Abstract
AIM: To evaluate the impact of an enteral feeding protocol on administration of nutrition to surgical intensive care unit (SICU) patients.
METHODS: A retrospective chart review was conducted on patients initiated on enteral nutrition (EN) support during their stay in a 14 bed SICU. Data collected over a seven-day period included date of tube feed initiation, rate initiated, subsequent hourly rates, volume provided daily, and the nature and length of interruptions. The six months prior to implementation of the feeding protocol (pre-intervention) and six months after implementation (post-intervention) were compared. One hundred and four patients met criteria for inclusion; 53 were pre-intervention and 51 post-intervention.
RESULTS: Of the 624 patients who received nutrition support during the review period, 104 met the criteria for inclusion in the study. Of the 104 patients who met criteria outlined for inclusion, 64 reached the calculated goal rate (pre = 28 and post = 36). The median time to achieve the goal rate was significantly shorter in the post-intervention phase (3 d vs 6 d; P = 0.01). The time to achieve the total recommended daily volume showed a non-significant decline in the post-intervention phase (P = 0.24) and the overall volume administered daily was higher in the post-intervention phase (61.6% vs 53.5%; P = 0.07). While the overall interruptions data did not reach statistical significance, undocumented interruptions (interruptions for unknown reasons) were lower in the post-intervention phase (pre = 23/124, post = 9/96; P = 0.06).
CONCLUSION: A protocol delineating the initiation and advancement of EN support coupled with ongoing education can improve administration of nutrition to SICU patients.
Keywords: Enteral nutrition, Surgical critical care, Protocol, Critical care, Nutrition support
Core tip: Surgical critical care patients are more prone to frequent feeding interruptions for unavoidable reasons. In this study we validated that implementation of a feeding protocol in a surgical intensive care unit (SICU) decreased time to achieve goal rate and increased the total volume administered daily, despite frequent interruptions. It also increased detailed documentation by unit staff of interruptions allowing us to identify a trend with regard to feeding interruptions to better understand which practices/procedures require further review. The median time to achieve the goal rate was significantly shorter in the post-intervention phase. The time to achieve the total recommended daily volume showed a non-significant decline in the post-intervention phase and the overall volume administered daily was higher in the post-intervention phase. While the overall interruptions data did not reach statistical significance, undocumented interruptions (interruptions for unknown reasons) were lower in the post-intervention phase. To our knowledge, we are the second largest single center study supporting the benefit of implementing a feeding protocol in a SICU.
INTRODUCTION
Nutrition support is an important element of managing surgical critical care patients. Perioperative malnourishment and prolonged catabolism can lead to multiple deleterious effects, including delayed or abnormal wound healing, secondary infections, muscle atrophy, and increased length of stay[1,2]. Providing early enteral nutrition (EN) helps meet the metabolic demands during the acute phase of surgery-associated critical illness, rebuilds nutritional stores during recovery, and reduces hospital mortality[3-6]. When oral feeding is not possible it is more physiologic to deliver nutrients through the gut to preserve its barrier role. EN is therefore preferred over parenteral nutrition (PN) as it has been shown to maintain gastrointestinal (GI) integrity and function and improve blood flow and peristalsis. It also prevents bacterial translocation, thereby decreasing the risk for systemic infections[7]. Existing literature shows that surgical patients are less likely to receive EN and more likely to receive PN compared to medical patients. Tube feeding is often delayed and patients are less likely to achieve nutritional adequacy following both elective and urgent surgery[8]. Patients undergoing gastrointestinal and cardiovascular surgeries receive the least amount of EN with no clear explanation[8]. Despite the known benefits, providing adequate nutrition early is challenging in the surgical intensive care unit (SICU) setting due to frequent interruptions from the scheduling of procedures and tests, perceived intolerance of tube feeding, ventilator weaning trials and routine nursing care. These lengthy and sometimes unnecessary interruptions lead to the inadequate administration of nutrition. Additionally, specific guidelines for controversial practices like checking gastric residual volume (GRV) can also lead to frequent and prolonged interruptions in feeding. Current literature on routine monitoring of GRV refutes the correlation between GRV and a patient’s risk for ventilator associated pneumonia, ICU-acquired infections, mechanical ventilation duration, ICU length of stay, or mortality rates[9] however, complete abandonment of this long-standing practice remains a challenge. Given the obstacles to optimal EN support for SICU patients, it is evident that there is a need for more structured processes that guide practitioners and standardize practice.
MATERIALS AND METHODS
A quality improvement project was conducted in the SICU to determine whether patients were being adequately fed. Results indicated that 65% of patients did not achieve goal rate during the seven-day period, and 65% of patients received less than half of the total volume recommended daily. The results of this quality review coupled with the frequency and duration of tube feed interruptions led to the development of an EN feeding protocol. The protocol outlined instructions for more timely advancement of tube feeding to goal rate and incorporated guidelines intended to decrease unnecessary feeding interruptions.
The aim of this study was to evaluate whether the EN feeding protocol improved the ability to meet nutritional goals in a timely fashion and increased overall administration of nutrition during SICU stay.
Patients and settings
This study was conducted in the SICU of a 1171-bed tertiary care teaching hospital. The SICU is a closed 14-bed unit that admits approximately 900 patients annually with an average length of stay of five days. Most SICU patients are post-operative from a variety of surgical specialties, including general surgery, surgical oncology, and liver and intestinal transplant. The charts of 624 adult patients over 18 years of age who received EN support for a one-year period were screened for inclusion in the study. Due to the retrospective nature of this study, the Institutional Review Board waived consent.
The pre-intervention phase was defined as the six months before the EN feeding protocol implementation and the post-intervention phase was the six months post implementation (Table 1).
Table 1.
Exclusion criteria
| Exclusion criteria | n | Pre-intervention phase | Post-intervention phase |
| Enteral nutrition not initiated | 10 | 8 | 2 |
| Intestinal transplant | 1 | 1 | 0 |
| Tube fed < 48 h | 30 | 13 | 17 |
| Tube feed initiated before ICU admission | 1 | 1 | 0 |
| Patient to or for GI surgery | 2 | 2 | 0 |
| Not tolerating | 1 | 1 | 0 |
| Withdrawal of care | 0 | 0 | 0 |
| Total | 45 | 26 | 19 |
Exclusion criteria with counts for pre-intervention, post-intervention and the total number of patients who met each criterion. ICU: Intensive care unit; GI: Gastrointestinal.
The primary hospital admission date, SICU admission date, formula name, date of initiation, rate initiated, subsequent hourly rates, and volume provided daily were recorded over a seven-day period. The nature and length of interruptions were noted for all patients included in the study.
Intervention
The EN protocol delineated steps for initiating, advancing and maintaining nutrition support in these patients. Following implementation of the protocol, EN was started at half the goal rate. Gastric residual volumes were checked 6 h after initiation. If GRV were less than 250 mL, EN feeds were advanced to goal rate with GRV and signs and symptoms of intolerance monitored every 6 h, for the first 24 h, or until confirmation of tolerance of tube feeding at the goal rate. In the event that GRV was more than 250 mL, the bedside nurse would inform the physician on call for further assessment of symptoms such as abdominal pain, distention, tenderness, vomiting or high GRV (≥ 500 mL). In the presence of any of these symptoms, EN feeding was held for 3 h with reevaluation thereafter. With implementation of the protocol, if symptoms were absent, the ICU team could start promotility agents, if not otherwise contraindicated. Promotility agents used included metoclopramide and erythromycin. The GRV was then rechecked after six hours and feeds advanced as indicated above. If EN was held due to intolerance or inability to advance to goal rate, PN support was considered. Stop rules for procedures were also developed to guide practitioners on the appropriate timing for holding EN support. For emergent procedures feeds would be held and NGT placed to suction to decompress the stomach. For non-emergent procedures, including planned surgery and elective tracheostomy, holding feeds 6 to 8 h prior to procedure was suggested, and for pressure support or weaning trials, holding feeds one hour prior to trial was advised. It was recommended that feeds be restarted upon return from procedure; pending confirmation from the primary team or upon determination that extubation was not possible (Figure 1). Nurses and physicians were educated on the protocol. The importance of clear and accurate documentation, including reason and duration of feeding interruptions was emphasized.
Figure 1.
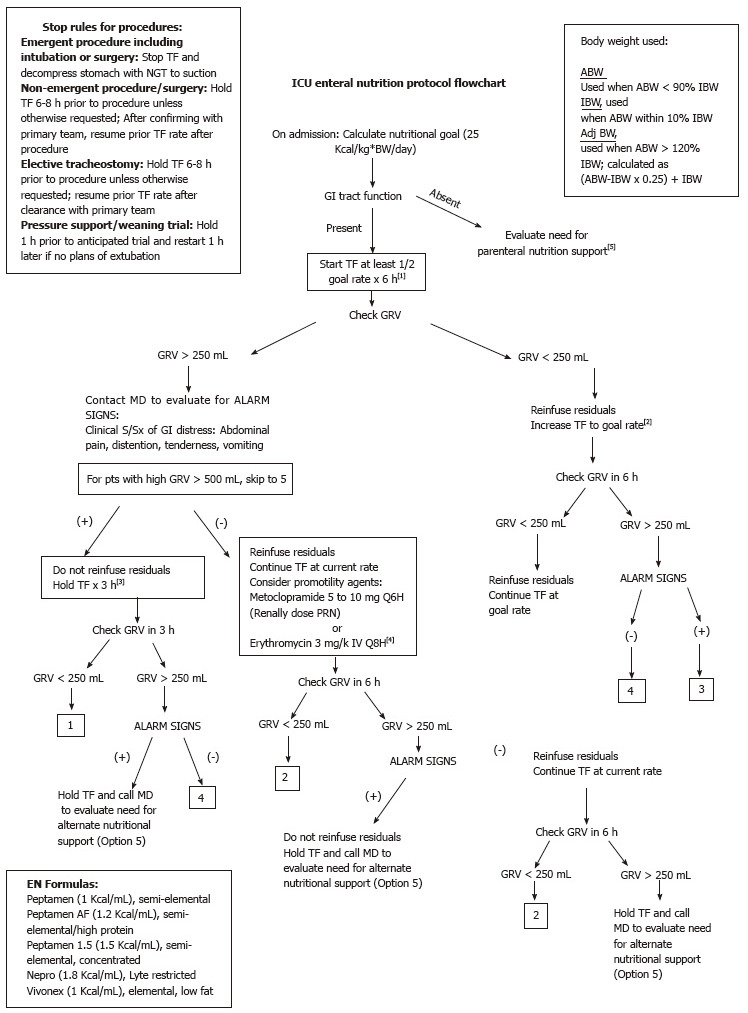
Intensive care unit enteral nutrition protocol flowchart. TF: Tube feeds; NGT: Nasogastric tube; GI: Gastrointestinal; PN: Parenteral nutrition; S/Sx: Symptoms and signs; pts: Patients; GRV: Gastric residual volume; ABW: Actual body weight; IBW: Ideal body weight; Adj BW: Adjusted body weight.
Statistical analysis
The Kaplan-Meier method was used to calculate the time to achieve goal rate and total recommended daily volume over the seven-day period. The Log-Rank test was used to compare the time to both of those events between the pre- and post-intervention phases. An aggregated average percent goal was calculated for each patient and compared. In addition, the percentage of patients who reached goal rate by day seven was compared. Interruptions were categorized by type into avoidable and unavoidable. Gastrointestinal surgeries, interventional radiology (procedures, access), tracheostomy/PEG tube placement, extubation/re-intubation, ventilator weaning trials, high GRV (> 500 mL), and abdominal imaging were considered unavoidable causes. Avoidable interruptions included imaging studies where the radiologist did not request fasting and GRV < 500 mL. The average length of interruptions by type in the pre- and post-intervention phases were also calculated and compared. The Statistical methods of this study were reviewed by John Doucette, Associate professor, preventive medicine at Icahn School of Medicine at Mt Sinai, New York.
RESULTS
Of the 624 patients who had nutrition support during the review period, 104 met criteria for inclusion in the study. Of the 104 who met criteria, 53 were pre- and 51 were post-intervention (Table 2).
Table 2.
Baseline characteristics and study cohort
| Patient demographics | Pre-intervention phase | Post-intervention phase | All patients |
| Age (yr) | 67 ± 16 | 66 ± 16 | 67 ± 16 |
| Male | 31 (58%) | 26 (51%) | 57 (55%) |
| Height (cm) | 166.79 ± 11.59 | 167.09 ± 12.37 | 166.93 ± 11.91 |
| Weight (kg) | 76.7 ± 22.8 | 81.7 ± 25.5 | 79.1 ± 24.2 |
| GI surgery | 18 (34%) | 27 (53%) | 45 (43%) |
| Vascular surgery | 8 (15%) | 3 (6%) | 11 (11%) |
| Transplant | 14 (26%) | 11 (22%) | 25 (24%) |
| Medicine | 3 (6%) | 5 (9%) | 8 (7.5%) |
| Surgical oncology | 7 (13%) | 1 (2%) | 8 (7.5%) |
| Other (ENT, HIV medicine, orthopedics, orthopedic surgery, oral and maxillofacial surgery) | 3 (6%) | 4 (8%) | 7 (7%) |
| Total | 53 | 51 | 104 |
Data are reported as mean ± SD or n (%). Patient demographics (average age, gender, average height and average admission weight) and primary service caring for patient upon admission to ICU. ICU: Intensive care unit; GI: Gastrointestinal; HIV: Human immunodeficiency virus.
The largest admitting service was GI surgery followed by transplant, vascular surgery, surgical oncology, orthopedics and medicine.
Of the 104 patients monitored during the seven-day period, 40 did not reach goal rate (pre = 25, post = 15). Among those who did not reach goal rate, 22 stopped enteral feeding before the seventh day due to extubation, transfer from ICU or hemodynamic instability (pre = 16/25, post = 6/15; P = 0.14). The remaining 18 patients continued on tube feeds for seven days without reaching goal rate.
The distribution of patients who reached goal rate was 55% (28/53) during the pre-intervention phase, and 71% (36/51) during the post-intervention phase. The median time to achieve goal was significantly shorter in the post-intervention phase (3 d vs 6 d; P = 0.01) (Figure 2). The overall time to achieve total recommended daily volume showed a non-significant decline in the post-intervention phase (P = 0.24) (Figure 3). The overall volume administered daily was higher in the post-intervention phase (61.6% vs 53.5%; P = 0.07).
Figure 2.
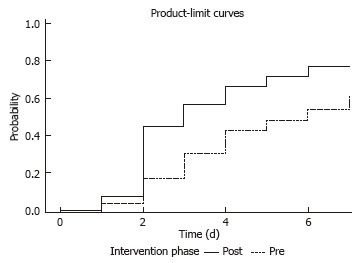
Days to achieve goal rate by intervention phase. The Kaplan-Meier method was used to calculate the time to achieve goal rate over the seven-day period. The log-rank test was used to compare the time to both of those events between the pre- and post-intervention phases.
Figure 3.
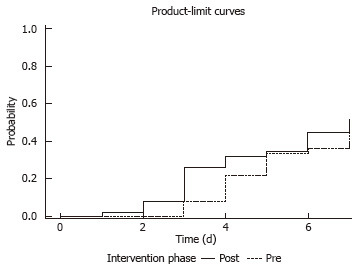
Days to achieve total recommended volume by intervention phase. The Kaplan-Meier method was used to calculate the time to achieve total recommended daily volume over the seven-day period. The log-rank test was used to compare the time to both of those events between the pre- and post-intervention phases.
There were 124 instances of TF interruptions in the pre-intervention phase and 96 in the post-intervention phase. The most common reason was tests and procedures (pre = 42/124, post = 49/96) followed by ventilator weaning (pre= 31/124, post = 19/96), GRV (pre = 22/124, post = 10/96), and “other” (which included nursing care, change in status and other miscellaneous reasons) (pre = 6/124, post = 9/96). Interruptions were categorized as “undocumented” when the reason could not be found in either the flow sheets or medical record. The overall interruption data did not reach statistical significance. However, undocumented interruptions were lower in the post-intervention phase (pre = 23/124, post = 9/96; P = 0.06) (Table 3).
Table 3.
Hold time (hours) median hold time and interquartile ranges by interruption type
| Interruption | Pre-intervention phase | Post-intervention phase |
| Procedures | 17.4 (9-19) | 20 (7-24) |
| Residuals | 17.5 (7-22) | 21.5 (4-29) |
| Weaning | 13.6 (4-15) | 12.6 (7-14) |
| Other1 | 22.9 (10-48) | 11.3 (3-15) |
| Undocumented | 5.7 (3-4) | 6.9 (4-10) |
| All interruptions | 14.6 (4-17.25) | 16.6 (5-22.5) |
Data are reported as median and interquartile range. Length of interruptions by type during the pre- and post-intervention phases.
Nursing care, change in status, etc.
DISCUSSION
To our knowledge, our study is the second largest single center study supporting the benefit of the EN protocol in a SICU[10]. We compared the timeliness to achieve goal rate, the amount of EN received, frequency of nutrition interruptions, and accuracy of documentation in critically ill surgical patients before and after implementation of the EN protocol. Guidelines recommend initiating enteral feeds within 24-48 h of ICU admission, yet up to 50% of patients do not even receive EN during their ICU stay[11,12]. Furthermore, EN interruption occurs more frequently in SICUs than their counterparts for multiple unavoidable reasons, including surgical procedures and imaging studies. Hence, these patients are at higher risk of iatrogenic malnutrition[13,14]. There is an overall lack of consensus on the duration of time to hold EN in preparation for a procedure among various specialists, including anesthesiologists, surgeons and intensivists[15]. Physicians are often reluctant to start EN in hemodynamically unstable patients, despite the overwhelming data showing improved outcomes[16]. Establishing criteria for when to interrupt tube feeding, and more importantly, when to restart feeding, may improve overall administration of nutrition support[17]. After conducting the QI project on enteral feeding in our SICU, we determined that 65% of patients on EN support did not achieve goal rate by the seventh day of administration and received less than 50% of the daily-recommended volume. The literature on developing protocols for EN administration suggests that outlining criteria for the initiation and advancement of EN support may improve nutrient delivery[17,18]. Moreover protocols also serve as an effective tool for the physicians in-training, registered nurses and other support staff. Multiple protocols have been introduced over the past years in different aspects of critical care medicine (ventilator weaning, spontaneous breathing and awakening trails, sedation and analgesia) leading to better outcomes[19].
Despite prolonged hold times our data supports the use of an EN protocol to decrease time to achieve goal rate and increase the volume of tube feeding delivered daily. Though data on interruptions varied between the pre- and post-intervention phases, it highlighted the extensive duration of interruptions for various reasons. During the post-intervention phase one of the greatest challenges faced when executing the feeding protocol was overcoming existing nursing and physician practices regarding holding tube feeding and inconsistent documentation. Creating awareness among physician and nursing staff of enteral feeding practices led to an increase in accurate documentation.
Future research should focus on patient outcomes and quality indicators to promote the use of protocols for EN administration in the SICU, and further extended to other ICUs throughout the hospital. Optimizing the EN protocol by providing distinct instructions for how to minimize feeding interruptions could improve the parameters where significant progress was lacking between the pre and post intervention phases. Guidelines and strategies for moving the location of the tip of the feeding tube more distal in the jejunum could also assist in reducing length of hold times for feeding intolerance. Incorporating volume-based practices that summarize how to adjust tube-feeding rates in order to “catch-up” may also assist in optimizing the protocol, and increasing the overall administration of nutrition daily. By developing standards of practice and guidelines for when to hold and restart enteral feeds, we improved the overall administration of nutrition provided.
Given the retrospective nature of our study, we are unable to establish cause and effect. The study does not draw solid conclusions, however the data can be used to provide descriptive characteristics, and add to the limited literature available.
In conclusion, this study suggests a user friendly EN protocol in conjunction with extensive ongoing education may lead to shorter time to achieve goal rate, and enhance overall administration of nutrition to surgical critical care patients.
ACKNOWLEDGMENTS
To John T Doucette, Associate Professor, Preventive Medicine at Icahn School of Medicine at Mount Sinai for his contribution to the manuscript by helping with the data analysis.
COMMENTS
Background
The benefits of enteral nutrition (EN) to critically ill patients are well cited in the reducing length of stay and hospital mortality. Clinical protocols serve as effective tools for guiding clinical practice and improving patient outcomes (e.g., ventilator weaning, spontaneous breathing and awakening).
Research frontiers
EN is preferred over parenteral nutrition, as it has been shown to maintain gastrointestinal integrity and function, and increase peristalsis and blood flow. Discrepancies between prescribed nutrition goal and actual nutrition delivered in critically ill patients are not uncommon; this is especially the case in the surgical population. Prior studies have established that feeding protocols can increase administration of nutrition to patients. The current research hotspot is to implement a feeding protocol in a surgical intensive care unit (ICU) setting where the number of interruptions are frequent and goal rates are often not achieved.
Innovations and breakthroughs
Few studies to date have been conducted on the use of feeding protocols in surgical ICU patients. Existing literature suggests patients are less likely to get EN compared to medical ICU patients due to concern of postoperative ileus, anastomotic leak, diagnostic testing and operative procedures. To our knowledge, this study is the second largest single center study supporting the benefit of implementing a feeding protocol in surgical ICU. The feeding protocol was introduced and data collected on the rate initiated and total volume provided daily. The authors monitored time to achieve goal rate and the total volume provided six months prior to and following implementation of the protocol. Overall time to achieve goal rate decreased, while the total volume administered daily increased. The protocol also led to an increase in detailed documentation of interruptions by the unit staff.
Applications
The study results suggest feeding protocols can lead to improved nutrient administration during the acute phase. Improved documentation may allow them to identify and trend with regard to feeding interruptions to better understand which practices or procedures require further review.
Terminology
EN is any method of feeding that utilizes the gastrointestinal tract to deliver nutrients. Parenteral nutrition, also referred to as intravenous feeding, is a method of providing nutrition into the body via the veins.
Peer-review
This is a well-written paper, focused on an interesting topic.
Footnotes
Institutional review board statement: The study was reviewed and approved by Icahn School of Medicine at Mt Sinai Institutional Review Board.
Informed consent statement: Waiver of informed consent was provided by Icahn School of Medicine at Mt Sinai Institutional Review Board.
Conflict-of-interest statement: All the authors involved in the study have no conflict of interest to declare.
Data sharing statement: Data is available from the corresponding author - nagendra.madisi@mountsinai.org.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 27, 2016
First decision: April 15, 2016
Article in press: June 3, 2016
P- Reviewer: Rossi RE, Willms DC S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
References
- 1.McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305–315. doi: 10.1177/0884533609335176. [DOI] [PubMed] [Google Scholar]
- 2.Singer P, Anbar R, Cohen J, Shapiro H, Shalita-Chesner M, Lev S, Grozovski E, Theilla M, Frishman S, Madar Z. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011;37:601–609. doi: 10.1007/s00134-011-2146-z. [DOI] [PubMed] [Google Scholar]
- 3.Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake! Crit Care Med. 2011;39:2619–2626. doi: 10.1097/CCM.0b013e318226641d. [DOI] [PubMed] [Google Scholar]
- 4.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 5.Heyland DK, Stephens KE, Day AG, McClave SA. The success of enteral nutrition and ICU-acquired infections: a multicenter observational study. Clin Nutr. 2011;30:148–155. doi: 10.1016/j.clnu.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, Ebner C, et al. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin Nutr. 2006;25:210–223. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Btaiche IF, Chan LN, Pleva M, Kraft MD. Critical illness, gastrointestinal complications, and medication therapy during enteral feeding in critically ill adult patients. Nutr Clin Pract. 2010;25:32–49. doi: 10.1177/0884533609357565. [DOI] [PubMed] [Google Scholar]
- 8.Drover JW, Cahill NE, Kutsogiannis J, Pagliarello G, Wischmeyer P, Wang M, Day AG, Heyland DK. Nutrition therapy for the critically ill surgical patient: we need to do better! JPEN J Parenter Enteral Nutr. 2010;34:644–652. doi: 10.1177/0148607110372391. [DOI] [PubMed] [Google Scholar]
- 9.Poulard F, Dimet J, Martin-Lefevre L, Bontemps F, Fiancette M, Clementi E, Lebert C, Renard B, Reignier J. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. JPEN J Parenter Enteral Nutr. 2010;34:125–130. doi: 10.1177/0148607109344745. [DOI] [PubMed] [Google Scholar]
- 10.Taylor B, Brody R, Denmark R, Southard R, Byham-Gray L. Improving enteral delivery through the adoption of the “Feed Early Enteral Diet adequately for Maximum Effect (FEED ME)” protocol in a surgical trauma ICU: a quality improvement review. Nutr Clin Pract. 2014;29:639–648. doi: 10.1177/0884533614539705. [DOI] [PubMed] [Google Scholar]
- 11.Yeh DD, Fuentes E, Quraishi SA, Cropano C, Kaafarani H, Lee J, King DR, DeMoya M, Fagenholz P, Butler K, et al. Adequate Nutrition May Get You Home: Effect of Caloric/Protein Deficits on the Discharge Destination of Critically Ill Surgical Patients. JPEN J Parenter Enteral Nutr. 2016;40:37–44. doi: 10.1177/0148607115585142. [DOI] [PubMed] [Google Scholar]
- 12.Heyland DK, Schroter-Noppe D, Drover JW, Jain M, Keefe L, Dhaliwal R, Day A. Nutrition support in the critical care setting: current practice in canadian ICUs--opportunities for improvement? JPEN J Parenter Enteral Nutr. 2003;27:74–83. doi: 10.1177/014860710302700174. [DOI] [PubMed] [Google Scholar]
- 13.Passier RH, Davies AR, Ridley E, McClure J, Murphy D, Scheinkestel CD. Periprocedural cessation of nutrition in the intensive care unit: opportunities for improvement. Intensive Care Med. 2013;39:1221–1226. doi: 10.1007/s00134-013-2934-8. [DOI] [PubMed] [Google Scholar]
- 14.Peev MP, Yeh DD, Quraishi SA, Osler P, Chang Y, Gillis E, Albano CE, Darak S, Velmahos GC. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39:21–27. doi: 10.1177/0148607114526887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider JA, Lee YJ, Grubb WR, Denny J, Hunter C. Institutional practices of withholding enteral feeding from intubated patients. Crit Care Med. 2009;37:2299–2302. doi: 10.1097/CCM.0b013e3181a007eb. [DOI] [PubMed] [Google Scholar]
- 16.Khalid I, Doshi P, DiGiovine B. Early enteral nutrition and outcomes of critically ill patients treated with vasopressors and mechanical ventilation. Am J Crit Care. 2010;19:261–268. doi: 10.4037/ajcc2010197. [DOI] [PubMed] [Google Scholar]
- 17.Heyland DK, Cahill NE, Dhaliwal R, Sun X, Day AG, McClave SA. Impact of enteral feeding protocols on enteral nutrition delivery: results of a multicenter observational study. JPEN J Parenter Enteral Nutr. 2010;34:675–684. doi: 10.1177/0148607110364843. [DOI] [PubMed] [Google Scholar]
- 18.Stewart ML. Interruptions in enteral nutrition delivery in critically ill patients and recommendations for clinical practice. Crit Care Nurse. 2014;34:14–21; quiz 22. doi: 10.4037/ccn2014243. [DOI] [PubMed] [Google Scholar]
- 19.Helwig A, Bower D, Wolff M, Guse C. Residents find clinical practice guidelines valuable as educational and clinical tools. Fam Med. 1998;30:431–435. [PubMed] [Google Scholar]


