Tangye and collaborators use a series of mutants to elucidate the pathways required to generate distinct subsets of human effector CD4+ T cells.
Abstract
Naive CD4+ T cells differentiate into specific effector subsets—Th1, Th2, Th17, and T follicular helper (Tfh)—that provide immunity against pathogen infection. The signaling pathways involved in generating these effector cells are partially known. However, the effects of mutations underlying human primary immunodeficiencies on these processes, and how they compromise specific immune responses, remain unresolved. By studying individuals with mutations in key signaling pathways, we identified nonredundant pathways regulating human CD4+ T cell differentiation in vitro. IL12Rβ1/TYK2 and IFN-γR/STAT1 function in a feed-forward loop to induce Th1 cells, whereas IL-21/IL-21R/STAT3 signaling is required for Th17, Tfh, and IL-10–secreting cells. IL12Rβ1/TYK2 and NEMO are also required for Th17 induction. Strikingly, gain-of-function STAT1 mutations recapitulated the impact of dominant-negative STAT3 mutations on Tfh and Th17 cells, revealing a putative inhibitory effect of hypermorphic STAT1 over STAT3. These findings provide mechanistic insight into the requirements for human T cell effector function, and explain clinical manifestations of these immunodeficient conditions. Furthermore, they identify molecules that could be targeted to modulate CD4+ T cell effector function in the settings of infection, vaccination, or immune dysregulation.
Naive CD4+ T cells from humans and mice differentiate into distinct populations of effector cells with specialized functions. CD4+ T cell differentiation is mediated by the microenvironment in which the cells encounter and integrate various signals provided by APCs in the form of MHC class II–peptide complexes, and co-stimulatory signals provided by interacting surface receptors, cytokines, and associated signaling pathways and transcription factors. Thus, the generation of Th1 cells is driven by IL-12 and IFN-γ, which activate STAT4 and STAT1, respectively, to induce T-bet and promote IFN-γ production. Similarly, IL-4 activates STAT6 to induce GATA3 and cMaf to imprint a Th2 fate on naive CD4+ T cells. Th17 cells require TGFβ, IL-6, and IL-23, which, through STAT3 and RORγt, induce the signature Th17 cytokines IL-17A, IL-17F, and IL-22 (O’Shea and Paul, 2010; Zhu and Paul, 2010; Deenick et al., 2011; Zielinski et al., 2011; Vahedi et al., 2013). There also exists a population of effector CD4+ T cells that shares features of both Th1 and Th17 cells (termed Th1* or Th1/17 cells), insomuch that they produce IFN-γ, IL-17, and IL-22, express RORγt and T-bet, and coexpress the chemokine receptors CXCR3 and CCR6, which typically define Th1 and Th17 cells, respectively (Annunziato et al., 2007; Morita et al., 2011; Becattini et al., 2015; Ma et al., 2015; Okada et al., 2015). Human Th1, Th17, and Th1/17 cells have important roles in host protection against different classes of pathogens. Indeed, patients with inborn errors of IFN-γ immunity are susceptible to infection with mycobacteria (Boisson-Dupuis et al., 2015; Kreins et al., 2015; Okada et al., 2015), whereas those with inborn errors of IL-17–mediated immunity develop chronic mucocutaneous candidiasis (CMC; de Beaucoudrey et al., 2008; Ma et al., 2008; Milner et al., 2008; Liu et al., 2011; Puel et al., 2012; Okada et al., 2015).
Another subset of effector CD4+ T cells, T follicular helper (Tfh) cells, mediates the differentiation of B cells into memory cells and plasma cells in response to T cell–dependent antigens (Crotty, 2011; Tangye et al., 2013). Tfh cells express CXCR5, the transcription factor Bcl-6, which is essential for Tfh generation, and a host of molecules involved in T cell–B cell interactions, including CD40L, inducible costimulator (ICOS), PD-1, SAP, and IL-21 (Crotty, 2011; Liu et al., 2013; Tangye et al., 2013). Many studies have addressed the requirements for Tfh formation. IL-6, IL-12, IL-21, and IL-27 can induce features of Tfh cells in human and murine naive CD4+ T cells in vitro (Crotty, 2011; Tangye et al., 2013). These findings were extended by demonstrating reduced murine Tfh cells in vivo in the absence of one or more of these cytokines (Crotty, 2011; Tangye et al., 2013). Studies in mice also identified receptor/ligand pairs (CD40/CD40L, ICOS/ICOS-L, SLAM family members, and CD28/B7), specific signaling pathways (SAP, PI3 kinase, STAT1, and STAT3), and transcription factors in addition to Bcl-6 (cMAF, IRF4, BATF, and Ascl2) that are involved in Tfh formation (Crotty, 2011; Tangye et al., 2013). More recently, an additional level of complexity has been added to Tfh biology, with several studies implicating roles for TGFβ and/or IL-23 in their formation in humans and mice (Schmitt et al., 2014; Marshall et al., 2015). However, TGFβ also represses murine Tfh formation in vivo and in vitro (Suto et al., 2008; McCarron and Marie, 2014; Schmitt et al., 2014), indicating that the precise requirements for Tfh cell development remains to be established. Despite this, the importance of Tfh cells in humoral immunity is apparent from their dysregulated production and function in numerous human immunopathologies, such as autoimmunity and primary immunodeficiencies (PIDs; Tangye et al., 2013). Furthermore, frequencies of circulating Tfh (cTfh) cells correlates with the efficacy of humoral immune responses elicited after natural infection and vaccination (Pallikkuth et al., 2012; He et al., 2013; Locci et al., 2013; Herati et al., 2014).
We and others have previously quantified CD4+ T cell subsets in individuals with monogenic PIDs. Biallelic mutations in IL12B1, IL12RB1, and ISG15 impaired the development of Th1 cells (de Beaucoudrey et al., 2010; Prando et al., 2013; Boisson-Dupuis et al., 2015; Ma et al., 2015), whereas heterozygous dominant-negative (DN) mutations in STAT3 or gain-of-function (GOF) mutations in STAT1 impaired development of Th17 cells (de Beaucoudrey et al., 2008; Ma et al., 2008, 2015; Milner et al., 2008; Liu et al., 2011). Notably, loss-of-function (LOF) mutations in RORC impaired both Th17 and Th1/17 cells (Okada et al., 2015). There was also a paucity of cTfh cells in patients with IL10R, IL21R, IL12RB1, CD40LG, ICOS, NEMO, or BTK LOF mutations, STAT3 DN mutations, or STAT1 GOF mutations (Bossaller et al., 2006; Martini et al., 2011; Ma et al., 2012, 2015; Schmitt et al., 2013). Although these findings shed light on the requirements for generating or maintaining CD4+ T cell subsets in vivo, they do not necessarily discriminate between intrinsic and extrinsic effects of the disease-causing mutations on these processes. We therefore set out to analyze the ability of naive CD4+ T cells from individuals with distinct disease-causing mutations to differentiate into effector populations in vitro. Specifically, this included individuals with heterozygous LOF or DN mutations in STAT1, STAT3 or IFNGR1, X-linked hemizygous LOF mutations in CD40LG, NEMO, or BTK; bi-allelic recessive LOF mutations in IL21, IL21R, IL10R, ICOS, IL12RB1, IFNGR1, or TYK2; or heterozygous dominant GOF mutations in STAT1 (Table S1; 88 patients total). In doing so, we have now determined the cell intrinsic requirements and signaling pathways necessary to generate human Th1, Th17, Tfh-type, and IL-10–producing effector cells. These findings provide insight into the pathophysiology of diseases characterized by impaired cell-mediated and humoral immunity and infectious susceptibility in the setting of human primary immune deficient states.
RESULTS
Although IL-12 is the key inducer of Th1 cells (O’Shea and Paul, 2010; Zhu and Paul, 2010; Deenick et al., 2011; Zielinski et al., 2011; Vahedi et al., 2013), it also induces IL-21, a feature of Tfh cells, in human activated naive CD4+ T cells, and endows these cells with the capacity to induce B cell differentiation in vitro (Ma et al., 2009, 2012; Schmitt et al., 2009). It was recently observed that both Bcl-6 and IL-21 were strongly induced in human naive CD4+ T cells after culture with IL-23 and/or TGF-β (Schmitt et al., 2014). Furthermore, human naive CD4+ T cells cultured with TGF-β and IL-23 or under Th17 conditions (i.e., TGF-β/IL-1β/IL-6/IL-23) were more efficient than IL-12–primed T cells at inducing B cell differentiation in vitro, implicating TGFβ as a key driver of Tfh development and function in humans (Schmitt et al., 2014). This is consistent with findings demonstrating that human and murine Th17 cells can promote B cell differentiation (Annunziato et al., 2007; Mitsdoerffer et al., 2010). To extend these findings, we systemically compared different in vitro culture conditions for their ability to induce key features of human Th1, Th17, and Tfh cells. This would then facilitate elucidation of the molecular requirements for generating these effector cells from naive CD4+ T cells with defined mutations in specific signaling pathways.
IL-12 potently and selectively induces IL-21 expression in human naive CD4+ T cells
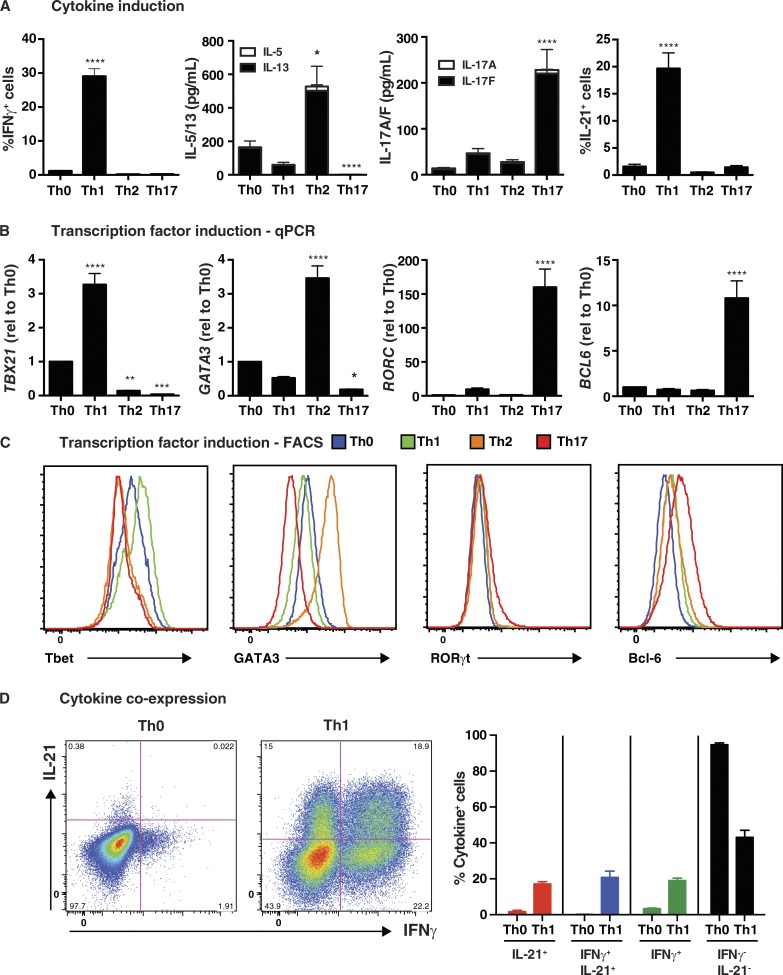
Naive CD4+ T cells from healthy donors were cultured under Th0-, Th1-, Th2-, or Th17-polarizing conditions for 5 d and then assessed for production and expression of cytokines and transcription factors associated with these effector fates. As expected, IL-12 in the Th1 culture induced the greatest proportion of cells expressing IFN-γ (Fig. 1 A) and highest levels of TBX21/T-bet, as determined both by qPCR (Fig. 1 B) and intracellular staining (Fig. 1 C and Table 1). Similarly, secretion of IL-5 and IL-13, and expression of GATA3 mRNA and protein, by naive CD4+ T cells were greatly increased by Th2 culture conditions, whereas this culture significantly reduced basal levels of T-bet observed in Th0 condition (Fig. 1, B and C; and Table 1). The Th17 culture induced significantly higher levels of IL-17A, IL-17F (Fig. 1 A), and RORC (Fig. 1 B) than the other cultures, and actively repressed Th2 cytokines and GATA3 (Fig. 1, A–C; and Table 1). RORγt protein was also detectable in a small but significant population of naive CD4+ T cells cultured under Th17, but not Th0, Th1, or Th2, conditions (Fig. 1 C and Table 1). Consistent with previous findings (Ma et al., 2009, 2012; Schmitt et al., 2009), IL-21 was most strongly induced by IL-12/Th1 culture, whereas the effect of Th2 and Th17 cultures on IL-21 expression did not exceed that of the Th0 culture (Fig. 1 A). However, induction of the signature Tfh cytokine IL-21 did not correlate with expression of Bcl6 (Fig. 1, B and C). Rather, Bcl6 was only induced in the Th17 culture (Fig. 1, B and C; and Table 1). The nature of IL-21–expressing cells in the Th1 culture was further explored by determining coexpression of IFN-γ. IL-12 induced approximately equal proportions of naive CD4+ T cells that were IFN-γ+, IL-21+, or IL-21+IFN-γ+ (Fig. 1 D). This demonstrates that IL-12 can induce IFN-γ+ Th1 cells, IL21+ Tfh-type cells, and Th1/Tfh transitional type cells expressing both cytokines.
Figure 1.
In vitro induction of features of Tfh cells in human naive CD4+ T cells. Naive CD4+ T cells were isolated from peripheral blood of healthy donors, and then cultured with T cell activation and expansion (TAE) beads alone (Th0) or under Th1 (+IL-12), Th2 (+IL-4), or Th17 (+TGF-β, IL-1β, IL-6, IL-21, IL-23, and PGE2) conditions for 5 d. After this time, the cells were harvested and analyzed for (A) production of IFN-γ, IL-5/IL-13, IL-17A/IL-17F, and IL-21 by intracellular staining or cytometric bead array (CBA; mean ± SEM; n = 8–17); and (B and C) expression of TBX21/Tbet, GATA3, RORC/Rorγt, or BCL6 by qPCR (B) or flow cytometry (C). The graphs in B correspond to the fold change (mean ± SEM; n = 10–17) in expression of the indicated transcription factor relative to Th0 culture. Histograms depicted in C are representative of three to five independent experiments. (D) Coexpression of IL-21 and IFN-γ by Th0- or Th1-stimulated human naive CD4+ T cells was determined by intracellular staining and flow cytometry. The graphs depicts the mean ± SEM of cytokine-expressing cells derived from nine different experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, compared with Th0 (ANOVA).
Table 1. Induction of transcriptional regulators of CD4+ T cell differentiation in vitro.
| Transcription factor | In vitro polarizing conditions | |||
|---|---|---|---|---|
| Th0 | Th1 | Th2 | Th17 | |
| Tbet (ΔMFI) | 1.0 | 1.4 ± 0.07a | 0.8 ± 0.09 | 0.65 ± 0.1a |
| GATA3 (ΔMFI) | 1.0 | 0.75 ± 0.05 | 3.1 ± 0.16 § | 0.4 ± 0.02b |
| RORγt (ΔMFI) | 1.0 | 1.25 ± 0.13 | 1.2 ± 0.03 | 1.5 ± 0.08a |
| RORγt (%) | 0.5 ± 0.2% | 0.66 ± 0.1% | 0.66 ± 0.15% | 6.3 ± 0.7%c |
| Bcl-6 (ΔMFI) | 1.0 | 1.41 ± 0.08a | 1.3 ± 0.1 | 2.4 ± 0.08c |
Naive CD4+ T cells were isolated from peripheral blood of healthy donors, and then cultured with TAE beads alone (Th0) or under Th1, Th2, or Th17 conditions for 5 d. After this time, the cells were harvested and expression of Tbet, GATA3, RORγt, or Bcl-6 was determined by intracellular staining and flow cytometry. The values represent either the fold change (ΔMFI, mean ± SEM; n = 3–5) in expression of the indicated transcription factor relative to Th0 culture, or the % of cells expressing RORγt (n = 3).
P < 0.05.
P < 0.01.
P < 0.0001 (ANOVA).
IL-12 induces key phenotypic and functional features of Tfh cells in human naive CD4+ T cells
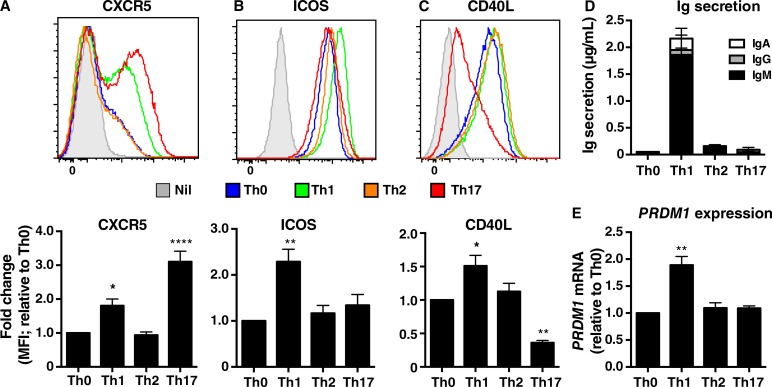
To extend these studies, we assessed in vitro cultures of naive CD4+ T cells for additional features of Tfh cells. After 3 d of in vitro culture, expression of CXCR5 (Fig. 2 A), ICOS (Fig. 2 B), CD40L (Fig. 2 C), and PD-1 (not depicted) was significantly greater on naive CD4+ T cells cultured under Th1 conditions compared with those subjected to Th0 or Th2 cultures. Interestingly, although Th17 conditions had the greatest effect on CXCR5, they had no effect on ICOS and actually down-regulated CD40L (Fig. 2, A–C), which is inconsistent with a Tfh fate despite high expression of Bcl6. In terms of B cell differentiation, naive CD4+ T cells cultured under Th1 conditions induced abundant amounts of Ig secretion by co-cultured B cells, whereas CD4+ T cells subjected to Th2- and Th17-polarizing conditions exhibited minimal, if any, ability to induce B cell differentiation (Fig. 2 D). This is consistent with selective high level IL-21 expression by IL-12–primed cells. Similarly, the lack of B cell help provided by Th17-induced CD4+ T cells mirrored not only their poor expression of IL-21 (Fig. 1 A), but also reduced expression of CD40L by these cells (Fig. 2 C). The unique ability of Th1-cultured naive CD4+ T cells to promote Ig secretion by co-cultured B cells was further demonstrated by the exclusive induction of PRDM1 (encoding Blimp-1), a master regulator of plasma cell formation (Nutt et al., 2011), in cultures of B cells and IL-12–treated CD4+ T cells, but not when B cells were cultured with Th0, Th2, or Th17 cells (Fig. 2 E). Collectively, these data establish that IL-12 strongly induces phenotypic and functional attributes of Tfh cells in human naive CD4+ T cells in vitro. One exception is Bcl-6, which is not required for these initial events in human Tfh differentiation. These findings parallel observations that murine CD4+ T cells express CXCR5 and IL-21 before expressing Bcl-6 (Liu et al., 2012, 2013). Thus, the in vitro culture we employed corresponds to early events in differentiation of human naive CD4+ T cells to a Tfh fate.
Figure 2.
IL-12 selectively induces Tfh phenotype and function in human naive CD4+ T cells. Peripheral blood naive CD4+ T cells were cultured in media only (Nil) or with TAE beads alone (Th0) or under Th1, Th2, or Th17 conditions. (A–C) After 3 d, the cells were harvested and expression of (A) CXCR5, (B) ICOS, and (C) CD40L were determined by flow cytometry. The histogram plots (top) depict expression of the indicated surface receptor by cells cultured in media (solid gray) or under Th0 (blue), Th1 (green), Th2 (orange), or Th17 (red) conditions. The graphs (bottom) represent fold change in expression of the indicated cell surface marker relative to the Th0 culture (mean ± SEM; n = 4). *, P < 0.05; **, P < 0.01; ****, P < 0.0001 compared with Th0 (ANOVA). (D and E) After 5 d, activated CD4+ T cells were treated with mitomycin C and co-cultured with sort-purified allogeneic naive B cells in the presence of TAE beads for 7 d. (D) Secretion of IgM, IgG, and IgA (n = 3) and (E) expression of PRDM1 (Blimp1; n = 2) relative to the Th0 cultures were determined by ELISA and qPCR, respectively. Values represent mean ± SEM. **, P < 0.01.
Differential induction of phosphorylation of STATs by in vitro–polarizing cultures
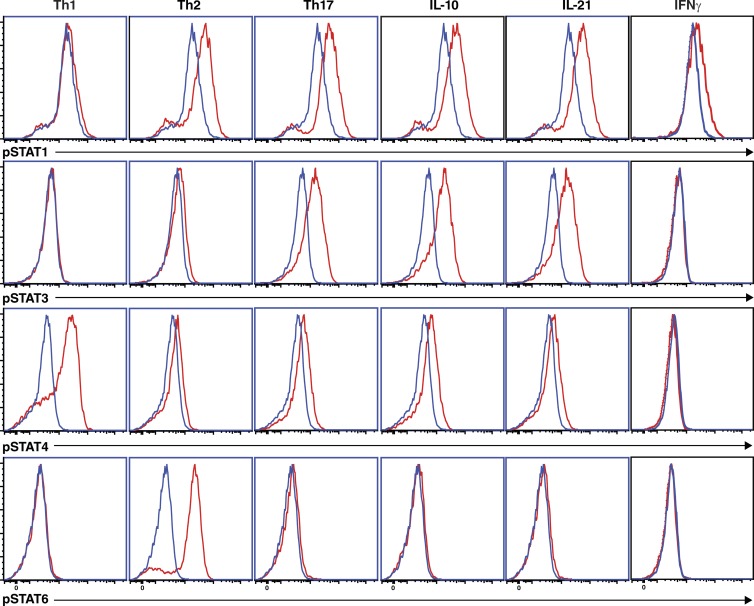
The focus of this study is to determine molecular requirements for human CD4+ T cell differentiation. To do this, we used CD4+ T cells from individuals with mutations in various signaling pathways, including cytokine receptors (IL10R, IL12RB1, IL21R, and IFNGR1) and downstream components (TYK2, STAT1, and STAT3; Table S1). Thus, it was necessary to establish which STAT molecules are activated in normal naive CD4+ T cells in response to the different stimulatory conditions used in our in vitro cultures. The rationale of this is to determine, first, direct effects of the polarizing conditions on STAT activation and, second, possible indirect effects after induction of cytokines that may act autonomously to activate specific STATs. Th1/IL-12 or Th2/IL-4 stimuli induced strong phosphorylation of STAT4 or STAT6, respectively (Fig. 3). STAT1 was also weakly phosphorylated in response to Th2 polarizing conditions, while in Th17 conditions, IL-10 or IL-21 activated STAT1 and STAT3, but had little effect on STAT4 or STAT6 (Fig. 3). IFN-γ only activated STAT1 (Fig. 3). Thus, consistent with previous studies (Leonard, 2001), different stimuli induced distinct but overlapping patterns of activation of STAT molecules.
Figure 3.
Differential induction of STAT activation by in vitro–polarizing conditions. Naive CD4+ T cells were cultured with TAE beads for 4 d. After this time, the cells were harvested, and then cultured in media alone (blue histogram) or under Th1 (+IL-12), Th2 (+IL-4), or Th17 (+TGF-β, IL-1β, IL-6, IL-21, IL-23, and PGE2) conditions, or with IL-10, IL-21, or IFN-γ (red histograms) for 30 min. Phosphorylation of STAT1, STAT3, STAT4, and STAT6 was determined by intracellular staining and flow cytometry. The data are representative of experiments performed on cells from two to three different donors.
Monogenic mutations impact the in vitro differentiation of naive CD4+ T cells into IL-21–expressing Tfh-type cells
We recently examined >110 individuals with defined monogenic mutations that underlie distinct PID states to identify key molecular regulators of human CD4+ T cell differentiation in vivo. This revealed that the generation and/or function of cTfh cells was compromised by STAT3 DN, IL21, IL21R, IL10R, CD40LG, NEMO, ICOS, or BTK LOF, or STAT1 GOF mutations (Ma et al., 2015). As expression of most of these genes is not restricted to CD4+ T cells, identifying perturbations in cTfh cells does not indicate whether this is T cell intrinsic or extrinsic. To gain further insight into the molecular requirements for generating human Tfh cells, we examined the ability of naive CD4+ T cells isolated from patients with mutations in key signaling pathways (Table S1) to differentiate into Tfh-type cells in vitro under appropriate culture conditions.
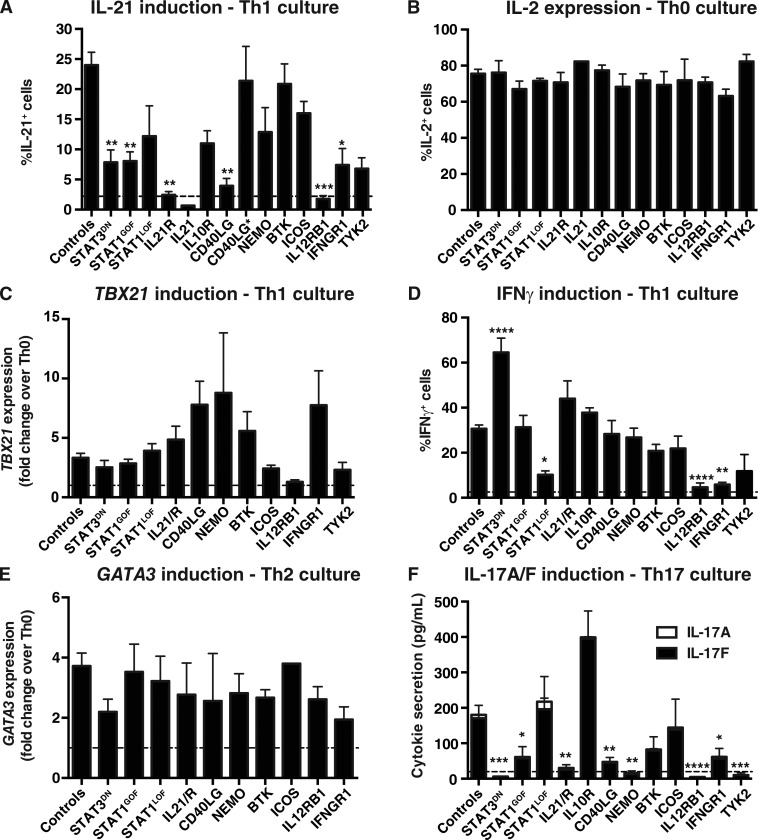
When naive CD4+ T cells from PID patients were examined in vitro, STAT3 DN, IL21R, CD40LG, or IFNGR1 LOF, or STAT1 GOF, mutations significantly impaired their ability to become IL-21+ cells in response to IL-12 in vitro (Fig. 4 A). The specificity of this response was established by demonstrating that IL12RB1 LOF mutations abolished IL-21 induction, whereas naive CD4+ T cells from the single IL21-deficient patient thus far reported (Salzer et al., 2014) failed to express detectable amounts of IL-21 (Fig. 4 A). Importantly, hematopoietic stem cell transplant of a CD40LG-deficient patient improved the ability of his naive CD4+ T cells to express IL-21 in response to IL-12 (i.e., pretransplant: 1.0% IL-21+ cells; after transplant: 21.0% IL-21+ cells). Mutations in IL10R, TYK2, or NEMO also reduced IL-21 expression by IL-12–stimulated naive cells (Fig. 4 A); however, these differences did not reach statistical significance due to the small sample size (IL10R and TYK2) or the variability of NEMO mutations on lymphocyte function. For instance, responses of three NEMO-deficient patients were intact, whereas responses of four other patients were greatly reduced (<25% of controls). This is consistent with extreme variability in the cellular phenotypes and clinical presentation of NEMO-mutated individuals (Orange et al., 2004; Hanson et al., 2008; Boisson-Dupuis et al., 2015). In contrast, STAT1, BTK, or ICOS LOF mutations had no significant effect on IL-12–induced IL-21 expression. In our cohort of patients with LOF CD40LG mutations, there were three individuals with hypomorphic mutations and milder disease. Interestingly, naive CD4+ T cells from these patients exhibited a normal capacity to differentiate into IL-21+ cells in response to IL-12 (CD40L*; Fig. 4 A), consistent with normal frequencies of cTfh cells in these individuals (Ma et al., 2015). Thus, pathways involving IL12RB1, STAT3, STAT1, IL21R, CD40LG, and possibly NEMO, IL10R, and TYK2, are key for inducing IL-21 expression in human naive CD4+ T cells under Tfh-inducing conditions in vitro.
Figure 4.
Impact of disease-causing mutations on differentiation of naive CD4+ T cells to Th1, Th2, Th17, and Tfh fates in vitro. Naive CD4+ T cells were isolated from peripheral blood of healthy donors or patients with mutations in STAT3, STAT1, IL21/R, IL10R, CD40LG, NEMO, BTK, ICOS, IL12RB1, IFNGR1, or TYK2, and then cultured with TAE beads alone (Th0) or under Th1/Tfh (+IL-12), Th2 (+IL-4), or Th17 (+TGF-β, IL-1β, IL-6, IL-21, and IL-23) conditions for 5 d. Cells were then harvested and analyzed for expression of cytokines and transcription factors. (A) Percentage of cells expressing IL-21 in response to Tfh/IL-12 stimulation. (B) Percentage of cells expressing IL-2 in Th0 culture. (C and D) Induction of TBX21 (C) and IFN-γ (D) expression after Th1/IL-12–polarizing culture. (E) Induction of GATA3 in response to Th2/IL-4 stimulation. (F) Induction of IL-17A and IL-17F secretion after Th17-polarizing culture. Controls, n = 17–48; STAT3DN, n = 2–10; STAT1GOF, n = 5–14; STAT1LOF, n = 3–8; IL21R, n = 3–5 experiments; IL21 n = 1; IL10R, n = 1–2; CD40LG, n = 3–9 (CD40LG* (MILD), n = 3); NEMO, n = 2–7; BTK, n = 5–8; ICOS, n = 2–7; IL12RB1, n = 3–8; IFNGR1, n = 2–6; TYK2, n = 2–3. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, compared with Th0 (ANOVA; Student’s t test). The dashed lines indicate the mean percentage of IL-21+ (A) or IFN-γ+ (D) cells, or secretion of IL-17A/F (F), detected under Th0 conditions. Note that data for IL-17A/F production by TYK2-deficient naive CD4+ T cells was presented in Kreins et al. (2015), but is shown here for comparison.
Effects of monogenic mutations on the in vitro generation of Th1, Th2, and Th17 cells
To establish whether the inability of naive CD4+ T cells from some PID patients to yield IL-21+ Tfh-type cells resulted from a global impairment in activation, we assessed these cells for IL-2 expression, as well as their ability to differentiate to other effector lineages in vitro. Comparable proportions of naive CD4+ T cells expressed IL-2, irrespective of genotype, in response to Th0 stimulation (Fig. 4 B). Similarly, although STAT3 DN, IL21R, CD40LG LOF, and STAT1 GOF mutations compromised the ability of naive CD4+ T cells to acquire IL-21 in response to IL-12 (Fig. 4 A), these same cells up-regulated TBX21 and produced IFN-γ comparably to normal naive CD4+ T cells under Th1/Tfh conditions (Fig. 4, C and D; Fig. S1 A). There was actually a significant increase in IFN-γ production by STAT3 mutant naive CD4+ T cells in response to IL-12 stimulation, indicating STAT3 signaling restrains Th1 differentiation (Fig. 4 D and Fig. S1 A). This indicates a selective impairment in differentiating into Tfh-type, but not Th1-type, cells in response to IL-12. IL-12–mediated IFN-γ induction was strongly reduced by LOF STAT1, IFNGR1, and TYK2 mutations, and abolished by IL12RB1 mutations (Fig. 4 D and Fig. S1 A). Induction of GATA3 was also intact in most populations of naive CD4+ T cells when subjected to Th2-polarizing conditions (Fig. 4 E).
Consistent with previous studies (de Beaucoudrey et al., 2008; Ma et al., 2008; Milner et al., 2008), STAT3 DN mutations abolished induction of IL-17A, IL-17F (Fig. 4 F), and RORC (Fig. S1 B) in naive CD4+ T cells cultured under Th17-polarizing conditions. We have now found that, in addition to compromising IL-12–mediated IL-21 induction in naive CD4+ T cells, LOF mutations in IL12RB1 and TYK2 abolished, whereas STAT1 GOF and IL21R, CD40LG, NEMO, and IFNGR1 LOF mutations impaired, Th17 generation from naive precursors (Fig. 4 F). In contrast, STAT1 LOF mutations had no effect, whereas IL-10R deficiency increased production of IL-17F (Fig. 4 F). Thus, signaling pathways involving IL-23/IL-12Rβ1/TYK2/STAT3, IL-21R/STAT3, and NEMO downstream of IL-1β or TCR (Hanson et al., 2008) are required to induce human Th17 cells, a process also compromised by STAT1 GOF mutations. To extend these findings, we assessed the effects of Th17-polarizing culture conditions on expression of IL-17A and IL-17F by memory CD4+ T cells. Consistent with our previous findings (Ma et al., 2015), the proportions of IL-17A/F+ memory CD4+ T cells detected under Th0 and Th17 culture conditions was reduced by STAT3 DN, STAT1 GOF, and IL21R, NEMO, and IL12RB1 LOF mutations (Fig. S1 C). In contrast LOF mutations in STAT1, CD40LG, BTK, ICOS, or IFNGR1 had no effect on amplifying Th17 responses of memory cells, yet lack of IL-10R resulted in an exaggerated response (Fig. S1 C). Thus, the consequences of pathogenic mutations on the ability of naive CD4+ T cells to differentiate into Th17 cells in vitro are shared by in vivo–generated memory CD4+ T cells.
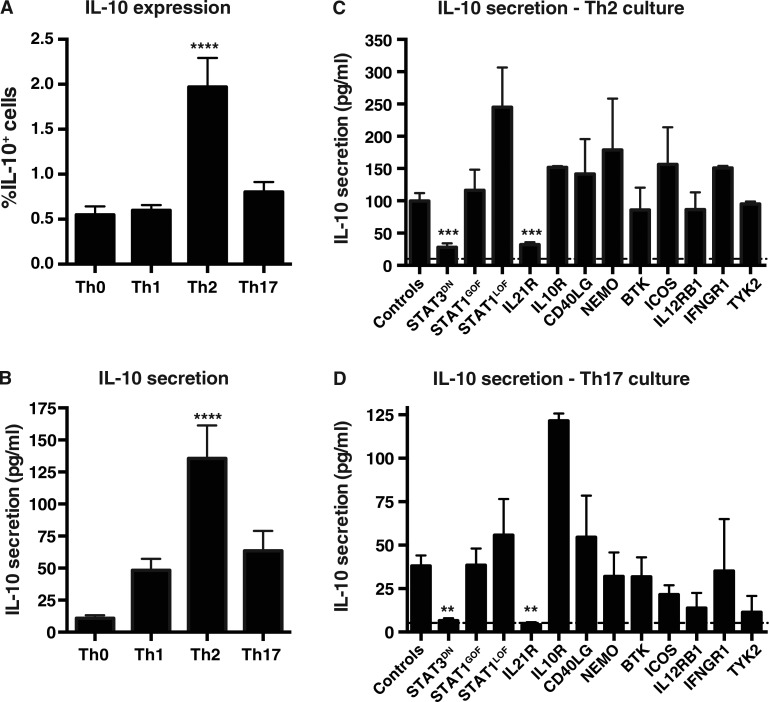
IL-21R/STAT3 signaling is required for IL-10 production by naive CD4+ T cells in vitro
IL-10 is a pleiotropic cytokine produced by numerous CD4+ T cell subsets and is involved in suppressing immune responses and promoting human B cell differentiation (Hirahara et al., 2013; Moens and Tangye, 2014). Thus, we investigated which conditions most efficiently induce IL-10 production in vitro. Th2 polarization induced a significantly increased proportion of IL-10+ cells compared with the other conditions (Fig. 5 A). This was also observed for IL-10 secretion (Fig. 5 B). Th17 conditions induced greater levels of IL-10 compared with the Th0 culture; however, the absolute levels were less than those induced by the Th2 culture (Fig. 5 B). Thus, naive CD4+ T cells produced substantial amounts of IL-10 after stimulation with Th2- or Th17-polarizing conditions, consistent with studies showing IL-10 production by human Th2 and Th17 cells (Sornasse et al., 1996; Boniface et al., 2009; Saraiva and O’Garra, 2010). When naive CD4+ T cells from PID patients were investigated, STAT3 DN or IL21R LOF mutations significantly impaired Th2 induction of IL-10, whereas none of the other mutations had any effect (Fig. 5 C). These same mutations abolished IL-10 secretion induced under Th17 conditions (Fig. 5 D). Thus, signaling through IL-21R/STAT3 represents the major pathway required for inducing IL-10 production by human CD4+ T cells.
Figure 5.
Induction of IL-10 by Th2- and Th17-polarizing cultures is compromised by mutations in IL21R and STAT3. (A and B) Naive CD4+ T cells from healthy donors were cultured with TAE beads alone (Th0) or under Th1, Th2, or Th17 conditions for 5 d. After this time, the cells were harvested and analyzed for IL-10 production by intracellular staining and flow cytometry (A; n = 15) or CBA (n = 24). Values represent mean ± SEM. ****, P < 0.0001 compared with Th0 (ANOVA). (C and D) Naive CD4+ T cells from healthy donors or patients were cultured with TAE beads alone (Th0) or under Th2 (C) or Th17 (D) conditions for 5 d. IL-10 secretion was determined by CBA. Values represent mean ± SEM; dashed line indicates the mean level of IL-10 secretion under Th0 conditions. Controls, n = 31–35; STAT3DN, n = 6–8; STAT1GOF, n = 8; STAT1LOF, n = 4–5; IL21R, n = 4–5; IL10R, n = 1–2; CD40LG, n = 5; NEMO, n = 2–3; BTK, n = 7; ICOS, n = 3; IL12RB1, n = 5–6; IFNGR1, n = 2–5; TYK2, n = 2–3. **, P < 0.01; ***, P < 0.001, compared with controls (ANOVA; Student’s t test).
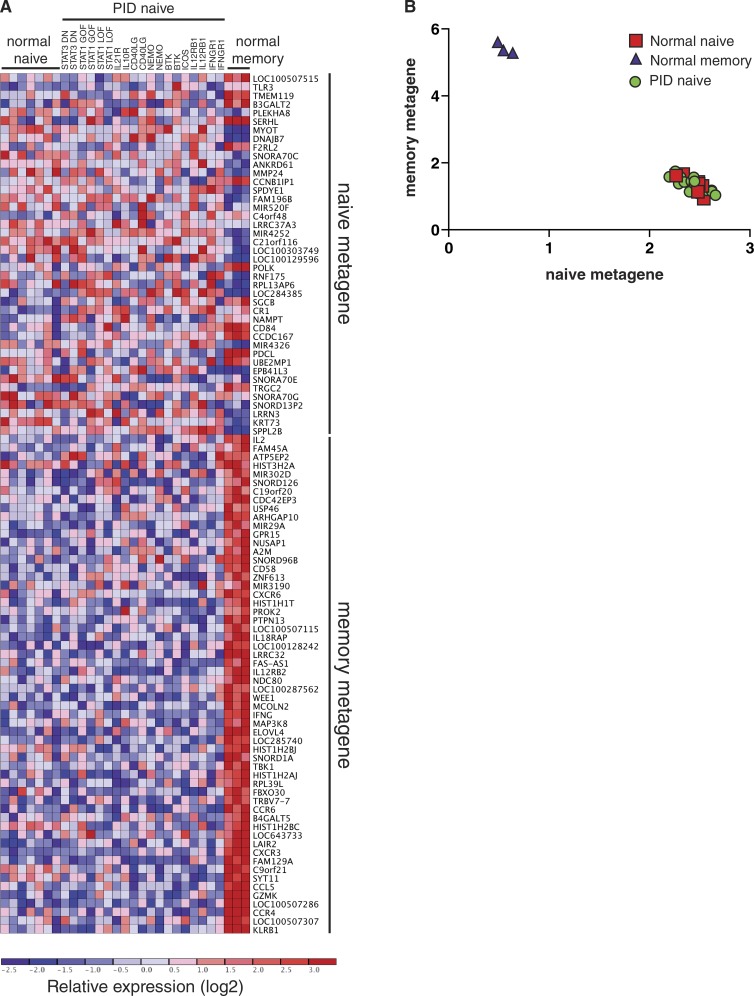
Transcriptional analysis reveals similar differentiation states between normal and PID naive CD4+ T cells
Although our in vitro functional studies revealed marked defects in the differentiation capacity of naive CD4+ T cells from PID patients with distinct disease-causing mutations, the finding that these same naive CD4+ T cells could be induced to express comparable levels of IL-2 as naive CD4+ T cells from healthy controls, or could differentiate normally under some polarizing conditions (Figs. 4 and 5), suggested that the differentiation defects did not result from global defects in lymphocyte activation. However, it remained possible that such mutations compromised the in vivo development of CD4+ T cells such that cells isolated from PID patients according to a naive surface phenotype were different to phenotypically matched cells from healthy donors. Thus, it was necessary to provide further evidence that naive CD4+ T cells from PID patients were of a comparable differentiation state as those from healthy controls. To formally investigate this, we performed microarray analysis on naive CD4+ T cells (CD4+CD45RA+CCR7+CD127hiCD25lo) from healthy donors (n = 7) and 19 PID patients corresponding to 11 different gene mutations (STAT3DN, STAT1GOF, STAT1LOF, IL21R, IL10R, CD40LG, NEMO, BTK, ICOS, IL12RB1, and IFNGR1). We also compared these to memory CD4+ T cells from three healthy donors. Gene expression profiles were subjected to unsupervised clustering analysis by nonnegative matrix factorization (NMF; Brunet et al., 2004; Suan et al., 2015). NMF identified two molecularly distinct populations in this dataset (k = 2; cophenetic coefficient = 0.9999). One consisted entirely of memory CD4+ T cells, characterized by high expression of a memory metagene or gene signature that included known memory CD4+ T cell markers such as CD58, CCR4, CCR6, CXCR3, and GZMK (Fig. 6 A; Weng et al., 2012). The second population comprised naive CD4+ T cells from both healthy controls and the different PID patients, marked by expression of a naive metagene that included microRNAs and small nucleolar RNAs (Fig. 6 A). Importantly, visualization of naive and memory metagene expression revealed that naive CD4+ T cells from healthy donors and PID patients clustered tightly together and were indistinguishable (Fig. 6 B). Taken together, these data show that naive CD4+ T cells from PID patients were not programmed differently to those from normal controls and could be justifiably used to interrogate the function of signaling pathways.
Figure 6.
Unsupervised clustering of CD4+ T cells from PID and normal healthy donors by nonnegative matrix factorization (NMF). (A) Heat map of log2-transformed expression data, with genes organized into naive and memory metagenes. Expression levels are indicated by the color scale, with red and blue respectively representing high and low relative expression. (B) Visualization of expression levels of naive and memory metagenes by memory CD4+ T cells and naive CD4+ T cells from normal healthy donors and PID patients. Naive CD4+ T cells clustered tightly together and could not be distinguished from each other irrespective of genotype.
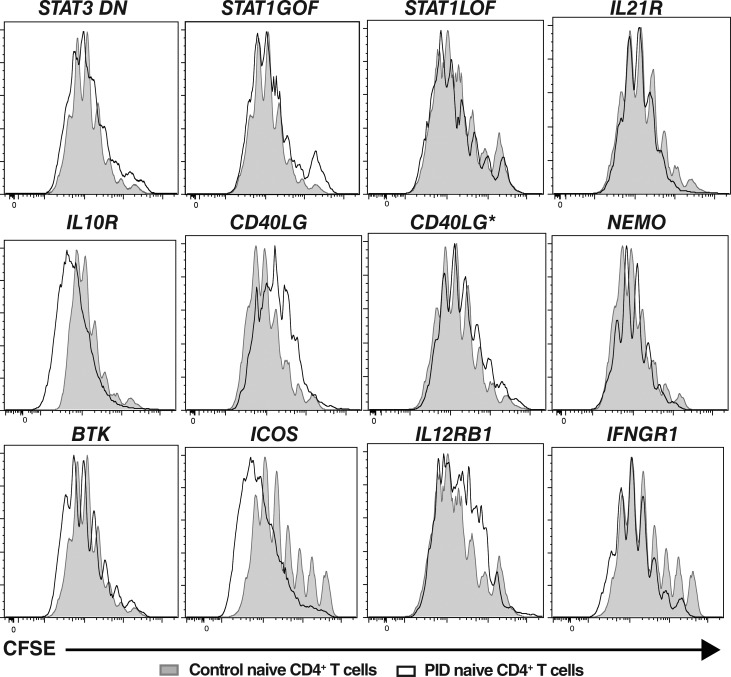
Impaired differentiation of mutant naive CD4+ T cells is not a result of compromised proliferation
Many aspects of lymphocyte differentiation, such as class switching by naive B cells and acquisition of cytokine expression by naive CD4+ T cells, require cell division (Tangye and Hodgkin, 2004; Ma et al., 2005). Thus, as several of the gene mutations examined could potentially influence T cell proliferation, another explanation for the poor differentiation of naive CD4+ T cells from PID patients into cytokine-producing could be impaired cell division in vitro. To test this, naive CD4+ T cells sorted from the peripheral blood of healthy controls and PID patients were labeled with the division tracking dye CFSE, and then cultured under Th0, Th1, Th2, or Th17 conditions. When stimulated under Th0 conditions, ∼70–90% of naive CD4+ T cells from healthy controls had undergone >1 division after 5 d of in vitro culture, with the majority of proliferating cells being in divisions four to seven (Fig. 7, gray filled histograms). Th1 or Th2 polarizing conditions slightly increased the proportions of divided cells (Fig. S2). Although Th17-polarizing conditions modestly reduced proliferation of naive CD4+ T cells, most likely due to TGFβ, the cells continued to undergo extensive rounds of division (Fig. S2). Importantly, naive CD4+ T cells from all patient groups tested—irrespective of genotype—exhibited proliferation (CFSE) profiles that largely overlapped those of normal controls (Fig. 7 and Fig. S2, black outline histograms). Thus, defective proliferation of naive CD4+ T cells harboring disease-causing mutations does not underlie impaired differentiation of these cells into distinct effector fates, as defined by cytokine expression. Rather, the mutations specifically impede the cytokine-driven differentiation process.
Figure 7.
Disease-causing mutations do not compromise proliferation of naive CD4+ T cells. Naive CD4+ T cells from healthy donors (solid gray histograms) and patients (overlay black histograms) with the indicated gene mutations were labeled with CFSE, and then cultured with TAE beads (Th0) for 5 d. After this time, the cells were harvested and analyzed for proliferation by assessing CFSE dilution. Histograms are representative of experiments performed using naive CD4+ T cells from nine different healthy controls, two patients with mutations in STAT3, STAT1, CD40LG, NEMO, BTK, IL12RB1, or IFNGR1, or individual patients with mutations in IL21R, IL10R, or ICOS.
Functional defects in in vitro–induced Tfh-type cells due to specific gene mutations
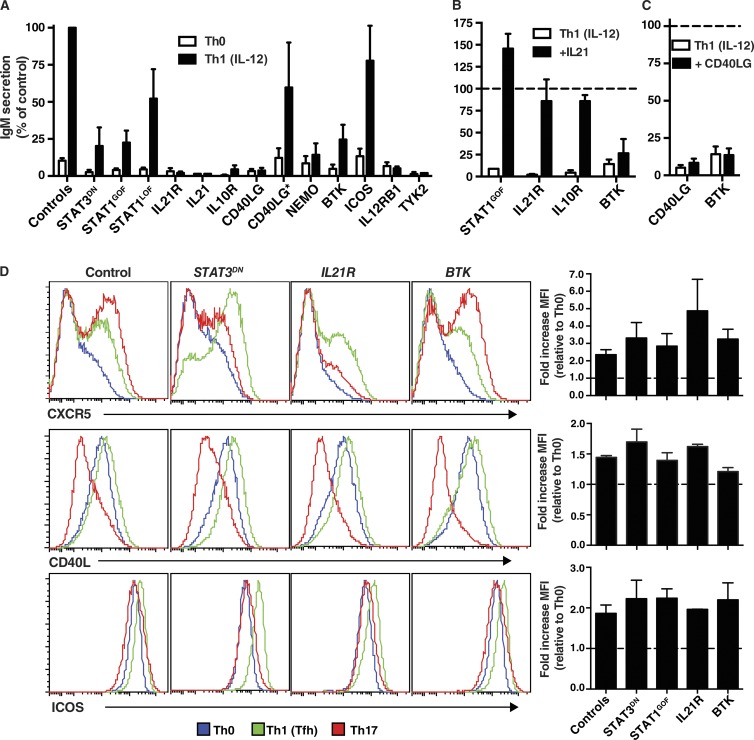
Although Tfh cells are typically defined by a canonical phenotype, the fundamental readout of Tfh biology is their ability to mediate differentiation of B cells into antibody-secreting cells (Crotty, 2011; Tangye et al., 2013). Thus, we next tested the capacity of IL-12–stimulated naive CD4+ T cells from PID patients to induce IgM secretion from co-cultured naive B cells isolated from allogeneic normal donors. When naive CD4+ T cells from normal donor are first cultured under Th0 conditions for 5 d, and then co-cultured with naive B cells, the levels of IgM secreted are very low (i.e., 39 ± 6.5 ng/ml; n = 30). However, these levels were significantly increased if the CD4+ T cells were first cultured under Th1 conditions that induced a Tfh phenotype (IgM, 590 ± 100 ng/ml; P < 0.0001). When naive CD4+ T cells from patients with STAT3 DN, IL21R, IL10R, CD40LG, NEMO, BTK, or TYK2 LOF, or GOF STAT1 GOF mutations were tested in these co-cultures, their ability to mediate B cell differentiation was greatly reduced (<25% of normal naive CD4+ T cells; Fig. 8 A). Consistent with the inability of IL12RB1 and IL21 mutant CD4+ T cells to produce IL-21 in response to IL-12 (Fig. 4 A), these cells failed to promote differentiation of co-cultured B cells (Fig. 8 A). In contrast, STAT1 or ICOS LOF mutations, or hypomorphic CD40LG mutations in individuals whose naive CD4+ T cells produced normal levels of IL-21 in vitro, did not impede B cell helper function of IL-12–primed naive CD4+ T cells (Fig. 8 A).
Figure 8.
Impaired Tfh function of naive CD4+ T cells caused by mutations affecting IL-21/STAT3 signaling or cognate T–B cell interactions. (A) Naive CD4+ T cells from healthy controls or PID patients cultured under Th0 or Th1 conditions that induce a Tfh phenotype for 5 d were then treated with mitomycin C and co-cultured with sort-purified normal allogeneic naive B cells in the presence of TAE beads. Secretion of IgM was determined after 7 d; values are expressed as IgM secretion (mean ± SEM) as a percentage of the response induced by normal naive CD4+ T cells assessed concurrently. Controls, n = 33; STAT3DN, n = 6; STAT1GOF, n = 8; STAT1LOF, n = 4; IL21R, n = 5; IL21, n = 1; IL10R, n = 2; CD40LG, n = 5; CD40LG*, n = 3; NEMO, n = 3; BTK, n = 7; ICOS, n = 4; IL12RB1, n = 4; TYK2, n = 1. (B and C) Co-cultures described in A were supplemented with exogenous IL-21 (B) or CD40L (C) on day 0; IgM secretion was determined after 7 d. The dotted line indicates the relative response (mean ± SEM) of control naive CD4+ T cells (normalized to 100%). (D) Naive CD4+ T cells from healthy controls or patients with STAT3DN, IL21R, or BTK LOF mutations or STAT1 GOF mutations were cultured under Th0, Th1/Tfh, or Th17 conditions. After 3 d, the cells were harvested, and expression of CXCR5, CD40L, or ICOS was determined by flow cytometry. The histogram plots depict expression of the indicated surface receptor on cells cultured under Th0 (blue), Th1/Tfh (green), or Th17 (red) conditions. The graphs represent fold change (mean ± SEM) in expression of the indicated cell surface marker normalized to the Th0 culture (dashed line). Controls, n = 5; STAT3LOF, n = 3; STAT1GOF, n = 2; IL21R, n = 2; BTK, n = 3.
Exogenous IL-21 rescues the functional Tfh defect in STAT1GOF mutant and IL21R- and IL10R-deficient naive CD4+ T cells
Given the extremely strong correlation between the inability of IL-12–primed mutant naive CD4+ T cells to acquire expression of IL-21 (Fig. 4 A) and of these cells to induce B cell differentiation (Fig. 8 A), we tested the hypothesis that impaired B cell help was a direct result of a lack of IL-21 production. For these experiments, we attempted to restore impaired B cell help of CD4+ T cells with STAT3 DN, IL21R, IL10R, or BTK LOF or STAT1 GOF mutations by supplementing the co-cultures with exogenous IL-21. Exogenous IL-21 greatly improved Ig secretion by IL-12–primed naive CD4+ T cells with STAT1 GOF or IL21R, IL10R LOF (Fig. 8 B), or STAT3 DN (not depicted; Ma et al., 2012) mutations. Remarkably, the level of B cell help was restored to that of normal in vitro–derived Tfh-type cells (Fig. 8 B), confirming that poor IL-21 production by these cells was responsible for ineffective B cell differentiation. The fact that exogenous IL-21 corrected defective B cell help by IL-21R–deficient CD4+ T cells (Fig. 8 B) demonstrated that it exerted this effect by directly stimulating the B cells and not co-cultured CD4+ T cells. IL-21 did not improve B cell help of naive CD4+ T cells from BTK-deficient patients (Fig. 8 B), consistent with intact production of IL-21 by these cells under Tfh-inducing conditions (Fig. 4 A). Similarly, exogenous CD40L failed to improve B cell help of IL-12–primed CD40LG-deficient naive CD4+ T cells (Fig. 8 C), consistent with poor production of IL-21 by, and a lack of expression of CD40L on, these cells (Fig. 4 A and not depicted). Impaired B cell help by BTK mutant CD4+ T cells was also unaffected by addition of CD40L to the co-cultures (Fig. 8 C).
To determine whether naive CD4+ T cells from PID patients exhibited additional defects after culture under Tfh-inducing conditions, we examined expression of signature molecules expressed by Tfh cells in vivo. The ability of naive CD4+ T cells to up-regulate CXCR5, CD40L, ICOS (Fig. 8 D), and PD-1 (not depicted) after culture under Tfh conditions compared with Th0 conditions was unaffected by STAT3 DN, IL21R, or BTK LOF or STAT1 GOF mutations. Thus, these mutations do not impair responsiveness of naive CD4+ T cells to IL-12 with respect to modifying their phenotype, akin to their intact production of IFN-γ. In contrast, STAT3 DN or IL21R LOF mutations did reduce Th17-mediated up-regulation of CXCR5 on naive CD4+ T cells (Fig. 8 D). However, CD40L expression by naive CD4+ T cells harboring these mutations continued to be repressed by Th17 cultures (Fig. 8 D). Thus, IL-21R/STAT3 signaling makes a dominant contribution to enhancing CXCR5 expression induced by Th17 stimuli, but this pathway does not regulate CD40L expression under these conditions.
B cell function in PID patients
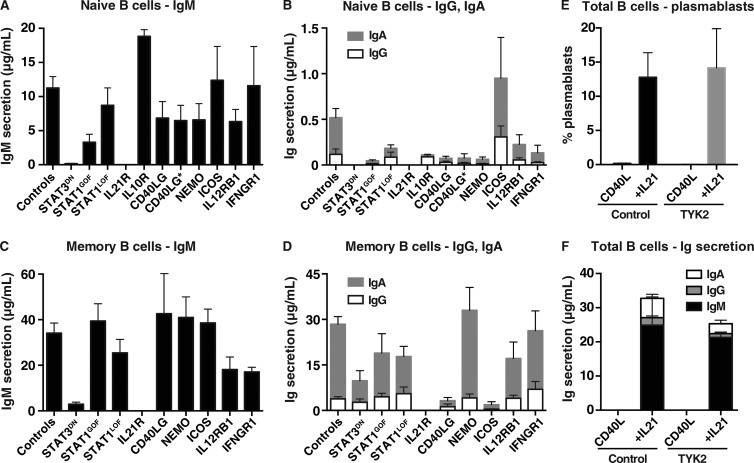
Many of the patient groups studied here—STAT3 DN, CD40LG, NEMO, IL21R, ICOS LOF, and STAT1 GOF—exhibit defects in humoral immunity (Table S1). The findings of altered naive CD4+ T cell differentiation for some of these individuals suggest the humoral immune defects are CD4+ T cell intrinsic. However, several of the genes investigated are broadly expressed in immune cells, including B cells. Thus, it was important to address the possibility that intrinsic B cell defects also contributed to poor antibody responses in some PIDs. Isolated naive B cells and, where possible, memory cells were cultured with CD40L/IL-21, a culture that induces high-rate differentiation of human B cells into antibody-secreting cells (Bryant et al., 2007; Avery et al., 2010). Consistent with our previous studies (Avery et al., 2010; Deenick et al., 2013), STAT3 DN and IL21R LOF mutant naive B cells were unresponsive to the stimulatory effects of IL-21 (Fig. 9, A and B). A similar but less severe phenotype was noted for STAT1 GOF mutant naive B cells, with 3–10-fold less secretion of IgM IgG and IgA (Fig. 9, A and B). Interestingly, although IgM production was intact for B cells with other disease-causing mutations, including severe or hypomorphic CD40LG mutations (Fig. 9 A), naive B cells with LOF NEMO mutations exhibited reduced secretion of IgG and IgA (Fig. 9 B), consistent with studies showing impaired class switching of NEMO-deficient B cells in vitro in response to CD40L and IL-2, IL-4, and/or IL-10 (Jain et al., 2001; Hanson et al., 2008). Similar results were observed for memory B cells from the different patient groups, with the exceptions of STAT3 DN, STAT1 GOF, and NEMO LOF mutant memory B cells, which exhibited greatly improved production of all Ig isotypes (Fig. 9, C and D). The ability of NEMO-deficient memory B cells to secrete normal levels of Ig in vitro may explain the variability in serum Ig in these patients (Döffinger et al., 2001; Hanson et al., 2008). The normal production of IgM, but reduced production of IgG and IgA, by memory B cells from CD40LG- and ICOS-deficient patients reflects the predominance of IgM+, and thus the lack of class switched, memory B cells in these individuals (Ma et al., 2015), rather than a B cell–intrinsic defect in class switching. Indeed, the finding of intact Ig secretion by CD40LG mutant B cells, irrespective of the severity of the mutation, highlights the B cell–extrinsic nature of the defect in humoral immunity in these individuals, consistent with the seminal work from Mayer et al. (1986). Lastly, as a result of the limited numbers of available cells, we could only perform cultures using total B cells from TYK2-deficient patients (Kreins et al., 2015). Despite this limitation, we found comparable proportions of Ig-secreting cells (Fig. 9 E) and levels of Ig secretion (Fig. 9 F) by normal and TYK2-deficient B cells in response to CD40L/IL-21 stimulation. However, TYK2 mutations did reduce B cell differentiation in response to CD40L/IL-10 (IgM, 540 ± 230 ng/ml vs. 100 ± 10 ng/ml; IgG, 75 ± 25 ng/ml vs. 14 ± 6 ng/ml; IgA, 345 ± 75 ng/ml vs. 67 ± 36 ng/ml; n = 3). Thus, although intrinsic B cell defects may impact humoral immunity in the setting of some PIDs, the inability of naive CD4+ T cells to acquire functional attributes of Tfh cells will have a predominant influence on generating productive antibody-mediated immune responses in many of the affected individuals studied here.
Figure 9.
Functional in vitro responses of naive and memory B cells from immunodeficient patients. Naive (A and B) and memory (C and D) B cells were isolated from the peripheral blood of healthy donors or patients with mutations in STAT3, STAT1 (LOF/GOF), IL21R, IL10R, CD40LG (null and hypomorphic [CD40LG*]), NEMO, ICOS, IL12RB1, or IFNGR1, and then cultured with CD40L/IL-21. Secretion of IgM (A and C), IgG and IgA (B and D) was determined after 7 d by Ig heavy chain specific ELISAs. Values represent mean ± SEM. Controls, n = 25; STAT3DN, n = 6; STAT1GOF, n = 9; STAT1LOF, n = 6; IL21R, n = 4; IL10R, n = 2; CD40LG, n = 4; NEMO, n = 5; ICOS, n = 4; IL12R, n = 5; IFNGR1, n = 6. (E and F) total B cells were isolated from healthy donors or patients with mutations in TYK2 (n = 3), and then cultured with CD40L alone or CD40L/IL-21. (E) The proportion of cells acquiring a plasmablast phenotype (CD38hiCD27hi) was determined after 5 d by flow cytometry. (F) Ig secretion was determined after 7 d.
DISCUSSION
Human CD4+ T cells contribute to immune-mediated protection against a broad array of infections. This reflects their dynamic ability to differentiate into distinct effector subsets that cooperate with other immune cells to protect the host against infection with specific pathogens (O’Shea and Paul, 2010; Zhu and Paul, 2010; Zielinski et al., 2011; Vahedi et al., 2013; Becattini et al., 2015). The relevance of these subsets is revealed by PIDs that are characterized by an inability to generate a protective immune response against defined pathogens and the associated lack of functional CD4+ T cells. An example of this is an absence of Th17 cells resulting from STAT3 DN (de Beaucoudrey et al., 2008; Ma et al., 2008; Milner et al., 2008), RORC LOF (Okada et al., 2015), or STAT1 GOF (Liu et al., 2011; Puel et al., 2011) mutations and CMC in these individuals. Thus, analyzing human CD4+ T cell function in PIDs provides opportunities to determine mechanisms underlying disease pathogenesis plus the molecular requirements for lymphocyte differentiation. By using this approach, we have now revealed the consequences of mutations responsible for several PIDs on human CD4+ T cell differentiation, with implications for the pathophysiology of these conditions.
IL21R, CD40LG, or NEMO LOF and STAT1 GOF mutations impaired the generation of IL-21+ Tfh-type cells from naive CD4+ T cells in response to IL-12, whereas such cells from BTK-deficient patients failed to induce B cell differentiation despite expressing IL-21. This substantially extends our previous findings that STAT3 DN and IL12RB1 LOF mutations diminish the ability of naive CD4+ T cells to become Tfh cells in vitro (Ma et al., 2012). These in vitro defects recapitulate the paucity of cTfh cells in patients with STAT3 DN, NEMO, BTK, IL21R, or CD40LG LOF or STAT1 GOF mutations (Ma et al., 2015) and correlated with impaired humoral immunity in these individuals. These findings provide novel insights into the generation of human Tfh cells (Fig. 10). First, the observation that IL-12–mediated induction of IL-21 in naive CD4+ T cells was comparably reduced by STAT3 or IL21R LOF mutations implied that IL-12 directly activated STAT3 to induce a Tfh-like fate, or STAT3 was required secondarily to induction of IL-21, which then promoted its own expression via STAT3. As IL-12 activated STAT4, but not STAT3, yet IL-21 activated STAT3, the latter scenario is more likely. Thus, IL-12 induces a low level of IL-21 in a STAT4-dependent manner, which then substantially up-regulates its own production in a STAT3-dependent manner (Fig. 10; Caprioli et al., 2008). This explains the reduction, but not complete abolition, of IL-12–induced IL-21 production in STAT3- and IL21R-deficient CD4+ T cells, with the low levels of IL-21 observed being induced by an IL-12/STAT4-dependent/STAT3-independent pathway. This is supported by findings in mice, where it was found that IL-12 could also induce expression of IL-21 and additional features of Tfh cells in vitro, and this was reduced by ∼75% by Stat3 deficiency but was completely abolished by Stat4 deficiency (Nakayamada et al., 2011). These findings in the setting of Stat-null murine CD4+ T cells indicate that although Stat4 is necessary for initiating IL-12–induced Tfh differentiation, it is not sufficient to achieve the maximal response; this also requires Stat3. Overall, these findings in mice (Nakayamada et al., 2011) and humans reveal that the sequential activation of STAT4 by IL-12 and STAT3 by IL-21 are required to induce IL-12–mediated differentiation of naive CD4+ T cells to a Tfh-fate in both species. Second, our finding that STAT3 DN, IL21R-deficient, or STAT1 GOF CD4+ T cells up-regulated CXCR5, CD40L, and ICOS normally separated the defect in acquiring IL-21 by IL-12–primed naive CD4+ T cells from their intact ability to exhibit phenotypic features of Tfh cells, with the former being IL-21/STAT3-dependent and the latter likely requiring IL-12/STAT4. This underscores the central requirement of STAT3 in regulating IL-21 production by Tfh cells, a prerequisite for the execution of their effector function. Indeed, impaired Tfh-type function of IL-12–primed STAT3 DN, IL21R LOF, or STAT1 GOF CD4+ T cells could be restored by exogenous IL-21. Third, NEMO mutations will limit Tfh formation by impairing TCR responses (Hanson et al., 2008), but also extrinsically by compromising CD40L-induced production of IL-12 and IL-6—inducers of human Tfh cells (Ma et al., 2009; Schmitt et al., 2009, 2014)—by human DCs (Döffinger et al., 2001; Filipe-Santos et al., 2006; Temmerman et al., 2006). Fourth, CD4+ T cells require CD40/CD40L interactions to differentiate into Tfh cells in vitro. A small subset of murine T cells has been found to express CD40, and this facilitates T cell activation in a CD40/CD40L-dependent manner (Hasbold et al., 1994; Bourgeois et al., 2002). Surprisingly, we found that activated human naive CD4+ T cells could be induced to transiently express low levels of CD40 in vitro (unpublished data). Interestingly, human CD4+ T cells can be activated by reverse signaling through CD40L to proliferate and secrete cytokines (Cayabyab et al., 1994). Thus, it is possible that CD40/CD40L signaling initiated by interactions between activated CD4+ T cells is required to generate Tfh-like cells in vitro. This would explain why CD40L-deficient human naive CD4+ T cells fail to produce IL-21 in response to IL-12, even in the absence of CD40-expressing APCs.
Figure 10.
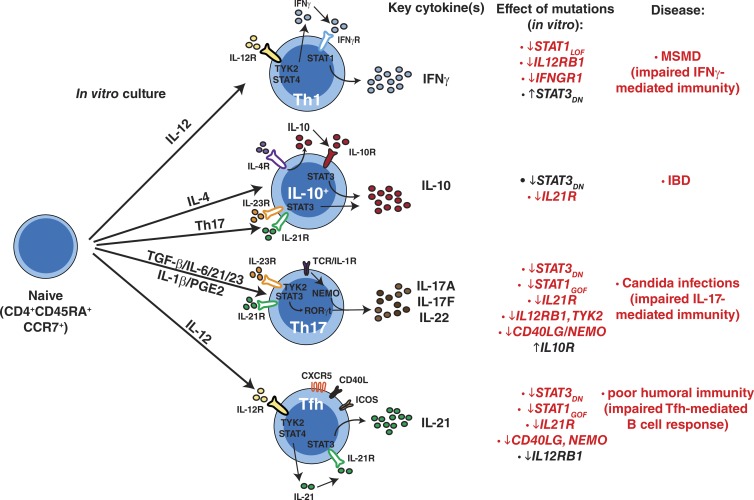
Impact of monogenic mutations on differentiation of human naive CD4+ T cells. Pathways of human naive CD4+ T cell differentiation in vitro and how specific mutations compromise these processes. MSMD, Mendelian susceptibility to mycobacterial disease; IBD, inflammatory bowel disease. The corresponding diseases listed occur in individuals with gene mutations that are shown in red type. The genes shown in black type do not result in the indicated disease states.
It was recently reported that TGF-β cooperates with IL-12 or IL-23 to induce a Tfh fate in human naive CD4+ T cells in vitro (Schmitt et al., 2014). Although there are some consistencies with our findings, i.e., co-induction of CXCR5, Bcl6, and RORγt in Th17 cultures, we did not find this culture influenced Tfh generation in vitro. Indeed, Th17 conditions failed to induce IL-21 in naive CD4+ T cells and actively repressed CD40L expression, the fundamental requirements for Tfh cells to mediate B cell differentiation, thereby explaining their inability to induce B cell differentiation in vitro. Thus, akin to our earlier findings (Ma et al., 2009, 2012), IL-12 is the single cytokine that most efficiently induces Tfh-like features in human naive CD4+ T cells, when features such as combined expression of IL-21, CXCR5, ICOS, PD-1, and induction of B cell differentiation are used as indicators of Tfh function. Although IL-12 strongly induces Tfh features in vitro, it is not required in vivo. This is evidenced from analysis of IL12B (Prando et al., 2013) or IL12RB1 mutant individuals (de Beaucoudrey et al., 2010; Schmitt et al., 2013; Ma et al., 2015), who have intact humoral immunity and only subtle reductions in cTfh cells ex vivo (Ma et al., 2012, 2015; Schmitt et al., 2013). Thus, inputs beyond IL-12—IL-6, IL-21, IL-27 (Ma et al., 2009, 2012; Schmitt et al., 2009; Batten et al., 2010), IL-23, and TGFβ (Schmitt et al., 2014)—compensate for impaired IL-12R signaling in vivo. This makes teleological sense because safeguards are needed to ensure effective humoral immune responses can be induced even in the absence of dominant signaling pathways.
Our findings also revealed important aspects of the biology of Th1, Th17, and IL-10–producing cells, and associated pathological conditions. STAT3 DN, IL12RB1, NEMO, and TYK2 LOF mutations abolished (whereas IL21R, CD40LG, and IFNGR1 LOF and STAT1 GOF mutations impaired) Th17 generation from naive CD4+ T cells. IL12RB1, TYK2, IL21R, and STAT3 deficiency all affect Th17 polarization by preventing instructive signals provided by IL-23 or IL-21 in this culture, whereas NEMO mutations impair Th17 differentiation by affecting responses through the TCR and/or IL-1R (Hanson et al., 2008; Fig. 10). CD40LG mutations likely compromise Th17 development by impairing CD40/CD40L-mediated activation of T cells in a similar manner, as the effect of these mutations on Tfh cells. Our findings of impaired Th17 differentiation by naive CD4+ T cells with STAT3 DN, IL21R, NEMO, IL12RB1, or TYK2 LOF or STAT1 GOF mutations mirrors the marked deficiency in IL-17A, IL-17F, and IL-22 production by memory CD4+ T cells from these individuals (Kreins et al., 2015; Ma et al., 2015). Poor Th17 function in vivo and in vitro caused by STAT3 DN, IL12RB1, CD40LG, or NEMO LOF, or STAT1 GOF mutations is consistent with CMC/Candida/fungal infections in some of these patients (Holland et al., 2007; Hanson et al., 2008; de Beaucoudrey et al., 2010; Picard et al., 2011; Cabral-Marques et al., 2012a,b, 2014; Puel et al., 2012; Lovell et al., 2016; Fig. 10). Similarly, several patients with IL-21R deficiency had peripheral Candida infections (Kotlarz et al., 2014), and mild CMC was reported in one of eight currently identified TYK2-deficient patient (Minegishi et al., 2006; Kreins et al., 2015).
IL12RB1, TYK2, IFNGR1, and STAT1 LOF mutations dramatically reduced IFN-γ–expressing cells induced by Th1 culture conditions. Although this was expected for IL12Rβ1 and TYK2 deficiency, the fact that these other mutations caused a similar phenotype highlights the importance of an autocrine IFN-γ/IFN-γR/STAT1 feed-forward loop in enhancing IFN-γ production downstream of IFN-γ induction by IL12R/TYK2 signaling in human CD4+ T cells (Fig. 10). This shared functional defect of IL12RB1-, TYK2-, IFNGR1-, and STAT1-deficient CD4+ T cells is consistent with these mutations causing Mendelian susceptibility to mycobacterial disease due to impaired IFN-γ–mediated immunity (Boisson-Dupuis et al., 2015). In contrast, STAT3 DN mutations increased IFN-γ induction, indicating STAT3 normally restrains Th1 differentiation. Heightened IFN-γ production by STAT3-deficient naive CD4+ T cells is consistent with an accumulation of CXCR3+ memory T cells in these patients (Ma et al., 2015) and their intact immunity against mycobacterial infection.
IL-10 was produced by naive CD4+ T cells after Th2 or Th17 culture and was compromised by STAT3 DN or IL21R LOF mutations. Because IL-4 induces IL-10 (Sornasse et al., 1996; Levings et al., 2001), and IL-10 promotes its own production (Levings et al., 2001), STAT3 DN mutations may abolish Th2-induced IL-10 production by impairing autocrine IL-10 from amplifying its own secretion via IL-10R/STAT3 (Fig. 10). However, reduced IL-10 production by IL21R-deficient naive CD4+ T cells invokes a requirement for endogenous IL-21 in inducing IL-10 by Th2 conditions. Although we did not detect IL-21 production in the Th2 culture (Fig. 1), low amounts may be present that function on naive CD4+ T cells but are consumed by these cells rendering them below the limit of detection. These in vitro findings are supported by studies in mice, which demonstrated IL-21 induced IL-10 in CD4+ T cells in an Il21r/Stat3-dependent manner (Spolski et al., 2009). They also recapitulate our recent finding that STAT3 DN or IL21R LOF mutations impaired IL-10 secretion by memory CD4+ T cells ex vivo (Ma et al., 2015), thereby highlighting the importance of this pathway for IL-10 production in vivo. Reductions in IL-10 production by Th2- or Th17-primed STAT3- and IL21R-deficient human naive CD4+ T cells were comparable, indicating IL-21 is the predominant STAT3-activating cytokine responsible for regulating IL-10 secretion by these cells. Early-onset colitis is a hallmark of patients with IL-10, IL-10R1, or IL-10R2 deficiency (Glocker et al., 2011). Thus, the relevance of reduced IL-10 production by IL-21R–deficient CD4+ T cells is consistent with colitis occurring in some patients with IL21/IL21R mutations (Kotlarz et al., 2013, 2014; Salzer et al., 2014; Erman et al., 2015). Thus, IL-21 may prevent inflammation at mucosal surfaces by inducing IL-10. Whereas STAT3 is required downstream of IL-21R for this effect, reduced IL-10 production by STAT3 DN CD4+ T cells does not cause colitis because these CD4+ T cells are unable to mediate IL-23R/STAT3-dependent inflammation, a process that is dysregulated by IL10R deficiency evidenced by hyperproduction of Th17 cytokines by IL-10R–deficient CD4+ T cells (Ma et al., 2015; Fig. S1 C). As IL-10 is also an inducer of human B cell differentiation (Moens and Tangye, 2014), reduced IL-10 production by IL-21R–deficient CD4+ T cells could contribute to humoral immune defects caused by IL21/IL21R mutations (Kotlarz et al., 2013, 2014; Salzer et al., 2014; Erman et al., 2015; Stepensky et al., 2015).
The remarkable parallels between observations made from ex vivo analysis of memory CD4+ T cells and those from assessment of in vitro differentiation of naive CD4+ T cells to defined effector fates underscores the utility and applicability of an in vitro approach to dissect human CD4+ T cell differentiation. Overlapping defects observed in vitro and ex vivo reveal the predominant pathways involved in particular biological processes in vivo. From the gene defects studied here, IL-21R/STAT3 signaling is required for Tfh, Th17, and IL-10–secreting cells, yet it represses Th1 responses. STAT3 also restrains Th2 responses, as shown by increased production of IL-4, IL-5, and IL-13 by cTfh cells from STAT3-deficient patients (Fig. S1 D), extending recent findings for Stat3-deficient murine Tfh cells (Wu et al., 2015). Similarly, IL12Rβ1/TYK2 and IFN-γR/STAT1 signaling are required for Th1 cells, whereas IL12Rβ1/TYK2, NEMO, and CD40L are also required for Th17 induction. There was considerable similarity in effects of STAT3 DN and STAT1 GOF mutations on CD4+ T cell differentiation, including impaired generation of Tfh- and Th17-type cells in vitro. This is consistent with our recent findings that these mutations comparably altered the phenotype and function of cTfh cells and reduced Th17 cells in vivo (Ma et al., 2015). Collectively, our study provides a detailed analysis of molecular requirements for generating specialized subsets of human effector CD4+ T cells and reveals the value of using experiments of nature to determine these pathways. These pathways are not only important for understanding how CD4+ T cells operate but also for identifying molecules or pathways that could be targeted to modulate the function of Th1, Th2, Th17, Tfh and IL-10-producing CD4+ T cells in the settings of immunodeficiency or autoimmunity, as well as vaccination.
MATERIALS AND METHODS
Human samples
PBMCs were isolated from healthy controls (Australian Red Cross) and PID patients with disease-causing mutations in STAT1, STAT3, IL10R, IL21R, IL21, CD40LG, NEMO, ICOS, BTK, IL12RB1, IFNGR1, or TYK2 (Table S1; Dorman et al., 2004; Orange et al., 2004; Warnatz et al., 2006; Holland et al., 2007; Hanson et al., 2008; Avery et al., 2010; de Beaucoudrey et al., 2010; Ma et al., 2012, 2015; Deenick et al., 2013; Kotlarz et al., 2013, 2014; Salzer et al., 2014; Erman et al., 2015; Kreins et al., 2015; Stepensky et al., 2015; Lovell et al., 2016). Human tonsils were obtained from healthy donors undergoing routine tonsillectomy (Mater Hospital, North Sydney, NSW, Australia). All studies were approved by Institutional Human Research Ethics Committees.
Antibodies and reagents
eFluor660-anti–IL-21, Alexa Fluor 488–anti–IL-10, PerCP-Cy5.5-anti–IFN-γ, FITC-anti-CD45RA, PE-anti-ICOS, and anti-Tbet, and biotin–PD-1 were from eBioscience. Alexa Fluor 488–anti-GATA3, Alexa Fluor 647–anti-CXCR5, anti-pSTAT4, anti-pSTAT5, anti-pSTAT6, APC-anti-CD10, APC-Cy7-anti-CD4, BV605-anti-IgG, BV421-CD40L, BV711-anti–IL-2, PE-anti-pSTAT1, anti-RORγt, and Bcl-6, PE-Cy7-anti-CD25, and anti-CD27, PerCP-Cy5.5-anti-CD127, anti-pSTAT3, and anti-Tbet, SA-PerCpCy5.5, and IFN-γ were obtained from BD. Pacific Blue–anti-CD20 and SA-BV605 were purchased from BioLegend. Recombinant human IL-12 was purchased from R&D Systems. TGF-β, IL-1β, IL-6, IL-21, and IL-23 were obtained from PeproTech. PGE2 was purchased from Sigma-Aldrich. Human IL-4 and IL-10 were provided by R. de Waal Malefyt (DNAX Research Institute, Palo Alto, CA).
Lymphocyte isolation
For T cells, PBMCs were incubated with mAbs to CD4, CD45RA, CCR7, CD127, and CD25. Naive CD4+ T cells were isolated by first excluding T reg cells (CD25hiCD127lo), and then sorting CD45RA+CCR7+ cells. For B cells, tonsil MNCs were incubated with mAbs to CD20, CD27, CD10, and IgG. Naive B cells were isolated by sorting CD20+CD10−CD27−IgG− cells.
Analysis of CD4+ T cell differentiation in vitro
Isolated naive CD4+ T cells were cultured in 96-well round bottomed well plates (30–40 × 103 cells/well) with T cell activation and expansion (TAE) beads (anti-CD2/CD3/CD28; Miltenyi Biotech) alone (Th0) or under Th1 (50 ng/ml IL-12), Th2 (100 U/ml IL-4), or Th17 (2.5 ng/ml TGFβ, 20 ng/ml IL-1β, 50 ng/ml IL-6, 50 ng/ml IL-21, 100 ng/ml IL-23, and 50 ng/ml PGE2) polarizing conditions. After 5 d, supernatants were harvested and production of IL-5, IL-10, IL-13, IL-17A, IL-17F, and IFN-γ determined by CBAs (BD). For cytokine expression, activated CD4+ T cells were restimulated with PMA (100 ng/ml)/ionomycin (750 ng/ml) for 6 h, with Brefeldin A (10 µg/ml) added after 2 h. Cells were then fixed and expression of intracellular cytokines (IL-2, IL-10, IL-21, and IFN-γ) detected (Ma et al., 2008, 2012). Due to variability in induction of IL-21 by IL-12, results are normalized to expression by corresponding control naive CD4+ T cells and then averaged for all experiments performed. In some experiments, naive CD4+ T cells were first labeled with CFSE, and proliferation was determined by assessing CFSE dilution (Ma et al., 2008, 2012). For gene expression, RNA was extracted, and transcribed into cDNA. Expression of TBX21, GATA3, RORC, and BCL6 was determined by qPCR and standardized to GAPDH (Ma et al., 2009, 2012). Expression of Tbet, GATA3, RORγt, and Bcl-6 protein was also assessed by intracellular staining using a Fix/Perm kit from eBioscience (GATA3, RORγt, and Tbet) or following fixing with 2% formaldehyde and permeabilization with saponin (Bcl-6).
Expression of phospho-STATs
Naive CD4+ T cells were isolated from healthy donors and preactivated with TAE beads for 4–5 d. After this time, the cells were stimulated under Th0, Th1, Th17 conditions, or with IL-10 (100 U/ml), IL-21 (50 ng/ml), or IFN-γ (1,000 U/ml) for 25 min at 37°C. Cells were fixed in formaldehyde for 30 min, permeabilized in 90% methanol, and then stained with mAbs specific for pSTAT1, pSTAT3, pSTAT4, or pSTAT6 (Avery et al., 2010).
T and B cell co-culture assays and Ig determination
Naive CD4+ T cells were cultured under Th0, Th1, Th2, or Th17 conditions for 5 d. After this time, the cells were treated with mitomycin C (100 µg/ml; Sigma-Aldrich), and then co-cultured at a 1:1 ratio (50 × 103/200 µl/well) with sort-purified allogeneic naive tonsillar B cells (Ma et al., 2009, 2012). In some experiments, exogenous IL-21 was added to the cultures. After 7 d, Ig secretion was determined by ELISA (Avery et al., 2010; Ma et al., 2012).
Microarray analysis
Naive CD4+ T cells were isolated from PBMCs of healthy controls or from different PID patients by sort-purifying CD4+CD45RA+CD127+CD25lo cells. Memory CD4+ T cells from healthy controls were isolated as CD4+CD45RA− cells. RNA was extracted, transcribed to cDNA, and then gene expression analysis was determined using Affymetrix Human Gene 2.0 or Human Gene 2.1 ST microarray chips. Normalization (robust multichip average), log2 transformation and probe set summarization were performed for each dataset using Bioconductor packages implemented in the R statistical computing environment, version 3.1.1. Subsequent processing and analyses were performed using GenePattern modules. The datasets were merged, quantile normalized, and batch corrected using the MergeColumns, NormaliseColumns, and ComBat modules. Differential gene expression analysis was assessed using LimmaGP. The top 100 differentially expressed genes between normal healthy naive and normal healthy memory T cells, as determined by LimmaGP analysis, were used to filter the combined and batch corrected dataset of naive CD4+ T cells from 7 healthy controls and 19 PID patients, and memory CD4+ T cells from three healthy controls. Unsupervised clustering was performed using NMFConsensus and NMF (Brunet et al., 2004). The GEO accession no. for the microarray data is GSE81408.
Statistical analysis
Significant differences were determined using Prism (GraphPad Software).
Online supplemental material
Fig. S1 shows the effect of monogenic mutations in Th1, Th17, and Th2 cells. Fig. S2 depicts CFSE profiles of naive CD4+ T cells obtained for healthy donors and PID patients after culture under Th1, Th2, or Th17 polarizing conditions. Table S1 lists the mutations identified in the indicated genes in each of the PID patients included in this study, as well as the nature of inheritance, features of each PID. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20151467/DC1.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients and families, without whom we would know much less, for their invaluable contributions to this project. We also thank Rob Salomon and the Garvan Flow Facility for cell sorting, and Andrew Williams and the Immunology laboratory at Children’s Hospital Westmead for genotyping some of the patients used in this study.
This work was supported National Health and Medical Research Council (NHMRC) of Australia grants 596813, 1016953, 1066694, 1027400, and 1004632 (to CSM, EKD, DAF, MCC, TGP, SGT), the German Federal Ministry of Education and Research (BMBF 01EO1303, to BG and KW), and Rockefeller University Center for 541 Clinical and Translational science (5UL1RR024143, to JLC). CSM is a recipient of a Career Development Fellowship (1008820); SGT is a recipient of a Principal Research Fellowship (1042925) from the NHMRC of Australia and a Senior Scholarship from the Fulbright Commission.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- Bcl-6
- B cell lymphoma/leukemia-6
- BTK
- Bruton’s tyrosine kinase
- CBA
- cytometric bead array
- CD40L
- CD40 ligand
- CMC
- chronic mucocutaneous candidiasis
- cTfh
- circulating T follicular helper
- DN
- dominant negative
- GOF
- gain of function
- ICOS
- inducible costimulator
- LOF
- loss of function
- NEMO
- NF-κB essential modulator
- NMF
- non-negative matrix factorization
- PD-1
- programmed death-1
- PID
- primary immunodeficiency
- TAE
- T cell activation and expansion
- Tfh
- T follicular helper
References
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F., et al. . 2007. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204:1849–1861. 10.1084/jem.20070663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery D.T., Deenick E.K., Ma C.S., Suryani S., Simpson N., Chew G.Y., Chan T.D., Palendira U., Bustamante J., Boisson-Dupuis S., et al. . 2010. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207:155–171. 10.1084/jem.20091706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M., Ramamoorthi N., Kljavin N.M., Ma C.S., Cox J.H., Dengler H.S., Danilenko D.M., Caplazi P., Wong M., Fulcher D.A., et al. . 2010. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J. Exp. Med. 207:2895–2906. 10.1084/jem.20100064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becattini S., Latorre D., Mele F., Foglierini M., De Gregorio C., Cassotta A., Fernandez B., Kelderman S., Schumacher T.N., Corti D., et al. . 2015. T cell immunity. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science. 347:400–406. 10.1126/science.1260668 [DOI] [PubMed] [Google Scholar]
- Boisson-Dupuis S., Bustamante J., El-Baghdadi J., Camcioglu Y., Parvaneh N., El Azbaoui S., Agader A., Hassani A., El Hafidi N., Mrani N.A., et al. . 2015. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol. Rev. 264:103–120. 10.1111/imr.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K., Bak-Jensen K.S., Li Y., Blumenschein W.M., McGeachy M.J., McClanahan T.K., McKenzie B.S., Kastelein R.A., Cua D.J., and de Waal Malefyt R.. 2009. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 206:535–548. 10.1084/jem.20082293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller L., Burger J., Draeger R., Grimbacher B., Knoth R., Plebani A., Durandy A., Baumann U., Schlesier M., Welcher A.A., et al. . 2006. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 177:4927–4932. 10.4049/jimmunol.177.7.4927 [DOI] [PubMed] [Google Scholar]
- Bourgeois C., Rocha B., and Tanchot C.. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 297:2060–2063. 10.1126/science.1072615 [DOI] [PubMed] [Google Scholar]
- Brunet J.P., Tamayo P., Golub T.R., and Mesirov J.P.. 2004. Metagenes and molecular pattern discovery using matrix factorization. Proc. Natl. Acad. Sci. USA. 101:4164–4169. 10.1073/pnas.0308531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant V.L., Ma C.S., Avery D.T., Li Y., Good K.L., Corcoran L.M., de Waal Malefyt R., and Tangye S.G.. 2007. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 179:8180–8190. 10.4049/jimmunol.179.12.8180 [DOI] [PubMed] [Google Scholar]
- Cabral-Marques O., Arslanian C., Ramos R.N., Morato M., Schimke L., Soeiro Pereira P.V., Jancar S., Ferreira J.F., Weber C.W., Kuntze G., et al. . 2012a Dendritic cells from X-linked hyper-IgM patients present impaired responses to Candida albicans and Paracoccidioides brasiliensis. J. Allergy Clin. Immunol. 129:778–786. 10.1016/j.jaci.2011.10.026 [DOI] [PubMed] [Google Scholar]
- Cabral-Marques O., Schimke L.F., Pereira P.V., Falcai A., de Oliveira J.B., Hackett M.J., Errante P.R., Weber C.W., Ferreira J.F., Kuntze G., et al. . 2012b Expanding the clinical and genetic spectrum of human CD40L deficiency: the occurrence of paracoccidioidomycosis and other unusual infections in Brazilian patients. J. Clin. Immunol. 32:212–220. 10.1007/s10875-011-9623-6 [DOI] [PubMed] [Google Scholar]
- Cabral-Marques O., Klaver S., Schimke L.F., Ascendino E.H., Khan T.A., Pereira P.V., Falcai A., Vargas-Hernández A., Santos-Argumedo L., Bezrodnik L., et al. . 2014. First report of the Hyper-IgM syndrome Registry of the Latin American Society for Immunodeficiencies: novel mutations, unique infections, and outcomes. J. Clin. Immunol. 34:146–156. 10.1007/s10875-013-9980-4 [DOI] [PubMed] [Google Scholar]
- Caprioli F., Sarra M., Caruso R., Stolfi C., Fina D., Sica G., MacDonald T.T., Pallone F., and Monteleone G.. 2008. Autocrine regulation of IL-21 production in human T lymphocytes. J. Immunol. 180:1800–1807. 10.4049/jimmunol.180.3.1800 [DOI] [PubMed] [Google Scholar]
- Cayabyab M., Phillips J.H., and Lanier L.L.. 1994. CD40 preferentially co-stimulates activation of CD4+ T lymphocytes. J. Immunol. 152:1523–1531. [PubMed] [Google Scholar]
- Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621–663. 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- de Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Jannière L., et al. . 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 205:1543–1550. 10.1084/jem.20080321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L., Samarina A., Bustamante J., Cobat A., Boisson-Dupuis S., Feinberg J., Al-Muhsen S., Jannière L., Rose Y., de Suremain M., et al. . 2010. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore). 89:381–402. 10.1097/MD.0b013e3181fdd832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick E.K., Ma C.S., Brink R., and Tangye S.G.. 2011. Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr. Opin. Immunol. 23:111–118. 10.1016/j.coi.2010.10.007 [DOI] [PubMed] [Google Scholar]
- Deenick E.K., Avery D.T., Chan A., Berglund L.J., Ives M.L., Moens L., Stoddard J.L., Bustamante J., Boisson-Dupuis S., Tsumura M., et al. . 2013. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J. Exp. Med. 210:2739–2753. 10.1084/jem.20130323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döffinger R., Smahi A., Bessia C., Geissmann F., Feinberg J., Durandy A., Bodemer C., Kenwrick S., Dupuis-Girod S., Blanche S., et al. . 2001. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-κB signaling. Nat. Genet. 27:277–285. 10.1038/85837 [DOI] [PubMed] [Google Scholar]
- Dorman S.E., Picard C., Lammas D., Heyne K., van Dissel J.T., Baretto R., Rosenzweig S.D., Newport M., Levin M., Roesler J., et al. . 2004. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 364:2113–2121. 10.1016/S0140-6736(04)17552-1 [DOI] [PubMed] [Google Scholar]
- Erman B., Bilic I., Hirschmugl T., Salzer E., Çagdas D., Esenboga S., Akcoren Z., Sanal O., Tezcan I., and Boztug K.. 2015. Combined immunodeficiency with CD4 lymphopenia and sclerosing cholangitis caused by a novel loss-of-function mutation affecting IL21R. Haematologica. 100:e216–e219. 10.3324/haematol.2014.120980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe-Santos O., Bustamante J., Haverkamp M.H., Vinolo E., Ku C.L., Puel A., Frucht D.M., Christel K., von Bernuth H., Jouanguy E., et al. . 2006. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J. Exp. Med. 203:1745–1759. 10.1084/jem.20060085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker E.O., Kotlarz D., Klein C., Shah N., and Grimbacher B.. 2011. IL-10 and IL-10 receptor defects in humans. Ann. N. Y. Acad. Sci. 1246:102–107. 10.1111/j.1749-6632.2011.06339.x [DOI] [PubMed] [Google Scholar]
- Hanson E.P., Monaco-Shawver L., Solt L.A., Madge L.A., Banerjee P.P., May M.J., and Orange J.S.. 2008. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J. Allergy Clin. Immunol. 122:1169–1177.e16. 10.1016/j.jaci.2008.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbold J., Johnson-Léger C., Atkins C.J., Clark E.A., and Klaus G.G.. 1994. Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur. J. Immunol. 24:1835–1842. 10.1002/eji.1830240817 [DOI] [PubMed] [Google Scholar]
- He J., Tsai L.M., Leong Y.A., Hu X., Ma C.S., Chevalier N., Sun X., Vandenberg K., Rockman S., Ding Y., et al. . 2013. Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 39:770–781. 10.1016/j.immuni.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Herati R.S., Reuter M.A., Dolfi D.V., Mansfield K.D., Aung H., Badwan O.Z., Kurupati R.K., Kannan S., Ertl H., Schmader K.E., et al. . 2014. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. J. Immunol. 193:3528–3537. 10.4049/jimmunol.1302503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K., Poholek A., Vahedi G., Laurence A., Kanno Y., Milner J.D., and O’Shea J.J.. 2013. Mechanisms underlying helper T-cell plasticity: implications for immune-mediated disease. J. Allergy Clin. Immunol. 131:1276–1287. 10.1016/j.jaci.2013.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.M., DeLeo F.R., Elloumi H.Z., Hsu A.P., Uzel G., Brodsky N., Freeman A.F., Demidowich A., Davis J., Turner M.L., et al. . 2007. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 357:1608–1619. 10.1056/NEJMoa073687 [DOI] [PubMed] [Google Scholar]
- Jain A., Ma C.A., Liu S., Brown M., Cohen J., and Strober W.. 2001. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat. Immunol. 2:223–228. 10.1038/85277 [DOI] [PubMed] [Google Scholar]
- Kotlarz D., Ziętara N., Uzel G., Weidemann T., Braun C.J., Diestelhorst J., Krawitz P.M., Robinson P.N., Hecht J., Puchałka J., et al. . 2013. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J. Exp. Med. 210:433–443. 10.1084/jem.20111229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarz D., Ziętara N., Milner J.D., and Klein C.. 2014. Human IL-21 and IL-21R deficiencies: two novel entities of primary immunodeficiency. Curr. Opin. Pediatr. 26:704–712. 10.1097/MOP.0000000000000160 [DOI] [PubMed] [Google Scholar]
- Kreins A.Y., Ciancanelli M.J., Okada S., Kong X.F., Ramírez-Alejo N., Kilic S.S., El Baghdadi J., Nonoyama S., Mahdaviani S.A., Ailal F., et al. . 2015. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J. Exp. Med. 212:1641–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W.J. 2001. Cytokines and immunodeficiency diseases. Nat. Rev. Immunol. 1:200–208. [DOI] [PubMed] [Google Scholar]
- Levings M.K., Sangregorio R., Galbiati F., Squadrone S., de Waal Malefyt R., and Roncarolo M.G.. 2001. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166:5530–5539. 10.4049/jimmunol.166.9.5530 [DOI] [PubMed] [Google Scholar]
- Liu L., Okada S., Kong X.F., Kreins A.Y., Cypowyj S., Abhyankar A., Toubiana J., Itan Y., Audry M., Nitschke P., et al. . 2011. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 208:1635–1648. 10.1084/jem.20110958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yan X., Zhong B., Nurieva R.I., Wang A., Wang X., Martin-Orozco N., Wang Y., Chang S.H., Esplugues E., et al. . 2012. Bcl6 expression specifies the T follicular helper cell program in vivo. J. Exp. Med. 209:1841–1852: S1–S24. 10.1084/jem.20120219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Nurieva R.I., and Dong C.. 2013. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol. Rev. 252:139–145. 10.1111/imr.12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci M., Havenar-Daughton C., Landais E., Wu J., Kroenke M.A., Arlehamn C.L., Su L.F., Cubas R., Davis M.M., Sette A., et al. International AIDS Vaccine Initiative Protocol C Principal Investigators . 2013. Human circulating PD-1+CXCR3−CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 39:758–769. 10.1016/j.immuni.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell J.P., Foruraghi L., Freeman A.F., Uzel G., Zerbe C.S., Su H.C., Hsu A.P., and Holland S.M.. 2016. Persistent nodal histoplasmosis in NF-κB essential modulator (NEMO) deficiency: Report of a case and review of infection in primary immunodeficiencies. J. Allergy Clin. Immunol. 10.1016/j.jaci.2016.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Hare N.J., Nichols K.E., Dupré L., Andolfi G., Roncarolo M.G., Adelstein S., Hodgkin P.D., and Tangye S.G.. 2005. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J. Clin. Invest. 115:1049–1059. 10.1172/JCI200523139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., and Cook M.C.. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205:1551–1557. 10.1084/jem.20080218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Suryani S., Avery D.T., Chan A., Nanan R., Santner-Nanan B., Deenick E.K., and Tangye S.G.. 2009. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol. Cell Biol. 87:590–600. 10.1038/icb.2009.64 [DOI] [PubMed] [Google Scholar]
- Ma C.S., Avery D.T., Chan A., Batten M., Bustamante J., Boisson-Dupuis S., Arkwright P.D., Kreins A.Y., Averbuch D., Engelhard D., et al. . 2012. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 119:3997–4008. 10.1182/blood-2011-11-392985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Wong N., Rao G., Avery D.T., Torpy J., Hambridge T., Bustamante J., Okada S., Stoddard J.L., Deenick E.K., et al. . 2015. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J. Allergy Clin. Immunol. 136:993–1006.e1. 10.1016/j.jaci.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H.D., Ray J.P., Laidlaw B.J., Zhang N., Gawande D., Staron M.M., Craft J., and Kaech S.M.. 2015. The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. eLife. 4:e04851 10.7554/eLife.04851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini H., Enright V., Perro M., Workman S., Birmelin J., Giorda E., Quinti I., Lougaris V., Baronio M., Warnatz K., and Grimbacher B.. 2011. Importance of B cell co-stimulation in CD4(+) T cell differentiation: X-linked agammaglobulinaemia, a human model. Clin. Exp. Immunol. 164:381–387. 10.1111/j.1365-2249.2011.04377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Kwan S.P., Thompson C., Ko H.S., Chiorazzi N., Waldmann T., and Rosen F.. 1986. Evidence for a defect in “switch” T cells in patients with immunodeficiency and hyperimmunoglobulinemia M. N. Engl. J. Med. 314:409–413. 10.1056/NEJM198602133140703 [DOI] [PubMed] [Google Scholar]
- McCarron M.J., and Marie J.C.. 2014. TGF-β prevents T follicular helper cell accumulation and B cell autoreactivity. J. Clin. Invest. 124:4375–4386. 10.1172/JCI76179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. . 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 452:773–776. 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y., Saito M., Morio T., Watanabe K., Agematsu K., Tsuchiya S., Takada H., Hara T., Kawamura N., Ariga T., et al. . 2006. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 25:745–755. 10.1016/j.immuni.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Mitsdoerffer M., Lee Y., Jäger A., Kim H.J., Korn T., Kolls J.K., Cantor H., Bettelli E., and Kuchroo V.K.. 2010. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA. 107:14292–14297. 10.1073/pnas.1009234107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens L., and Tangye S.G.. 2014. Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front. Immunol. 5:65 10.3389/fimmu.2014.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G., Foucat E., Dullaers M., Oh S., Sabzghabaei N., et al. . 2011. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 34:108–121. 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayamada S., Kanno Y., Takahashi H., Jankovic D., Lu K.T., Johnson T.A., Sun H.W., Vahedi G., Hakim O., Handon R., et al. . 2011. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 35:919–931. 10.1016/j.immuni.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt S.L., Taubenheim N., Hasbold J., Corcoran L.M., and Hodgkin P.D.. 2011. The genetic network controlling plasma cell differentiation. Semin. Immunol. 23:341–349. 10.1016/j.smim.2011.08.010 [DOI] [PubMed] [Google Scholar]
- O’Shea J.J., and Paul W.E.. 2010. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 327:1098–1102. 10.1126/science.1178334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., Markle J.G., Deenick E.K., Mele F., Averbuch D., Lagos M., Alzahrani M., Al-Muhsen S., Halwani R., Ma C.S., et al. . 2015. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 349:606–613. 10.1126/science.aaa4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J.S., Jain A., Ballas Z.K., Schneider L.C., Geha R.S., and Bonilla F.A.. 2004. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J. Allergy Clin. Immunol. 113:725–733. 10.1016/j.jaci.2004.01.762 [DOI] [PubMed] [Google Scholar]
- Pallikkuth S., Parmigiani A., Silva S.Y., George V.K., Fischl M., Pahwa R., and Pahwa S.. 2012. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 120:985–993. 10.1182/blood-2011-12-396648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Casanova J.L., and Puel A.. 2011. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IκBα deficiency. Clin. Microbiol. Rev. 24:490–497. 10.1128/CMR.00001-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prando C., Samarina A., Bustamante J., Boisson-Dupuis S., Cobat A., Picard C., AlSum Z., Al-Jumaah S., Al-Hajjar S., Frayha H., et al. . 2013. Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine (Baltimore). 92:109–122. 10.1097/MD.0b013e31828a01f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Cypowyj S., Bustamante J., Wright J.F., Liu L., Lim H.K., Migaud M., Israel L., Chrabieh M., Audry M., et al. . 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 332:65–68. 10.1126/science.1200439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Cypowyj S., Maródi L., Abel L., Picard C., and Casanova J.L.. 2012. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr. Opin. Allergy Clin. Immunol. 12:616–622. 10.1097/ACI.0b013e328358cc0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer E., Kansu A., Sic H., Májek P., Ikincioğullari A., Dogu F.E., Prengemann N.K., Santos-Valente E., Pickl W.F., Bilic I., et al. . 2014. Early-onset inflammatory bowel disease and common variable immunodeficiency-like disease caused by IL-21 deficiency. J. Allergy Clin. Immunol. 133:1651–9.e12. 10.1016/j.jaci.2014.02.034 [DOI] [PubMed] [Google Scholar]
- Saraiva M., and O’Garra A.. 2010. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10:170–181. 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- Schmitt N., Morita R., Bourdery L., Bentebibel S.E., Zurawski S.M., Banchereau J., and Ueno H.. 2009. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 31:158–169. 10.1016/j.immuni.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N., Bustamante J., Bourdery L., Bentebibel S.E., Boisson-Dupuis S., Hamlin F., Tran M.V., Blankenship D., Pascual V., Savino D.A., et al. . 2013. IL-12 receptor β1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 121:3375–3385. 10.1182/blood-2012-08-448902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N., Liu Y., Bentebibel S.E., Munagala I., Bourdery L., Venuprasad K., Banchereau J., and Ueno H.. 2014. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat. Immunol. 15:856–865. 10.1038/ni.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornasse T., Larenas P.V., Davis K.A., de Vries J.E., and Yssel H.. 1996. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J. Exp. Med. 184:473–483. 10.1084/jem.184.2.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolski R., Kim H.P., Zhu W., Levy D.E., and Leonard W.J.. 2009. IL-21 mediates suppressive effects via its induction of IL-10. J. Immunol. 182:2859–2867. 10.4049/jimmunol.0802978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepensky P., Keller B., Abuzaitoun O., Shaag A., Yaacov B., Unger S., Seidl M., Rizzi M., Weintraub M., Elpeleg O., and Warnatz K.. 2015. Extending the clinical and immunological phenotype of human interleukin-21 receptor deficiency. Haematologica. 100:e72–e76. 10.3324/haematol.2014.112508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suan D., Nguyen A., Moran I., Bourne K., Hermes J.R., Arshi M., Hampton H.R., Tomura M., Miwa Y., Kelleher A.D., et al. . 2015. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity. 42:704–718. 10.1016/j.immuni.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Suto A., Kashiwakuma D., Kagami S., Hirose K., Watanabe N., Yokote K., Saito Y., Nakayama T., Grusby M.J., Iwamoto I., and Nakajima H.. 2008. Development and characterization of IL-21-producing CD4+ T cells. J. Exp. Med. 205:1369–1379. 10.1084/jem.20072057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye S.G., and Hodgkin P.D.. 2004. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology. 112:509–520. 10.1111/j.1365-2567.2004.01950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye S.G., Ma C.S., Brink R., and Deenick E.K.. 2013. The good, the bad and the ugly - TFH cells in human health and disease. Nat. Rev. Immunol. 13:412–426. 10.1038/nri3447 [DOI] [PubMed] [Google Scholar]
- Temmerman S.T., Ma C.A., Borges L., Kubin M., Liu S., Derry J.M., and Jain A.. 2006. Impaired dendritic-cell function in ectodermal dysplasia with immune deficiency is linked to defective NEMO ubiquitination. Blood. 108:2324–2331. 10.1182/blood-2006-04-017210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi G., C Poholek A., Hand T.W., Laurence A., Kanno Y., O’Shea J.J., and Hirahara K.. 2013. Helper T-cell identity and evolution of differential transcriptomes and epigenomes. Immunol. Rev. 252:24–40. 10.1111/imr.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatz K., Bossaller L., Salzer U., Skrabl-Baumgartner A., Schwinger W., van der Burg M., van Dongen J.J., Orlowska-Volk M., Knoth R., Durandy A., et al. . 2006. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 107:3045–3052. 10.1182/blood-2005-07-2955 [DOI] [PubMed] [Google Scholar]
- Weng N.P., Araki Y., and Subedi K.. 2012. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 12:306–315. 10.1038/nri3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Xu L.L., Teuscher P., Liu H., Kaplan M.H., and Dent A.L.. 2015. An inhibitory role for the transcription factor Stat3 in controlling IL-4 and Bcl6 expression in follicular helper t cells. J. Immunol. 195:2080–2089. 10.4049/jimmunol.1500335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., and Paul W.E.. 2010. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 238:247–262. 10.1111/j.1600-065X.2010.00951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski C.E., Corti D., Mele F., Pinto D., Lanzavecchia A., and Sallusto F.. 2011. Dissecting the human immunologic memory for pathogens. Immunol. Rev. 240:40–51. 10.1111/j.1600-065X.2010.01000.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.