Abstract
Remote sensing and geographic analysis of woody vegetation provide means of evaluating the distribution of natural resources, patterns of biodiversity and ecosystem structure, and socio-economic drivers of resource utilization. While these methods bring geographic datasets with global coverage into our day-to-day analytic spheres, many of the studies that rely on these strategies do not capitalize on the extensive collection of existing field data. We present the methods and maps associated with the first spatially-explicit models of global tree density, which relied on over 420,000 forest inventory field plots from around the world. This research is the result of a collaborative effort engaging over 20 scientists and institutions, and capitalizes on an array of analytical strategies. Our spatial data products offer precise estimates of the number of trees at global and biome scales, but should not be used for local-level estimation. At larger scales, these datasets can contribute valuable insight into resource management, ecological modelling efforts, and the quantification of ecosystem services.
Subject terms: Ecological modelling, Forest ecology, Forestry, Macroecology
Background & Summary
In this paper we detail the background, methods, and data associated with the first spatially-continuous model of global tree density1. This research was motivated by (i) a gap in the publically available forest-based geospatial data products, (ii) a specific request from Plant for the Planet Foundation, and (iii) recently published estimates of tree density in the Amazon basin2 implying that the previous estimate of the number of trees globally3 was potentially an order of magnitude too low.
Forests cover approximately one-third of the world’s terrestrial land surface4. They are fundamental in dictating ecosystem structure5,6, biogeochemical processes7,8, animal habitat9, biomass and carbon sequestration10,11, and anthropogenic demand for building materials, pulp products, and fuelwood12. An understanding of the extent of forest resources plays a critical role in sustainable forest management4, helping to guide policy and to provide key targets for initiatives like the Convention on Biological Diversity’s Strategic Plan for Biodiversity 2011–2020 (ref. 13), the United Nations Collaborative Programme on Reducing Emissions from Deforestation and Forest Degradation in Developing Countries (REDD)14, and the landmark 2015 United Nations Conference of Parties Agreement15–17.
A number of recent studies inform our understanding of the distribution and extent of forest resources2,18–21. However, until recently, global scale models have not focused on estimating forest population parameters such as total tree numbers or tree density1. These variables complement existing data (e.g., refs 19,22–24) and lend themselves to modelling biogeochemical processes8,25, nutrient cycling26, habitat suitability9, forest biodiversity27, and drivers of forest structure and heterogeneity28,29. Furthermore, the number and density of trees are intuitive metrics of interest to public and non-governmental organizations30,31, particularly those focused on tree planting, such as New York City’s MillionTreesNYC32 and the United Nations Environment Programme’s (UNEP) ‘Billion Tree Campaign’33.
To quantify the proportional impact of these reforestation campaigns and to establish meaningful reforestation targets, a baseline understanding of current tree numbers was essential. We initially developed the global tree density analysis to address this uncertainty. Further, we hypothesized that available data would demonstrate the extent to which biophysical and social variables interact to regulate global patterns in tree abundance. The previous estimate for the global number of trees was approximately 400.25 billion3—a mere 10 billion more than has been estimated for the Amazon basin during a recent broad-scale inventory2—highlighting a critical gap in our understanding of global tree densities.
We emphasize that the modelling approach described herein provides precise estimates of total number of trees and mean tree density at global and biome scales. However, this precision does not necessarily apply to smaller scales and our data products should not be used for local- or regional-scale analysis without further assessment (see Usage Notes).
Methods
Overview
To model global tree density we employed a spatially-explicit approach in which (i) field measurements were first linked to a suite of remote sensing and GIS covariates; (ii) predictive regression models were then developed using model selection criteria; and (iii) these models were then applied in a pixel-level map algebraic framework to develop spatially-explicit predictions of global tree density (Fig. 1).
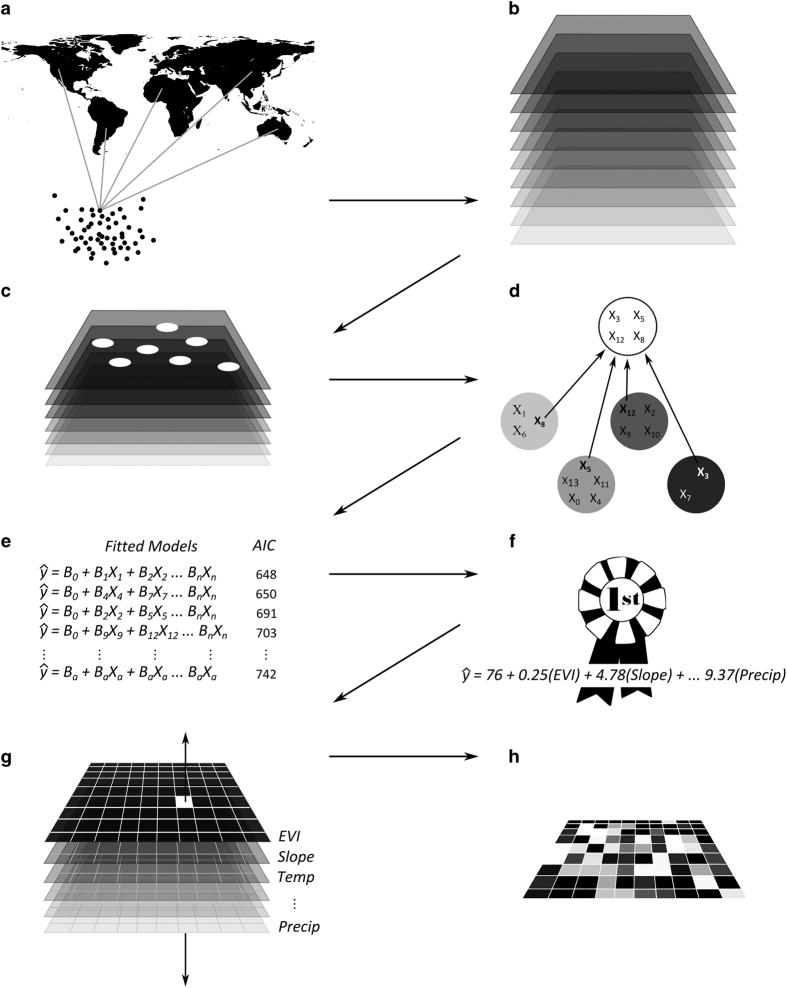
Figure 1. A conceptual model of our analytical process.
(a) We amassed over 420,000 forest inventory plot records from every continent except Antarctica. (b) We acquired and unified an initial pool of four-dozen spatial covariates to use in model development. (c) We selected a subset of spatial covariates, extracted their values at field plot locations, and bound these values to the plot records. (d) For each of 14 biomes we subjected the enhanced plot records to hierarchical (agglomerative) clustering to identify the least collinear collection of covariates. (e) Generalized linear models were fit to every possible combination of clustered covariates. (f) A top ranking predictive model was selected or created through model averaging. (g) Each biome’s top ranking model was applied in a pixel-level map algebraic framework. (h) We scaled a penultimate spatial model of tree density using land cover data to arrive at our final predictions.
Data collection and standardization
To model tree density across large geographic extents we collected field-based forest inventory plot records from around the globe. Plot-level data was obtained through three channels: (i) major forestry databases, (ii) peer-reviewed studies, and (iii) correspondence with individual scientists. We used both national and international forestry databases, including National Forest Inventory (NFI) analyses from 21 countries, the Global Index of Vegetation-Plot Database (GIVD http://www.givd.info), the Smithsonian Tropical Research Institute’s in-house database (http://www.stri.si.edu), and ICP-Level-I plot data for most of Europe (http://www.icp-forests.org). These sources provided the vast majority of our data, but were supplemented with inventory data reported through peer-reviewed publications during the last 10 years2,34,35. Small, unpublished collections of field data were obtained through several contacts where we lacked broad-scale inventory data (P. Umunay, DRC; R. Tavani, European NFI).
Although we defined trees as those larger than 10 cm diameter at breast height (DBH; i.e., to separate established trees from seedlings and saplings), the minimum-diameter thresholds for what constitutes a tree vary by country and inventory purpose. In the U.S. NFI—the Forest Inventory and Analysis National Program (FIA)—a tree is defined as a plant with a woody stem and DBH equal to or greater than 12.7 cm (i.e., 5 in). However, the 10 cm DBH threshold is used across most international forest inventory analyses, and the U.S. FIA was easily adapted to this level. After threshold identification, plot data was cleaned and collated using R (v. 3.1.x, Core R Development Team 2015), providing a total of 429,775 independent records for which we had, at a minimum: (i) latitude, (ii) longitude, and (iii) tree density (trees per hectare). Density measurements were derived through a number of proven field methods, including both fixed and variable radius plot sampling.
In the U.S., FIA data had been subjected to ‘jiggering’ or ‘fuzzing’, and ‘swapping’, in which plot locations had been spatially relocated by the US Forest Service to prohibit direct knowledge of plot locations36,37. A jiggered plot is generally less than 0.5 mi, but up to 1 mi, from the original plot location, and is to be placed in a stand that shares comparable structural attributes (i.e., the density of a jiggered plot must match the density of its true plot). McRoberts et al.36 suggest that plot jiggering should not introduce extensive bias in regression modelling when working with large pixel sizes (here ~1 km2), large sample sizes, and across broad spatial extents (see Acquisition and pre-processing of spatial data). Swapping describes the swapping of up to 20% of private plot coordinates between comparable plots within the same county, making local-level estimate impossible. Neither jiggering nor swapping should have affected our results given the spatial extents of our models.
Acquisition and pre-processing of spatial data
To generate spatially-explicit estimates of global tree density, we first acquired or developed 48 map-based covariates to consider during model development (Table 1 (available online only)). These datasets were pre-processed using R’s ‘raster’ package38, ArcMap 10.1 (ESRI, Redlands, CA), and conventional spatial data management strategies, including, as necessary: mosaicking raster tiles and unifying projections, ensuring precise pixel-level spatial coincidence using environmental processing controls and nearest neighbor resampling, using map algebraic operators and ArcGIS geoprocessing tools to create derivatives, performing spatial extractions (masking) to ensure a common spatial extent across datasets, and performing spatial joins to extract covariate values (Table 2). Each of the 48 datasets was obtained and initially managed using the World Geodetic System 1984 (WGS84) at a spatial resolution of 30-arc seconds (0.008333 degrees). Covariate pixel values were bound to spatially coincident field plot locations stored in a vector point file, ultimately producing a single tabular dataset from which to generate statistical models. During preliminary data exploration we discarded 28 less useful covariates based on (i) multicollinearity and (ii) mismatches between spatial resolution and scale. The remaining 20 covariates captured a range of topographic, climatic, vegetative, and anthropogenic factors (Table 1 (available online only)). We present the full complement of covariates and their primary sources, including those omitted in final analyses, to provide a clear sense of the data considered throughout model development.
Table 1. All spatial covariate datasets considered and/or used in analysis, as well as their sources and processing notes.
| Layer | Source | Initial Nominal Resolution | Inclusion | Notes |
|---|---|---|---|---|
|
1Crowther, T. W. et al. Mapping tree density at a global scale. Nature. 525, 201–2015 (2015). |
||||
|
52Danielson, J. J., and Gesch, D. B. 2011. Global Multi-resolution Terrain Dlevation Data 2010 (GMTED2010). U.S. Geological Survey, Reston, VA. (https://lta.cr.usgs.gov/GMTED2010). |
||||
|
53FAO/IIASA/ISRIC/ISS-CAS/JRC, 2012. Harmonized World Soil Database (version 1.2). FAO, Rome, Italy and IIASA, Laxenburg, Austria. (http://webarchive.iiasa.ac.at/Research/LUC/External-World-soil-database/HTML/). |
||||
|
54Hijmans, R.J., S.E. Cameron, J.L. Parra, P.G. Jones, and A. Jarvis. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25: 1965–1978. (http://www.worldclim.org/current). |
||||
|
55Zomer, R. J., Trabucco, A., and van Straaten, O. 2007. A Global Analysis on the Hydrologic Dimensions of Climate Change Mitigation through Afforestation/Reforestation. International Water Management Institute, Research Report 101. (http://www.cgiar-csi.org/data/global-aridity-and-pet-database). |
||||
|
56Zomer, R. J., Trabucco, A., Bossio, D. A., and Verchot, L. V. 2008. Climate change mitigation: A spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Agriculture Ecosystems and Environment, 126: 67–80 (http://www.cgiar-csi.org/data/global-aridity-and-pet-database). |
||||
|
57EarthEnv: Global environmental layers for climate, ecosystem, and biodiversity research. (http://www.earthenv.org/). |
||||
|
58Shunlin, L., and Zhiqiang, X. 2012. Global Land Surface Products: Leaf Area Index Product Data Collection(1985-2010). Beijing Normal University. (http://glcf.umd.edu/data/) |
||||
|
59Xiao, Z., Liang, S., Wang, J., et al., 2013. Use of general regression neural networks for generating the GLASS Leaf Area Index product from time series MODIS surface reflectance. IEEE Transactions on Geoscience and Remote Sensing. (http://glcf.umd.edu/data/). |
||||
| 60Center for International Earth Science Information Network: Socioeconomic Data and Applications Center. (http://sedac.ciesin.columbia.edu/data/sets/browse). | ||||
| Topographic |
||||
| Elevation | GMTED201052 | 250 m | + | |
| Slope | Derived from GMTED2010 above | 250 m | + | |
| Northness | Derived from GMTED2010 above | 250 m | + | Northness values inverted for southern hemisphere |
| Eastness | Derived from GMTED2010 above | 250 m | + | |
| Terrain Ruggedness Index (TRI) | Derived from GMTED2010 above | 250 m | + | Mean of absolute differences between a cell and its adjacent neighbors |
| Absolute Value of Latitude | Author generated using 500 m fishnet (ArcMap 10.1) | 500 m | + | |
| Global Soils | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil Clay Fraction (% wt.) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil Silt Fraction (% wt.) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil Sand Fraction (% wt.) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil Base Saturation (%) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil Bulk Density (kg/dm3) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil Calcium Carbonate (% wt.) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil Cation Exchange Capacity (cmol/kg) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil Organic Carbon (% wt.) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Topsoil pH (H2O) (−log(H+) | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Reference Soil Depth | Harmonized World Soils Database v. 1.253 | 1 km | − | |
| Climatic |
||||
| Annual Mean Temperature (Bio1) | WorldClim v. 154 | 1 km | + | |
| Mean Diurnal Range (Mean of monthly (max temp - min temp)) (Bio2) | WorldClim v. 154 | 1 km | − | |
| Isothermality (Bio2/Bio7) (* 100) (Bio3) | WorldClim v. 154 | 1 km | − | |
| Temperature Seasonality (standard deviation *100) (Bio4) | WorldClim v. 154 | 1 km | − | |
| Max Temperature of Warmest Month (Bio5) | WorldClim v. 154 | 1 km | − | |
| Min Temperature of Coldest Month (Bio6) | WorldClim v. 154 | 1 km | − | |
| Temperature Annual Range (Bio1-Bio2) (Bio7) | WorldClim v. 154 | 1 km | + | |
| Mean Temperature of Wettest Quarter (Bio8) | WorldClim v. 154 | 1 km | − | |
| Mean Temperature of Driest Quarter (Bio9) | WorldClim v. 154 | 1 km | − | |
| Mean Temperature of Warmest Quarter (Bio10) | WorldClim v. 154 | 1 km | − | |
| Mean Temperature of Coldest Quarter (Bio11) | WorldClim v. 154 | 1 km | − | |
| Annual Precipitation (Bio12) | WorldClim v. 154 | 1 km | + | |
| Precipitation of Wettest Month (Bio13) | WorldClim v. 154 | 1 km | − | |
| Precipitation of Driest Month (Bio14) | WorldClim v. 154 | 1 km | + | |
| Precipitation Seasonality (Coefficient of Variation) (Bio15) | WorldClim v. 154 | 1 km | + | |
| Precipitation of Wettest Quarter (Bio16) | WorldClim v. 154 | 1 km | − | |
| Precipitation of Driest Quarter (Bio17) | WorldClim v. 154 | 1 km | + | |
| Precipitation of Warmest Quarter (Bio18) | WorldClim v. 154 | 1 km | − | |
| Precipitation of Coldest Quarter (Bio19) | WorldClim v. 154 | 1 km | − | |
| Potential Evapotranspiration per Hectare per Year (1950–2000) | CGIARCSI Global Aridity and PET Database55,56 | 1 km | + | |
| Indexed Annual Aridity (1950−2000) | CGIARCSI Global Aridity and PET Database55,56 | 1 km | + | |
| Vegetative |
||||
| Enhanced Vegetation Index (EVI) | Crowther et al1; Global Habitat Heterogeneity57 | 1 km | + | 90th percentile of composited MOD13Q1 v. 5 (250 m) 16-day composites from 2001–2005, inclusive |
| Dissimilarity of EVI | Global Habitat Heterogeneity57 | 1 km | + | Difference in EVI between adjacent pixels |
| Contrast of EVI | Global Habitat Heterogeneity57 | 1 km | + | Exponentialy weighted difference in EVI between adjacent pixels |
| Uniformity (Angular Second Moment) of EVI | Global Habitat Heterogeneity57 | 1 km | + | Orderliness of EVI among adjacent pixels |
| Leaf Area Index | Global Land Cover Facility58,59 | 1 km | + | MOD09A1 derivation; 8-day composites from 2005, days 017 (s. hemisphere) and 193 (n. hemisphere) |
| Surface Reflectance Bands 1–7 | Global Land Cover Facility58,59 | 500 m | − | MOD44C derivation; 32-day composites of 16-day MOD44C composites (Collection 3), beginning days 193 (2005 n. hemisphere) and 361 (2004 s. hemisphere) |
| Normalized Difference Vegetation Index (NDVI) | Derived from Surface Reflectance above | 500 m | − | |
| Anthro. |
||||
| Urban/Built-up, and Cultivated and Managed Vegetation | Consensus Land Cover v. 157 | 1 km | + | Including GlobCover; Classes 7 and 9 |
| Human Appropriation of Net Primary Productivity | CIESIN: SEDAC60 | − | ||
| Global Human Footprint (1995–2004) | CIESIN: SEDAC60 | 1 km | − | v. 2 |
Table 2. Basic methods used to manage and pre-process spatial datasets.
| Spatial data pre-processing method | Description |
|---|---|
| Environmental Controls | Used in conjunction with other operations to control geospatial products. |
| Processing extent | Used to process all datasets at a common extent to eliminate unexpected data loss around land mass peripheries prior to controlled masking. |
| Snap raster | Used to ensure all datasets of a common resolution had precise pixel-level spatial coincidence. |
| Projection | Used to ensure all datasets held a common coordinate system for processing (WGS84) and area-dependent tabulation (Interrupted Goode Homolosine). |
| Construction | |
| Mosaicking | Used to spatially mosaic datasets delivered in tiled format. |
| Nearest neighbor resampling | Used to unify raster cell size across datasets without introducing new data values. |
| Map algebra and geoprocessing tools | Used to produce derivative covariates (e.g., slope, aspect, etc.). |
| Spatial extraction/masking | Used to reduce all datasets to the smallest common extent prior to model fitting. |
| Spatial joining | Used to bind covariate values to coincident plot locations. |
Given the inherent variability of plot-based tree density estimates, we developed spatial models for large geographic extents ensuring a high degree of confidence in mean tree density estimates. We relied on two maps of ecologically unique regions delineated by The Nature Conservancy (TNC): Biomes and Ecoregions (Terrestrial Ecoregions map - http://maps.tnc.org/gis_data.html). Biomes are large geographic areas (i.e., continental scale) linked through similarities in biodiversity and associated drivers, originally developed by the World Wildlife Fund (WWF; www.worldwildlife.org/biomes) as the Terrestrial Ecoregions component of their Global Ecoregions dataset. TNC’s Terrestrial Ecoregions are smaller, more localized regions that share a similar habitat type. Individual predictive models were developed for each of the 14 unique terrestrial biomes and 806 unique ecoregions that possibly contained forested land. These two models—hereafter biome-level and ecoregion-level—were used to create two estimates of global forest density corresponding to two different spatial scales of inquiry.
Statistical modelling
Using the above-mentioned tabular data, we produced statistical models through a multi-step process: (i) hierarchical clustering; (ii) model selection; (iii) model pairing. Given the interactive nature of many biophysical factors, we suspected strong interactions and/or multicollinearity among the selected set of 20 variables (Table 1 (available online only)). To account for this we used ascendant (agglomerative) hierarchical clustering for each biome-level model. Hierarchical clustering is an unsupervised learning algorithm in which groups of values are iteratively split and merged, ultimately dividing them into clusters whose inter-group distance or within-group homogeneity is maximized39. In this way, the strategy is similar to other iterative clustering algorithms such as ISODATA or k-means40. The product of clustering is a series of discrete groups (clusters) that contain values from one or more covariates that are better correlated with one another than with covariates in another cluster.
We employed ascendant hierarchical clustering using the hclustvar function in the ClustOfVar R package41. Homogeneity of clusters was defined as the sum of the squared correlation between the variables in a cluster and their respective cluster center (here a synthetic quantitative variable equivalent to the first principal component of a PCA mix analysis). To maximize predictive model strength and reduce collinearity we selected a single best ‘indicator’ variable from each cluster based on the squared loading values. This process produced, for each set of field plot records associated with a given biome or ecoregion, a single set of top-ranking covariates to consider during model development.
To estimate tree density in each biome we used the reduced set of covariates to construct generalized linear regression models42 with a negative binomial error structure (to accommodate count data that cannot extend below zero). To optimize model strength we used dredge, a multi-model dredging function in R’s MuMln package43. This function evaluates and ranks all possible candidate models from a set of predictors in a global model according to Akaike Information Criterion (AICc) and AIC likelihood weights (AICw). Where no single covariate was overwhelmingly influential, there were, in most cases, a number of candidate models nested within the global model that performed comparably well. We therefore employed weighted model averaging of the dredged models with cumulative AIC weights≥0.95 (ref. 44).
We constructed a unique regression model for each biome or ecoregion that contained at least 50 tree density measurements (for rationale see Model validation and testing). We lacked sufficient plot data for two of the forested biomes: ‘Mangroves’ and ‘Tropical and subtropical coniferous forests’, primarily due to the relative rarity of these biomes worldwide (representing 0.23 and 0.48% of the global land surface, respectively). In both cases we used models from the most analogous biomes for which we had sufficient data, relying on similarity in geography and general ecological conditions (e.g., moist environment, broadleaf species). The ‘Tropical and subtropical moist broadleaf’ biome was substituted for the ‘Mangroves biome’, and ‘Temperate coniferous biome’ for the ‘Tropical and subtropical coniferous’ biome. Because we used ecological analogy, the biome-level estimates for these areas should be considered less reliable than those of other biomes. At the ecoregion level, the distribution of plot-level data prevented us from modelling a large number of global ecoregions. For each of the missing ecoregion models we used the spatially coincident biome-level model in its place, such that the final global ecoregion model of tree density is largely driven by biome-level regression models.
Spatial modelling
Our final biome- and ecoregion-level negative binomial regression models were applied in a map algebraic framework45 using an iterative looping structure in R. We relied on the doSnow and foreach packages46,47 to perform computations in an embarrassingly parallel manner, such that each computational task bore no dependency on any other computational task. For both models, random access memory (RAM) limitations were bypassed by individually processing more than 10,000 geographically distinct regions and mosaicking the results to create a final map of predicted global tree density.
Prior to making area-dependent calculations, mosaicked datasets were reprojected to the Interrupted Goode Homolosine projected coordinate system48 and outlying predictions were truncated to 10,000 trees · ha−1 based on biome-level variability and expert knowledge of forest structure. Density estimates were then scaled from per-hectare units to per-pixel units where each pixel was nominally 1 km2 (897.27 m×897.27 m, or 0.805 km2 under Goode Homolosine projection). Since forest reference plots were predominantly located in moderately forested areas (63% +/− 35% [1 s.d.] forested, on average), the original model had minimal predictive power in regions with markedly different land cover types than the reference plots (i.e., in grasslands, deserts, or densely forested areas). To improve the spatial mapping, we therefore used a basic ratio estimation approach49 to scale the raw model means by an auxiliary independent data set—the global 1-km consensus land cover data set of 2014 (ref. 21)—which provides an estimate of the percent forested area for each pixel globally. First, we scaled the raw model means by the average percent forest area in the reference plots, on a biome-by-biome basis. We then multiplied this ratio by the percent forested area in the non-reference pixels. This estimation improved the final map characteristics by ensuring that pixels with 0% forests were assigned a value of zero trees, and that the difference in tree totals between two pixels with identical covariate values was directly proportional to their relative difference in percent forest cover. More importantly, this approach ensured that the global and biome-level marginal tree totals were approximately unbiased49 (see Fig. 2, Table 3).
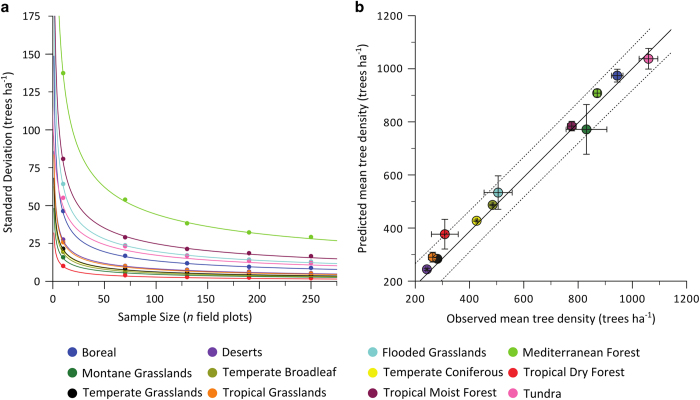
Figure 2. Statistical and spatial model validation.
(a) The standard deviation of the predicted mean number of trees per biome as a function of sample size. As sample size increases, the variability of the predicted mean tree density reaches a threshold, beyond which an increase in sample size results in a minimal increase in precision. Standard deviations were calculated using a bootstrapping approach (see Statistical model validation), and smooth curves were modeled using standard linear regression with a log–log transformation. After Crowther et al (2015) Fig. 3b. (b) Biome-level regression models predict the mean values of the omitted validation plot measurements in 12 biomes. Overall, the models underestimated mean tree density by ~3% (slope=0.97) but this difference was not statistically significant (P=0.51). Bars show±one s.d. for the predicted mean and the dotted boundaries represent the 95% confidence interval for the mean. The values plotted here represent mean densities for the plot measurements (that is, for forested ecosystems), rather than those predicted for each entire biome. Figure is modified from Crowther et al (2015) Fig. 3a.
Table 3. Summary table showing the results of model validation.
| Terrestrial Biome | n | Pred. Mean | Obs. Mean | SD | Pred. Sum | Obs. Sum | SD Sum |
|---|---|---|---|---|---|---|---|
| n= number of withheld plots, ~20% of total; Pred. Mean=Predicted mean number of trees per pixel, post scaling, at locations of withheld 20% of plots; Obs. Mean=Observed mean number of trees per plot x 100, for withheld 20% of plots; s.d.=Standard deviation of pixel-level predictions at withheld 20% of plot locations; Pred. Sum=Sum of predicted number of trees per pixel at all plot locations within each biome, post scaling; Obs. Sum=Sum of observed number of trees per plot×100, for all plots within each biome; SD Sum=Standard deviation of predicted number of trees per pixel for all plot locations within each biome; % Difference=Percent difference in the total number of trees predicted (by pixel) and observed (plot×100) at all plot locations within each biome. | |||||||
| Boreal forests | 1,116 | 98,157 | 94,459 | 1,168 | 109,542,928 | 105,416,204 | 1,303,045 |
| Deserts | 2,921 | 28,115 | 24,337 | 235 | 82,122,745 | 71,089,686 | 685,260 |
| Flooded grasslands | 55 | 47,691 | 50,576 | 2,894 | 2,623,006 | 2,781,658 | 159,169 |
| Mangroves | — | — | — | — | — | — | — |
| Mediterranean forests | 3,333 | 99,681 | 87,080 | 902 | 332,235,677 | 290,238,564 | 3,006,751 |
| Montane grasslands | 28 | 88,356 | 83,125 | 6,583 | 2,473,968 | 2,327,500 | 184,337 |
| Temperate broadleaf | 54,681 | 49,524 | 48,548 | 108 | 2,708,012,198 | 2,654,674,881 | 5,892,683 |
| Temperate conifer | 16,808 | 43,864 | 42,661 | 132 | 737,265,239 | 717,049,203 | 2,224,412 |
| Temperate grasslands | 3,415 | 30,406 | 28,215 | 264 | 103,835,175 | 96,353,092 | 900,426 |
| Tropical coniferous | — | — | — | — | — | — | — |
| Tropical dry | 17 | 48,525 | 30,938 | 4,083 | 824,925 | 525,938 | 69,415 |
| Tropical grasslands | 148 | 32,038 | 26,504 | 1,130 | 4,741,584 | 3,922,520 | 167,309 |
| Tropical moist | 1,017 | 80,839 | 77,722 | 795 | 82,212,834 | 79,043,004 | 808,476 |
| Tundra | 430 | 105,216 | 105,973 | 1,815 | 45,242,812 | 45,568,300 | 780,448 |
| Total | 83,969 | 752,410 | 700,137 | 4,211,133,091 | 4,068,990,550 | ||
| % Difference | 3.5% |
Code availability
We used custom scripting to automate a variety of tasks in the production of our global models of tree density. Covariate pre-processing was partially automated using R (v. 3.1.x, R Core Development Team 2015) and the raster38 package. Hierarchical clustering and model selection were fully automated using R’s ClustOfVar41 and MuMln43 packages, as was the spatial application of statistical models in parallel using the raster38, doSnow46, and foreach47 packages. See the above portions of Methods for additional details. At the time of publication, we have no plans to distribute the scripts used in our analysis.
Technical Validation
Statistical model validation
Using two cross-validation schemes to assess the bias and precision of our tree density estimates at plot locations, we evaluated biome-level regression models prior to applying them in a spatial context. In the first scheme, 20% of plot locations within each biome were withheld at random as an independent testing dataset and regression models were generated (dredged) from the remaining 80% using the hierarchically clustered results noted above. These models were then used to predict tree density at the withheld plot locations and the predicted densities were regressed against the observed densities (see Fig. 2 in (ref. 1)). Table 3 provides a summary of model validation results. The 80% models were constructed independently of the full models (see Statistical Modeling), however the additional 20% of plot locations in the full models only reduced bias in relation to the 80% models.
A second validation scheme evaluated the number of field plots required to maximize the precision of density estimates. Following ref. 50, we used a bootstrapping function to evaluate the incremental decrease in standard deviation of our density estimates as a function of sample size for each biome. From the 20% pool of withheld plots noted above, we used simple random sampling with replacement51 to obtain a sample of size n (n=10, 20, …, 500 plots). We next applied the fitted regression models from the retained 80% of plots to the sample to model density at the n omitted plots, from which we computed and stored the standard deviation of estimated densities. To obtain a reliable estimate of the standard deviation of tree densities for each n, this process was repeated 10,000 times for each sample size. We then plotted standard deviation as a function of sample size to evaluate the point at which an increase in the number of field plots no longer increased the precision of estimated densities (Fig. 2a). Beyond 50 field plots the inclusion of additional data produced only minor increases in precision. This led us to use 50 field plots as the threshold for whether or not to develop a unique regression model for a given geographic area (see Statistical modelling).
Spatial model validation
To evaluate the effect of scale on global predictions, spatial models were generated at both the biome and ecoregion levels (Fig. 3). Where our models were sensitive to the proportion of forested land cover within each pixel, we generated independent biome-level models using the consensus land cover dataset21 and the map of Global Forest Change 2000–2013 (ref. 19). The former was available at 1 km2 spatial resolution while the latter required spatial aggregation from 30 m2 to 1 km2 to make it compatible with our models. We also compared our predicted tree densities to published country-scale estimates to ensure agreement (Fig. 4).
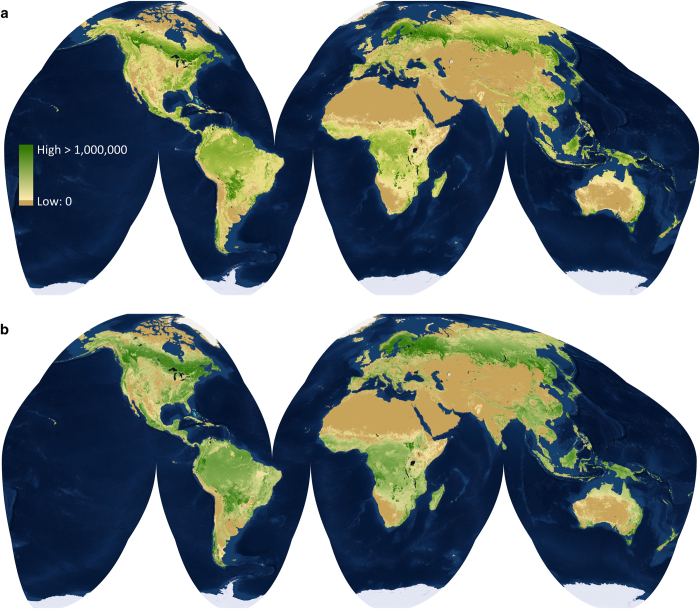
Figure 3. Global models of tree density.
Tree density as portrayed through biome- (a) and ecoregion-level (b) models where values represent number of trees per ~1 km2 pixel. Actual pixel size, 897.27 m by 897.27 m in the Goode Homolosine projection. All computations based on areal measurements were made using Goode Homolosine. Maps were produced using ESRI basemap imagery.
Figure 4. Correlation of predicted and published numbers of trees per country.
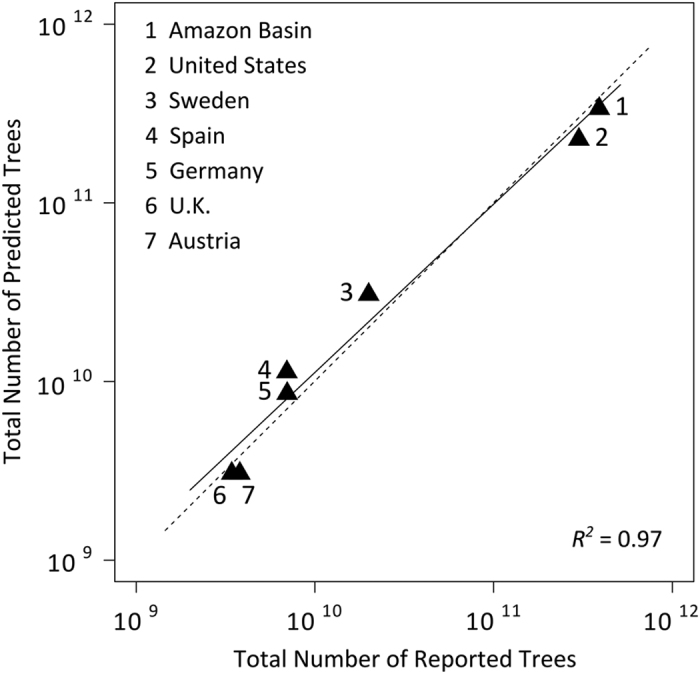
The dotted line is a 1:1 line, while the solid line is the ordinary least squares line of best fit. Figure is modified from Crowther et al (2015) Fig. 4d.
The close agreement of the biome- and ecoregion-level global models of tree density led us to compute margins of error associated with the biome-level model, which we believed to be more robust given the broader geographic regions over which it was built. We used a Taylor series approximation to estimate the variance in the global and biome-specific totals, accounting for collinearity among predicted values and the log-link negative binomial regression structures (Fig. 2b, Table 4).
Table 4. Summary table showing the number of field plots, estimates for the total number of predicted trees at those plots, and 95% confidence intervals on the estimates at biome and global scales.
| Terrestrial Biome | Number of field plots | Predicted number of trees (billions) | ±2 s.d. (billions) |
|---|---|---|---|
| Boreal forests | 8,688 | 749.3 | 50.1 |
| Deserts | 14,637 | 53.0 | 2.9 |
| Flooded grasslands | 271 | 64.6 | 14.2 |
| Mangroves | 21 | 8.2* | 0.3* |
| Mediterranean forests | 16,727 | 53.4 | 1.2 |
| Montane grasslands | 138 | 60.3 | 24.0 |
| Temperate broadleaf | 278,395 | 362.6 | 2.9 |
| Temperate coniferous | 85,144 | 150.6 | 1.3 |
| Temperate grasslands | 17,051 | 148.3 | 4.9 |
| Tropical coniferous | — | 22.2* | 0.4* |
| Tropical dry | 115 | 156.4 | 63.4 |
| Tropical grasslands | 999 | 318.0 | 35.5 |
| Tropical moist | 5,321 | 799.4 | 24.0 |
| Tundra | 2,268 | 94.9 | 6.3 |
| Global | 429,775 | 3041.2 | 96.1 |
*Mangroves and Tropical coniferous biome predictions rely on models derived from Tropical moist and Temperate coniferous biomes, respectively. Given data limitations, figures associated with these biomes should be considered less reliable than those for the other biomes.
Data Records
Due to data sharing agreements, we are unable to provide direct access to the forest inventory plot data used in model development. However, biome- and ecoregion-level spatial models (maps) of tree density can be downloaded from two locations (see Data Citation). Yale University’s EliScholar repository record (Data Citation 1) does not contain a formal DOI at the time of publication and was released with Crowther et al (2015) under a CC BY-ND license that prohibits distribution of derivatives made with the data. The Figshare repository record (Data Citation 2) offers the same data under a CC-BY license, which permits distribution of derivatives. The authors intend to maintain both repositories in parallel, but refer the reader to the Figshare repository as the primary source of new versions of these datasets.
Usage Notes
To increase the number of field plots within each ecologically-meaningful region, and to account for local-level variability in vegetative structure, we modeled tree density at large spatial scales. Through this approach we were able to obtain high precision in our global estimates of mean tree density and total number of trees. However, it is important to note that precision decreases with a concomitant reduction in spatial extent. Both biome- and ecoregion-level models are less accurate than their global counterparts, country-level estimates are less accurate than their biome- and ecoregion-level counterparts, and pixel-level estimates are less accurate than all other scales of estimation. With this in mind, we are explicit in stating that the spatial models we present here are not intended to provide accurate or precise estimates of tree density at the 1 km2 scale, nor even at the scale of small countries.
Spatial models are presented without restriction. See Data collection and standardization for known limitations in field plot information.
Additional Information
How to cite this article: Glick, H. B. et al. Spatially-explicit models of global tree density. Sci. Data 3:160069 doi: 10.1038/sdata.2016.69 (2016).
Supplementary Material
Acknowledgments
We thank Plant for the Planet for preliminary discussions and for collaboration during our initial study. The main project was funded by grants to T.W.C. from the Yale Climate and Energy Institute, the British Ecological Society, and a personal grant from Marie Curie. We acknowledge various sources for tree density measurements and estimates: the Canadian National Forest Inventory (https://nfi.nfis.org/index.php), the US Department of Agriculture Forest Service for their National Forest Inventory and Analysis (http://fia.fs.fed.us/), the Taiwan Forestry Bureau (which provided the National Vegetation Database of Taiwan), the DFG (German Research Foundation), BMBF (Federal Ministry of Education and Science of Germany), the Floristic and Forest Inventory of Santa Catarina (IFFSC), the National Vegetation Database of South Africa, and the Chilean research grants FONDECYT no. 1151495. For Europe NFI plot data were brought together with input from J. Rondeux and M. Waterinckx, Belgium, T. Bélouard, France, H. Polley, Germany, W. Daamen and H. Schoonderwoerd, Netherlands, S. Tomter, Norway, J. Villanueva and A. Trasobares, Spain, G. Kempe, Sweden. New Zealand Natural Forest plot data were collected by the LUCAS program for the Ministry for the Environment (New Zealand) and sourced from the National Vegetation Survey Databank (New Zealand) (http://nvs.landcareresearch.co.nz). We also acknowledge the BCI forest dynamics research project, which was funded by National Science Foundation grants to S. P. Hubbell, support from the Center for Tropical Forest Science, the Smithsonian Tropical Research Institute, the John D. and Catherine T. MacArthur Foundation, the Mellon Foundation, the Small World Institute Fund, numerous private individuals, the Ucross High Plains Stewardship Initiative, and the hard work of hundreds of people from 51 countries over the past two decades. The plot project is part of the Center for Tropical Forest Science, a global network of large-scale demographic tree plots. The authors acknowledge that we had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The authors declare no competing financial interests.
Data Citations
- Crowther T. W. 2015. EliScholar. http://elischolar.library.yale.edu/yale_fes_data/1
- Crowther T. W. 2016. Figshare. http://dx.doi.org/10.6084/m9.figshare.3179986
References
- Crowther T. W. et al. Mapping tree density at a global scale. Nature 525, 201–205 (2015). [DOI] [PubMed] [Google Scholar]
- ter Steege H. et al. Hyperdominance in the Amazonian tree flora. Science 342, 1243092 (2013). [DOI] [PubMed] [Google Scholar]
- Nadkarni N. Between Earth and Sky: Our Intimate Connections to Trees (University of California Press, 2008). [Google Scholar]
- FAO. Global Forest Resources Assessment 2010 - Main Report. (Rome, Italy (2010).
- Chisholm R. A. et al. Scale-dependent relationships between tree species richness and ecosystem function in forests. J. Ecol. 101, 1214–1224 (2013). [Google Scholar]
- Tuanmu M.-N. & Jetz W. A global, remote sensing-based characterization of terrestrial habitat heterogeneity for biodiversity and ecosystem modelling. Global Ecol. Biogeogr. 24, 1329–1339 (2015). [Google Scholar]
- Slik J. W. F. et al. Environmental correlates of tree biomass, basal area, wood specific gravity and stem density gradients in Borneo’s tropical forests. Global Ecol. Biogeogr. 19, 50–60 (2010). [Google Scholar]
- Walker A. P. et al. Predicting long-term carbon sequestration in response to CO2 enrichment: How and why do current ecosystem models differ? Glob. Biogeoch. Cycles 29, 476–495 (2015). [Google Scholar]
- Gouveia S. F., Villalobos F., Dobrovolski R., Beltrão-Mendes R. & Ferrari S. F. Forest structure drives global diversity of primates. J. Anim. Ecol. 83, 1523–1530 (2014). [DOI] [PubMed] [Google Scholar]
- Fauset S. et al. Hyperdominance in Amazonian forest carbon cycling. Nature communications 6, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindermann G. E., McCallum I., Fritz S. & Obersteiner M. A global forest growing stock, biomass and carbon map based on FAO statistics. Silva Fenn. 42, 387–396 (2008). [Google Scholar]
- Chimeli A. B., Boyd R. G. & Adams D. M. International timber markets and tropical deforestation: The evidence from prices. Appl. Eco 44, 1303–1314 (2011). [Google Scholar]
- UNEP .X/2. The Strategis Plan for Biodiversity 2011–2020 and the Aichi Biodiversity Targets (UNEP, 2010). [Google Scholar]
- FAO, UNDP & UNEP. The UN-REDD Programme Strategy, 2011–2015. United Nations Collaborative Programme on Reducing Emissions from Deforestation and Forest Degradation in Developing Countries, (2011). [Google Scholar]
- United Nations. Conference of the Parties: Twenty-first session, Paris, 30 November to 11 December 2015, Agenda item 4(b) (2015).
- Tollefson J. & Weiss K. R. Nations approve historic global climate accord. Nature 528, 315–316 (2015). [DOI] [PubMed] [Google Scholar]
- Tolleson J. Is the 2° C world a fantasy? Nature 527, 436–438 (2015). [DOI] [PubMed] [Google Scholar]
- Pfeifer M., Disney M., Quaife T. & Marchant R. Terrestrial ecosystems from space: A review of earth observation products for macroecology applications. Global Ecol. Biogeogr. 21, 603–624 (2012). [Google Scholar]
- Hansen M. C. et al. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- Kim D.-H. et al. Global, Landsat-based forest-cover change from 1990 to 2000. Remote Sens. Environ. 155, 178–193 (2014). [Google Scholar]
- Tuanmu M. N. & Jetz W. A global 1-km consensus land-cover product for biodiversity and ecosystem modelling. Global Ecol. Biogeogr. 23, 1031–1045 (2014). [Google Scholar]
- Saatchi S. S. et al. Benchmark map of forest carbon stocks in tropical regions across three continents. PNAS 108, 9899–9904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccini A. et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nature Clim. Change 2, 182–185 (2012). [Google Scholar]
- Harris N. L. et al. Baseline map of carbon emissions from deforestation in tropical regions. Science 336, 1573–1576 (2012). [DOI] [PubMed] [Google Scholar]
- Asner G. P. et al. A universal airborne LiDAR approach for tropical forest carbon mapping. Ecos. Ecol. 168, 1147–1160 (2012). [DOI] [PubMed] [Google Scholar]
- Melvin A. M. et al. Difference in ecosystem carbon distribution and nutrient cycling linked to forest tree species composition in a mid-successional boreal forest. Ecosystems 18, 1472–1488 (2015). [Google Scholar]
- Liang J. et al. Effects of productivity on biodiversity in forest ecosystems across the United States and China. Conserv. Biol. 30, 308–317 (2016). [DOI] [PubMed] [Google Scholar]
- Oliver C. D. & Larson B. C. Forest Stand Dynamics (McGraw-Hill, Inc., 1996). [Google Scholar]
- Riginos C. & Grace J. B. Savanna tree density, herbivores, and the herbaceous community: Bottom-up versus top-down effects. Ecology 89, 2228–2238 (2008). [DOI] [PubMed] [Google Scholar]
- Amos J. Earth’s trees number ‘three trillion’. British Broadcasting Corporation. BBC, http://www.bbc.com/news/science-environment-34134366 (2015). [Google Scholar]
- Greenfieldboyce N. Tree Counter Is Astonished By How Many Trees There Are. National Public Radio (NPR, 2015). [Google Scholar]
- Oldfield E. E. et al. Growing the urban forest: Tree performance in response to biotic and abiotic land management. Restor. Ecol. 23, 707–718 (2015). [Google Scholar]
- UNEP. United Nations Environment Programme (UNEP, 2011). [Google Scholar]
- Brus D. J. et al. Statistical mapping of tree species over Europe. Eur. J. of For. Res 131, 145–157 (2011). [Google Scholar]
- Lewis S. L. et al. Above-ground biomass and structure of 260 African tropical forests. Phil. Trans. of the Royal Soc. B 368, 20120295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts R. E. et al. Estimating and circumventing the effects of perturbing and swapping inventory plot locations. J. For 103, 275–279 (2005). [Google Scholar]
- Woudenberg S. et al. Inventory and Analysis Database: Database Description and Users Manual Version 4.0 for Phase 2.0. U.S. Department of Agriculture, Forest Service, Fort Collins, CO, (2010). [Google Scholar]
- Hijmans R. J. et al. Package ‘raster’. CRAN, Comprehensive R Archive Network, (2015). [Google Scholar]
- Rokach L., Maimon O. in Data Mining and Knowledge Discovery Handbook (eds Maimon Oded & Rokach Lior 321–352 (SpringerLink, 2005). [Google Scholar]
- Tou J. & Gonzalez R. C. Pattern Recognition Principles (Addision-Wesley Publishing Company, 1974). [Google Scholar]
- Chavent M., Kuentz V., Benoit L. & Saracco J. Package ‘ClustOfVar’ v. 0.8. CRAN, Comprehensive R Archive Network, (2015). [Google Scholar]
- Nelder J. A. & Wedderburn R. W. M. Generalized Linear Models. J.of the Royal Stat. Soc. Ser. A 135, 370–384 (1972). [Google Scholar]
- Bartoń K. Package ‘MuMln’ v. 1.15.1 (CRAN, Comprehensive R Archive Network, 2015). [Google Scholar]
- MacKenzie D. I. et al. Occupancy Estimation and Modeling (Academic Press, 2005). [Google Scholar]
- Tomlin C. D. GIS and Cartographic Modeling (Esri Press, 2012). [Google Scholar]
- Weston S. Package ‘doSNOW’ (Revolution Analytics, CRAN, Comprehensive R Archive Network, 2015). [Google Scholar]
- Weston S. Package ‘foreach’ (Revolution Analytics, CRAN, Comprehensive R Archive Network, 2015). [Google Scholar]
- Usery L. E. & Seong J. C. All equal-area map projections are created equal, but some are more equal than others. Cart. and Geo. Inf. Sci 28, 183–194 (2001). [Google Scholar]
- Thompson S. K. Sampling (John Wiley & Sons, 2002). [Google Scholar]
- Maclean M. G., Campbell M. J., Maynard D. S., Ducey M. J. & Congalton R. G. Requirements for labelling forest polygons in an object-based image analysis classification. Int. J. Remote Sens. 34, 2531–2547 (2012). [Google Scholar]
- Gregoire T. G. & Valentine H. T. Sampling Strategies for Natural Resources and the Environment (Chapman and Hall/CRC, 2004). [Google Scholar]
- Danielson J. J. & Gesch D. B. Global Multi-resolution Terrain Elevation Data 2010 (GMTED2010) (U.S. Geological Survey). https://lta.cr.usgs.gov/GMTED2010 (2011). [Google Scholar]
- FAO, IIASA, ISRIC, ISS-CAS & JRC. Harmonized World Soil Database (version 1.2). (FAO, and IIASA, http://webarchive.iiasa.ac.at/Research/LUC/External-World-soil-database/HTML/ 2012). [Google Scholar]
- Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G. & Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. of Clim 25, 1965–1978; http://www.worldclim.org/current (2005). [Google Scholar]
- Zomer R. J., Trabucco A. & van Straaten O. Carbon, Land, and Water: A Global Analysis on the Hydrologic Dimensions of Climate Change Mitigation through Afforestation/Reforestation. International Water Management Institute, Research Report 101 (http://www.cgiar-csi.org/data/global-aridity-and-pet-database, 2007). [Google Scholar]
- Zomer R. J., Trabucco A., Bossio D. A. & Verchot L. V. Climate change mitigation: A spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Ag. Eco. and Env. 126, 67–80 (http://www.cgiar-csi.org/data/global-aridity-and-pet-database, 2008). [Google Scholar]
- EarthEnv. Global environmental layers for climate, ecosystem, and biodiversity research (http://www.earthenv.org/landcover.html).
- Shunlin L. & Zhiqiang X. Global Land Surface Products: Leaf Area Index Product Data Collection (1985–2010) (Beijing Normal University http://glcf.umd.edu/data/ 2012). [Google Scholar]
- Xiao Z. et al. Use of general regression neural networks for generating the GLASS Leaf Area Index product from time series MODIS surface reflectance. IEEE Trans. on Geosc. and Rem. Sens. 52, 209–223(http://glcf.umd.edu/data/, 2013). [Google Scholar]
- Center for International Earth Science Information Network. Socioeconomic Data and Applications Center (http://sedac.ciesin.columbia.edu/data/sets/browse).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Crowther T. W. 2015. EliScholar. http://elischolar.library.yale.edu/yale_fes_data/1
- Crowther T. W. 2016. Figshare. http://dx.doi.org/10.6084/m9.figshare.3179986