Harris and collaborators show that neutropenia results in increased formation of plasma cells and elevated antibody production.
Abstract
Prolonged infections or adjuvant usage can trigger emergency granulopoiesis (EG), leading to dysregulation in neutrophil blood counts. However, the impact of EG on T and B cell function remains largely unknown. In this study, to address this question, we used a mouse model of neutropenia and studied immune activation after adjuvant administration. The initial neutropenic state fostered an environment of increased dendritic cell activation and T cell–derived IL-17 production. Interestingly, neutropenic lysozyme 2–diphtheria toxin A mice exhibited striking EG and amplified neutrophil recruitment to the lymph nodes (LNs) that was dependent on IL-17–induced prostaglandin activity. The recruited neutrophils secreted a B cell–activating factor that highly accelerated plasma cell generation and antigen-specific antibody production. Reduction of neutrophil functions via granulocyte colony-stimulating factor neutralization significantly diminished plasma cell formation, directly linking EG with the humoral immune response. We conclude that neutrophils are capable of directly regulating T cell–dependent B cell responses in the LN.
Neutrophils are an important innate immune cell type in first-line defense against pathogens such as bacteria and viruses (Rogers and Unanue, 1993; Appelberg, 2007). Neutrophils rapidly respond to inflammatory stimuli with effector functions such as phagocytosis, bacterial killing, and neutrophil extracellular trap formation (Brinkmann et al., 2004; Soehnlein and Lindbom, 2010). Neutrophil innate effector functions additionally include production of inflammatory cytokines such as TNF (Cassatella, 1995), degranulation (Borregaard et al., 2007), the production of reactive oxygen species (Leto and Geiszt, 2006), and the secretion of antimicrobial peptides (Mócsai, 2013).
During an inflammatory response, neutrophils perform innate effector functions before undergoing apoptosis, resulting in neutrophil consumption. If the demand for neutrophils is not met, steady-state granulopoiesis is switched to emergency granulopoiesis (EG) or reactive granulopoiesis. The latter is defined by an increase of serum granulocyte CSF (G-CSF), de novo generation of mature neutrophils in the BM, and an increased abundance of circulating myeloid progenitors. The overall objective of such EG is thus to maintain sufficient peripheral neutrophil numbers (Manz and Boettcher, 2014).
In addition to live infections, EG can be induced using heat-killed microorganisms, either alone or in adjuvant formulations (Kwak et al., 2015) and even during sterile inflammation (Manz and Boettcher, 2014). The use of adjuvants, such as CFA, is well established in the induction of adaptive T and B cell responses in immune-competent mice and has proven useful in circumventing peripheral tolerance to induce preclinical autoimmunity (Abdul-Majid et al., 2000, 2002, 2003; Svensson et al., 2002; Djerbi et al., 2003).
Although innate immune responses involving neutrophils have been extensively studied (Silva, 2010; Soehnlein and Lindbom, 2010; Mócsai, 2013), the emerging role of neutrophils in regulating adaptive immunity and in particular during EG remains to be fully elucidated. It has been reported that neutrophils migrate to draining LNs (dLNs) and that neutrophils regulate T cell activation (Chtanova et al., 2008; Pelletier et al., 2010; Yang et al., 2010; Brackett et al., 2013; Yang and Unanue, 2013). Although the involvement of neutrophils in mediating B cell responses has traditionally been limited to removal of antibody-opsonized pathogens (Tsuboi et al., 2008), more recent studies have addressed neutrophil support of B cells in the spleen (Cerutti et al., 2012, 2013; Puga et al., 2012). However, whether there is a consequence of elevated neutrophil abundance during EG and whether this type of regulation occurs in dLNs has not been investigated to date.
Using several neutropenic mouse strains and adjuvant-induced EG, we analyzed the mechanisms underlying neutrophil-mediated regulation of B cell activation, subsequent plasma cell formation, neutrophil kinetics, and regulation of adaptive immunity. We found that neutropenia at the time of CFA immunization enhanced DC migration and IL-23 production and potentiated the subsequent state of EG. This state dramatically amplifies IL-17–induced prostaglandin-dependent infiltration of neutrophils into the dLN. Neutrophilia in the dLN was associated with enhanced B cell activity, with the neutrophils localizing close to B cells and plasma cells in the LN and secreting B cell–activating factor (BAFF), fueling increased antibody production. Collectively, these results reveal a hitherto unreported mechanism of neutrophil regulation of B cell activation, plasma cell generation, and antibody production via secreted factors that are up-regulated during EG.
RESULTS
Mice depleted of lysozyme 2–expressing cells are neutropenic
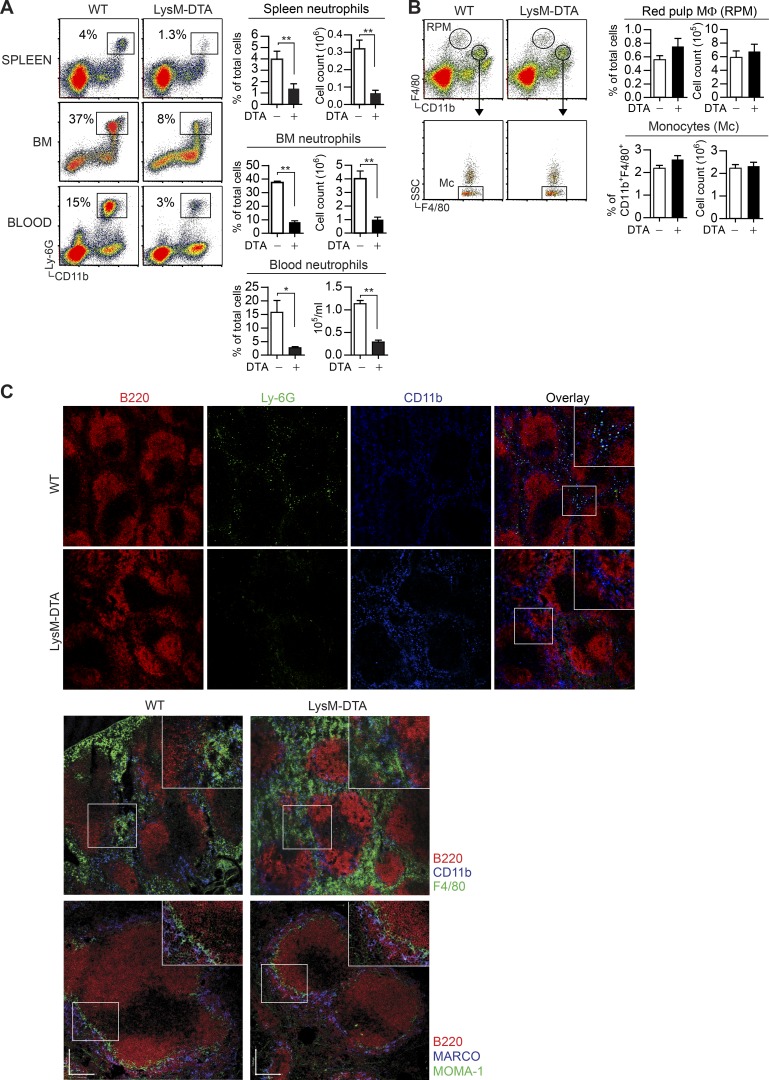
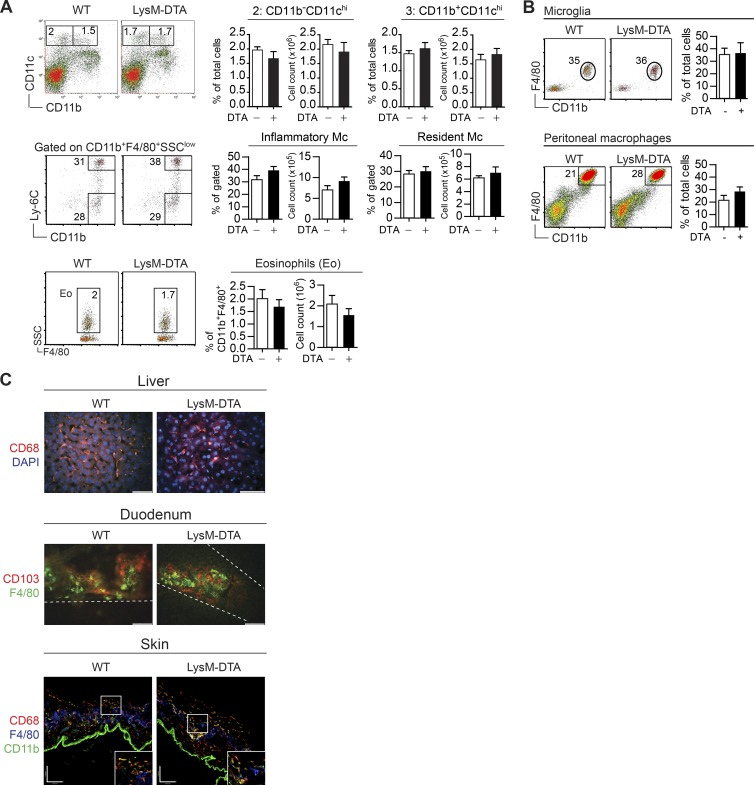
To address the role of neutrophils in the regulation of inflammatory responses, we generated neutropenic mice by crossing lysozyme 2 (LysM)–CRE and ROSA26–diphtheria toxin A (DTA; LysM-DTA mice; Wu et al., 2006). The majority of neutrophils expressed LysM (not depicted), and analyses of the spleen, BM, and blood of LysM-DTA mice demonstrated an 85% reduction in neutrophils compared with WT littermate controls (Fig. 1 A). Because LysM is also expressed in monocytes and macrophages, we assessed whether these subsets were affected in LysM-DTA mice. Analysis of the spleen revealed that monocytes and red pulp macrophages were not altered compared with controls (Fig. 1 B). Immunohistochemical analyses of the spleen in the steady state confirmed a lack of neutrophils (CD11b+Ly6G+) in LysM-DTA mice, whereas numbers of marginal zone macrophages (MARCO+) and metallophillic macrophages (MOMA-1+) were not affected (Fig. 1 C). Additionally, there were no differences in the abundance of splenic DCs, monocyte subsets, or eosinophils (Fig. 2 A). The numbers of resident peritoneal macrophages, brain microglia, and liver, duodenal, and skin macrophages were all unaltered in LysM-DTA mice (Fig. 2, B and C). We did not detect any general increase in apoptosis or necrosis in LysM-DTA mice based on annexin V and dead cell dye staining (not depicted). In summary, LysM-DTA mice represent a largely neutropenic mouse model with an 85% reduction in mature neutrophils without detectable changes in other phagocyte populations.
Figure 1.
LysM-DTA mice display a marked reduction of neutrophils in the steady state without changes in other myeloid cell populations. (A and B) Flow cytometry analysis of the percentage and absolute numbers of spleen, BM, and blood neutrophils (CD11b+Ly6G+; A), as well as splenic red pulp macrophages (RPM; F4/80hi; Mφ) and monocytes (SSClowCD11b+F4/80+; B) in naive WT and LysM-DTA mice. (C) Immunohistochemistry of spleens from naive WT and LysM-DTA mice to visualize neutrophils (CD11b+Ly6G+; top), red pulp macrophages (F4/80+; middle), and MARCO+ and MOMA-1+ macrophages (bottom). Representative data are from a minimum of three independent experiments. n = 4–6/group. Results are mean ± SEM. Immunohistochemistry was performed on four separate mice. Bar,100 µm. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
Figure 2.
Specific neutrophil reduction in LysM-DTA mice. (A) No differences were observed in the numbers of splenic CD11chi DCs (CD11b+ and CD11b−), monocyte (Mc) subsets (inflammatory Ly6C+ and resident Ly6C−), or eosinophils (SSChi). (B) The numbers of tissue macrophages such as brain microglia (CD11b+F4/80+) or peritoneal macrophages (CD11b+F4/80hi) were not altered in LysM-DTA mice. (C) No differences were seen in the numbers of liver (CD68+; bar, 50 μm), duodenum (F4/80+CD103−; bar, 50 μm), or skin (CD68+CD11b+ and F4/80+; bar, 100 μm) macrophages in LysM-DTA mice in comparison to WT mice. Data in A and B are representative of three independent experiments. n = 4–5/group. Results are mean ± SEM. The images in C are representative of three separate mice.
Adjuvant stimulation in LysM-DTA mice induces EG
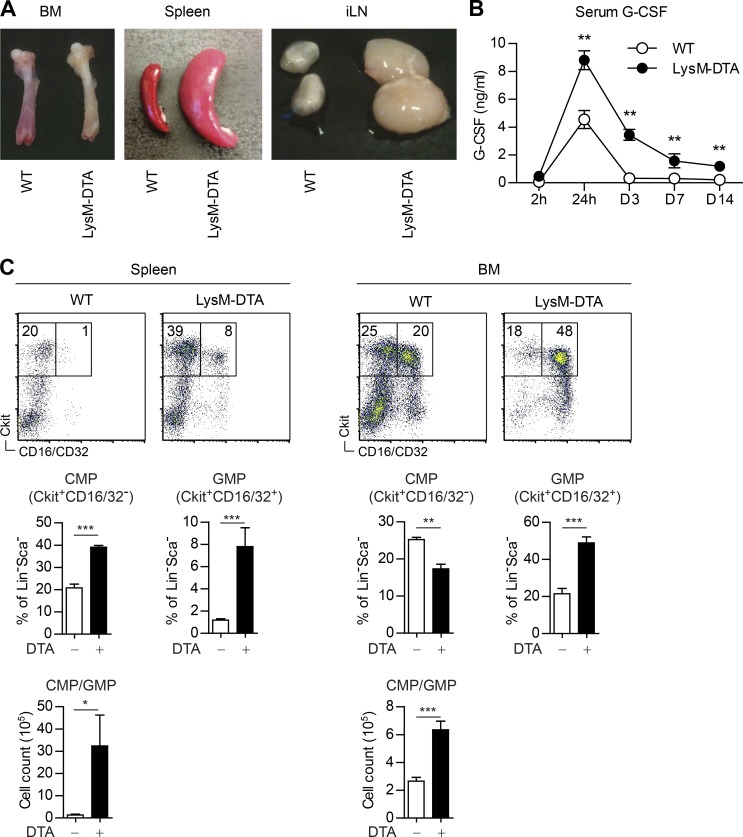
We immunized LysM-DTA mice s.c. with CFA to determine the immunological consequences of the neutropenic state of these mice on this model of induced EG. Dissection of BM, spleen, and draining inguinal LNs (iLNs) at day 7 postimmunization (p.i.) indicated a state of EG with a loss of pigmentation in the femur, splenomegaly, and lymphadenopathy in LysM-DTA compared with WT mice (Fig. 3 A). Furthermore, serum G-CSF levels, which are also linked to EG, were elevated by twofold at 24 h and by 10–20-fold at days 3–14 p.i. in LysM-DTA mice (Fig. 3 B). Another characteristic of this activation state is the increased numbers of common myeloid progenitors (CMPs) and granulocyte–macrophage progenitors (GMPs) in the BM and spleen. Analyses of LysM-DTA spleens revealed a twofold increase in CMPs and an approximately four- to eightfold increase in GMPs in comparison with WT spleens (Fig. 3 C). We also detected a significant increase of GMPs in the BM of LysM-DTA mice compared with WT mice, although we did not detect any progenitors in the iLN. No differences in CMP/GMP populations were observed in either unchallenged LysM-DTA or WT mice (not depicted). CFA immunization thus induced a state of EG in LysM-DTA mice.
Figure 3.
EG is induced in LysM-DTA mice after CFA immunization. (A) BM, spleen, and iLNs 7 d after CFA immunization. (B) Cytometric bead array flow cytometry analysis of serum G-CSF levels in WT and LysM-DTA mice at the indicated time points after CFA immunization. (C) Analysis of CMPs and GMPs in the spleen and BM. Lineage negative (Lin−) is defined by CD11b−CD11c−CD3−NK1.1−B220−F4/80−. The images in A are representative of three independent experiments. n = 4–5/group. Data in B and C are representative of two independent experiments. n = 5/group. Results are mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test).
Neutrophils are recruited to dLNs after adjuvant stimulation
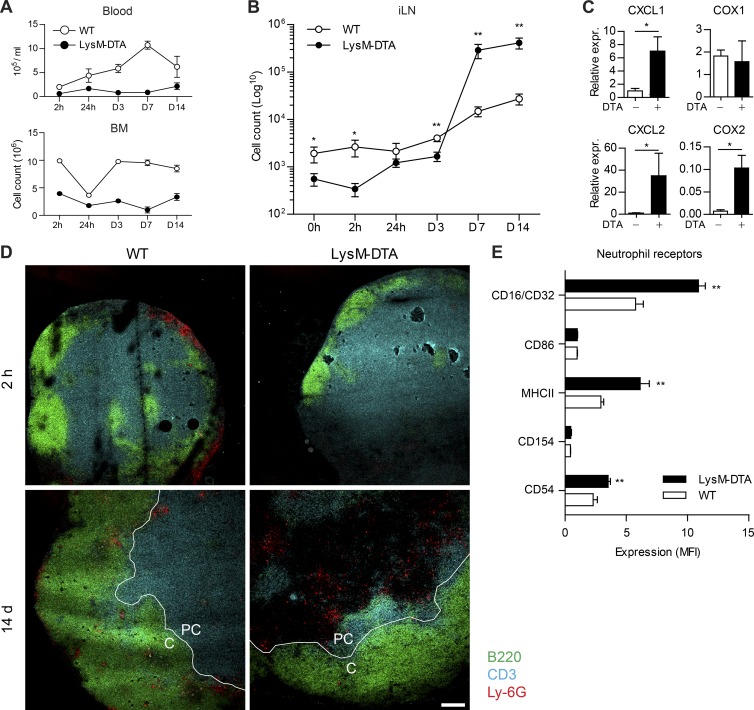
The s.c. application of adjuvants has been previously described to rapidly induce reactive neutrophilia (Manz and Boettcher, 2014). To investigate neutrophil migration after immunization-induced EG, we challenged LysM-DTA and WT mice with CFA and longitudinally analyzed the number of neutrophils in the blood, BM, and draining iLNs from 2 h up to 14 d p.i. Although WT mice exhibited a steady recruitment of neutrophils into the blood that peaked at 7 d p.i., LysM-DTA mice were neutropenic in the blood and BM throughout the 14 d of analysis (Fig. 4 A). Two waves of neutrophil infiltration after immunization have previously been reported (Yang et al., 2010; Yang and Unanue, 2013). Within iLNs from WT mice, neutrophil numbers were increased at 2 h p.i. and further increased steadily between 7 and 14 d p.i. (Fig. 4 B). As expected, neutropenic LysM-DTA mice lacked the early increase but surprisingly displayed a 20-fold higher neutrophil influx into the draining iLN during the later time point compared with WT littermate controls (Fig. 4 B). Because neutrophil numbers were still decreased in the blood and BM in LysM-DTA mice at this time point, we hypothesized that there was a selective recruitment of neutrophils to the iLN caused by a higher expression of neutrophil-attracting factors. In concordance with this, we observed increased expression of KC (CXCL1), MIP-2 (CXCL2), and cyclooxygenase 2 (Cox2) in whole iLNs in LysM-DTA mice (Fig. 4 C). The early absence and then later abundance of neutrophils were confirmed by immunohistochemistry at 2 h and 14 d p.i., respectively (Fig. 4 D). Early neutrophils were observed in the cortex of WT iLNs but were absent in LysM-DTA mice (Fig. 4 D). Neutrophils infiltrating the iLN during the later time point localized to the cortex/paracortex border, and their numbers were increased in LysM-DTA mice (Fig. 4 D). Analysis of receptor expression on infiltrating neutrophils from LysM-DTA and WT mice revealed increased levels of CD16/32, MHC II, and CD54 on LysM-DTA neutrophils, indicating an enhanced activation state (Fig. 4 E). In summary, LysM-DTA mice are characterized by systemic neutropenia in the steady state, but after CFA-induced EG, neutrophils are recruited to draining iLNs.
Figure 4.
Enhanced neutrophil recruitment to the draining iLN of LysM-DTA mice after CFA immunization. (A and B) Flow cytometry analysis of percentages and total numbers of neutrophils (CD11b+Ly6G+) in the blood or BM (A) and in the iLN (B) after s.c. immunization with CFA. (C) Quantitative RT-PCR analysis of CXCL1, CXCL2, COX1, and COX2 expression (expr.) in lymphocytes at day 7 p.i. Expression is normalized to HPRT. (D) Immunohistochemistry visualizing neutrophils (Ly6G+, red) in the cortex (C) or paracortex (PC) of iLNs at 2 h or 14 d p.i. B cells: B220, green; T cells: CD3, blue. (E) Analysis of receptor expression on neutrophils in iLNs at day 7 after CFA. Cells were gated on CD11b+Ly-6G+ cells. MFI, mean fluorescence intensity. Data in A, B, C, and E are representative of three independent experiments. n = 4–5/group. Results are mean ± SEM. The images in D are representative of four separate mice. Bar, 100 µm. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
Increased T cell activation and plasma cell formation after adjuvant stimulation in neutropenic mice
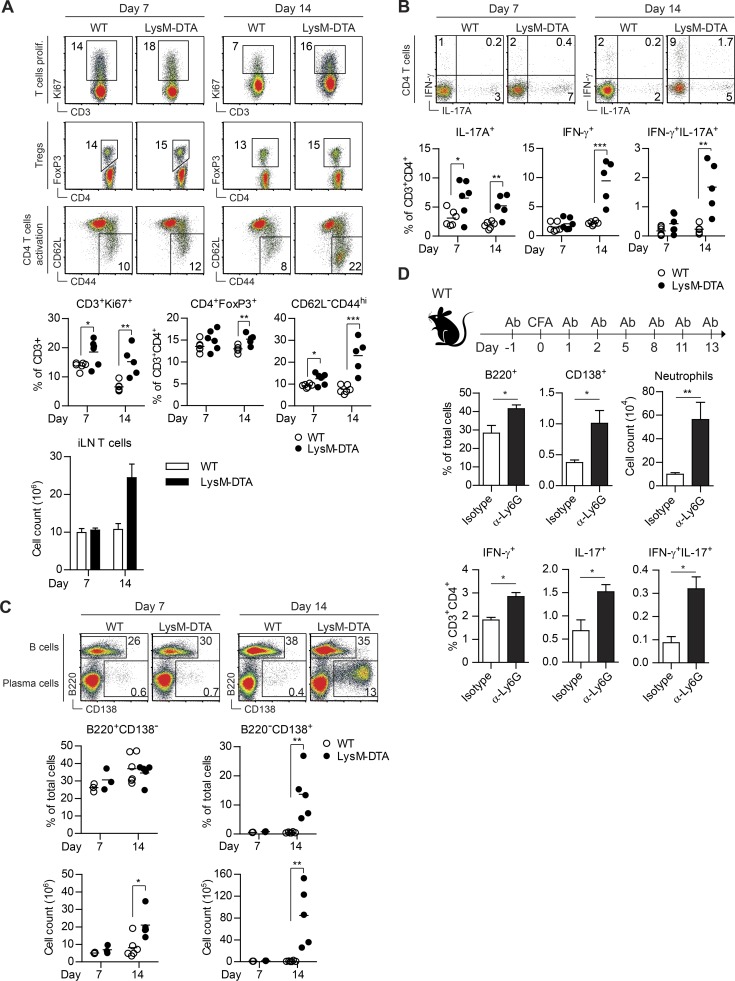
We next investigated whether the selective influx of neutrophils into iLNs impacted T and B cells. We observed an activation of CD4+ T cells in LysM-DTA mice (Fig. 5 A), with significantly increased numbers of IL-17–producing T cells already evident at day 7, coinciding with neutrophil influx (Fig. 5 B). At day 14, there were increased numbers of both IFN-γ– and IL-17–producing T cells (Fig. 5 B). Moreover, we observed a fourfold increase in B220+ B cells and a 25-fold increase in numbers of B220−CD138+ plasma cells in LysM-DTA iLNs at day 14 compared with WT mice (Fig. 5 C). In unchallenged mice, we did not detect any differences in T, myeloid, or B cell subsets in iLN, spleen, or BM (not depicted), indicating that our findings after adjuvant stimulation were not a steady-state phenotype of LysM-DTA mice.
Figure 5.
Profound number of plasma cells and increased IL-17–producing T cells in LysM-DTA mice. (A) The frequency of proliferating (prolif.) T cells (CD3+Ki67+), regulatory T cells (CD3+CD4+FoxP3+; Tregs), and activated CD4 T cells (CD62L−CD44hi) in the iLN from LysM-DTA and WT mice. (B) IL-17– and IFN-γ–producing CD4+ T cells in WT and LysM-DTA iLNs at 7 and 14 d after CFA immunization. (C) Analysis of B cells (B220+CD138−) and plasma cells (B220−CD138+) in the iLN at 7 and 14 d. (D) Analysis of B cells (B220+), plasma cells (B220−CD138+), neutrophils (SSC+CD11b+Ly6Cint), and IFN-γ/IL-17–producing T helper cells (CD3+CD4+) in the iLN in WT mice treated with neutrophil-depleting anti-Ly6G (1A8) or isotype control (2A3) antibodies (Ab). Mice were injected i.p. with 500 µg of antibody on days −1, 1, 2, 5, 8, 11, and 13 and sacrificed for analysis on day 14 p.i. Data in A–C are representative data from three independent experiments. n = 4–5/group. Data in D are representative of two independent experiments. n = 5/group. Results are mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test).
To investigate whether induced depletion of neutrophils could reproduce the phenotype of increased IL-17+ T cell and CD138+ plasma cell numbers, we injected CFA-immunized WT mice with an anti-Ly6G–depleting antibody (1A8) every 2–3 d and analyzed the draining iLN at day 14 p.i. This treatment induced a 40–50% reduction of circulating neutrophils compared with the isotype control antibody-injected animals, and this protocol has been previously used to induce a state of EG (Cain et al., 2011; not depicted). In agreement with our findings using LysM-DTA mice, transient antibody-mediated depletion of neutrophils induced significantly higher subsequent numbers of B220+ B cells, CD138+ plasma cells, and IL-17+ T cells and neutrophilia in the draining iLN, thus replicating the phenotype observed in CFA-immunized LysM-DTA mice (Fig. 5 D).
Collectively, these results indicate that after adjuvant stimulation, the cooperative activity of IL-17–producing CD4+ T cells and infiltrating neutrophils result in an expansion of plasma cells in the iLN of LysM-DTA mice.
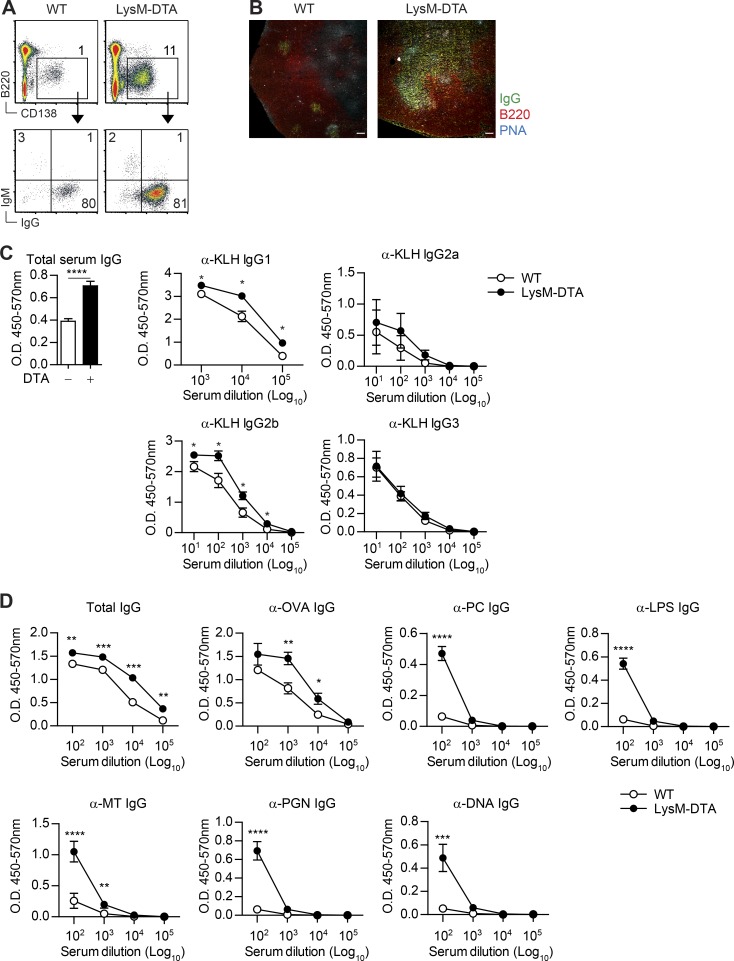
Neutrophil-induced plasma cells are IgG positive and antigen specific
To investigate whether the induced CD138+ cells in the iLN of LysM-DTA mice were indeed bona fide plasma cells and to determine the degree of isotype switching, we performed intracellular staining for IgG and IgM after 14 d p.i. with CFA. The majority of the plasma cells were IgG+, and there were no differences in antibody isotype composition between WT and LysM-DTA plasma cells (Fig. 6 A). However, LysM-DTA iLNs had significantly larger germinal centers as defined by peanut agglutinin (PNA) staining and an increased number of IgG+ cells in the germinal centers of LysM-DTA mice (Fig. 6 B).
Figure 6.
Plasma cells in LysM-DTA iLNs are IgG+ and have higher titers of antigen-specific serum antibodies after immunization. (A) Intracellular staining of iLN plasma cells (B220−CD138+) against IgG and IgM in WT and LysM-DTA iLNs at day14 p.i. (B) Immunofluorescent staining of iLNs to visualize B cell follicles (B220+) and germinal centers (PNA+IgG+). Bars, 100 µm. (C) LysM-DTA and WT mice were immunized with 100 µg KLH in CFA, and serum antibodies were analyzed at day 14. Total serum IgG was diluted 100,000-fold. (D) LysM-DTA and WT mice were immunized with 100 µg OVA in CFA, and serum antibodies were analyzed at day 14. Data in A are representative of three independent experiments. n = 4–5/group. The images in B are representative of four separate mice. Data in C and D are representative of two independent experiments. n = 5/group. Results are mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (unpaired Student’s t test).
To determine whether the plasma cell response could be directed toward a specific antigen, we immunized mice with KLH in CFA and analyzed serum IgG antibody specificity. We recorded significantly elevated IgG1 and IgG2b anti-KLH responses in the serum of LysM-DTA mice compared with WT littermate controls (Fig. 6 C). In contrast, there were no differences in overall Ig titers in sera of unchallenged LysM-DTA and WT mice (not depicted). Furthermore, this was unlikely to be an effect of skewing of the T follicular helper cell compartment because we could not detect any difference in T follicular helper cells in LysM-DTA iLNs compared with WT at 7 d p.i. (not depicted). To confirm that this was an antigen-specific response, we also immunized WT and LysM-DTA mice with OVA in CFA and analyzed serum IgG antibodies toward OVA and a panel of other molecules (Fig. 6 D). We detected high IgG titers specific for OVA but also recorded low IgG antibody titers toward LPS, phosphorylcholine, double-stranded DNA, peptidoglycan, and Mycobacterium tuberculosis. These data indicate that the majority of the expanded B cell clones are specific for the immunized antigen (KLH and OVA) but that other clones also expand to a small extent, a phenomenon that has been previously reported in other inflammatory models (Enoksson et al., 2011). Collectively, we demonstrated that plasma cells in the draining iLN of LysM-DTA mice were IgG producers responsible for elevated antigen-specific responses.
Early neutropenia enhances DC migration and IL-23 production
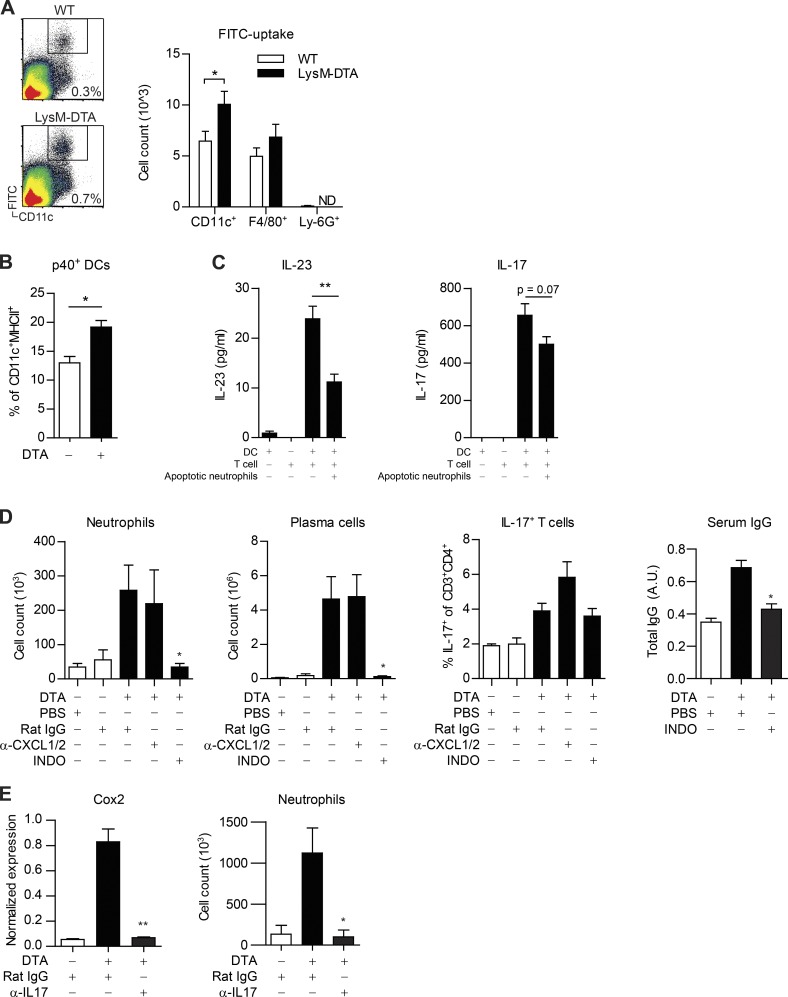
To determine the underlying mechanism responsible for the enhanced T cell activation observed in LysM-DTA iLNs, we analyzed DC migration and activation. It is established that apoptotic neutrophils have an important function in the regulation of tissue macrophage and DC activation through down-regulating IL-23 production (Stark et al., 2005). Analysis of FITC-painted mice revealed increased DC migration to the iLN in LysM-DTA mice compared with WT mice (Fig. 7 A). Furthermore, LysM-DTA CD11c+ DCs in the iLN had increased production of IL-23/IL-12 (p40) in contrast to WT DCs (Fig. 7 B). We set up a DC/T cell in vitro model to test whether apoptotic neutrophils could down-regulate IL-23 production by DCs. As previously reported (Stark et al., 2005), apoptotic neutrophils did indeed down-regulate IL-23 production by DCs, and there was an evident trend of suppressed subsequent IL-17 production by T cells (Fig. 7 C). In summary, LysM-DTA mice exhibited increased DC migration and enhanced DC IL-23 production that in turn could induce IL-17 production by T cells.
Figure 7.
Neutropenia at the time of immunization enhances DC migration and IL-23 production. (A–E) Subsequent IL-17 production enhances LN Cox2 expression that is crucial for late iLN neutrophilia. (A) Analysis of the iLN 24 h after FITC painting. (B) Analysis of intracellular levels of p40 in CD11c+ MHC II+ DCs 24 h after CFA. (C) LPS-stimulated GM-CSF–derived BMDCs were co-cultured with sorted transgenic 2D2 CD4+ T cells and apoptotic neutrophils for 72 h. (D) LysM-DTA mice treated with 5 µg anti-CXCL1 and anti-CXCL2 i.p. every second day after CFA or treated with 40 µg INDO i.p. on days 4, 6, 8, 10, 12, and 14. iLNs were analyzed at day 14 after CFA. A.U., arbitrary units. (E) LysM-DTA mice treated with 100 µg anti–IL-17 antibody i.p. on days 2, 4, and 6. iLNs were analyzed at day 7 after CFA. Data are representative of two independent experiments. n = 5/group. Results are mean ± SEM. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
Plasma cell–inducing neutrophils are recruited by prostaglandins in an IL-17–dependent manner
Gene expression analysis of the iLN indicated that CXCL1, CXCL2, and/or prostaglandins (Cox2) could be responsible for the specific recruitment of neutrophils in LysM-DTA mice. To determine whether these factors were important for the selective recruitment of neutrophils, we either treated mice with neutralizing antibodies against CXCL1 and CXCL2 or used the Cox1/Cox2 inhibitor indomethacin (INDO). Interestingly, anti-CXCL1/2 treatment did not block neutrophil recruitment to the LN or plasma cell formation in LysM-DTA mice compared with isotype-treated LysM-DTA mice, indicating that CXCL1 and CXCL2 are dispensable in our model (Fig. 7 D). However, neutrophil recruitment and plasma cell formation were completely abolished in INDO-treated LysM-DTA mice, indicating that prostaglandins had a crucial role for the observed selective neutrophil recruitment. Analysis of the serum in LysM-DTA mice revealed a decrease in circulating IgG after INDO treatment. T cell IL-17 production was similar in INDO-treated mice to controls, indicating there was ongoing immune activation in the neutropenic mice.
It has been reported that IL-17 can directly up-regulate Cox2 in stromal cells (Stamp et al., 2004; Lemos et al., 2009). We investigated whether neutropenia-induced IL-17 secretion by T cells could induce Cox2 expression in iLNs. Neutralization of IL-17 in LysM-DTA mice significantly reduced Cox2 expression in the iLN to the levels recorded in WT iLNs (Fig. 7 E). Furthermore, neutrophilia observed in LysM-DTA mice at this time point was abrogated by anti–IL-17 treatment.
Collectively, these data demonstrate that in LysM-DTA mice, IL-17 induces Cox2 expression in the iLN and that subsequent prostaglandin production is crucial for neutrophil influx and plasma cell formation.
Neutrophils in LNs produce BAFF and are proximal to plasma cells
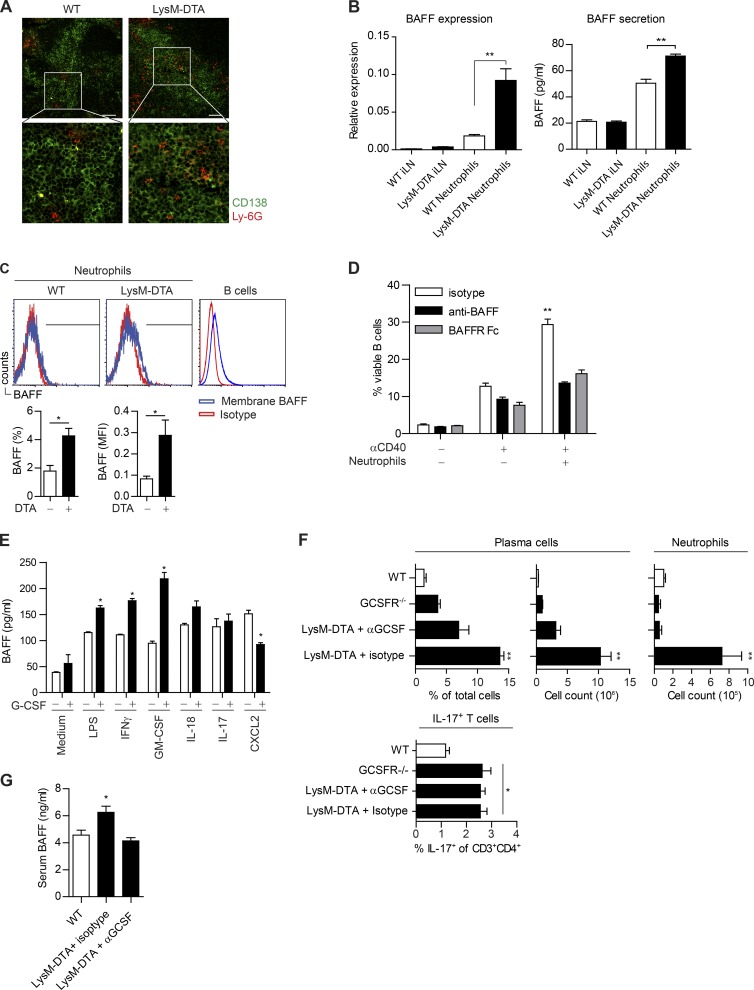
Because LysM-DTA mice had increased numbers of neutrophils and plasma cells in the iLN, our next goal was to determine whether neutrophils in LysM-DTA mice directly regulated B cell activation and plasma cell formation. Immunohistochemical investigations demonstrated that the neutrophils resided close to the plasma cells in the iLN at day 14 (Fig. 8 A).
Figure 8.
Neutrophils are localized close to plasma cells and produce BAFF in a G-CSF–dependent manner. (A) Immunofluorescent staining of iLNs at day 14 after CFA to visualize plasma cells (CD138+; green) and neutrophils (Ly-6G+; red). Bars, 50 µm. (B) Sorted neutrophils (purity >90%) from day 14 iLNs. WT iLNs and LysM-DTA iLNs are the neutrophil-depleted cell fraction. BAFF expression was normalized to HPRT. Measurement of BAFF in supernatants from sorted LysM-DTA and WT iLN neutrophils 14 d p.i. Neutrophils were cultured at a density of 105 cells/well for 24 h. (C) Neutrophils (CD11b+Ly-6G+) were analyzed for membrane-bound BAFF in the iLN at day 14. Isotype mean fluorescence intensity (MFI) was subtracted from BAFF mean fluorescence intensity. B220+ B cells were used as a positive control. (D) B cells were sorted from WT spleen, and neutrophils were sorted from WT BM. 106 B cells were co-cultured at a 1:1 ratio with neutrophils and 30 µg/ml anti-CD40 for 72 h. 5 µg anti-BAFF or 5 µg BAFF receptor (BAFFR) Fc was used to neutralize BAFF. (E) Sorted BM neutrophils (>95% purity) were cultured in a 48-well plate at a density of 5 × 105 cells in 500 µl of medium and stimulated for 6 h with the respective stimuli. (F) Analysis of the presence of plasma cells and neutrophils in iLNs at day 14 p.i. in WT, GCSFR−/−, and LysM-DTA mice treated with an anti–G-CSF neutralizing antibody (67604) or isotype control. Mice were given i.p. injections of 5 µg of antibody on day 0 and every second day until day 14. (G) BAFF-ELISA of serum samples collected at 14 d after CFA in WT and LysM-DTA mice treated with neutralizing anti–G-CSF or rat isotype antibody. Data in A are representative of four separate mice. Data in B–G are representative of two separate experiments. n = 5/group. Results are mean ± SEM. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
To assess neutrophil BAFF production, we sorted neutrophils from day 14 iLN and analyzed both BAFF gene expression and protein secretion compared with the neutrophil-depleted fraction in both WT and LysM-DTA mice. We observed increased BAFF gene expression and protein production in sorted LysM-DTA neutrophils compared with sorted WT iLN neutrophils (Fig. 8 B). Furthermore, the mean expression and secretion of BAFF was low in the neutrophil-depleted iLN cell fraction (Fig. 8 B, LysM-DTA iLN and WT iLN). This provides indirect evidence that the observed high numbers of neutrophils in our in vivo model are a significant source of BAFF. In addition to being secreted, BAFF can also be presented in a membrane-bound form measurable using flow cytometry. Similar to the increase in BAFF secretion, we could detect an increase in membrane-bound BAFF expression on LysM-DTA neutrophils at day 14 (Fig. 8 C).
To determine whether BAFF released from neutrophils could directly affect B cell activation and survival, we co-cultured neutrophils with B cells and measured B cell viability after CD40 stimulation in vitro. B cells cultured with neutrophils exhibited an almost threefold increased viability in comparison with B cells alone (Fig. 8 D). This increase was diminished when anti-BAFF or BAFF receptor Fc chimera protein was added to the co-culture.
In summary, we demonstrated that neutrophils migrated to iLNs, where they localized close to plasma cells. Furthermore, neutrophils from LysM-DTA iLNs exhibited a higher secretion of BAFF that could directly increase B cell viability.
Neutralization of G-CSF reduces plasma cell formation and serum BAFF levels
G-CSF has been suggested to be an important growth factor for neutrophils to produce and store intracellular BAFF, which is released upon secondary stimulation (Scapini et al., 2008). To determine whether secondary triggering could activate BAFF release, we sorted WT BM neutrophils and stimulated them for 6 h with G-CSF together with GM-CSF, IL-17, IFN-γ, LPS, IL-18, or CXCL2. The release of BAFF by neutrophils was enhanced by G-CSF when combined with GM-CSF, IFN-γ, or LPS (Fig. 8 E). Consequently, we expected that neutralizing G-CSF should limit the B cell helper functions of neutrophils. We therefore treated LysM-DTA mice with an anti–G-CSF antibody, which indeed limited plasma cell development and neutrophil influx in the draining iLN compared with isotype-treated LysM-DTA mice (Fig. 8 F). Furthermore, this result could be reproduced in G-CSF receptor–deficient mice (GCSFR−/−; Fig. 8 F). Importantly, IL-17 production by T cells was not altered in either anti–G-CSF–treated LysM-DTA mice or G-CSFR−/− mice (Fig. 8 F). Analysis of sera from anti–G-CSF–treated LysM-DTA mice revealed normalization of circulating BAFF to WT levels (Fig. 8 G).
In summary, we demonstrated that G-CSF stimulates neutrophils to produce increased levels of BAFF, which is released upon secondary stimulation by cytokines produced locally in the LN. Neutralization of G-CSF in vivo after immune activation limited the circulating levels of BAFF as well as the excessive formation of plasma cells in LysM-DTA mice.
DISCUSSION
Neutrophils are the predominant circulating blood leukocyte in humans and are primarily regarded as having immune activities during innate immune responses. We generated a severely neutropenic mouse and used it to study several aspects of adaptive immune functionality. LysM-DTA mice were neutropenic in the steady state. However, after proinflammatory immune activation, EG was induced, and a selective recruitment of the remaining neutrophil pool into draining iLNs was observed. This significantly amplified neutrophil influx peaked at 7 d p.i. and exceeded the numbers characteristic of the late neutrophil wave observed in WT mice. The neutrophil influx was associated with an increased number of IL-17–producing CD4+ T cells and a significant expansion of plasma cells with subsequent antibody production and was dependent on the actions of IL-23, IL-17, Cox2, and G-CSF. This highlights a previously unreported role of neutrophils in the regulation of B cell responses, linking innate immune cell activity to the development of an adaptive immune response.
LysM-Cre mice have been widely used to study macrophage functionality (Clausen et al., 1999). Yet, LysM is also highly expressed in neutrophils. Previous studies used a diphtheria toxin receptor system that results in significant loss of macrophage populations as well as neutrophils, and the desirability of a neutrophil-specific depletion model has been highlighted (Mócsai, 2013). In contrast, we used a DTA system, and our extensive characterization of LysM-DTA mice revealed a selective depletion of up to 85% of neutrophils with no significant changes in other myeloid cell populations. We thus concluded that this mouse model could be useful to assess the role of neutrophils in the development of immune responses and addressed this using CFA alone or CFA-antigen immunizations. The most striking finding in these experiments was a greatly elevated activity of B cells and plasma cells in the draining iLN.
We conducted a systematic analysis of both the initial and later-developing immunological events in the draining iLN after immunization. A previous study described a bimodal action of neutrophils, with an immediate and a later phase of activity (Yang et al., 2010). We used early (2 h) and late (day 7–14) time points to study LysM-DTA mice and to compare the effects of both neutrophil depletion and neutralization of G-CSF in WT mice. These experiments indicated that the immunological phenotypes of gene-deleted or manipulated WT mice were essentially identical. We determined that in the absence of neutrophils (i.e., significantly reduced numbers), there was an elevation in the development of IL-17–producing T cells associated with increased IL-23 production by DCs, which agrees with and extends the findings of the increased antigen-specific proliferative activity previously described (Yang et al., 2010; Yang and Unanue, 2013). Similarly, enhancement of induced Th1 responses through neutrophil depletion have recently been reported in both an ear Bacillus Calmette–Guérin immunization model (Kozakiewicz et al., 2013) and in a Leishmania major infection setting (Ribeiro-Gomes et al., 2012).
Despite being severely neutropenic at steady state, LysM-DTA mice had a >20-fold elevated infiltration of neutrophils into draining iLNs by 14 d after CFA immunization, and this was also evident in antibody-mediated neutrophil-depleted WT mice. This scenario is thus reminiscent of an EG response caused by an increased demand for neutrophils during infection postulated to be the result of chemokine amplification within the LN, with neutrophils attracting themselves through both their own chemokine production as well as that being induced in other cells by neutrophil-released cytokines (Chou et al., 2010). Although we had predicted that neutrophil attractant chemokines CXCL1 and CXCL2 would likely cause this effect, as has been previously reported via the action of IL-17 (Onishi and Gaffen, 2010; Brackett et al., 2013; Jin and Dong, 2013), our analyses rather indicated the critical involvement of prostaglandins in this process. Considering that there are such low numbers of circulating neutrophils in LysM-DTA mice, this neutrophil mobilization is thus extremely efficient. Further investigations should address what specific cell type produces the prostaglandins in the LN. It has been reported that stromal fibroblasts in the LN express Cox2 (Kawamura et al., 2013) and that this could be induced by IL-17 (Stamp et al., 2004). It has also been demonstrated that prostaglandins play an important role in neutrophil recruitment to the inflamed joints during rheumatoid arthritis (Lemos et al., 2009).
The infiltrating neutrophils localized close to B cells and plasma cells within the LN and produced both soluble and membrane-bound BAFF upon stimulation with G-CSF in combination with a secondary secretagogue-like signal (Scapini et al., 2008). We were able to reproduce these interactions in vitro, and inhibition of G-CSF in vivo attenuated the observed highly activated plasma cell phenotype. Human neutrophils have similarly been reported to secrete BAFF in a time-dependent manner and to increase levels of membrane-expressed BAFF after G-CSF stimulation, both in vitro and in vivo (Scapini et al., 2003; Assi et al., 2007). Other studies have also demonstrated the capacity of neutrophils to produce B cell cytokines such as BAFF, APRIL, and IL-21 (Scapini et al., 2008; Puga et al., 2012; Coquery et al., 2014; Magri et al., 2014). BAFF/APRIL-mediated interaction between neutrophils and splenic marginal zone B cells permits T cell–independent antibody responses to microbial antigens (Puga et al., 2012), and neutrophils producing BAFFs have been demonstrated to maintain autoantibody production in settings of both autoimmunity and cancer (Roosnek et al., 2009).
Plasmablast formation is linked to both the number of antigen-specific B cells recruited as well as to microenvironmental factors required for their survival (Mohr et al., 2009). The excessive recruitment of neutrophils into the B cell regions of iLNs in LysM-DTA mice would thus provide for an increased induction and proliferation of B cells and subsequent plasma cell antibody production, exactly as we observed. Neutrophil infiltration into the LN cortex has been characterized in a setting of Toxoplasma gondii infection, the neutrophils forming swarms of varying size and duration with coordinated and cooperative migration (Chtanova et al., 2008). Although this behavior is desired in an immune response aimed at eliminating an infective agent, the same cooperative neutrophil behavior in our model could lead to the significant local accumulation of plasmablast-promoting factors, which result in such a significant antibody response.
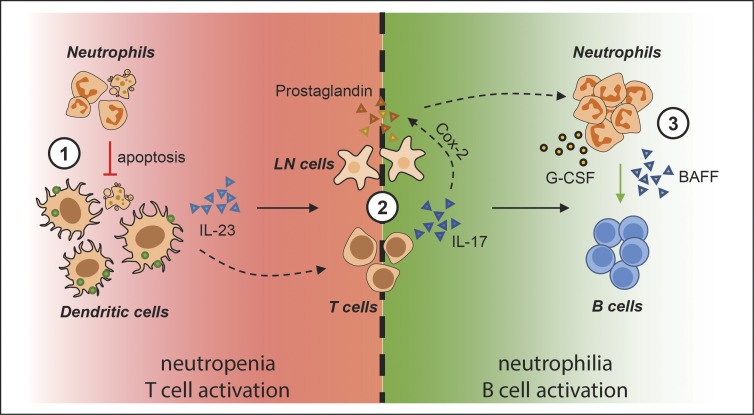
A prolonged lifespan or alternatively a dramatically increased local infiltration of neutrophils (as evident in our study) would increase the efficiency of innate neutrophil functionality, such as elimination of microbes, but will also permit more extensive cross talk with other immune cells. The current view is that neutrophils are capable of such cross talk with a multiplicity of immune cell types including T and B cells, either potentiating or suppressing their activities during both initiation and resolution of inflammation (Mantovani et al., 2011). We conclude that T cell–dependent B cell responses can now also be regarded as being neutrophil regulated. The sequence of events in CFA-immunized LysM-DTA mice (Fig. 9) we propose is thus (a) enhanced DC migration and IL-23 production caused by neutropenia, leading to (b) enhanced activation of IL-17–producing T cells and (c) the sequential IL-17–induced prostaglandin-dependent attraction of neutrophils, which in turn (d) produce an excess of BAFF that (e) efficiently promotes B cell activation and plasma cell formation. As neutrophilia has been demonstrated to adversely affect the efficacy of Bacillus Calmette–Guérin vaccination with respect to Th1 cell–induced activities (Kondratieva et al., 2010), vaccinations aimed at induction of antigen-specific antibody responses might be made more efficient by transient neutrophil depletion at the time of vaccination. However, one would need to ensure that antigen specificity prevails using this protocol, and this will be the focus of future studies.
Figure 9.
Proposed model for neutrophil regulation of T and B cells during EG. (1) Neutropenia at the time of CFA immunization triggers induced EG by enhanced serum G-CSF levels. Lack of apoptotic neutrophils enhances IL-23 production by DCs and increases DC migration to the dLNs. (2) Increased IL-23 production by DCs induces IL-17 production by CD4+ T cells in the LN. IL-17 induces Cox2 expression in stromal LN cells, and the subsequent prostaglandin synthesis by Cox2 specifically recruits neutrophils to the LNs. (3) The recruited neutrophils migrate close to the B cells and with the support of G-CSF produce BAFF that directly promotes B cell activation and survival.
MATERIALS AND METHODS
Mice
All mice were bred and maintained under specific pathogen-free conditions at the Karolinska Institutet. All animal experiments were approved and performed in accordance with national animal care guidelines and ethical permits (local ethical committee, Stockholm North). The R26RDTA, LysM-CRE, GCSFR−/−, and B6.2D2 mice were obtained from The Jackson Laboratory. R26REYFP was a gift from C. Johansson (Karolinska Institutet, Stockholm, Sweden) and was originally obtained from The Jackson Laboratory.
Immunizations and treatments
Mice were s.c. immunized with 100 µl of emulsified CFA (Sigma-Aldrich; 200 µg/mouse M. tuberculosis H37 RA; DIFCO). KLH (100 µg/mouse; Sigma-Aldrich) or OVA (100 µg/mouse; Worthington Biochemical Corporation) was emulsified in CFA and immunized s.c. in a volume of 100 µl/mouse. To deplete neutrophils, mice were i.p. injected with 500 µg of rat anti-Ly6G antibody (1A8; Bio X Cell) or with isotype control rat IgG (2A3; Bio X Cell) in PBS (Sigma-Aldrich). G-CSF neutralization was conducted by i.p. injection of 5 µg of rat anti–G-CSF antibody (67604; R&D Systems). Rat anti-CXCL1 and rat anti-CXCL2 antibodies were injected i.p. at a concentration of 10 µg/mouse. IL-17 neutralization was conducted by i.p. injection of 100 µg/mouse of rat anti–IL-17 antibody (50104; R&D Systems). INDO was used at a concentration of 40 µg/mouse i.p. Isotype rat IgG (R&D Systems) was injected as a control.
Cellular assays and reagents
LN and spleen cell suspensions were obtained by mechanical dissociation of the organs in ice-cold PBS. BM cell suspensions were obtained by dissection of both femurs. Blood cells were obtained by puncturing the retro orbital sinus postmortem. Erythrocyte lysis was performed on blood, spleen, and BM cells with ice-cold ammonium-chloride-potassium (ACK) lysis buffer (Sigma-Aldrich). Cells were counted using the Scepter cell counter (EMD Millipore) with cell discriminator set at 4.8–15 µm. Cells were cultured in complete high glucose DMEM (Sigma-Aldrich) supplemented with 10% FBS (Sigma-Aldrich). Isolation of untouched BM and LN neutrophils was achieved using a magnetic-activated cell-sorting (MACS) neutrophil isolation kit (BM >95% purity, LN >90% purity; Miltenyi Biotec). BMDCs were differentiated with 20 ng/ml GM-CSF (R&D Systems) for 6 d. CD4 T cells were MACS sorted from TCR transgenic 2D2 splenocyte preparations. DC–T cell co-cultures were pulsed with MOG35–55 peptide at a concentration of 30 µg/ml. Neutrophils were defined as being apoptotic (30–40% based on annexin V/potassium iodide staining) after 6–8 h in culture. The following reagents were used at 100 ng/ml: LPS (Sigma-Aldrich), G-CSF, GM-CSF, IFN-γ, IL-17, CXCL2, and IL-18 (R&D Systems). Stimulatory anti-CD40 (1C10; BioLegend) was used at a concentration of 30 µg/ml. Anti-BAFF (AF2106; R&D Systems) or BAFF receptor Fc chimera protein (1357-BR; R&D Systems) was used to neutralize soluble BAFF. FITC (isomer 1 FITC, 1%; Sigma-Aldrich) was dissolved in an acetone/dibutyl phthalate (1:1, vol/vol; Sigma-Aldrich) for contact sensitization solution. Mice were shaved closed to the base of the tail, and 20 µl FITC solution was painted onto the skin. Draining iLNs were dissected 24 h after painting.
Flow cytometry and reagents
Staining procedures were always performed at 4°C except for staining of the blood, which was at room temperature. Dead cells were always excluded using a LIVE/DEAD Fixable Dead Cell Stain kit (Invitrogen). The Annexin V Apoptosis Detection Kit II (BD) was added to determine the apoptosis/necrosis ratio. Forward scatter area and forward scatter time-of-flight were used to remove doublets. Intracellular cytokine staining with an intracellular staining kit (eBioscience) was performed after 4-h treatment with 50 ng/ml PMA (Sigma-Aldrich), 1 µg/ml ionomycin (Sigma-Aldrich), and 1 µl/ml GolgiPlug (BD) in complete DMEM (Sigma-Aldrich). Antibody dilutions were generally 1/100, and 106 cells were stained in a 50-µl volume. The following antibodies were used: B220 (RA3-6B2; BioLegend), BAFF (121808; R&D Systems), CD3 (17A2; BioLegend), CD4 (GK1.5; BioLegend), CD8 (53-6.7; eBioscience), CD11b (M1/70; BioLegend), CD11c (N418; BioLegend), CD16/CD32 (93; BioLegend), CD21 (7E9; BioLegend), CD23 (B3B4; BioLegend), CD44 (IM7; BD), CD54 (YN1/1.7.4; BioLegend), CD62L (MEL-14; eBioscience), CD68 (927; BioLegend), CD86 (GL-1; BioLegend), CD93 (AA4.1; BioLegend), CD138 (281-2; BioLegend), CD154 (MR1; BioLegend), CXCR5 (L138D7; BioLegend), F4/80 (BM8; BioLegend), FoxP3 (FJK-16s; eBioscience), IFN-γ (XMG1.2; BD), IL-17 (TC11-18H10; BD), IgD (11-26c.2a; BioLegend), IgG (STAR120F; AbD Serotec), IgM (RMM-1; BioLegend), Ki67 (B56; BD), Ly-6C (HK1.4; BioLegend), Ly-6G (1A8; BD), MHC II (M5/113.15; BioLegend), NK1.1 (PK136; BD), and PD1 (RMP1-30; BioLegend). Cells were acquired with a flow cytometer (Gallios; Beckman Coulter) and were analyzed by Kaluza software (Beckman Coulter).
Immunofluorescence
Tissue was embedded in optimal cutting temperature medium (TissueTec) and directly snap frozen in isopentane cooled by dry ice. The tissue was then cut into 8- or 14-µm sections by a cryostat and mounted on superfrost glass slides, which were dried for at least 1 h before storage at −70°C. Tissue sections were fixed and permeabilized with ice-cold acetone. The following antibodies were used: B220 (RA3-6B2; BioLegend), CD3 (pc; Abcam), CD68 (FA-11; AbD Serotec), CD103 (2E7; BioLegend), CD138 (281-2; BioLegend), F4/80 (BM8; AbD Serotec), IgG (STAR120F; AbD Serotec), Ly-6G (1A8; BioLegend), MARCO (ED31; AbD Serotec), MOMA1 (Abcam), and PNA (Vector). Tissue was mounted with Prolong Gold mountant (Invitrogen), and images were captured using a confocal scanning microscope (DMI6000; Leica Biosystems) connected to a True Confocal scanner (SP5; Leica Biosystems).
ELISA and cytometric bead array
Cell-free supernatants were analyzed for soluble BAFF using BAFF ELISA (R&D Systems) after neutrophil stimulation. Serum was collected by heart puncture postmortem. Serum antibodies toward KLH or OVA were analyzed with anti–mouse IgG, IgG1, IgG2a, IgG2b, and IgG3 (SouthernBiotech) horseradish peroxidase–coupled antibodies, and the serum was incubated in KLH-, LPS-, peptidoglycan (Sigma-Aldrich)-, phosphorylcholine (Sigma-Aldrich)-, calf thymus DNA (Sigma-Aldrich)–, M. tuberculosis (heat-killed bacteria resuspended in PBS and 0.2-µm filtered)–, or OVA-coated ELISA plates as previously described (Aarntzen et al., 2012). Serum G-CSF was measured using a cytometric flex bead array (BD). Data collection and analysis were performed using a Gallios flow cytometer and FCAP array software (BD).
RT-PCR
For the quantification of gene products, RNA was extracted and reverse transcribed into cDNA as described previously (Parsa et al., 2012). Quantitative RT-PCR was done with specific primer pairs: CXCL1/KC (forward): 5′-CTGGGATTCACCTCAAGAACATC-3′; CXCL1/KC (reverse): 5′-CAGGGTCAAGGCAAGCCTC-3′; CXCL2/MIP-2 (forward): 5′-CCAACCACCAGGCTACAGG-3′; CXCL2/MIP-2 (reverse): 5′-GCGTCACACTCAAGCTCTG-3′; BAFF (forward): 5′-ACACTGCCCAACAATTCCTG-3′; BAFF (reverse): 5′-TCGTCTCCGTTGCGTGAAATC-3′; COX1 (forward): 5′-ATGAGTCGAAGGAGTCTCTCG-3′; COX1 (reverse): 5′-GCACGGATAGTAACAACAGGGA-3′; COX2 (forward): 5′-TGAGCAACTATTCCAAACCAGC-3′; and COX2 (reverse): 5′-GCACGTAGTCTTCGATCACTATC-3′. Gene expression was normalized to that of the gene encoding hypoxanthine-guanine phosphoribosyltransferase (HPRT) for each sample.
Statistical analysis
Statistical significance was assessed using the two-tailed unpaired Student’s t test performed in Prism (version 5; GraphPad Software).
ACKNOWLEDGMENTS
This work was supported by the Swedish Medical Research Council (2014-02087).
The authors declare no competing financial interests.
Author contributions: R. Parsa conceived the idea for this project, designed experiments, performed experiments, analyzed the data, and wrote the paper. H. Lund performed experiments, analyzed the data, and wrote the paper. R.A. Harris designed experiments, analyzed the data, secured funding, and wrote the paper. A.-M. Georgoudaki, X.-M. Zhang, A. Ortlieb Guerreiro-Cacais, A. Warnecke, D. Grommisch, and A.L. Croxford performed experiments and discussed the data. M. Jagodic, B. Becher, and M.C.I. Karlsson designed experiments and discussed the data. All authors contributed to the writing of the paper.
Footnotes
Abbreviations used:
- BAFF
- B cell–activating factor
- CMP
- common myeloid progenitor
- COX
- cyclooxygenase
- dLN
- draining LN
- DTA
- diphtheria toxin A
- EG
- emergency granulopoiesis
- G-CSF
- granulocyte CSF
- GMP
- granulocyte–macrophage progenitor
- HPRT
- hypoxanthine-guanine phosphoribosyltransferase
- iLN
- inguinal LN
- MACS
- magnetic-activated cell sorting
- p.i.
- postimmunization
- PNA
- peanut agglutinin
References
- Aarntzen E.H.J.G., de Vries I.J.M., Göertz J.H., Beldhuis-Valkis M., Brouwers H.M.L.M., van de Rakt M.W.M.M., van der Molen R.G., Punt C.J.A., Adema G.J., Tacken P.J., et al. 2012. Humoral anti-KLH responses in cancer patients treated with dendritic cell-based immunotherapy are dictated by different vaccination parameters. Cancer Immunol. Immunother. 61:2003–2011. 10.1007/s00262-012-1263-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Majid K.-B., Jirholt J., Stadelmann C., Stefferl A., Kjellén P., Wallström E., Holmdahl R., Lassmann H., Olsson T., and Harris R.A.. 2000. Screening of several H-2 congenic mouse strains identified H-2(q) mice as highly susceptible to MOG-induced EAE with minimal adjuvant requirement. J. Neuroimmunol. 111:23–33. 10.1016/S0165-5728(00)00360-X [DOI] [PubMed] [Google Scholar]
- Abdul-Majid K.-B., Stefferl A., Bourquin C., Lassmann H., Linington C., Olsson T., Kleinau S., and Harris R.A.. 2002. Fc receptors are critical for autoimmune inflammatory damage to the central nervous system in experimental autoimmune encephalomyelitis. Scand. J. Immunol. 55:70–81. 10.1046/j.1365-3083.2002.01024.x [DOI] [PubMed] [Google Scholar]
- Abdul-Majid K.-B., Wefer J., Stadelmann C., Stefferl A., Lassmann H., Olsson T., and Harris R.A.. 2003. Comparing the pathogenesis of experimental autoimmune encephalomyelitis in CD4−/− and CD8−/− DBA/1 mice defines qualitative roles of different T cell subsets. J. Neuroimmunol. 141:10–19. 10.1016/S0165-5728(03)00210-8 [DOI] [PubMed] [Google Scholar]
- Appelberg R. 2007. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 15:87–92. 10.1016/j.tim.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Assi L.K., Wong S.H., Ludwig A., Raza K., Gordon C., Salmon M., Lord J.M., and Scheel-Toellner D.. 2007. Tumor necrosis factor α activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum. 56:1776–1786. 10.1002/art.22697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Sørensen O.E., and Theilgaard-Mönch K.. 2007. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 28:340–345. 10.1016/j.it.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Brackett C.M., Muhitch J.B., Evans S.S., and Gollnick S.O.. 2013. IL-17 promotes neutrophil entry into tumor-draining lymph nodes following induction of sterile inflammation. J. Immunol. 191:4348–4357. 10.4049/jimmunol.1103621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., and Zychlinsky A.. 2004. Neutrophil extracellular traps kill bacteria. Science. 303:1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Cain D.W., Snowden P.B., Sempowski G.D., and Kelsoe G.. 2011. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS One. 6:e19957 10.1371/journal.pone.0019957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M.A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today. 16:21–26. 10.1016/0167-5699(95)80066-2 [DOI] [PubMed] [Google Scholar]
- Cerutti A., Puga I., and Cols M.. 2012. New helping friends for B cells. Eur. J. Immunol. 42:1956–1968. 10.1002/eji.201242594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A., Puga I., and Magri G.. 2013. The B cell helper side of neutrophils. J. Leukoc. Biol. 94:677–682. 10.1189/jlb.1112596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R.C., Kim N.D., Sadik C.D., Seung E., Lan Y., Byrne M.H., Haribabu B., Iwakura Y., and Luster A.D.. 2010. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 33:266–278. 10.1016/j.immuni.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T., Schaeffer M., Han S.-J., van Dooren G.G., Nollmann M., Herzmark P., Chan S.W., Satija H., Camfield K., Aaron H., et al. 2008. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 29:487–496. 10.1016/j.immuni.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen B.E., Burkhardt C., Reith W., Renkawitz R., and Förster I.. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265–277. 10.1023/A:1008942828960 [DOI] [PubMed] [Google Scholar]
- Coquery C.M., Wade N.S., Loo W.M., Kinchen J.M., Cox K.M., Jiang C., Tung K.S., and Erickson L.D.. 2014. Neutrophils contribute to excess serum BAFF levels and promote CD4+ T cell and B cell responses in lupus-prone mice. PLoS One. 9:e102284 10.1371/journal.pone.0102284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerbi M., Abdul-Majid K.B., Abedi-Valugerdi M., Olsson T., Harris R.A., and Grandien A.. 2003. Expression of the long form of human FLIP by retroviral gene transfer of hemopoietic stem cells exacerbates experimental autoimmune encephalomyelitis. J. Immunol. 170:2064–2073. 10.4049/jimmunol.170.4.2064 [DOI] [PubMed] [Google Scholar]
- Enoksson S.L., Grasset E.K., Hägglöf T., Mattsson N., Kaiser Y., Gabrielsson S., McGaha T.L., Scheynius A., and Karlsson M.C.I.. 2011. The inflammatory cytokine IL-18 induces self-reactive innate antibody responses regulated by natural killer T cells. Proc. Natl. Acad. Sci. USA. 108:E1399–E1407. 10.1073/pnas.1107830108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W., and Dong C.. 2013. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2:e60 10.1038/emi.2013.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M., Tada Y., Kadoya Y., Obata S., and Harada Y.. 2013. COX-2 expression in stromal fibroblasts self-limits their numbers in lymph node inflammatory responses. Prostaglandins Other Lipid Mediat. 106:79–90. 10.1016/j.prostaglandins.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Kondratieva T.K., Rubakova E.I., Linge I.A., Evstifeev V.V., Majorov K.B., and Apt A.S.. 2010. B cells delay neutrophil migration toward the site of stimulus: tardiness critical for effective bacillus Calmette-Guérin vaccination against tuberculosis infection in mice. J. Immunol. 184:1227–1234. 10.4049/jimmunol.0902011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozakiewicz L., Chen Y., Xu J., Wang Y., Dunussi-Joannopoulos K., Ou Q., Flynn J.L., Porcelli S.A., Jacobs W.R. Jr., and Chan J.. 2013. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog. 9:e1003472 10.1371/journal.ppat.1003472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H.-J., Liu P., Bajrami B., Xu Y., Park S.-Y., Nombela-Arrieta C., Mondal S., Sun Y., Zhu H., Chai L., et al. 2015. Myeloid cell-derived reactive oxygen species externally regulate the proliferation of myeloid progenitors in emergency granulopoiesis. Immunity. 42:159–171. 10.1016/j.immuni.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos H.P., Grespan R., Vieira S.M., Cunha T.M., Verri W.A. Jr., Fernandes K.S.S., Souto F.O., McInnes I.B., Ferreira S.H., Liew F.Y., and Cunha F.Q.. 2009. Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNγ production. Proc. Natl. Acad. Sci. USA. 106:5954–5959. 10.1073/pnas.0812782106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T.L., and Geiszt M.. 2006. Role of Nox family NADPH oxidases in host defense. Antioxid. Redox Signal. 8:1549–1561. 10.1089/ars.2006.8.1549 [DOI] [PubMed] [Google Scholar]
- Magri G., Miyajima M., Bascones S., Mortha A., Puga I., Cassis L., Barra C.M., Comerma L., Chudnovskiy A., Gentile M., et al. 2014. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat. Immunol. 15:354–364. 10.1038/ni.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M.A., Costantini C., and Jaillon S.. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11:519–531. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- Manz M.G., and Boettcher S.. 2014. Emergency granulopoiesis. Nat. Rev. Immunol. 14:302–314. 10.1038/nri3660 [DOI] [PubMed] [Google Scholar]
- Mócsai A. 2013. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 210:1283–1299. 10.1084/jem.20122220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr E., Serre K., Manz R.A., Cunningham A.F., Khan M., Hardie D.L., Bird R., and MacLennan I.C.. 2009. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J. Immunol. 182:2113–2123. 10.4049/jimmunol.0802771 [DOI] [PubMed] [Google Scholar]
- Onishi R.M., and Gaffen S.L.. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 129:311–321. 10.1111/j.1365-2567.2009.03240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa R., Andresen P., Gillett A., Mia S., Zhang X.-M., Mayans S., Holmberg D., and Harris R.A.. 2012. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 61:2881–2892. 10.2337/db11-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M., Maggi L., Micheletti A., Lazzeri E., Tamassia N., Costantini C., Cosmi L., Lunardi C., Annunziato F., Romagnani S., and Cassatella M.A.. 2010. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 115:335–343. 10.1182/blood-2009-04-216085 [DOI] [PubMed] [Google Scholar]
- Puga I., Cols M., Barra C.M., He B., Cassis L., Gentile M., Comerma L., Chorny A., Shan M., Xu W., et al. 2012. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 13:170–180. 10.1038/ni.2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Gomes F.L., Peters N.C., Debrabant A., and Sacks D.L.. 2012. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog. 8:e1002536 10.1371/journal.ppat.1002536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H.W., and Unanue E.R.. 1993. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect. Immun. 61:5090–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosnek E., Burjanadze M., Dietrich P.Y., Matthes T., Passweg J., and Huard B.. 2009. Tumors that look for their springtime in APRIL. Crit. Rev. Oncol. Hematol. 72:91–97. 10.1016/j.critrevonc.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Scapini P., Nardelli B., Nadali G., Calzetti F., Pizzolo G., Montecucco C., and Cassatella M.A.. 2003. G-CSF–stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 197:297–302. 10.1084/jem.20021343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapini P., Bazzoni F., and Cassatella M.A.. 2008. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol. Lett. 116:1–6. 10.1016/j.imlet.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Silva M.T. 2010. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 87:93–106. 10.1189/jlb.0809549 [DOI] [PubMed] [Google Scholar]
- Soehnlein O., and Lindbom L.. 2010. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 10:427–439. 10.1038/nri2779 [DOI] [PubMed] [Google Scholar]
- Stamp L.K., Cleland L.G., and James M.J.. 2004. Upregulation of synoviocyte COX-2 through interactions with T lymphocytes: role of interleukin 17 and tumor necrosis factor-alpha. J. Rheumatol. 31:1246–1254. [PubMed] [Google Scholar]
- Stark M.A., Huo Y., Burcin T.L., Morris M.A., Olson T.S., and Ley K.. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 22:285–294. 10.1016/j.immuni.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Svensson L., Abdul-Majid K.B., Bauer J., Lassmann H., Harris R.A., and Holmdahl R.. 2002. A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. Eur. J. Immunol. 32:1939–1946. [DOI] [PubMed] [Google Scholar]
- Tsuboi N., Asano K., Lauterbach M., and Mayadas T.N.. 2008. Human neutrophil Fcγ receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity. 28:833–846. 10.1016/j.immuni.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Wu Y., and Capecchi M.R.. 2006. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 133:581–590. 10.1242/dev.02236 [DOI] [PubMed] [Google Scholar]
- Yang C.-W., and Unanue E.R.. 2013. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J. Exp. Med. 210:375–387. 10.1084/jem.20122183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-W., Strong B.S.I., Miller M.J., and Unanue E.R.. 2010. Neutrophils influence the level of antigen presentation during the immune response to protein antigens in adjuvants. J. Immunol. 185:2927–2934. 10.4049/jimmunol.1001289 [DOI] [PMC free article] [PubMed] [Google Scholar]