SUMMARY
MRI remains the backbone of measuring disease burden and treatment response in individuals with malignant gliomas. Traditional radiographic approaches, however, are largely limited to depicting anatomic changes and are not a direct measure of disease burden. For example, contrast enhancement is related to blood–brain barrier integrity rather than actual tumor size. Without accurate measures of disease, common clinical dilemmas include ‘pseudo-progression’ (e.g., after chemoradiation) or ‘pseudo-response’ (e.g., with steroid treatment and antiangiogenic agents), which can lead to delays in therapy, premature discontinuation of successful treatments and to unnecessary surgical procedures. This overview focuses on novel, minimally invasive approaches in the area of imaging and blood-based biomarkers that aim to more accurately determine disease status and response to treatment in malignant brain tumors.
Practice Points.
Contrast-enhanced MRI is suboptimal for assessment of disease burden and response to therapy in malignant gliomas as it cannot reliably identify tumor burden and differentiate between active tumor and treatment effect.
Further developments of MRI (diffusion-tensor imaging, diffusion-weighted images, perfusion and magnetic resonance spectroscopy) appear promising; however, they need to be evaluated in larger prospective studies before their actual clinical value will be known.
Blood-based biomarkers are being investigated as potential adjunct tests in clinical decision-making. The research to date is still at an early stage and a promising blood-based biomarker has yet to be identified and prospectively validated.
The development of better minimally or non-invasive techniques to assess disease status and response to therapy should be one of the highest priorities in neuro-oncology as it would accelerate clinical development of new treatments that are urgently required for these devastating malignancies.
Assessment of disease burden and response to therapy is particularly challenging in patients with malignant gliomas owing to the infiltrative growth and complex anatomy of these cancers. Currently, the most commonly used technique for detecting these tumors has remained contrast-enhanced MRI. In individuals unable to obtain MRI scans, contrast-enhanced computed tomography is often used. Traditional contrast-based imaging, however, is dependent on changes in blood–brain barrier (BBB) integrity that is frequently altered with the multimodality treatment that patients with these cancers commonly receive. Treatment-related changes often cannot be reliably differentiated from actual tumor growth, leading to the well-described phenomena of ‘pseudo-progression’ (e.g., after chemoradiation) and ‘pseudo-response’ (e.g., with steroids and bevacizumab) [1]. It has been described that pseudo-progression is more commonly found in glioblastomas with methylated than with unmethylated MGMT promoters [2]. Nevertheless, it frequently requires repeat surgery to provide clarity on disease status and to differentiate treatment effect from active disease, and pathology has remained the gold standard for assessment of disease activity. The need for improved methods to measure disease and assess response to treatment has been highlighted by the development of brain cancer-specific response criteria that integrate neurological status, steroid dose and noncontrast-enhancing disease in the assessment [3,4]. These criteria, although an improvement, highlight the need for the development of further minimally invasive or noninvasive approaches to accurately determine disease status and tumor burden. This review provides an overview of several emerging concepts that are aimed at more accurate measurement of disease and that, if validated, could help streamline treatment decisions and avoid unnecessary repeat resections in patients. This overview is focused on new approaches in imaging, the current status in the search for a blood-based biomarker for malignant gliomas and imaging techniques that can detect disease-specific molecular alterations within these cancers.
Imaging biomarkers in gliomas
Gadolinium-enhanced MRI: the current state of the art of response assessment in malignant gliomas
Gadolinium-enhanced MRI is the current gold standard for diagnosis and monitoring of high-grade gliomas. Enhancement is specific to disruption in the BBB but not to active tumor. Contrast agents that extravasate into the brain tissue alter T1, T2 and/or T2* relaxation rates of tissues that can be detected by MRI imaging as contrast enhancement [5]. Contrast MRI is unable to differentiate active tumor from tumor necrosis or so-called treatment effect after radiation. Abnormal contrast enhancement is seen in up to 40% of the first scans after radiation therapy, and it is believed to be due to increased vessel permeability, a phenomenon known as pseudo-progression [1]. Alterations to the BBB due to the effects of steroids and antiangiogenic drugs (i.e., bevacizumab) can further complicate the picture. In order to bring uniformity to radiographic response assessment in these tumors, brain cancer-specific response criteria have been developed. The Macdonald criteria integrated clinical status as well as steroid dose as part of the assessment (Table 1) [3]. Limitations of these criteria included interobserver variability, inability to measure nonenhancing tumors and problems with measuring enhancing lesions in the walls of cysts and surgical cavities. This led to the development of the Revised Assessment in Neuro-oncology criteria that incorporate the nonenhancing T2W/fluid-attenuated inversion recovery portions of the MRI in order to overcome some of these drawbacks (Table 2) [4]. The utility of changes in areas of T2 has been shown to be a potentially sensitive marker of tumor progression in patients treated with bevacizumab in whom measurement of enhancement is not reliable [6]. These criteria have not yet been prospectively validated.
Table 1. Criteria for response assessment in malignant gliomas: Macdonald criteria.
| Response | Criteria |
|---|---|
| Complete response | Complete disappearance of all enhancing measurable and nonmeasurable disease sustained for at least 4 weeks; no new lesions; no corticosteroids; and stable or clinically improved |
| Partial response | 50% decrease in enhancing lesion volume compared with baseline, sustained for at least 4 weeks; no new lesions with stable or reduced corticosteroid dose; and stable or clinical improvement |
| Stable disease | Does not qualify for complete response, partial response or progression; and clinically stable |
| Progression | 25% increase in enhancing lesion volume or any new lesion or clinical deterioration |
Data taken from [3].
Table 2. Revised Assessment in Neuro-Oncology criteria.
| Criterion | T1 gadolinium-enhancing disease | T2/FLAIR | New lesion | Corticosteroids | Clinical status | Requirement for response |
|---|---|---|---|---|---|---|
| Complete response | None | Stable or improved | None | Off steroids or on physiological replacement doses only | Stable or improved | All |
| Partial response | ≥50% decrease | Stable or improved | None | Stable or decreased | Stable or worse | All |
| Stable disease | <50% decrease or <25% increase | Stable or improved | None | Stable or decreased | Stable or worse | All |
| Progression | ≥25% increase | Increased | Present | NA | Worse | Any |
FLAIR: Fluid-attenuated inversion recovery; NA: Not available.
Data taken from [4].
Novel techniques in MRI
Contrast-enhanced MRI can detect changes in BBB integrity; however, the etiology of the changes often remains unclear. Several novel MRI-based approaches are being designed to add new dimensions to existing imaging protocols. These advances may provide more information on the underlying disease process itself. Some of these techniques are already clinically available and can be ordered as additional sequences to standard brain MRI. However, these approaches have not been formally evaluated in larger prospective studies. The following discussion provides an overview of some of the novel imaging-based methods for monitoring malignant gliomas and the current evidence for their application in clinical practice (Table 3).
Table 3. Frequently performed novel imaging techniques.
| Novel imaging techniques | Concept | Ref. |
|---|---|---|
| DWI | Measures the rate of Brownian motion of water molecules in tissue. Identification of areas of high cellularity/active tumor growth | [7–11] |
| DTI | As a refined form of DWI imaging, DTI helps to visualize white matter tracts. May be helpful in preoperative planning and differentiating tumor growth from pseudo-progression | [12–18] |
| MRI perfusion imaging | Measures tumor vascular permeability. Active tumors tend to have higher relative cerebral blood volumes | [19–21,23–26] |
| Magnetic resonance spectroscopy | Measures tissue metabolic activity. May be helpful in preoperative grading as well as differentiating tumor growth from pseudo-progression | [26–31] |
| PET imaging | Measures biochemical activity of tumor. May be helpful in preoperative grading as well as for assessment of disease progression | [32–37] |
DTI: Diffusion-tensor imaging; DWI: Diffusion-weighted imaging.
Diffusion-weighted images
The apparent diffusion coefficient (ADC) is the rate of water movement measured in mm2/s and is visualized using the diffusion-weighted image sequences with each voxel representing the rate of Brownian motion of water molecules or their diffusion rate in tissue. Increased cellularity is often associated with reduced ADC values. Although ADC values could not reliably predict tumor types in individual cases, lower ADCs suggest a higher grade [7]. Higher ADC values were also reported in 25 patients with MGMT promoter methylation and a linear correlation was demonstrated with progression-free and overall survival [8]. Small studies have suggested ADC values to be helpful in differentiating pseudo-progression from true progression [9–11], but this needs confirmation in larger prospective trials.
Diffusion-tensor imaging
Diffusion-tensor imaging (DTI) is a refined version of diffusion-weighted imaging that visualizes white matter tracts (trachography) utilizing the magnitude and directionality of water diffusion along a vector (eigenvectors) in 3D space. It can improve target volume delineation for image-guided biopsies and help predict the extent of resection, which in turn, can have prognostic value [12,13]. Fractional anisotropy values measured using DTI were shown to be predictors of cellularity in astrocytic tumors, such as glioblastoma, anaplastic astrocytoma and pilocytic astrocytoma [14]. With the incorporation of DTI into intraoperative neuronavigational systems, some groups have demonstrated its utility during tumor resections in eloquent areas. DTI has been used for preoperatively identifying tract projections and association fibers, such as those associated with vision and motor function. A recent study suggested that differences in brain stem fiber tract response to radiation can be detected with DTI and may be a helpful tool in radiation planning [15]. Previous studies suggested that DTI may play a role in the differentiation of pseudo-progression and true tumor growth based on the assumption that recurrent tumors compress or displace the white matter tracts while radiation necrosis leads to destruction of these tracts [16,17]. However, more recent studies have failed to confirm these findings [18].
MRI perfusion
MRI perfusion provides information about tumor blood volume and vascular permeability by measuring the relative cerebral blood volume (rCBV). rCBV can be calculated using dynamic contrast enhancement and dynamic susceptibility contrast (DSC) methods. DSC is more commonly used clinically, and relies on T2* signal intensity changes that occur with passage of contrast agents through the tissue to generate rCBV maps [19]. Relationships between rCBV and tumor vascularity, VEGF expression, tumor grade cellular density and proliferation, as well as time to progression, have been demonstrated [12,20,21]. In addition, a study of 65 patients with high-grade gliomas showed that mitotic activity and endothelial proliferation rates correlated with the rCBV (p < 0.01) [22]. Another study found that rCBV of 1.5 or higher was associated with a higher grade and also reduced survival in a cohort of 46 consecutive patients [23]. It is thought that the relationship between rCBV and survival could be explained by higher tumor grade [24]. New evidence suggests that quantitative parameters such as 10% maximum values of the transfer coefficient (also called K trans-T2*:10%) derived from pretreatment T2*-weighted dynamic perfusion MR scans may be a potential predictive biomarker. A small study with 39 patients demonstrated that high maximum transfer coefficient values had an independent statistical relationship with low survival in high-grade glioma patients [25]. Larger studies are required to further validate these associations and to define the role of MRI perfusion in high-grade gliomas.
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) adds to the information provided by conventional MRI by measuring the metabolic activity in the imaging area of interest. Apart from providing presurgical guidance regarding tumor grade, MRS is increasingly used as an adjunct to try to differentiate true progression from treatment effect, however, this has not yet been validated prospectively. Spectral patterns or specific metabolite intensities can be compared with traditional MRI to identify changes in spectra from adjacent voxels or to obtain a distribution pattern of a particular metabolite within the tumor. Standard brain proton MRS uses proton and measures choline (assessment of membrane turnover and proliferation), creatine (energy homeostasis), N-acetyl aspartate (glioneural structures) and lactate or lipids (necrosis) [26]. A direct correlation between choline and cellular proliferation has been shown [27–29]. In a blinded prospective study with 14 patients, detection of new choline accumulation was shown to suggest residual or recurrent tumor after treatment, especially if a pretreatment choline level was available [30]. Glutamine peaks are detected more frequently in tumors than in controls, and a rise in glutamine peak may relate to a role of glutamate as an excitotoxin in accelerated cell proliferation of malignant brain tumors. Lactate is normally not found in the brain, and its presence indicates anaerobic or nonoxidative metabolism, which is expected to be elevated in areas of recurrent tumor, but it can also be elevated in areas of necrosis and abscess [31]. Detection of new choline accumulation may indicate residual or recurrent tumor after treatment. Necrotic areas are devoid of any spectroscopic activity and a decrease in the level of choline and modest increases in N-acetyl aspartate indicate decrease in tumor burden [26]. Even though multiple metabolites and their ratios have been studied, to date none have been demonstrated to have sufficient diagnostic specificity to differentiate active tumor from treatment effect. MRS is a promising candidate marker for treatment response assessment, follow-up and early detection of tumor recurrence; however, larger studies are still required to define its role in clinical practice and in clinical trials.
PET
PET scans measure biochemical activity of short-lived positron-emitting radionucleotides (radiotracers). 18F-fluorodeoxyglucose PET (18F-FDG-PET) scans have been used in the evaluation of gliomas since the 1980s and are still frequently performed. Higher uptake of tracer is thought to be present in tumors as compared with surrounding normal tissue or necrosis due to its higher metabolic activity [32]. The ability of FDG-PET scans to grade and prognosticate survival in patients with brain tumors was demonstrated in a retrospective study of 331 patients with brain tumors where over 90% of patients with high uptake were found to have a high-grade glioma and approximately 30% of those patients survived over 12 months and none survived over 5 years [33]. FDG-PET was one of the first tracers that has been studied regarding its ability to differentiate recurrent tumor from radiation necrosis, however, the sensitivity and specificity of this test in this setting was 75 and 81%, respectively [34]. It has been argued that the sensitivity of FDG-PET is lower in the brain than in other organs due to the physiologically higher uptake of 18F-FDG in the brain. Furthermore, it may have limited ability to distinguish residual tumor after therapy owing to its uptake into macrophages in areas with inflammation after radiation [35,36]. Amino acid tracers, such as 11C-methionine, and newer agents, such as 18F-fluroethyltyrosine and 18F-flurothymidine, are promising agents owing to improved specificity due to their low physiological uptake in normal brain tissue. In a small study with 24 patients (five patients with WHO grade III and 19 with WHO grade II gliomas), 11C-methionine PET scans were reported to detect tumor progression with a sensitivity and specificity of over 90% [37]. Another study with 45 patients using 18F-fluoro-ethyl-tyrosine-PET in a similar population was able to achieve a sensitivity of over 90% and specificity of 100% in patients with suspected disease progression based on contrast-enhanced MRI [38]. The specificity of MRI was only approximately 50% in this study.
PET agents can be tailored for specific applications. For example, PET imaging with 18F-arginine–glycine-aspartic acid was used as a surrogate imaging biomarker to detect αvβ3 integrin expression in a randomized clinical trial using the targeted agent cilengitide (integrin-targeted therapy) in patients with newly diagnosed glioblastoma [39–41]. Other potential uses of this technique that are being explored include presurgical tumor grading, biopsy and margin delineation, as well as radiation planning [42–49]. Current studies are clustered around diagnosis at time of presentation, tumor grading, particularly at recurrence. Unfortunately, limited information is available to recommend using it for guiding tumor biopsy or planning local therapy [50]. Differential uptake within the tumor is the key parameter with any radioactive tracer and the selection of an appropriate cutoff value for background activity is crucial to the interpretation of studies utilizing PET scans. At this time small sample sizes and heterogeneous study designs limit our ability to derive straightforward conclusions for adoption of PET scans into routine clinical practice.
Multimodality imaging
Coregistration of images from MRI or CT scans with the metabolic signature of target tissue using PET scans have shown potential in the diagnosis and monitoring of brain tumors [51]. There is an emerging trend towards incorporating MRI and PET scanners to achieve better morphologic as well as metabolic images in brain tumors by avoiding sequential scanning [52,53]. This may be of particular benefit if these techniques are used for high precision biopsies or to monitor very small lesions. Most standard MRI scanners have the ability to incorporate diverse imaging sequences and even MRS with the addition of appropriate software. Hybrid MRI/PET scanners incorporate a specially designed PET detector with good results [53].
Another approach uses currently available MRI voxel-based techniques using serial ADC maps (functional diffusion maps), and rCBV derived from DSC-MRI parametric response map have been described as potential early indicators of survival [54–57]. A strong correlation was demonstrated in the 1-year and overall survival rates with 3-week midtreatment scans that used a composite of ADC and rCBV as analyzed by parametric response map [56]. Such early biomarkers may help clinicians to reassess treatment options in a timely manner, but these techniques are still in the early stages of development.
Selected other novel imaging concepts for malignant gliomas
Studies on the MRI contrast agent ferumoxytol, a novel superparamagnetic iron oxide nanoparticle, pointed towards a potential role in differentiation between tumor progression and pseudo-progression in a group of 19 patients with glioblastoma [58]. Ferumoxytol is a blood pool agent that does not require contrast agent leakage correction for calculation of rCBV. A survival advantage was observed in patients with rCBV less than or equal to 1.75 using ferumoxytol, suggesting pseudo-progression rather than active tumor in these patients. Recent advancements have also enabled development of nanoparticles with engineered physical and chemical properties, including shape, size and surface properties, which can reduce the T2 relaxation time of tumor cells. For example, encouraging results were shown in animal studies with iron-based single-domain antibodies against tumor blood vessel-specific antibodies [59]. Another novel magnetic resonance probe utilizes superparamagnetic iron oxide nanoparticles linked to a short DNA sequence, complementary to the cerebral mRNA of GFAP found in glial cells and astrocytes [60,61]. In addition, studies are under way to image transcription-related events in tumors cells using MRI scans. ‘Transcription MRI’ techniques use mRNA-linked radiotracers that bind to intracellular mRNA in tumor cells with high specificity. A recent report of self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine in rat models also shows promise in tracking as few as 1000 neural stem cells [62]. These small and hypothesis-generating studies will certainly require further validation in larger prospective studies.
Amide proton transfer scans are another novel MRI-based imaging technique that detects endogenous mobile proteins and peptides in tissue via saturation of the amide protons in their peptide bonds. Tumor cells proliferate rapidly and have higher content of proteins and peptides than radiation necrosis as demonstrated in rat glioma models that had been treated with radiation [63,64]. A study in humans is currently ongoing [65].
In summary, there are several novel imaging techniques that are being developed to provide more disease-specific assessment of disease status and tumor burden in malignant gliomas. However, contrast-enhanced MRI still remains the current standard imaging technique in clinical practice and in therapeutic clinical trials. Carefully designed larger, prospective trials are required to identify the actual value of these individual novel imaging techniques.
Blood-based tumor markers in malignant gliomas
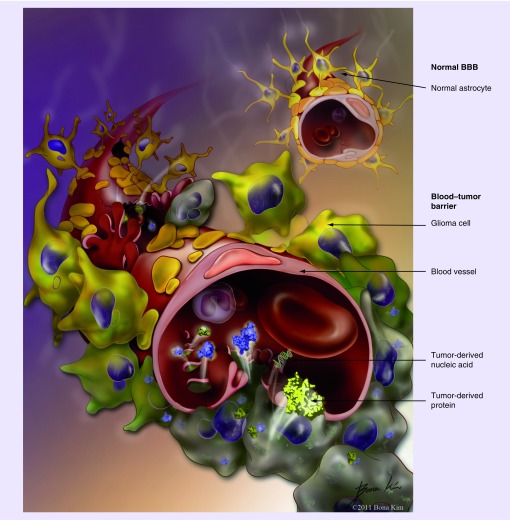
Over the past years there has been an increasing interest in the search for blood-based tumor markers for malignant gliomas, which is in part a result of the limitations of the currently available imaging studies as detailed above. A valid blood-based tumor marker would serve as an adjunct in clinical decision-making if it was sufficiently sensitive and disease specific and if it accurately reflected changes in tumor burden. As an example, it could function as a ‘tie breaker’ when the patient‘s disease status is unclear based on ambiguity of the imaging findings (e.g., pseudo-progression). If imaging showed findings concerning for progression versus treatment effect, an increase in the marker levels (compared with baseline) would suggest progression, whereas a decrease of the marker level would be more consistent with treatment-related changes. A complicating factor in the search for a blood-based biomarker for malignant gliomas is certainly the BBB. As evidenced by contrast enhancement on MRI, however, the BBB is commonly altered and more permeable in high-grade gliomas which leads to shedding of cellular components of the tumor (Figure 1). As blood-based tumor markers in other diseases, the marker may not need to be ‘perfect‘, i.e., have 100% sensitivity and specificity, in order to be clinically meaningful. Examples of such clinically relevant, but ‘suboptimal’ markers that are currently in use are PSA in prostate cancer, CEA in colorectal and CA-125 in ovarian cancer.
Figure 1. Blood–brain barrier versus blood–tumor barrier.
Schematic illustration of the differences between the normal BBB (upper right) and the altered BBB in high-grade gliomas (blood–tumor barrier; lower left). It is hypothesized that cells, proteins and nucleic acids ‘leak’ through gaps in the blood–tumor barrier into the blood stream.
BBB: Blood–brain barrier.
The following overview focuses on three tumor-derived blood-based biomarker classes that are evaluated in malignant gliomas: circulating tumor cells, circulating nucleic acids and circulating proteins. Other circulating markers that are not directly derived from the malignant cells themselves, including circulating hematogenic progenitor cells and circulating endothelial cells, have been investigated and may also be useful for response to certain treatments, however, they are not part of this review that focuses on markers that are comprised of components of the tumor itself.
Circulating tumor cells
CTCs have been detected and are under clinical development in a variety of solid tumors, including lung, breast and prostate cancer [66]. CTCs have not yet been successfully isolated in patients with malignant gliomas, although there is biological evidence that CTCs exist in these cancers. Although rarely seen in clinical practice, there are numerous reports in the literature of metastatic glioblastomas and gliosarcomas, indicating that hematogenous tumor dissemination of these cancers does occur, reportedly in approximately 0.4–0.5% of cases [67–71]. In addition, to date there have been 17 reports of organ transplant-related glioblastoma multiforme (GBM) transmission in patients [72,73]. Recipients of organs from donors with GBM grew metastatic GBM, proving that GBM cells must have been present in the distant organs at time of transplant and that they presumably made their way through the bloodstream as CTCs.
Circulating nucleic acids
Circulating tumor DNA is a particularly attractive tumor marker with a significant amount of promising data in other solid tumors including colorectal and breast cancer [74–76]. Tumor-specific mutations can be detected even in minimal concentrations in plasma using novel ‘digital’ PCR-based methods that differentiate mutant tumor-derived DNA from wild-type DNA in the blood of patients. Techniques including BEAMing and personalized analysis of rearranged ends can detect and quantify tumor-derived DNA with a sensitivity of up to one in 10,000 and 100,000, respectively [77,78]. Two pilot studies described that DNA with tumor-specific mutations can be detected in patients with gliomas. One study showed detection of mutated IDH-1 DNA in plasma of patients with IDH-1-positive gliomas [79]. The other recently published report demonstrated detectability of EGFRvIII-mutated DNA in plasma of patients with glioblastomas using personalized analysis of rearranged ends analysis. Presence or absence of EGFRvIII in plasma matched that in tissue [80]. In addition to circulating genetic alterations, there have been several reports on the detectability of methylated DNA in blood of patients with gliomas with reported sensitivities of 67–83% and specificity of 75–100% [81–83]. The results were, however, not quantitative. In addition to DNA, there have been some early data on miRNA that was detected in blood of patients with GBM. In a screening study of 20 patients with GBM compared with 20 age-matched controls, there were two miRNAs that appeared significantly altered in GBM, miR-128 that was upregulated and miR-342–3p that was downregulated [84]. Another study indentified miRNA-21 as a potentially promising marker for GBM [85].
Circulating proteins
The third group of tumor-derived candidate markers for malignant gliomas are circulating proteins. Many of the proteins that have been investigated as potential glioma biomarkers were initially found as potential markers of hypoxic or traumatic brain injury. The major limitation of proteins as candidate markers for gliomas is their lack of specificity, and to date no truly glioma-specific protein marker has been identified. Protein-based markers that have previously been studied in malignant gliomas can be grouped into three categories: glial- or neuronal-specific markers (e.g., GFAP), proangiogenic proteins (e.g., EGFR, VEGF and β-FGF) and inflammatory markers (e.g., TGF-B and MMP-9). The most extensively studied protein as a potential circulating marker for malignant gliomas is GFAP. Several studies showed that GFAP is detectable in most glioblastomas and to lesser degree in lower grade gliomas [86–89]. A study that showed a significant increase in plasma GFAP levels within 1–2 days after resection, irrespective of tumor grade, illustrates that GFAP is not a truly specific marker for gliomas but rather a marker of brain injury [88]. An exploratory investigation of eight circulating proteins (GFAP, BDNF, GDNF, PGF, S100B, secretagogin, IL-8 and Neuropeptide Y) in 105 patients with various brain tumors assessed, concluded that none of the investigated markers was suitable to substitute histological diagnosis, but that there was a statistically significant association of GFAP with a diagnosis of GBM [89].
In addition to analyzing plasma, there has been a growing interest in the potential utility of circulating microvesicles in patients with malignant gliomas. Microvesicles are small, membrane-bound compartments that are shed in large quantities from glioblastomas into the circulation. These vesicles contain, in part, tumor-derived molecules and may give indirect information on tumor biology, providing a potentially noninvasive way to assess for presence or absence of certain molecular changes in the tumor that may be important as molecular targets or a prerequisite for enrollment in certain clinical trials [90,91].
It appears likely that the BBB (or the altered blood–tumor barrier) limits the amount of the respective molecules that are shed into the bloodstream. This raises the question of whether, instead of blood, cerebrospinal fluid may be the more appropriate compartment to test for tumor-specific biomarkers and for changes in their levels over time. This work clearly needs to be carried out; however, a major barrier is the need for nontherapeutic lumbar punctures that could provide an additional burden for patients.
In summary, these preliminary observations show that glioma-specific blood-based markers do exist and they can in part be detected and quantified with novel analytical methods. However, to date there has been no candidate marker that appears clearly superior and ready for further development and clinical use. Further work needs to be performed to more systematically screen for candidate markers that deserve further validation in larger data sets and eventually in prospective clinical trials. Further technological advances are required to enhance sensitivity and additional comprehensive, high-throughput screening studies will be necessary to identify disease-specific molecular alterations that can be exploited as cancer-specific biomarkers.
Conclusion
Despite recent advances and the introduction of novel therapies, survival of patients with malignant gliomas has remained poor and these cancers have remained incurable. Contrast MRI, the current standard imaging modality for these tumors, cannot reliably determine tumor burden or differentiate active tumor from treatment effect. More robust markers of disease burden and response to therapy are urgently required, both for standard therapy and for clinical trials. This is of ever greater importance due to the increased pace at which novel treatment approaches are introduced into and tested in clinical practice. Interesting and hypothesis-generating data exist for novel imaging techniques and blood-based markers for assessment of disease burden; however, the stage of development of these markers is still early. To date, there has been no imaging technique for malignant gliomas that proved to be superior to contrast MRI. However, several promising novel imaging techniques are actively being tested in trials to determine their clinical utility. Research on blood-based biomarkers is still in its infancy and to date there has been no marker or marker class identified that appears to be the most promising candidate for further development.
In summary, there exist several promising approaches for disease monitoring in malignant gliomas; however, a more comprehensive evaluation of these techniques within larger prospective studies is urgently required. We argue that development of novel biomarkers of disease burden and treatment response should be among the highest priorities in neuro-oncology. Superior methods to measure disease would accelerate the development of new treatment paradigms and directly impact patient care.
Future perspective
It is anticipated that some of the imaging techniques mentioned in this article will at least, in part, be validated in larger clinical trials and a fraction of these will find their place in standard clinical practice. There will be more clarity on which circulating tumor markers deserve further evaluation, and it is likely that multimodality assessment of disease burden (standard plus novel imaging plus tumor biomarkers) will enhance our current standard of care that largely relies on MRI. This would lead to more prudent clinical decision-making, improved patient outcomes and more rapid implementation of new treatment paradigms.
Acknowledgements
The authors wish to thank B Kim for the illustration in Figure 1.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:▪ of interest ▪▪ of considerable interest
- 1.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]; ▪Provides a thorough overview of the phenomenon and mechanism of so-called pseudo-progression in malignant gliomas.
- 2.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 2008;26(13):2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]; Describes an association between MGMT promoter methylation and a higher rate of pseudo-progression in patients with newly diagnosed glioblastoma.
- 3.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for Phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]; ▪Describes the so-called Revised Assessment in Neuro-Oncology criteria, response criteria for treatment of high-grade gliomas that are now widely used in clinical trials.
- 5.Zeng QS, Li CF, Liu H, Zhen JH, Feng DC. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int. J. Radiat. Oncol. Biol. Phys. 2007;68(1):151–158. doi: 10.1016/j.ijrobp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Hattingen E, Jurcoane A, Daneshvar K, et al. Quantitative T2 mapping of recurrent glioblastoma under bevacizumab improves monitoring for non-enhancing tumor progression and predicts overall survival. Neuro Oncol. 2013 doi: 10.1093/neuonc/not105. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am. J. Neuroradiol. 2001;22(6):1081–1088. [PMC free article] [PubMed] [Google Scholar]
- 8.Romano A, Calabria LF, Tavanti F, et al. Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: correlation with MGMT promoter methylation status. Eur. Radiol. 2013;23(2):513–520. doi: 10.1007/s00330-012-2601-4. [DOI] [PubMed] [Google Scholar]
- 9.Chan YL, Yeung DK, Leung SF, Chan PN. Diffusion-weighted magnetic resonance imaging in radiation-induced cerebral necrosis. Apparent diffusion coefficient in lesion components. J. Comput. Assist. Tomogr. 2003;27(5):674–680. doi: 10.1097/00004728-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am. J. Neuroradiol. 2004;25(2):201–209. [PMC free article] [PubMed] [Google Scholar]
- 11.Asao C, Korog Y, Kitajima M, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am. J. Neuroradiol. 2005;26(6):1455–1460. [PMC free article] [PubMed] [Google Scholar]
- 12.Price SJ, Jena R, Burnet NG, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am. J. Neuroradiol. 2006;27(9):1969–1974. [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullah KG, Lubelski D, Nucifora PG, Brem S. Use of diffusion tensor imaging in glioma resection. Neurosurg. Focus. 2013;34(4):E1. doi: 10.3171/2013.1.FOCUS12412. [DOI] [PubMed] [Google Scholar]
- 14.Beppu T, Inoue T, Shibata Y, et al. Measurement of fractional anisotropy using diffusion tensor MRI in supratentorial astrocytic tumors. J. Neurooncol. 2003;63(2):109–116. doi: 10.1023/a:1023977520909. [DOI] [PubMed] [Google Scholar]
- 15.Uh J, Merchant TE, Li Y, et al. Differences in brainstem fiber tract response to radiation: a longitudinal diffusion tensor imaging study. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(2):292–297. doi: 10.1016/j.ijrobp.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat. Rev. Clin. Oncol. 2009;6(11):648–657. doi: 10.1038/nrclinonc.2009.150. [DOI] [PubMed] [Google Scholar]
- 17.Kashimura H, Inoue T, Beppu T, Ogasawara K, Ogawa A. Diffusion tensor imaging for differentiation of recurrent brain tumor and radiation necrosis after radiotherapy–three case reports. Clin. Neurol. Neurosurg. 2007;109(1):106–110. doi: 10.1016/j.clineuro.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Kumar S, Narang J, et al. Morphologic MRI features, diffusion tensor imaging and radiation dosimetric analysis to differentiate pseudo-progression from early tumor progression. J. Neurooncol. 2013;112(3):413–420. doi: 10.1007/s11060-013-1070-1. [DOI] [PubMed] [Google Scholar]
- 19.Maia AC, Jr, Malheiros SM, da Rocha AJ, et al. Stereotactic biopsy guidance in adults with supratentorial nonenhancing gliomas: role of perfusion-weighted magnetic resonance imaging. J. Neurosurg. 2004;101(6):970–976. doi: 10.3171/jns.2004.101.6.0970. [DOI] [PubMed] [Google Scholar]
- 20.Law M, Yang S, Babb JS, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am. J. Neuroradiol. 2004;25(5):746–755. [PMC free article] [PubMed] [Google Scholar]
- 21.Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247(2):490–498. doi: 10.1148/radiol.2472070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadeghi N, Salmon I, Decaestecker C, et al. Stereotactic comparison among cerebral blood volume, methionine uptake, and histopathology in brain glioma. AJNR Am. J. Neuroradiol. 2007;28(3):455–461. [PMC free article] [PubMed] [Google Scholar]
- 23.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected] AJNR Am. J. Neuroradiol. 2004;25(2):214–221. [PMC free article] [PubMed] [Google Scholar]
- 24.Mills SJ, Soh C, O‘Connor JP, et al. Tumour enhancing fraction (EnF) in glioma: relationship to tumour grade. Eur. Radiol. 2009;19(6):1489–1498. doi: 10.1007/s00330-008-1288-z. [DOI] [PubMed] [Google Scholar]
- 25.Sanz-Requena R, Revert-Ventura A, Marti-Bonmati L, Alberich-Bayarri A, Garcia-Marti G. Quantitative MR perfusion parameters related to survival time in high-grade gliomas. Eur. Radiol. 2013 doi: 10.1007/s00330-013-2967-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Fan G. Comments and controversies: magnetic resonance spectroscopy and gliomas. Cancer. Imaging. 2006;6:113–115. doi: 10.1102/1470-7330.2006.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am. J. Neuroradiol. 2003;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu H, Kumabe T, Shirane R, Yoshimoto T. Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. AJNR Am. J. Neuroradiol. 2000;21(4):659–665. [PMC free article] [PubMed] [Google Scholar]
- 29.Tamiya T, Kinoshita K, Ono Y, Matsumoto K, Furuta T, Ohmoto T. Proton magnetic resonance spectroscopy reflects cellular proliferative activity in astrocytomas. Neuroradiology. 2000;42(5):333–338. doi: 10.1007/s002340050894. [DOI] [PubMed] [Google Scholar]
- 30.Rabinov JD, Lee PL, Barker FG, et al. In vivo 3-T MR spectroscopy in the distinction of recurrent glioma versus radiation effects: initial experience. Radiology. 2002;225(3):871–879. doi: 10.1148/radiol.2253010997. [DOI] [PubMed] [Google Scholar]
- 31.Fulham MJ, Bizzi A, Dietz MJ, et al. Mapping of brain tumor metabolites with proton MR spectroscopic imaging: clinical relevance. Radiology. 1992;185(3):675–686. doi: 10.1148/radiology.185.3.1438744. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J. Nucl. Med. 2005;46(12):1948–1958. [PubMed] [Google Scholar]
- 33.Padma MV, Said S, Jacobs M, et al. Prediction of pathology and survival by FDG PET in gliomas. J. Neurooncol. 2003;64(3):227–237. doi: 10.1023/a:1025665820001. [DOI] [PubMed] [Google Scholar]
- 34.Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int. J. Cancer. 2001;96(3):191–197. doi: 10.1002/ijc.1016. [DOI] [PubMed] [Google Scholar]
- 35.Brock CS, Young H, O‘Reilly SM, et al. Early evaluation of tumour metabolic response using [18F] fluorodeoxyglucose and positron emission tomography: a pilot study following the Phase II chemotherapy schedule for temozolomide in recurrent high-grade gliomas. Br. J. Cancer. 2000;82(3):608–615. doi: 10.1054/bjoc.1999.0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T, Nishizawa S, Maruyama I, et al. Acute effects of stereotactic radiosurgery on the kinetics of glucose metabolism in metastatic brain tumors: FDG PET study. Ann. Nucl. Med. 2001;15(2):103–109. doi: 10.1007/BF02988599. [DOI] [PubMed] [Google Scholar]
- 37.Ullrich RT, Kracht L, Brunn A, et al. Methyl-L-11C-methionine PET as a diagnostic marker for malignant progression in patients with glioma. J. Nucl. Med. 2009;50(12):1962–1968. doi: 10.2967/jnumed.109.065904. [DOI] [PubMed] [Google Scholar]
- 38.Rachinger W, Goetz C, Popperl G, et al. Positron emission tomography with O-(2-[18F] fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57(3):505–511; discussion 505–511. doi: 10.1227/01.neu.0000171642.49553.b0. [DOI] [PubMed] [Google Scholar]
- 39.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin. Investig. Drugs. 2008;17(8):1225–1235. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS. Micro-PET imaging of alphavbeta3-integrin expression with 18F-labeled dimeric RGD peptide. Mol. Imaging. 2004;3(2):96–104. doi: 10.1162/15353500200404109. [DOI] [PubMed] [Google Scholar]
- 41.Schnell O, Krebs B, Carlsen J, et al. Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol. 2009;11(6):861–870. doi: 10.1215/15228517-2009-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman S, Levivier M, Pirotte B, et al. Regional glucose metabolism and histopathology of gliomas. A study based on positron emission tomography-guided stereotactic biopsy. Cancer. 1996;78(5):1098–1106. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1098::AID-CNCR21>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 43.Kaschten B, Stevenaert A, Sadzot B, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J. Nucl. Med. 1998;39(5):778–785. [PubMed] [Google Scholar]
- 44.Grosu AL, Weber WA, Riedel E, et al. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005;63(1):64–74. doi: 10.1016/j.ijrobp.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 45.Pauleit D, Stoffels G, Schaden W, et al. PET with O-(2–18F-Fluoroethyl)-L-Tyrosine in peripheral tumors: first clinical results. J. Nucl. Med. 2005;46(3):411–416. [PubMed] [Google Scholar]
- 46.Pirotte B, Goldman S, Massager N, et al. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J. Nucl. Med. 2004;45(8):1293–1298. [PubMed] [Google Scholar]
- 47.Pirotte BJ, Levivier M, Goldman S, et al. Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery. 2009;64(3):471–481; discussion 481. doi: 10.1227/01.NEU.0000338949.94496.85. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka Y, Nariai T, Momose T, et al. Glioma surgery using a multimodal navigation system with integrated metabolic images. J. Neurosurg. 2009;110(1):163–172. doi: 10.3171/2008.4.17569. [DOI] [PubMed] [Google Scholar]
- 49.la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13(8):806–819. doi: 10.1093/neuonc/nor054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nihashi T, Dahabreh IJ, Terasawa T. PET in the clinical management of glioma: evidence map. AJR Am. J. Roentgenol. 2013;200(6):W654–W660. doi: 10.2214/AJR.12.9168. [DOI] [PubMed] [Google Scholar]
- 51.Heiss WD, Raab P, Lanfermann H. Multimodality assessment of brain tumors and tumor recurrence. J. Nucl. Med. 2011;52(10):1585–1600. doi: 10.2967/jnumed.110.084210. [DOI] [PubMed] [Google Scholar]
- 52.Heiss WD. The potential of PET/MR for brain imaging. Eur. J. Nucl. Med. Mol. Imaging. 2009;36(Suppl. 1):S105–S112. doi: 10.1007/s00259-008-0962-3. [DOI] [PubMed] [Google Scholar]
- 53.Boss A, Bisdas S, Kolb A, et al. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. J. Nucl. Med. 2010;51(8):1198–1205. doi: 10.2967/jnumed.110.074773. [DOI] [PubMed] [Google Scholar]
- 54.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc. Natl Acad. Sci. USA. 2005;102(15):5524–5529. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galban CJ, Chenevert TL, Meyer CR, et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat. Med. 2009;15(5):572–576. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galban CJ, Chenevert TL, Meyer CR, et al. Prospective analysis of parametric response map-derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin. Cancer Res. 2011;17(14):4751–4760. doi: 10.1158/1078-0432.CCR-10-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsien C, Galban CJ, Chenevert TL, et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J. Clin. Oncol. 2010;28(13):2293–2299. doi: 10.1200/JCO.2009.25.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology. 2013;266(3):842–852. doi: 10.1148/radiol.12111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomanek B, Iqbal U, Blasiak B, et al. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neuro Oncol. 2012;14(1):53–63. doi: 10.1093/neuonc/nor183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu CH, You Z, Ren J, Kim YR, Eikermann-Haerter K, Liu PK. Noninvasive delivery of gene targeting probes to live brains for transcription MRI. FASEB J. 2008;22(4):1193–1203. doi: 10.1096/fj.07-9557com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu CH, Ren JQ, You Z, et al. Noninvasive detection of neural progenitor cells in living brains by MRI. FASEB J. 2012;26(4):1652–1662. doi: 10.1096/fj.11-199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thu MS, Bryant LH, Coppola T, et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat. Med. 2012;18(3):463–467. doi: 10.1038/nm.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reson. Med. 2003;50(6):1120–1126. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 64.Zhou J, Tryggestad E, Wen Z, et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat. Med. 2011;17(1):130–134. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blakeley JO, Ye X, Lim M, et al. The role of amide proton transfer imaging in detecting active malignant glioma. J. Clin. Oncol. 2011;29 Abstract 2024. [Google Scholar]
- 66.Armstrong AJ, Eisenberger MA, Halabi S, et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur. Urol. 2012;61(3):549–559. doi: 10.1016/j.eururo.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lun M, Lok E, Gautam S, Wu E, Wong ET. The natural history of extracranial metastasis from glioblastoma multiforme. J. Neurooncol. 2011;105(2):261–273. doi: 10.1007/s11060-011-0575-8. [DOI] [PubMed] [Google Scholar]; Reviews literature related to metastasis from glioblastoma.
- 68.Beauchesne P. Letter to the editor: the natural history of extra-cranial metastasis from glioblastoma multiform. J. Neurooncol. 2012;109(3):593–594. doi: 10.1007/s11060-012-0921-5. author reply 595. [DOI] [PubMed] [Google Scholar]
- 69.Pasquier B, Pasquier D, N‘Golet A, Panh MH, Couderc P. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer. 1980;45(1):112–125. doi: 10.1002/1097-0142(19800101)45:1<112::aid-cncr2820450121>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 70.Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. J. Neurosurg. 1969;31(1):50–58. doi: 10.3171/jns.1969.31.1.0050. [DOI] [PubMed] [Google Scholar]
- 71.Smith DR, Hardman JM, Earle KM. Contiguous glioblastoma multiforme and fibrosarcoma with extracranial metastasis. Cancer. 1969;24(2):270–276. doi: 10.1002/1097-0142(196908)24:2<270::aid-cncr2820240210>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 72.Armanios MY, Grossman SA, Yang SC, et al. Transmission of glioblastoma multiforme following bilateral lung transplantation from an affected donor: case study and review of the literature. Neuro Oncol. 2004;6(3):259–263. doi: 10.1215/S1152851703000474. [DOI] [PMC free article] [PubMed] [Google Scholar]; Includes an overview of previously reported cases of organ transplant-related glioblastoma transmission.
- 73.Healey PJ, Davis CL. Transmission of tumours by transplantation. Lancet. 1998;352(9121):2–3. doi: 10.1016/S0140-6736(98)22027-7. [DOI] [PubMed] [Google Scholar]
- 74.Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008;14(9):985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holdhoff M, Schmidt K, Donehower R, Diaz LA., Jr Analysis of circulating tumor DNA to confirm somatic KRAS mutations. J. Natl Cancer Inst. 2009;101(18):1284–1285. doi: 10.1093/jnci/djp240. [DOI] [PubMed] [Google Scholar]
- 76.Higgins MJ, Jelovac D, Barnathan E, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin. Cancer Res. 2012;18(12):3462–3469. doi: 10.1158/1078-0432.CCR-11-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat. Methods. 2006;3(7):551–559. doi: 10.1038/nmeth898. [DOI] [PubMed] [Google Scholar]
- 78.Leary RJ, Kinde I, Diehl F, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci. Transl. Med. 2010;2(20):20ra14. doi: 10.1126/scitranslmed.3000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boisselier B, Perez-Larraya JG, Rossetto M, et al. Detection of IDH1 mutation in the plasma of patients with glioma. Neurology. 2012;79(16):1693–1698. doi: 10.1212/WNL.0b013e31826e9b0a. [DOI] [PubMed] [Google Scholar]
- 80.Salkeni MA, Zarzour A, Ansay TY, et al. Detection of EGFRvIII mutant DNA in the peripheral blood of brain tumor patients. J. Neurooncol. 2013 doi: 10.1007/s11060-013-1209-0. [DOI] [PubMed] [Google Scholar]
- 81.Balana C, Ramirez JL, Taron M, et al. O6-methyl-guanine-DNA methyltransferase methylation in serum and tumor DNA predicts response to 1,3-bis(2-chloroethyl)-1-nitrosourea but not to temozolamide plus cisplatin in glioblastoma multiforme. Clin. Cancer Res. 2003;9(4):1461–1468. [PubMed] [Google Scholar]
- 82.Weaver KD, Grossman SA, Herman JG. Methylated tumor-specific DNA as a plasma biomarker in patients with glioma. Cancer Invest. 2006;24(1):35–40. doi: 10.1080/07357900500449546. [DOI] [PubMed] [Google Scholar]
- 83.Lavon I, Refael M, Zelikovitch B, Shalom E, Siegal T. Serum DNA can define tumor-specific genetic and epigenetic markers in gliomas of various grades. Neuro Oncol. 2010;12(2):173–180. doi: 10.1093/neuonc/nop041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roth P, Wischhusen J, Happold C, et al. A specific miRNA signature in the peripheral blood of glioblastoma patients. J. Neurochem. 2011;118(3):449–457. doi: 10.1111/j.1471-4159.2011.07307.x. [DOI] [PubMed] [Google Scholar]
- 85.Ilhan-Mutlu A, Wagner L, Wohrer A, et al. Blood alterations preceding clinical manifestation of glioblastoma. Cancer Invest. 2012;30(9):625–629. doi: 10.3109/07357907.2012.725443. [DOI] [PubMed] [Google Scholar]
- 86.Jung CS, Foerch C, Schanzer A, et al. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain. 2007;130(Pt 12):3336–3341. doi: 10.1093/brain/awm263. [DOI] [PubMed] [Google Scholar]
- 87.Brommeland T, Rosengren L, Fridlund S, Hennig R, Isaksen V. Serum levels of glial fibrillary acidic protein correlate to tumour volume of high-grade gliomas. Acta Neurol. Scand. 2007;116(6):380–384. doi: 10.1111/j.1600-0404.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 88.Husain H, Savage W, Grossman SA, et al. Pre- and post-operative plasma glial fibrillary acidic protein levels in patients with newly diagnosed gliomas. J. Neurooncol. 2012;109(1):123–127. doi: 10.1007/s11060-012-0874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ilhan-Mutlu A, Wagner L, Widhalm G, et al. Exploratory investigation of eight circulating plasma markers in brain tumor patients. Neurosurg. Rev. 2012;36(1):45–55, discussion 55–56. doi: 10.1007/s10143-012-0401-6. [DOI] [PubMed] [Google Scholar]
- 90.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noerholm M, Balaj L, Limperg T, et al. RNA expression patterns in serum microvesicles from patients with glioblastoma multiforme and controls. BMC Cancer. 2012;12:22. doi: 10.1186/1471-2407-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]