injured spinal cords show a decrease in anisotropy due to disruption of longitudinally aligned axons, and exhibit a decrease in FA. The ADC or mean diffusivity (MD) is the mathematical average of the diffusivities in the three principal axes, and its value may increase or decrease based on the histopathological progression of the lesion. The lADC represents rostro-caudal diffusivity along white matter fibers, and is often decreased in the presence of axonal injury.11 tADC measures radial diffusivity and is characteristically increased in the presence of demyelination.11, 12 Overall, DTI indices are affected by microstructural alterations that affect the diffusion of water molecules, and this forms the basis for using DTI indices to identify spinal cord pathology.
DTI studies in rat models
DTI measurements of rat spinal cord
DTI measurements of the rat spinal cord were initially performed either ex vivo,13–15 or in vivo using implantable coils.16, 17 The majority of these studies used scanners with field strengths from 4.7 T to 7 T. With improved technology, in vivo measurements were possible with higher field strength scanners,18, 19 and without implantable coils.18, 19 Studies with animal spinal cords indicate that DTI values clearly differentiate white (WM) and gray matter (GM) (Figure 1).13, 17, 18, 20 Since diffusion occurs preferentially along axonal bundles, WM is significantly more anisotropic as compared to GM.10, 20 Significant differences in DTI indices are described between spinal levels (cervical, thoracic, and caudal) in rat studies.18 This is probably a result of microstructural variations in the gray and white matter along the spinal cord.21 These results indicate that diffusion properties are not uniform throughout the length of the cord, and vary according to the level being studied. These results further establish the usefulness of DTI to delineate neural structures in the spinal cord.
Figure 1.
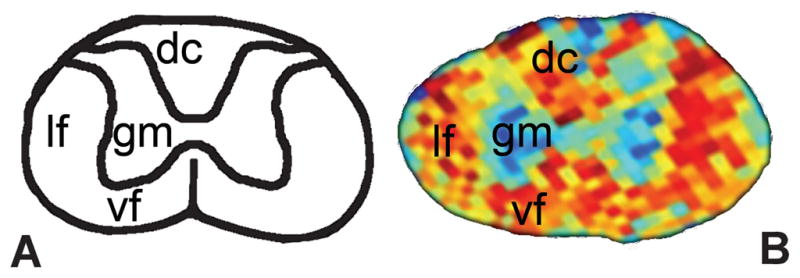
A: Schematic diagram of a cross-section of a rat cervical spinal cord showing location of white matter funiculi and gray matter; B: Corresponding structure is shown on an axial FA map of the ex vivo rat cervical spinal cord obtained with a 9.4 T MR scanner. (vf- ventral funiculus, lf- lateral funiculus, dc- dorsal columns, gm- gray matter)
DTI measurements after spinal cord injury (SCI)
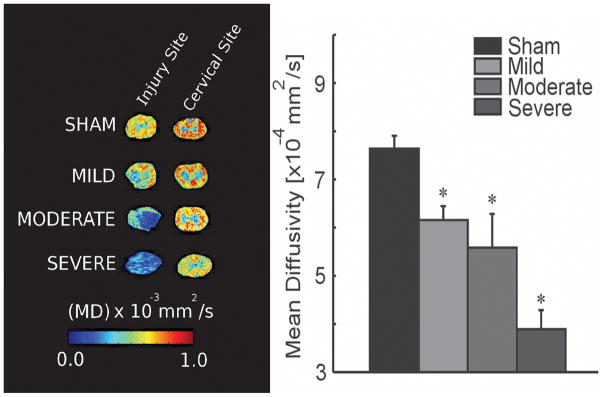
One of the important applications of DTI is the evaluation of SCI in animal models. DTI demonstrates a significant decrease in anisotropy and increase in radial diffusivity at the level of injury16, 22, 23 as well as in areas of the cord that are apparently normal on conventional T2-weighted images.24 In hyperacute SCI (0–6 hours), diffusion measurements are able to distinguish SCI based on severity.25 However, the unique feature of DTI is its ability to detect changes in diffusion metrics at regions rostral and caudal to the lesion.16, 26–28 A decrease in diffusivity remote from the lesion is observed during recovery from SCI (Figure 2).27 These findings are possibly related to cytotoxic edema, axonal loss or chronic atrophy.29–31 Interestingly, changes in DTI indices away from the lesion correlate with the injury severity, indicating that they may be used as surrogate markers of neural injury (Figure 2). Moreover, these changes are not limited to the white matter tracts only. At our center, we find that motor neurons rostral to the lesion are enlarged after SCI and this is associated with an increase in the FA of the rostral gray matter (unpublished data). Studies show that spinal cord gray matter is affected by ischemia due to impaired microvascular perfusion32 and is characterized by astrogliosis during recovery.33 Using DTI to track these remote changes will help us better understand the pathophysiology of SCI. Since there are changes in diffusivities throughout the cord after SCI, it is apparent that microstructural recovery from SCI is not limited to the epicenter alone.
Figure 2.
Axial DTI images obtained from ex vivo rat spinal cord specimens at the injury site (thoracic cord), and at a rostral site in the cervical spinal cord, 10 weeks after contusive SCI of varying severity. Fractional anisotropy (FA) maps demonstrate loss of anisotropy at the injury site. Sham spinal cords showed intact cord structure with normal central gray matter morphology. Bar graph showing significant differences between severity groups in mean diffusivity of the cervical spinal cord sections. * P<0.05
Several animal studies show correlations between DTI indices and histological changes during recovery from SCI.25, 34–37 The hyperacute phase following SCI is associated with edema, hemorrhage and inflammation. Following this, there is an intermediate phase characterized by a robust glial response and revascularization process. The chronic phase of SCI shows wallerian degeneration, astroglial scar formation and progressive cavitation of the cord with rostral-caudal spreading.34, 38 Identifying specific changes in DTI metrics to characterize particular histological events during recovery from SCI remains a challenge. While an increase in MD after injury can map the extent of degeneration, a decrease in FA is sensitive to cavity formation within the cord.34 DTI is also able to characterize the orientation of the glial scar as well as the degree of axonal dieback and preservation.14, 15 Changes in DTI measurements possibly reflect a combination of histopathological changes.28, 39, 40 DTI values have been shown to be more affected by axonal injury than demyelination,28, 40 suggesting that the diverse tissue damage as a result of SCI may not be completely captured by diffusion measurements.
DTI and functional correlates in SCI
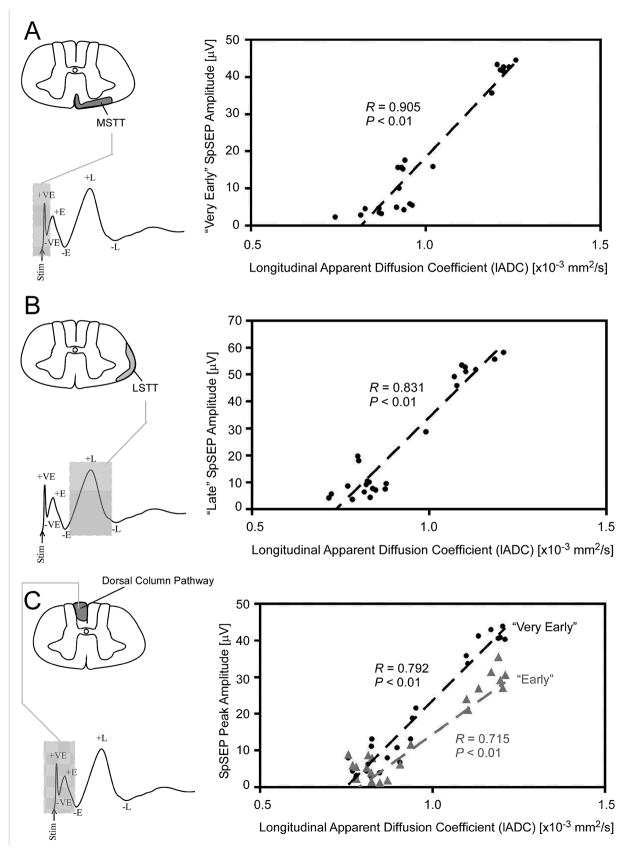
DTI metrics correlate with electophysiological measures, indicating that specific diffusion measures could be used as predictors of neurological function. The use of cortical sensory evoked potentials (SEPs) to assess cord integrity in SCI models has been limited by its sensitivity to anesthetic agents41, 42 and changes in body temperature43. Spinal SEPs (SpSEPs) represent a reliable technique to obtain repeated recordings,44, 45 and these correlate well with the Basso, Beattie, and Bresnahan (BBB) score.46 DTI measurements of the medial spinothalamic tracts and dorsal columns correlate with very early and early components of the SpSEPs, while diffusion measures of the lateral spinothalamic tracts are linked to the late components (Figure 3).47 Other studies show that the lADC of the rostral white matter correlates with the BBB score,16 while the radial diffusivity caudal to the lesion correlates with the grid walk test.28 The lADC of the spared ventrolateral white matter can also predict hindlimb motor recovery using the Basso mouse scale.48 Since axonal structure and integrity are closely linked to MR diffusion measurements21, 23 the above correlations emphasize the utility of DTI to measure both the structural and functional properties of axons.
Figure 3.
Scatter plots showing correlations between spinal sensory evoked potentials (SpSEPs) amplitude and longitudinal apparent diffusion co-efficient (lADC) of the spinal cord rostral to the injury site in a rat SCI model. Significant correlations were observed for the medial (A) and lateral (B) spinothalamic tracts as well as the dorsal columns (C). MSTT- medial spinothalamic tract, LSTT- lateral spinothalamic tract.
The role of DTI in therapeutic interventions for SCI is the focus of a few animal studies. The radial diffusivity around the injured site correlates with behavioral recovery in rats that are transplanted with fibroblasts following SCI.26 At our center, we find significantly increased diffusivity rostral to the injury site in rat SCI models following stem cell transplants, as compared to rats that received placebo. In the future, it is expected that spinal cord DTI will be used to monitor transplants and other therapeutic interventions for SCI.
DTI studies in humans
DTI in the intact human spinal cord
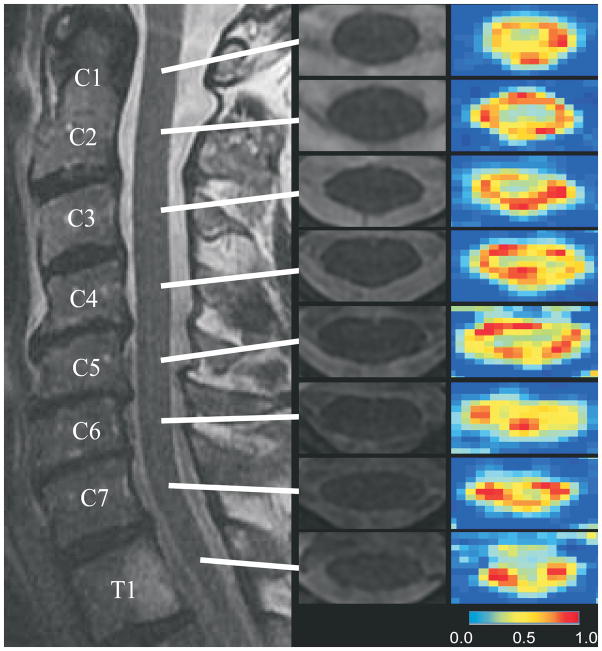
Spinal cord DTI studies in healthy human subjects show feasibility and reliability of this procedure.49–54 Good contrast is observed between gray and white regions, with the highly anisotropic white matter showing much higher FA values than the central gray matter (Figure 4). While the magnitude of FA of the whole cord decreases in the rostral-caudal direction, the MD is relatively constant throughout the cord. DTI indices are age-dependent, and reflect microstructural changes in the spinal cord associated with ageing.55–59 These results show that DTI is sensitive to degenerative changes within the spinal cord that are not visualized on conventional MRI. Moreover, they also emphasize the need to compare DTI measurements in patients with age-matched controls.
Figure 4.
Axial fractional anisotropy (FA) maps and T2-weighted images at individual levels of the cervical spinal cord in a healthy subject. Images were obtained using a standard 1.5 T clinical MR scanner. FA maps show higher anisotropy in the white matter funiculi and lower anisotropy in the central gray matter. (from 59, published with permission from Journal of Magnetic Resonance Imaging, John Wiley & Sons Inc.)
DTI in human SCI
In acute human SCI, DTI shows a reduction in diffusivity, particularly FA and lADC, around the injury site.60, 61 Choosing a DTI parameter that best characterizes SCI remains a challenge and authors suggest that diffusivity along the individual axes are more useful than DTI indices in representing microstructural changes.13 Similar to animal studies, human SCI is characterized by changes in diffusivity rostral to the injury site, in regions of the cord that appear normal on conventional MRI,61, 62 and possibly reflect retrograde neural injury. Axial FA maps and tractography are also sensitive to asymmetric cord damage in acute SCI, and can supplement conventional MR imaging in this setting.63, 64
The prognostic value of DTI indices in acute SCI is still unclear. Higher ADC values at the injured site is shown to be associated with better postoperative neurosurgical cervical spine scale (NCSS) scores but not Frankel scale measures.65 Another report shows that the DTI indices are correlated with the ASIA motor score in patients with non-hemorrhagic contusions.62 Correlations between DTI parameters and other outcome scales such as the functional independence measure (FIM), walking index for spinal cord injury (WISCI), and spinal cord injury measure (SCIM) have not been explored. There is a need to use a standardized functional outcome score in order to define the prognostic value of DTI indices. Moreover, if diffusivities of individual white matter tracts within the spinal cord are measured, it becomes essential to correlate the diffusion indices to scales that measure sensory and motor function separately. Chronic SCI is associated with a number of microstructural neural changes including demyelination,66, 67 remyelination,68, 69 axonal loss68 and atrophy70 that affect the diffusion of water molecules. As opposed to acute SCI, the injury site is characterized by increased diffusivity in patients with chronic SCI. FA at the injury site, however, is greatly reduced and appears to depend on both the level of injury and the completeness of the injury.71 FA values and connection rates of fiber tracking have also been shown to correlate with motor score in patients with chronic cervical cord injury.72 Similar to acute SCI, diffusivity within the high cervical spinal cord, rostral to the chronic injury site, is significantly altered.71, 73, 74 Importantly, rostral DTI indices correlate with functional measures in this group of patients,73, 74 thereby demonstrating that these indices may be non-invasive imaging biomarker for spinal cord injury. Additionally, spinal cord DTI indices rostral to the injury site correlate with DTI indices within cranial white matter tracts, and could be utilized as a marker of neural reorganization and plasticity.74 Since spinal fixation hardware around the injury site creates artifacts on diffusion images, DTI of the spinal cord, rostral to the injury site, allows us to evaluate neural injury without directly imaging the injury site. This may be a useful approach for future studies that investigate longitudinal changes in diffusivity during recovery from SCI.
DTI applications in cervical spondylotic myelopathy (CSM)
The complex pathophysiology of CSM includes mechanical spinal cord compression due to disc protrusion, osteophytes or ossified posterior longitudinal ligament as well as secondary cord ischemia.75, 76 Histopathological changes within the cervical cord in CSM include cavitation, demyelination and regions of cord infarction.77 Diffusion MRI is able to detect cord changes in patients with narrow cervical canals, in spite of normal T1W and T2W images.5, 49, 78–80 Across studies, FA is shown to be lower at the affected level in patients compared to corresponding levels in controls. DTI indices in CSM patients appear to depend on the degree of cord damage. Symptomatic CSM patients have lower FA values and higher ADC measures at the compressed level, as compared to asymptomatic patients with radiological features of cord compression.81 However, DTI measurements do not have consistent correlations with clinical scores of patients with CSM.80, 82–84 It therefore appears that DTI has a role to play in the preoperative planning of CSM patients, but the use of DTI to decide on surgical intervention or monitor recovery is yet to be investigated in detail.
DTI for spinal cord tumors
Diffusion tensor tractography is presently used to describe the orientation and location of white matter fibers around brain tumors.85–87 Recent studies have employed tractography for intradural spinal cord tumors.88, 89 The use of fiber tracking to delineate displaced white matter tracts seems to be particularly useful in solid tumors. In cystic tumors and tumors with considerable vasogenic edema, the increased diffusion of water molecular can lead to erroneous fiber tracking. A recent study showed that diffusion tensor tractography has a sensitivity of 87.5% and a specificity of 100% for predicting tumor resectability preoperatively.90 Measurement of diffusion indices within spinal cord tumors suggests that higher tumor mass is characterized by a decrease in FA and increase in ADC. However, studies have yet to evaluate the utility of DTI indices as predictors of tumor histology. In this regard, DTI indices may be able to differentiate spinal cord lesions on conventional MR images, and provide surgeons with an idea as to the possible pathology. Overall, the use of DTI shows much promise in planning surgical approaches for spinal cord tumors, as it has in brain tumor resection.
DTI has been used in a variety of other spinal cord disorders including multiple sclerosis,91, 92 syringomyelia,93, 94, and transverse myelitis.95 Although many of these studies are able to characterize DTI parameters in diseased states, the routine use of spinal cord DTI in the clinical setting is yet to be realized.
Limitations of DTI
Spinal cord DTI in humans still has a number of limitations. Adequate spatial resolution remains a problem and it is difficult to visualize the individual funiculi on diffusion-weighted images, particularly in the lower thoracic cord.54 DTI of these segments is affected more by artifacts arising from cardiac and respiratory motion as well as CSF pulsation.96 The use of faster imaging techniques such as parallel imaging, single shot echo-planar imaging as well as the use of cardiac pulse-gating have helped to reduce these artifacts. However, scan acquisition time is still a limitation for patients with acute SCI since these patients often cannot withstand additional scanning time in the MRI suite. Also, the signal to noise ratio is not uniform throughout the cervical spinal cord and is significantly decreased in caudal segments.59, 97 A low signal to noise ratio can lead to overestimation of anisotropy measures, particularly in low-anisotropic tissues such as the central gray matter.98 The use of 3T MR scanners does improve the SNR,99 but is still not used universally. The use of DTI postoperatively is hampered significantly by the use of spinal instrumentation, which creates numerous artifacts. Additionally, standardized software to process tensor images is essential to make this a feasible option for routine clinical use.
Conclusion
DTI provides a unique insight into the pathophysiology and microstructural alterations associated with spinal cord disorders. While initial studies in rat models have primed this modality for human research, more data are required on the accuracy and reliability of DTI indices in defining cord pathology. DTI of the spinal cord does show promise in certain neurosurgical conditions such as traumatic SCI, CSM and spinal cord tumors. However, scanning protocols and image processing need to be refined and standardized. Once these challenges are overcome, we can expect the use of DTI in mainstream clinical practice, both to prognosticate as well as monitor patients with spinal cord disease.
Acknowledgments
Funding: VA Rehab R&D grant #1 I01 RX000113-01, Bryon Riesch Paralysis Foundation Endowment
Abbreviations
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- ADC
apparent diffusion co-efficient
- lADC
longitudinal apparent diffusion co-efficient
- tADC
transverse apparent diffusion co-efficient
Footnotes
Disclosure: The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986 Nov;161(2):401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 2.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994 Jan;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark CA, Werring DJ. Diffusion tensor imaging in spinal cord: methods and applications - a review. NMR Biomed. 2002 Nov-Dec;15(7–8):578–586. doi: 10.1002/nbm.788. [DOI] [PubMed] [Google Scholar]
- 4.Barker GJ. Diffusion-weighted imaging of the spinal cord and optic nerve. J Neurol Sci. 2001 May 1;186(Suppl 1):S45–49. doi: 10.1016/s0022-510x(01)00490-7. [DOI] [PubMed] [Google Scholar]
- 5.Demir A, Ries M, Moonen CT, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003 Oct;229(1):37–43. doi: 10.1148/radiol.2291020658. [DOI] [PubMed] [Google Scholar]
- 6.Shen H, Tang Y, Huang L, et al. Applications of diffusion-weighted MRI in thoracic spinal cord injury without radiographic abnormality. Int Orthop. 2007 Jun;31(3):375–383. doi: 10.1007/s00264-006-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996 Jun;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 8.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995 Nov-Dec;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31(4):394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 11.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003 Nov;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002 Nov;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz ED, Hackney DB. Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp Neurol. 2003 Dec;184(2):570–589. doi: 10.1016/S0014-4886(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz ED, Duda J, Shumsky JS, Cooper ET, Gee J. Spinal cord diffusion tensor imaging and fiber tracking can identify white matter tract disruption and glial scar orientation following lateral funiculotomy. J Neurotrauma. 2005 Dec;22(12):1388–1398. doi: 10.1089/neu.2005.22.1388. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz ED, Chin CL, Shumsky JS, et al. Apparent diffusion coefficients in spinal cord transplants and surrounding white matter correlate with degree of axonal dieback after injury in rats. AJNR Am J Neuroradiol. 2005 Jan;26(1):7–18. [PMC free article] [PubMed] [Google Scholar]
- 16.Deo AA, Grill RJ, Hasan KM, Narayana PA. In vivo serial diffusion tensor imaging of experimental spinal cord injury. J Neurosci Res. 2006 Apr;83(5):801–810. doi: 10.1002/jnr.20783. [DOI] [PubMed] [Google Scholar]
- 17.Madi S, Hasan KM, Narayana PA. Diffusion tensor imaging of in vivo and excised rat spinal cord at 7 T with an icosahedral encoding scheme. Magn Reson Med. 2005 Jan;53(1):118–125. doi: 10.1002/mrm.20304. [DOI] [PubMed] [Google Scholar]
- 18.Ellingson BM, Kurpad SN, Li SJ, Schmit BD. In vivo diffusion tensor imaging of the rat spinal cord at 9.4T. J Magn Reson Imaging. 2008 Mar;27(3):634–642. doi: 10.1002/jmri.21249. [DOI] [PubMed] [Google Scholar]
- 19.Bilgen M, Al-Hafez B, Berman NEJ, Festoff BW. Magnetic resonance imaging of mouse spinal cord. Magn Reson Med. 2005;54(5):1226–1231. doi: 10.1002/mrm.20672. [DOI] [PubMed] [Google Scholar]
- 20.Moseley ME, Cohen Y, Kucharczyk J, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990 Aug;176(2):439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz ED, Cooper ET, Fan Y, et al. MRI diffusion coefficients in spinal cord correlate with axon morphometry. Neuroreport. 2005 Jan 19;16(1):73–76. doi: 10.1097/00001756-200501190-00017. [DOI] [PubMed] [Google Scholar]
- 22.Shi R, Pryor JD. Pathological changes of isolated spinal cord axons in response to mechanical stretch. Neuroscience. 2002;110(4):765–777. doi: 10.1016/s0306-4522(01)00596-6. [DOI] [PubMed] [Google Scholar]
- 23.Ford JC, Hackney DB, Lavi E, Phillips M, Patel U. Dependence of apparent diffusion coefficients on axonal spacing, membrane permeability, and diffusion time in spinal cord white matter. J Magn Reson Imaging. 1998 Jul-Aug;8(4):775–782. doi: 10.1002/jmri.1880080405. [DOI] [PubMed] [Google Scholar]
- 24.Ford JC, Hackney DB, Alsop DC, et al. MRI characterization of diffusion coefficients in a rat spinal cord injury model. Magn Reson Med. 1994 May;31(5):488–494. doi: 10.1002/mrm.1910310504. [DOI] [PubMed] [Google Scholar]
- 25.Loy DN, Kim JH, Xie M, Schmidt RE, Trinkaus K, Song S-K. Diffusion Tensor Imaging Predicts Hyperacute Spinal Cord Injury Severity. J Neurotrauma. 2007 Jun 01;24(6):979–990. doi: 10.1089/neu.2006.0253. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz ED, Shumsky JS, Wehrli S, Tessler A, Murray M, Hackney DB. Ex vivo MR determined apparent diffusion coefficients correlate with motor recovery mediated by intraspinal transplants of fibroblasts genetically modified to express BDNF. Exp Neurol. 2003 Jul;182(1):49–63. doi: 10.1016/s0014-4886(03)00036-0. [DOI] [PubMed] [Google Scholar]
- 27.Ellingson BM, Kurpad SN, Schmit BD. Ex vivo diffusion tensor imaging and quantitative tractography of the rat spinal cord during long-term recovery from moderate spinal contusion. J Magn Reson Imaging. 2008 Nov;28(5):1068–1079. doi: 10.1002/jmri.21578. [DOI] [PubMed] [Google Scholar]
- 28.Sundberg LM, Herrera JJ, Narayana PA. In vivo longitudinal MRI and behavioral studies in experimental spinal cord injury. J Neurotrauma. 2010 Oct;27(10):1753–1767. doi: 10.1089/neu.2010.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loubinoux I, Volk A, Borredon J, et al. Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke. 1997 Feb;28(2):419–426. doi: 10.1161/01.str.28.2.419. [DOI] [PubMed] [Google Scholar]
- 30.Lu H, Sun SQ. A correlative study between AQP4 expression and the manifestation of DWI after the acute ischemic brain edema in rats. Chin Med J (Engl) 2003 Jul;116(7):1063–1069. [PubMed] [Google Scholar]
- 31.Ellingson BM, Ulmer JL, Prost RW, Schmit BD. Morphology and morphometry in chronic spinal cord injury assessed using diffusion tensor imaging and fuzzy logic. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1885–1888. doi: 10.1109/IEMBS.2006.259379. [DOI] [PubMed] [Google Scholar]
- 32.Koyanagi I, Tator CH, Theriault E. Silicone rubber microangiography of acute spinal cord injury in the rat. Neurosurgery. 1993;32(2):260–268. doi: 10.1227/00006123-199302000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Barrett CP, Guth L, Donati EJ, Krikorian JG. Astroglial reaction in the gray matter of lumbar segments after midthoracic transection of the adult rat spinal cord. Exp Neurol. 1981;73(2):365–377. doi: 10.1016/0014-4886(81)90272-7. [DOI] [PubMed] [Google Scholar]
- 34.Ellingson BM, Schmit BD, Kurpad SN. Lesion growth and degeneration patterns measured using diffusion tensor 9.4-T magnetic resonance imaging in rat spinal cord injury. J Neurosurg Spine. 2010 Aug;13(2):181–192. doi: 10.3171/2010.3.SPINE09523. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Jones M, DeBoy CA, et al. Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. J Neurosci. 2009 Mar 11;29(10):3160–3171. doi: 10.1523/JNEUROSCI.3941-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozlowski P, Raj D, Liu J, Lam C, Yung AC, Tetzlaff W. Characterizing white matter damage in rat spinal cord with quantitative MRI and histology. J Neurotrauma. 2008 Jun;25(6):653–676. doi: 10.1089/neu.2007.0462. [DOI] [PubMed] [Google Scholar]
- 37.Farrell JA, Zhang J, Jones MV, et al. q-space and conventional diffusion imaging of axon and myelin damage in the rat spinal cord after axotomy. Magn Reson Med. 2010 May;63(5):1323–1335. doi: 10.1002/mrm.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. Journal of Neurotrauma. 2004 Apr;21(4):429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 39.Herrera JJ, Chacko T, Narayana PA. Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J Neurosci Res. 2008;86(2):443–447. doi: 10.1002/jnr.21481. [DOI] [PubMed] [Google Scholar]
- 40.Budde MD, Kim JH, Liang HF, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007 Apr;57(4):688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- 41.Mongan PD, Peterson RE. Intravenous anesthetic alterations on the spinal-sciatic evoked response in swine. Anesth Analg. 1993 Jul;77(1):149–154. [PubMed] [Google Scholar]
- 42.Rojas MJ, Navas JA, Rector DM. Evoked response potential markers for anesthetic and behavioral states. Am J Physiol Regul Integr Comp Physiol. 2006 Jul;291(1):R189–196. doi: 10.1152/ajpregu.00409.2005. [DOI] [PubMed] [Google Scholar]
- 43.Oro J, Haghighi SS. Effects of altering core body temperature on somatosensory and motor evoked potentials in rats. Spine (Phila Pa 1976) 1992 May;17(5):498–503. doi: 10.1097/00007632-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Nordwall A, Axelgaard J, Harada Y, Valencia P, McNeal DR, Brown JC. Spinal cord monitoring using evoked potentials recorded from feline vertebral bone. Spine (Phila Pa 1976) 1979 Nov-Dec;4(6):486–494. doi: 10.1097/00007632-197911000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Lueders H, Gurd A, Hahn J, Andrish J, Weiker G, Klem G. A new technique for intraoperative monitoring of spinal cord function: multichannel recording of spinal cord and subcortical evoked potentials. Spine (Phila Pa 1976) 1982 Mar-Apr;7(2):110–115. doi: 10.1097/00007632-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Ellingson BM, Kurpad SN, Schmit BD. Characteristics of mid- to long-latency spinal somatosensory evoked potentials following spinal trauma in the rat. J Neurotrauma. 2008 Nov;25(11):1323–1334. doi: 10.1089/neu.2008.0575. [DOI] [PubMed] [Google Scholar]
- 47.Ellingson BM, Kurpad SN, Schmit BD. Functional correlates of diffusion tensor imaging in spinal cord injury. Biomed Sci Instrum. 2008;44:28–33. [PubMed] [Google Scholar]
- 48.Kim JH, Loy DN, Wang Q, et al. Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. J Neurotrauma. 2010 Mar;27(3):587–598. doi: 10.1089/neu.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ries M, Jones RA, Dousset V, Moonen CT. Diffusion tensor MRI of the spinal cord. Magn Reson Med. 2000 Dec;44(6):884–892. doi: 10.1002/1522-2594(200012)44:6<884::aid-mrm9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 50.Clark CA, Barker GJ, Tofts PS. Magnetic resonance diffusion imaging of the human cervical spinal cord in vivo. Magn Reson Med. 1999 Jun;41(6):1269–1273. doi: 10.1002/(sici)1522-2594(199906)41:6<1269::aid-mrm26>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 51.Holder CA, Muthupillai R, Mukundan S, Jr, Eastwood JD, Hudgins PA. Diffusion-weighted MR imaging of the normal human spinal cord in vivo. AJNR Am J Neuroradiol. 2000 Nov-Dec;21(10):1799–1806. [PMC free article] [PubMed] [Google Scholar]
- 52.Bammer R, Fazekas F, Augustin M, et al. Diffusion-weighted MR imaging of the spinal cord. AJNR Am J Neuroradiol. 2000 Mar;21(3):587–591. [PMC free article] [PubMed] [Google Scholar]
- 53.Nagayoshi K, Kimura S, Ochi M, et al. Diffusion-weighted echo planar imaging of the normal human cervical spinal cord. J Comput Assist Tomogr. 2000 May-Jun;24(3):482–485. doi: 10.1097/00004728-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 54.Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging of the neurologically intact human spinal cord. AJNR Am J Neuroradiol. 2008 Aug;29(7):1279–1284. doi: 10.3174/ajnr.A1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agosta F, Lagana M, Valsasina P, et al. Evidence for cervical cord tissue disorganisation with aging by diffusion tensor MRI. Neuroimage. 2007 Jul 1;36(3):728–735. doi: 10.1016/j.neuroimage.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 56.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005 Jul;22(1):38–43. doi: 10.1002/jmri.20357. [DOI] [PubMed] [Google Scholar]
- 57.Lindberg PG, Feydy A, Maier MA. White matter organization in cervical spinal cord relates differently to age and control of grip force in healthy subjects. J Neurosci. 2010 Mar 17;30(11):4102–4109. doi: 10.1523/JNEUROSCI.5529-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Hecke W, Leemans A, Sijbers J, Vandervliet E, Van Goethem J, Parizel PM. A tracking-based diffusion tensor imaging segmentation method for the detection of diffusion-related changes of the cervical spinal cord with aging. J Magn Reson Imaging. 2008 May;27(5):978–991. doi: 10.1002/jmri.21338. [DOI] [PubMed] [Google Scholar]
- 59.Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, Kurpad SN. Characterization and limitations of diffusion tensor imaging metrics in the cervical spinal cord in neurologically intact subjects [published online ahead of print Feb 8 2013] [Accessed [March 1, 2013]];J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24039. http://www.ncbi.nlm.nih.gov/pubmed/23389869. [DOI] [PubMed]
- 60.Facon D, Ozanne A, Fillard P, Lepeintre J-F, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005 Jun 1;26(6):1587–1594. [PMC free article] [PubMed] [Google Scholar]
- 61.Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol. 2008 Apr;29(4):655–659. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheran S, Shanmuganathan K, Zhuo J, et al. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma. 2011 Sep;28(9):1881–1892. doi: 10.1089/neu.2010.1741. [DOI] [PubMed] [Google Scholar]
- 63.Vedantam A, Jirjis MB, Schmit BD, et al. Diffusion tensor imaging and tractography in brown-sequard syndrome. Spinal Cord. 2012 Dec;50(12):928–930. doi: 10.1038/sc.2012.94. [DOI] [PubMed] [Google Scholar]
- 64.Rajasekaran S, Kanna RM, Karunanithi R, Shetty AP. Diffusion tensor tractography demonstration of partially injured spinal cord tracts in a patient with posttraumatic Brown Sequard syndrome. J Magn Reson Imaging. 2010 Oct;32(4):978–981. doi: 10.1002/jmri.22320. [DOI] [PubMed] [Google Scholar]
- 65.Endo T, Suzuki S, Utsunomiya A, Uenohara H, Tominaga T. Prediction of neurological recovery using apparent diffusion coefficient in cases of incomplete spinal cord injury. Neurosurgery. 2011 Feb;68(2):329–336. doi: 10.1227/NEU.0b013e3182031ce7. [DOI] [PubMed] [Google Scholar]
- 66.Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005 Jun 13;486(4):373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 67.Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 68.Blight AR, Decrescito V. Morphometric analysis of experimental spinal cord injury in the cat: the relation of injury intensity to survival of myelinated axons. Neuroscience. 1986 Sep;19(1):321–341. doi: 10.1016/0306-4522(86)90025-4. [DOI] [PubMed] [Google Scholar]
- 69.Harrison BM, McDonald WI. Remyelination after transient experimental compression of the spinal cord. Ann Neurol. 1977 Jun;1(6):542–551. doi: 10.1002/ana.410010606. [DOI] [PubMed] [Google Scholar]
- 70.Potter K, Saifuddin A. Pictorial review: MRI of chronic spinal cord injury. Br J Radiol. 2003 May;76(905):347–352. doi: 10.1259/bjr/11881183. [DOI] [PubMed] [Google Scholar]
- 71.Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol. 2008 Nov;29(10):1976–1982. doi: 10.3174/ajnr.A1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang Y, Jung TD, Yoo DS, Hyun JK. Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma. 2010 Nov;27(11):2033–2040. doi: 10.1089/neu.2009.1265. [DOI] [PubMed] [Google Scholar]
- 73.Petersen JA, Wilm BJ, von Meyenburg J, et al. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma. 2012 May 20;29(8):1556–1566. doi: 10.1089/neu.2011.2027. [DOI] [PubMed] [Google Scholar]
- 74.Freund P, Schneider T, Nagy Z, et al. Degeneration of the injured cervical cord is associated with remote changes in corticospinal tract integrity and upper limb impairment. [Accessed [March 1, 2013]];PLoS One. 2012 7(12):e51729. doi: 10.1371/journal.pone.0051729. http://www.ncbi.nlm.nih.gov/pubmed/23251612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006 Nov-Dec;6(6 Suppl):190S–197S. doi: 10.1016/j.spinee.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 76.Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007 Jan;60(1 Supp1 1):S35–41. doi: 10.1227/01.NEU.0000215383.64386.82. [DOI] [PubMed] [Google Scholar]
- 77.Ono K, Ota H, Tada K, Yamamoto T. Cervical myelopathy secondary to multiple spondylotic protrusions: a clinicopathologic study. Spine. 1977;2(2):109–125. [Google Scholar]
- 78.Song T, Chen WJ, Yang B, et al. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011 Mar;20(3):422–428. doi: 10.1007/s00586-010-1587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kara B, Celik A, Karadereler S, et al. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology. 2011 Aug;53(8):609–616. doi: 10.1007/s00234-011-0844-4. [DOI] [PubMed] [Google Scholar]
- 80.Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011 Feb;21(2):426–433. doi: 10.1007/s00330-010-1927-z. [DOI] [PubMed] [Google Scholar]
- 81.Kerkovsky M, Bednarik J, Dusek L, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine. 2012 Jan 1;37(1):48–56. doi: 10.1097/BRS.0b013e31820e6c35. [DOI] [PubMed] [Google Scholar]
- 82.Lee JW, Kim JH, Park JB, et al. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol. 2011 Dec;40(12):1543–1551. doi: 10.1007/s00256-011-1161-z. [DOI] [PubMed] [Google Scholar]
- 83.Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol. 2012 Feb;34(2):471–478. doi: 10.3174/ajnr.A3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uda T, Takami T, Tsuyuguchi N, et al. Assessment of Cervical Spondylotic Myelopathy using Diffusion Tensor MRI Parameter at 3.0 Tesla. Spine. 2012 Mar 1;38(5):407–414. doi: 10.1097/BRS.0b013e31826f25a3. [DOI] [PubMed] [Google Scholar]
- 85.Wieshmann U, Symms M, Parker G, et al. Diffusion tensor imaging demonstrates deviation of fibres in normal appearing white matter adjacent to a brain tumour. J Neurol Neurosurg Psychiatry. 2000 Apr;68(4):501–503. doi: 10.1136/jnnp.68.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price S, Burnet N, Donovan T, et al. Diffusion tensor imaging of brain tumours at 3 T: A potential tool for assessing white matter tract invasion? Clin Radiol. 2003;58(6):455–462. doi: 10.1016/s0009-9260(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 87.Witwer BP, Moftakhar R, Hasan KM, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg. 2002;97(3):568–575. doi: 10.3171/jns.2002.97.3.0568. [DOI] [PubMed] [Google Scholar]
- 88.Vargas MI, Delavelle J, Jlassi H, et al. Clinical applications of diffusion tensor tractography of the spinal cord. Neuroradiology. 2008 Jan;50(1):25–29. doi: 10.1007/s00234-007-0309-y. [DOI] [PubMed] [Google Scholar]
- 89.Ducreux D, Lepeintre JF, Fillard P, Loureiro C, Tadie M, Lasjaunias P. MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol. 2006 Jan;27(1):214–216. [PMC free article] [PubMed] [Google Scholar]
- 90.Setzer M, Murtagh RD, Murtagh FR, et al. Diffusion tensor imaging tractography in patients with intramedullary tumors: comparison with intraoperative findings and value for prediction of tumor resectability. J Neurosurg Spine. 2010;13(3):371–380. doi: 10.3171/2010.3.SPINE09399. [DOI] [PubMed] [Google Scholar]
- 91.Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999 May 12;52(8):1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 92.van Hecke W, Nagels G, Emonds G, et al. A diffusion tensor imaging group study of the spinal cord in multiple sclerosis patients with and without T2 spinal cord lesions. J Magn Reson Imaging. 2009 Jul;30(1):25–34. doi: 10.1002/jmri.21817. [DOI] [PubMed] [Google Scholar]
- 93.Roser F, Ebner F, Maier G, Tatagiba M, Nägele T, Klose U. Fractional anisotropy levels derived from diffusion tensor imaging in cervical syringomyelia. Neurosurgery. 2010 Oct;67(4):901–905. doi: 10.1227/NEU.0b013e3181ecfcdd. [DOI] [PubMed] [Google Scholar]
- 94.Hatem SM, Attal N, Ducreux D, et al. Assessment of spinal somatosensory systems with diffusion tensor imaging in syringomyelia. J Neurol Neurosurg Psychiatry. 2009 Dec;80(12):1350–1356. doi: 10.1136/jnnp.2008.167858. [DOI] [PubMed] [Google Scholar]
- 95.Renoux J, Facon D, Fillard P, Huynh I, Lasjaunias P, Ducreux D. MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. AJNR Am J Neuroradiol. 2006 Oct;27(9):1947–1951. [PMC free article] [PubMed] [Google Scholar]
- 96.Thurnher MM, Law M. Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magn Reson Imaging Clin N Am. 2009 May;17(2):225–244. doi: 10.1016/j.mric.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Wheeler-Kingshott CA, Hickman SJ, Parker GJ, et al. Investigating cervical spinal cord structure using axial diffusion tensor imaging. Neuroimage. 2002 May;16(1):93–102. doi: 10.1006/nimg.2001.1022. [DOI] [PubMed] [Google Scholar]
- 98.Bastin ME, Armitage PA, Marshall I. A theoretical study of the effect of experimental noise on the measurement of anisotropy in diffusion imaging. Magn Reson Imaging. 1998 Sep;16(7):773–785. doi: 10.1016/s0730-725x(98)00098-8. [DOI] [PubMed] [Google Scholar]
- 99.Carballido-Gamio J, Xu D, Newitt D, Han ET, Vigneron DB, Majumdar S. Single-shot fast spin-echo diffusion tensor imaging of the lumbar spine at 1.5 and 3 T. Magn Reson Imaging. 2007 Jun;25(5):665–670. doi: 10.1016/j.mri.2006.10.005. [DOI] [PubMed] [Google Scholar]