Abstract
OBJECTIVES
Deep sternal wound infection poses a serious problem in cardiac surgery, with an up to 40% risk of mortality. Massive loss of sternum bone tissue and adjacent ribs results in major chest wall instability causing respiratory insufficiency and defects of soft tissue healing. Proposals for managing the situation have been published but the complexity of the issue precludes unequivocal resolution. Capitalizing on orthopaedic experience, we used allogeneic bone graft of sternum as a viable option.
METHODS
We performed the transplantation of allogeneic bone graft in 10 patients. In 9 cases, an allograft of sternum was used and in 1 case an allograft of calva bone. After the primary cardiac surgery, a massive post-sternotomy defect of the chest wall had developed in all 10 patients. Vacuum wound drainage was applied in the treatment of all patients. To stabilize the transverse, titanium plates were used. Bone allograft was prepared by the official Tissue Centre. Crushed allogeneic spongy bone was applied to reinforce the line of contact of the graft and the edges of residual skeleton. In 9 cases, the soft tissue was closed by direct suture of mobilized pectoral flaps. In 1 case, V-Y transposition of pectoral flap was performed.
RESULTS
In 6 cases, healing of the reconstructed chest wall occurred without further complications. In 3 cases, additional re-suture of the soft tissues and skin in the lower pole of the wound was necessary. Excellent chest wall stability along with the adjustment of respiratory insufficiency and good cosmetic effect was achieved in all cases. In 1 case, severe concomitant complications and no healing of the wound resulted in death within 6 months after the reconstruction. Median follow-up of all patients in the series was 14.1 months (1–36 months). In 4 patients, scintigraphy of the chest wall was performed.
CONCLUSIONS
Our existing results show that allogeneic bone graft transplantation is a promising and easily applied method in the management of serious tissue loss in sternal dehiscence with favourable functional and cosmetic effects. The relatively small number of patients with such severe healing complications of sternotomy however puts critical limits to a more detailed comparison with other practices and evaluation of long-term results.
Keywords: Sternotomy, Deep sternal wound infection, Massive post-sternotomy defect, Allogeneic bone graft of sternum
INTRODUCTION
Longitudinal median sternotomy remains the most widely used approach in cardiac surgery [1]. It is fast to carry out, provides a good overview of the operating field and the incidence of healing disorders is low. However, early sternotomy wound complications are a major cause of morbidity and mortality in cardiac surgery [2, 3]. Deep sternal wound infection (DSWI) is life-threatening, particularly in cases of concomitant loss of sternal bone and adjacent ribs due to osteomyelitis. Large defects of the anterior chest wall increase the risk of injury to the right ventricle and sewn bypasses. Mechanical instability of the chest wall impairs respiratory functions, often necessitating prolonged mechanical ventilation. An inadequate mechanical basis also hampers the healing of soft tissue and skin.
Using titanium plates, following the principles of AO osteosynthesis fixed to the surface of the sternum and adjacent ribs, a high degree of ribcage stability can be safely achieved while minimizing the risk of injury [4]. In cases of extensive post-sternal bone defects, however, the missing bone material fails to provide adequate support for plates and this increases the risk of osteosynthesis failure [5].
On the basis of the experience of orthopaedic surgery in the treatment of bone defects, we began to treat extensive post-sternal chest wall defects using the combined method of applying an allogeneic bone graft, crushed allogeneic spongy bone and transversal titanium plates.
MATERIALS AND METHODS
In the period between August 2011 and September 2014, our Cardiosurgery Department treated 10 patients (4 men, 6 women) with the development of a major post-sternotomy defect of the chest wall formed on the basis of DSWI. Seven patients were operated for coronary heart disease, 4 of them urgently. In all 7 cases, the left internal mammary artery (LIMA) was removed for left anterior descending artery construction of the bypass. Bilateral internal mammary artery harvesting was not carried out in any case. Two patients underwent aortic valve replacement procedure. One patient was treated by a combination of mitral valve replacement and tricuspid valve plastic surgery. All surgeries were performed using extracorporeal circulation (ECC). Standard antibiotic prophylaxis as Unasyn® (Haupt Pharma Latina S.r.L., Borgo San Michele, Latina, Italy) 4 × 1.5 g intravenously in coronary artery surgery or Cefazolin® (Sandoz GmbH, Kundl, Austria) 3 × 1 g intravenously in valve surgery was administered in every case.
In all patients, a DSWI developed along with dehiscence and advanced osteomyelitis of the sternum and adjacent costal cartilages. Clinical signs of the DSWI developed within the first week after the surgery in all cases. Microbiological agents of primary early wound infection are summarized in Table 2.
Table 2:
Results of the reconstruction of the chest wall and follow-up
| Patient no. | VAC procedures (n) | Microbiology pathogens | Type of graft | Soft tissue suture | ICU stay (days) | MPV (h) | Total hospitalization (days) | Hospitalization after chest wall reconstruction (days) | Stability of chest wall/wound healing | Follow-up (months) | Financial cost (Euro) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | Staph. epidermidis | C | V-Y m. pectoralis transposition | 16 | 22 | 112 | 56 | Stable/soft tissue resuture of the distal part of the wound | 15 | 65 578 |
| 2 | 8 | Staph. epidermidis | S | Direct suture | 20 | 168 | 56 | 28 | Stable/healed completely | 36 | 78 458 |
| 3 | 5 | Staph. epidermidis | S | Direct suture | 2 | 8 | 42 | 14 | Stable/healed completely | 36 | 21 218 |
| 4 | 14 | Kl. pneumonie | S | Direct suture | 24 | 24 | 66 | 24 | Stable/healed completely | 10 | 63 057 |
| 5 | 8 | Staph. epidermidis | S | Direct suture | 3 | 6 | 39 | 8 | Stable/healed completely | 18 | 16 768 |
| 6 | 7 | Kl. pneumonie | S | Direct suture | 36 | 48 | 91 | 36 | Stable/soft tissue resuture of the distal part of the wound | 1 | 82 228 |
| 7 | 6 | Morg. morganii | S | Direct suture | 14 | 216 | 60 | 37 | Stable/healed completely | 10 | 31 884 |
| 8 | 5 | Staph. epidermidis | S | Direct suture | 11 | 12 | 43 | 14 | Stable/healed completely | 6 | 31 823 |
| 9 | 5 | Staph. epidermidis | S | Direct suture | 3 | 3 | 75 | 50 | Stable/soft tissue resuture of the distal part of the wound | 6 | 18 602 |
| 10 | 9 | Staph. aureus Staph.epidermidis | S | Direct suture | 6 | 6 | 140 | 96 | Stable/secondary m. latissimus dorsi flap covered/VAC | 3 | 102 627 |
C: calva; ICU: intensive care unit; MPV: mechanical pulmonary ventilation; S: sternum; VAC: vacuum-assisted closure.
Determination of sternotomy wound infectious complications was based on the following criteria [6, 7]:
the presence of wound secretions and subsequent clinical and microbiological confirmation of early infection. Microbiological screening was done in a swab from the wound surface and a tissue sample;
early superficial infection was located within the area of skin and/or dermis;
deep sternal infection affects the sternum; there is possible presence of sternal instability; the mediastinum is not exposed;
to confirm sternal osteomyelitis, we used the clinical findings during surgery confirmed by positive microbiological examination of bone or cartilage;
the diagnosis of mediastinitis was based on the clinical findings of a complete sternotomy dehiscence, positive microbiological examination of secretions or tissue samples from the mediastinum;
systemic symptoms of infection were assessed as follows: fever over 38° C, chest wall pain, chest wall instability, suppurating wound secretion and evidence of micro-organisms in blood cultures taken; and
according to the recommendations, abnormality on the X-ray, computed tomography (CT) scan/magnetic resonance imaging and scintigraphy of the sternum was considered.
Given the number of patients with such severe post-sternotomy complication in our series, an average incidence of all wound healing complications after the primary sternotomy was 2.94% in the period from 2011 to 2014. The incidence of DSWI was 1.32%. The incidence of massive post-sternotomy bone defects was 0.39%. Total number of patients operated in our centre was 2549 within this period.
Preoperative risk factors included: obesity (body mass index ≥30), diabetes mellitus (DM; insulin/oral antidiabetics), chronic renal insufficiency, chronic obstructive pulmonary disease (COPD) and smoking.
Intraoperative risk factors included 3 patients diagnosed with severe osteoporosis of the sternum. No paramedian sternotomies or intraoperative fragmentations of sternum were recorded. In 6 cases, a classic cerclage using six to seven wire loops was used for closure of the sternotomy, whereas in 4 cases a newly available ZipFix® (Synthes-DePuy, Johnson & Johnson, USA) system of sternal straps was used. ECC and aortic-clamp times were also considered.
Early postoperative course data after the primary surgery were included: revision for bleeding or cardiac tamponade, total blood loss, number of units of blood transfusions, average duration of mechanical pulmonary ventilation and length of stay in the intensive care unit (ICU).
Details of the patient age, cardiac surgery, pre-, intra- and postoperative risk factors are summarized in Table 1.
Table 1:
Patients and risk factors
| Patient no. | Sex (M/F) | Age (years) | Operation | BMI | DM (i.d./p.a.) | RI | COPD | Smoker | ECC (min) | Aortic clamp (min) | Sternal pathology | Sternal closure | Revision (n) | Blood loss (ml) | Blood units (n) | MPV (h) | ICU stay (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | CABG + maze | 32 | i.d. | Yes | Yes | Yes | 108 | 65 | No | W | 0 | 1200 | 2 | 11 | 3 |
| 2 | M | 70 | CABG urg | 33 | p.a. | No | No | No | 55 | 35 | No | W | 2 | 6200 | 19 | 168 | 15 |
| 3 | F | 67 | AVR | 40 | p.a. | No | No | No | 56 | 37 | No | W | 2 | 2800 | 6 | 17 | 4 |
| 4 | M | 65 | CABG | 34 | i.d. | No | No | No | 52 | 33 | No | W | 0 | 2200 | 5 | 12 | 2 |
| 5 | M | 47 | CABG | 39 | i.d. | Yes | Yes | No | 59 | 44 | No | W | 0 | 970 | 2 | 16 | 2 |
| 6 | F | 62 | AVR + maze | 40 | i.d. | Yes | No | No | 123 | 89 | No | ZF | 0 | 870 | 2 | 11 | 11 |
| 7 | F | 74 | CABG | 26 | No | No | No | Yes | 55 | 23 | Osteoporosis | W | 0 | 500 | 1 | 14 | 4 |
| 8 | F | 73 | MVR, TVP | 26 | No | Yes | Yes | No | 117 | 90 | Osteoporosis | ZF | 1 | 2950 | 10 | 27 | 7 |
| 9 | F | 83 | CABG urg | 27 | p.a. | Yes | No | No | 90 | 48 | Osteoporosis | ZF | 0 | 850 | 2 | 24 | 5 |
| 10 | F | 82 | CABG urg | 38 | i.d. | Yes | No | No | 58 | 34 | No | ZF | 0 | 1150 | 2 | 10 | 5 |
AVR: aortic valve replacement; BMI: body mass index; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus, i.d.: insulin dependent, p.a.: peroral antidiabetics; ECC: extracorporeal circulation; ICU: intensive care unit; MPV: mechanical pulmonary ventilation; MVR: mitral valve replacement; RI: renal insufficiency; TVP: tricuspid valve plasty; W: wires; Z: ZipFix.
Deep sternal wound infection treatment protocol
In our department, vacuum-assisted closure system of wound drainage (VAC®, KCI, Inc., San Antonio, TX, USA) is used as a standard and on a long-term basis [8]. In most cases, revision of the wound is carried out on the day early complications are diagnosed, or the next day at the latest. Wound dressings in the DSWI category are performed under general anaesthesia in the operating room, based on the current clinical report two to three times a week. At each dressing change, scrapings from the base of the wound and samples of the soft tissue, bone and rib cartilage are taken for microbiological examination. In the case of irreversible sternal dehiscence, caused by release or cut-through of the material used for sternotomy closure, the material is removed. Devitalized edges of soft tissues, bone and cartilage are carefully removed. Sterilization of the wound is done using diluted solution of povidone-iodine (Betadine®, Mundipharma AG, Basel, Switzerland).
VAC® system polyurethane foam is formed following the current shape of the wound and is applied in one or two layers. In the case of exposed right ventricle and sewn bypasses, the wound bed is covered with one or two layers of Mepilex® (Mölnlycke Health Care, Sweden). The entire system is covered with sterile foil and is connected to a suction device with a negative pressure in the range −75 to −125 mmHg.
An average of 8.2 (5–15) dressings using the VAC® system were performed (Table 2). In all cases, this necessitated radical removal of infected tissues. Bone defect, comprising more than 3/4 of the original area of the sternum, developed. There was also loss of cartilage of adjacent ribs, mainly on the LIMA harvest side.
Within the holistic approach to DSWI treatment, general intravenous administration of one or more antibiotics is indicated, in strict accordance with the recommendations of our antibiotic centre.
The aim of our treatment is to achieve three consecutive negative results of all microbiological tests on the wound. Negative results of these microbiological tests are the main criteria for timing the chest wall reconstruction. The status of the soft tissues and bone is assessed macroscopically. To proceed with treatment, the absence of fever and drop in levels of C-reactive protein and leucocytes in the blood are required. Mean interval from the sternotomy dehiscence diagnosis to decision of chest wall reconstruction was 38.6 days (28–56 days) in our series.
An allogenous graft must meet the legislative criteria of the Czech Republic and the European Association of Tissue Banks [9, 10]. Prior to performing an allogeneic bone graft transplant, informed consent of the patient is always required. Sternal bone recovery is performed as multitissue procurement and limited to viable cases of sternum bone graft harvesting before the autopsy. Prior to graft harvesting, each donor is cross-checked for registration within the national registry for organ donation refusal. All deceased donors treated for infectious disease, sepsis, malignant tumours or systemic and autoimmune diseases at the time of death are withdrawn from the donor list. Donor blood serum samples are tested for antibodies and HIV types 1 and 2, hepatitis B surface antigen (HbsAg), hepatitis C antibodies (anti-HCV) and human T-cell lymphotropic virus I and II antibodies. Harvest of a sternal bone graft is performed under strictly sterile conditions by a team from the National Cell and Tissue Centre in Brno. The graft is harvested under sterile conditions and stored in a freezer at −80°C. Prior to clinical use, the graft is thawed at 4–6°C for 12 h, soaked in a 1% gentamicin solution, prepared for its final shape and stored in the freezer again at −80°C. If bacterial sampling is negative, the graft is thawed for 12 h before transplantation and submerged in a bath with 1% gentamicin solution immediately before surgery (Fig. 1).
Figure 1:
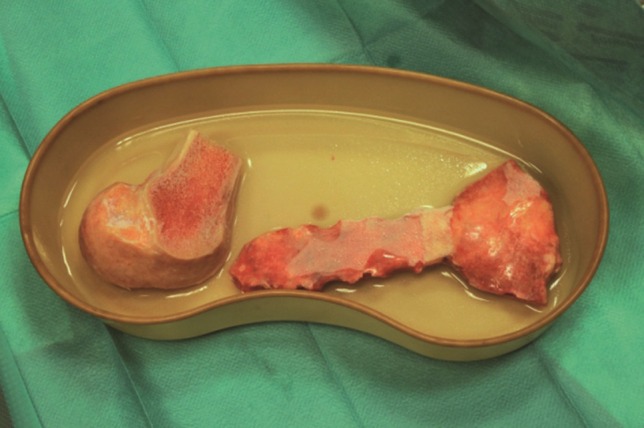
Allogeneic bone grafts of sternum and head of femur (source of crushed spongy bone) in a bath with 1% gentamicin solution before surgery.
Surgical technique
Prior to the transplantation of allogeneic graft, it is necessary to perform bilateral release of pectoral musculocutaneous flaps, usually to the level of midclavicular line. If possible, we try to preserve the blood supply of muscle flap, provided by intercostal vascular perforators. The resection of residual edges of the sternum and the ribs is then performed within the precautionary line reaching 1–2 cm into the healthy tissue.
Bone graft is adjusted following the contour of the defect. To fix the allogeneic bone graft and simultaneously stabilize the whole chest wall, two transversal two-way malleable titanium plates (Synthes-DePuy, Johnson & Johnson) are used. To attach the plates to the surface of the residual skeleton of the sternum and ribs, we use bicortical self-drilling screws of adjusted length. To secure the fixation, we apply at least four screws to both ends of each plate. The bone graft is fixed to each plate using one or two screws of the shortest possible length. The line of contact between residual edge of the sternum or ribs and bone graft is filled using crushed spongy bone obtained from the remnants of the original graft and partly from another enclosed allogeneic graft—usually the head or femoral or tibial condyle (Figs 2A and B and 3A and B).
Figure 2:
(A) Massive bone loss of sternum. (B) Stabilization of allogeneic sternal graft and crushed spongy bone.
Figure 3:
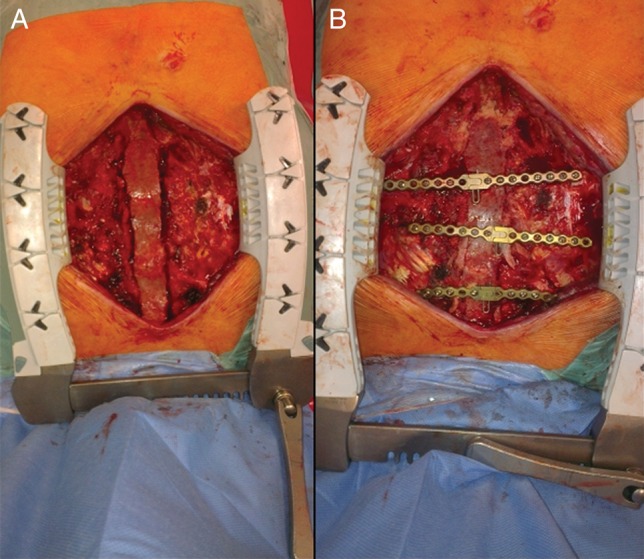
(A) Allogeneic graft of sternum before AO osteosynthesis. (B) Bone graft of sternum after stabilization using transverse plates.
We normally introduce two stronger sub-pectoral Redon drains under both pectoral musculocutaneous flaps. Another weaker Redon drain is introduced within the lower part of the wound under the graft. The closure of soft tissue can be performed by direct suture of bilaterally resected pectoral flaps. In the absence of soft tissues, more extensive surgery in cooperation with a plastic surgeon is necessary.
Postoperative care
After the surgery, patients are monitored at the ICU. Extubation is also performed at the ICU. The postoperative monitoring emphasizes cough avoidance, temporary restriction on the load of the upper limbs and respiratory rehabilitation. In the case of direct suture of resected pectoral flaps, a chest strap can be used. Drains remain in place for 6–7 days in order to prevent the formation of serous exudate. With regard to complex sternal osteomyelitis treatment, the antibiotic therapy continues for a period of 3 weeks from the reconstruction of the chest wall.
Follow-up after release from hospital
Further monitoring takes the form of clinical check-ups at 2, 6 and 12 months. In patients monitored for a longer time, this is performed once a year. Three years after the reconstruction of the chest wall, living patients are removed from follow-up.
To obtain further information about the dynamics of the healing of allogeneic bone graft, scintigraphic examination was performed in four cooperating patients at intervals of 2–3 and 18–22 months after reconstruction of chest wall. A planar whole-body bone scan with single-photon emission computed tomography (SPECT)/CT of the thorax was performed following the administration of 750 MBq Technetium 99m-bisphosphonate on a GE Infinia Hawkeye gamma camera equipped with low-energy, high-resolution parallel-hole collimators. Planar images were acquired in 1024 × 256 matrix. SPECT data with low-dose CT for attenuation correction and morphological correlation were acquired in 128 × 128 matrix.
RESULTS
In the closure procedure of the chest wall defect, we chose an allogeneic bone graft. In the first case, we used an allograft of calva bone, currently, freely available from the Tissue Bank. For the following 9 cases, technology using an allogeneic graft of sternum was developed. In all patients, the lines of contact between the graft and the bone edges were filled with crushed spongy bone using a graft from allogeneic femoral or tibial condyle. If a graft of calva bone was used, the closure of soft tissues was performed using V-Y transposition of the musculocutaneous pectoral flap. In 9 patients, direct suture of the bilaterally resected muscular pectoral flaps was successfully performed.
Details of the time of mechanical ventilation, length of stay in the ICU, length of hospitalization after the chest wall reconstruction and duration of total hospital stay are summarized in Table 2.
In 6 patients, we report a successful and complete healing of the wound with entirely stable thoracic wall. However, in 3 patients (nos. 1, 6 and 9) further treatment of the wound was necessary during hospitalization due to partial dehiscence of the lower pole requiring extra resuture of soft tissues and skin. The chest wall was confirmed as completely stable in all cases. In 1 case, an unmanageable wound healing complication occurred during hospitalization.
The median follow-up after release was 14.1 months (1–36 months). Two patients with proven complete wound healing were removed from follow-up after 36 months. In 2 cases, deaths resulting from other causes occurred (Patient no. 1 died after 15 months of extensive stroke; Patient no. 4 died after 10 months of heart failure). In both, however, the wound was healed and the chest wall was stable. In 1 case (Patient no. 6), a poorly rehabilitating and passive patient, death occurred 36 days after the reconstruction of the chest wall, with sudden onset of severe bronchopneumonia. However, the autopsy revealed complete healing of the reconstructed chest wall (Fig. 4A and B).
Figure 4:
Biopsy of the chest wall. (A) View from above (pectoral) side. (B) View from lower (mediastinal) side.
Among other postoperative complications, pneumothorax occurred in 2 cases (Patient nos. 4 and 7) and a transient decrease in skin sensitivity within the front of the chest occurred in 1 case (Patient no. 3).
Details of the healing of the chest wall and follow-up are summarized in Table 2.
In 5 patients assessed for quality of life during the follow-up after discharge from the hospital, there was a normal range of daily activities compared with the condition prior to the primary operation. The overall condition of the other 3 patients was affected by the progression of comorbidities and slower overall rehabilitation; the quality of life was rated as fair. However, in all 8 cases, we found an excellent cosmetic effect of the chest wound and contours, with no pathological movement of the chest wall during breathing or activity, no signs of respiratory insufficiency or any significant limitation of movement or weakness of the upper limbs.
A very complicated progress was recorded in Patient no. 10: 23 days after the reconstruction of the chest wall, the patient fell off the bed hitting the front wall of the thorax and upper limbs. The plate binding broke loose off the chest bones injuring the soft tissues. Wound revision indicated the necessity of removing the plates. However, the sternal graft was found to be healed and thus was left in situ. The defect of soft tissues was managed by a plastic surgeon, who transposed the latissimus dorsi muscle-cutaneous flap. The wound needed to be revised twice for haemorrhage within the flap. Another early infection then developed, necessitating repeated and long-term VAC® system treatment. The residual soft tissue defect in the distal pole of the original sternotomy was solved by secondary healing. The patient, not thriving well and suffering from associated complications (melaena resulting from a bleeding gastric ulcer, renal insufficiency requiring permanent haemodialysis), died of overall septic complications 6 months after the reconstruction of the chest wall.
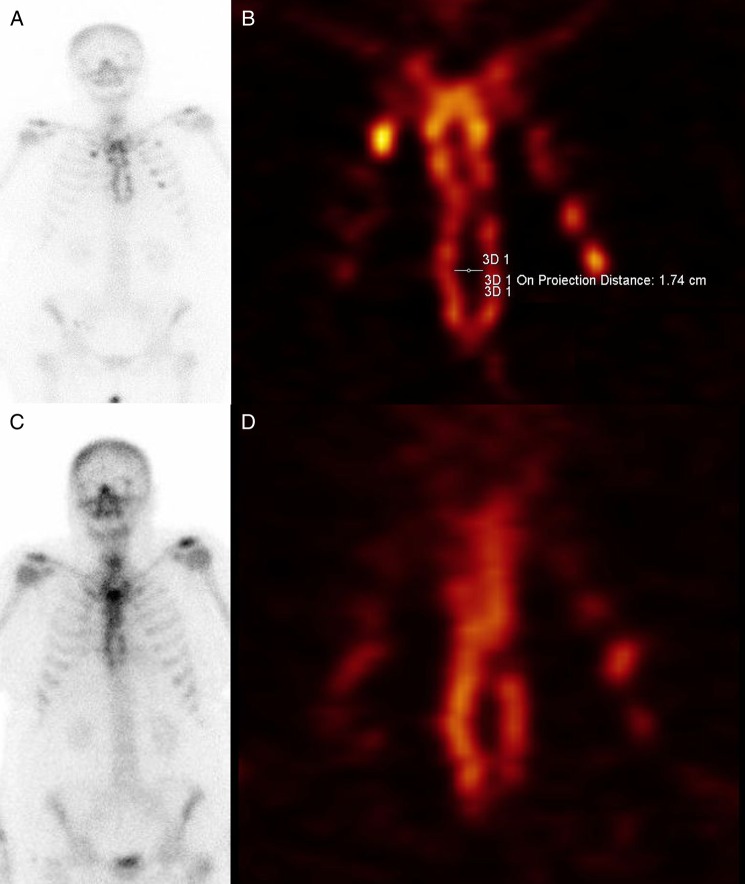
During the next follow-up, whole-body planar and SPECT/CT bone scintigraphic examination of the chest wall were performed in 4 cooperating patients. High healing activity of graft was proven in all cases both after the operation and during the check-up. In 1 case, there was actually a reduction of osteopaenic defect of allogeneic graft by 42% (Fig. 5A–D).
Figure 5:
Whole-body planar scintigraphy and SPECT; 3 (A and B) and 18 (C and D) months after the chest wall reconstruction.
The average increase in the financial expenses of management of early wound complication (calculated from the date of diagnosis to the end of our treatment) amounted to 51 224 Euros (16 768–102 627 Euros). In our local conditions, the average cost of complete care of an uncomplicated patient ranges from 5500 to 7500 Euros. This includes for each patient, total length of stay in the ICU, transfer of blood, wound dressing in the operating room, use of the VAC® system, materials used to reconstruct the chest wall (osteosynthetic material, tissue grafts) and stay in the hospital and rehabilitation centre. The costs do not include physician and nursing fees.
DISCUSSION
Longitudinal median sternotomy has been the dominant operating approach in cardiac surgery from the time of its introduction into practice by Julian in 1957 [1]. It is generally a safe surgical procedure, with an incidence of early postoperative complications ranging from 0.5 to 5% (according to the literature). Sternotomy dehiscence caused by early wound infection, however, is a serious complication with a mortality rate of 14–47% [3].
The current literature includes reports on a number of risk factors associated with the healing of sternotomy. Preoperative risks include obesity, DM, COPD, smoking, chronic renal insufficiency and prolonged use of steroids as the most frequently mentioned. The literature also mentions excessive breast size in women as a preoperative risk factor in healing of sternotomy [11].
Of perioperative risk factors in sternotomy healing, the following are important: paramedian sternotomy, fragmentation of fragile sternum, bilateral mammary artery harvesting for construction of artery composite bypasses, mainly in the case of an obese patient with diabetes, an operation with prolonged extracorporeal circuit, excessive use of bone wax, excessive overall use of diathermy in haemostasis and suboptimal position of both halves of sternum during closure.
Among the most significant postoperative risk factors, these are mainly increased need for blood transfusions, necessity of surgical revision for bleeding or tamponade, prolonged mechanical ventilation and prolonged length of stay in the ICU [3, 12].
The spectrum of defects of healing of sternotomy includes sterile mechanical dehiscence of sternotomy surface, early infection involving the skin and subcutaneous soft tissues, DSWI affecting the bone surface without sternal instability and DSWI with sternal instability [3].
Unstable and mutual movement of sternal edges are breeding grounds for further progression of inflammatory complications of healing, increased wound secretion, occurrence of exudates and secondary wound infection with easier transmission to the deeper wound layers. A complicating factor could also be fragmentation of the sternum into small pieces in the case of cut-through of wire loops. In particular serious cases, a DSWI with osteomyelitis progression can be accompanied by extensive loss of bone and cartilage of adjacent ribs. This results in a large anterior chest wall defect of varying extent. Besides early infectious complications, these patients are also compromised by severe respiratory insufficiency, often resulting in pneumonia and difficulty in being disconnected from mechanical ventilation due to unstable chest wall.
Since the mid-1990s, the VAC® method has proved effective in treating surgical wound complications. The therapeutic effects are due to reduction in bacterial colonization of the wound base by stimulating blood flow within the soft tissues and the growth of granulation and by reducing the wound volume by continuously removing secretions and reducing the swelling of soft tissues. Mechanical stabilization of the chest wall is also vital as it improves respiratory parameters. There is the added advantage of reduced need for dressing changes which can then be performed, according to the clinical condition of the wound, two to three times a week [13].
In case of a resistant early infection, the necessity for repeated VAC treatment sessions under general anaesthesia is a relative disadvantage. However, we emphasize that the absence of microbial infection in the wound, preferably confirmed two to three times repeatedly, is an indispensable precondition to further treatment success.
In the case of a larger post-sternal defect, as there is risk of injury to the right ventricle and sewn bypasses, we always protect the surface of the right ventricle with a layer of Mepilex®. We also always very carefully examine and remove any sharp sternum edges, ribs and cartilage affecting the wound.
General intravenous application of one or more types of antibiotics is a standard and integral part of treatment of severe wound complications of sternotomy. Antibiotic therapy is always administered strictly following the recommendation of the antibiotic centre.
As a part of proper timing of the chest wall reconstruction, complex patient care focuses on overall fitness, nutrition, diabetes control, renal insufficiency management, pulmonary and other associated complications.
For the closure of sternal dehiscence, a variety of simple cerclage—mostly the Robicsek one—is still widely used [14]. However, the success of this fast and cheap method is limited by the quality of the sternum, which is often largely fragmented, fragile or missing. The downside of this method is the risk of injuring the heart and sewn bypasses in the case of very hard tissue adhesions within the space below the sternum.
Two-way shapeable titanium plates following AO osteosynthesis principles are used these days to manage sternal dehiscences without significant bone tissue loss. To stabilize the sternum, the plates can be applied both transversely and longitudinally. The plates are fixed using bicortically placed self-cutting or self-drilling screws of a length adequate for the thickness of the sternum or ribs. From experience, we prefer self-drilling screws. They are faster to apply and without the need of predrilling. The benefits of AO osteosynthesis use are its easy and safe application with significant reduction in the risk of injury to the heart and bypasses, along with the achievement of high stability of the chest wall. It allows the patients high-quality breathing comfort. A perfectly stable chest wall represents an important moment in the healing of subcutaneous soft tissues and skin. The risks of this method are as follows: bleeding after extensive dissection of soft tissues of pectoral lobes, postoperative pneumothorax caused by the use of too long screws to fix the plate, the risk of injury of in situ LIMA or intercostal artery. During the postoperative course, formation of sub-pectoral reactive exudates may occur; therefore, we leave the Redon drains within the sub-pectoral area for 6–7 days. In some cases, decreased skin sensitivity of anterior chest wall may occur. If the transverse plates extend laterally to the sides, it may cause persisting painful respiratory excursion of the chest wall, possibly resulting in the necessity of removing the plates [4, 5].
However, in the case of extensive post-sternal defects, an AO osteosynthesis by itself may fail. Complex shear, caused by breathing movements of the chest wall combined with insufficient bone tissue base for fixation, represents a high risk of collapse and detachment of the plates. The loose osteosynthetic material and consequent formation of further bone fragments dramatically increase the risk of injury to the exposed heart and the risk of serious bleeding. On the basis of the newly developed mechanical instability of the chest wall, worsening respiratory insufficiency, relapse of dehiscence of previously resutured soft tissues and the formation of serious reactive exudates can be expected.
In the literature, a number of methods have been described. These contribute to the management of extensive defects of thoracic wall occurring under different circumstances. However, given their small incidence, and the individual circumstances of a particular patient, it is unlikely that any of the methods listed below will become the gold standard of treatment.
The seemingly simplest solution described is overlay of the bone defect using a musculocutaneous flap; e.g. using one or both muscular flaps or muscular flap of the rectus abdominis muscle [15, 16]. This however, does not stabilize the skeleton of the chest wall and the result is an increased risk of respiratory function deterioration and difficulties in disconnecting from mechanical ventilation. Instability of the chest wall may cause further dehiscences and fistulas of soft tissue. There may also be the question of the cosmetic effect of abnormalities of thoracic contours.
Another possibility for filling the defect is using a large lobe of the omentum, capitalizing on the high degree of angiogenic and healing effect of its tissue [17]. The downside, however, is the necessity of intervention in the peritoneal cavity with the need to extend the surgical wound, which may result in fatal consequences if any infectious complications occur. Until the formation of a sufficiently healed fibrous layer between the edge of the sternum and the ribs, the chest wall remains unstable with the risk of all the consequences described.
Minor defects of the sternum can be dealt with using an autologous graft. Studies using bone graft of fibula, ribs and Achilles tendon have been published [18–20]. The advantage of this technique is the use of patient's own tissues with good healing potential. The disadvantage, however, is the limited amount of material available. Another is the additional operation to harvest the autologous material.
Among synthetic materials used are e.g. methyl methacrylate and polythetrafluorethylene. The use of a tailor-made titanium implant has been reported after resection of sternum affected by primary tumour or metastasis [21].
However, synthetic materials are generally rather too rigid, increasing the risk of erosion or even injury during contact with surrounding tissues. There is also a concern about increased risk of infectious complications or immunological reaction in the presence of excessive amounts of foreign material. The risk of rejection of foreign material by the wound too cannot be omitted [22]. The high cost of the material used in ‘tailor-made’ titanium-based implants is also a consideration.
The use of allogeneic bone graft appears to be a smart option. The literature has described sporadic use of allogeneic bone graft for reconstruction of extensive chest wall defects arising after resection of a malignant tumour or metastases [23, 24]. There are also some initial reports of the use of allogeneic bone graft sternum in the treatment of extensive post-sternotomy defect [25]. Allogeneic bone graft of sternum, prepared by the Tissue Bank, is very easy to use. It is easy to apply and fixes within the wound easily. It also provides sufficient amount of material for reconstruction of chest wall defects of larger extent, with very satisfactory cosmetic effects of preserved chest contour. Despite being an avital bone, allograft serves as ‘scaffold structure’ for ingrowth of recipient’s fibroblasts and tissue angiogenesis. Moreover, despite dealing with a tissue transplant, it is not necessary to address the question of immunocompatibility.
In our series of patients, we started using allogeneic sternal grafts prepared by the Tissue Bank in the primary form without any original soft tissues left. This method accelerates the application of the graft without the need of additional cleanse in the operating room. To reinforce the line of contact between the graft and the residual edges of the chest skeleton of the recipient, we use crushed spongy bone prepared using the remnants of allogeneic sternal graft or also parts of extra graft, usually a condyle of the femur or tibia. On the basis of skeletal scintigraphy, we found great potential for healing in the areas where crushed spongy bones was applied. This consequently accelerates the engraftment to the surrounding tissues.
However, the strict absence of microbial contamination of the wound at the time of application is a prerequisite of implantation of allogeneic bone graft. For this reason, despite repeated confirmations of the absence of microbial contamination of the wound, we perform prophylactic resection of residual margins of sternum and ribs. No microbial infection of the wound as a strict precondition explains the reason for extended VAC® therapy in some cases within our series.
One caveat in the case of transplantation of biological material and despite the strictest preventive measures possibly taken in the management of allogeneic bone graft, the eventuality of viral/bacterial infection transmitted from donor to recipient, albeit minimal, exists.
CONCLUSION
Proposals for managing extensive post-sternotomy defects of the chest wall have been published. However, given the complexity of the issue, none of the methods advanced can be unequivocally declared as the gold standard of treatment. Our experience with transplantation of allogeneic sternal graft and the existing results of our patients so far is a promising treatment method in particularly serious cases. It is also simple and easy to apply, providing acceptable functional and cosmetic results. The relatively small number of patients with such severe healing complications as sternotomy put critical limits to a more detailed comparison with other practices and evaluation of long-term results.
Conflict of interest: none declared.
REFERENCES
- 1.Julian OC, Lopez-Belio M, Dye WS, Javid H, Grove WJ. The median sternal incision in intracardiac surgery with extracorporeal circulation: a general evaluation of its use in heart surgery. Surgery 1957;42:753. [PubMed] [Google Scholar]
- 2.Losanoff JE, Richman BW, Jones JW. Disruption and infection of median sternotomy: a comprehensive review. Eur J Cardiothorac Surg 2002;21:831–9. [DOI] [PubMed] [Google Scholar]
- 3.El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61:1030–6. [DOI] [PubMed] [Google Scholar]
- 4.Gaudreau G, Costache V, Houde C, Cloutier D, Montalin L, Voisine P et al. Recurrent sternal infection following treatment with negative pressure wound therapy and titanium transverse plate fixation. Eur J Cardiothorac Surg 2010;37:888–92. [DOI] [PubMed] [Google Scholar]
- 5.Voss B, Bauernschmitt R, Will A, Krane M, Kröss R, Brockmann G et al. Sternal reconstruction with titanium plates in complicated sternal dehiscence. Eur J Cardiothorac Surg 2008;34:139–45. [DOI] [PubMed] [Google Scholar]
- 6.Tarzia V, Carrozzini M, Bortolussi G, Buratto E, Bejko J, Comisso M et al. Impact of vacuum-assisted closure therapy on outcomes of sternal wound dehiscence. Interact CardioVasc Thorac Surg 2014;19:70–5. [DOI] [PubMed] [Google Scholar]
- 7.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definitive of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- 8.Simek M, Hajek R, Fluger I, Molitor M, Grulichova J, Langova K et al. Superiority of topical negative pressure over closed irrigation therapy of deep sternal wound infection in cardiac surgery. J Cardiovasc Surg (Torino) 2012;53:113–20. [PubMed] [Google Scholar]
- 9.Collection of Laws of The Czech Republic. Act No. 296/2008 Collegium on Human Tissues and Cells Prague 2008.
- 10.European Association of Tissue Banks. General Standards for Tissue Banking, ÖBIG-Transplant, Vienna, 1995. [Google Scholar]
- 11.Copeland M, Senkowski C, Ulcickas M, Mendelson M, Griepp RB. Breast size as a risk factor for sternal wound complications following cardiac surgery. Arch Surg 1994;129:757–9. [DOI] [PubMed] [Google Scholar]
- 12.Loop FD, Lytle BW, Cosgrove DM, Mahfood S, Mc Henry MC, Goormastic M et al. Maxwell Chamberlain memorial paper: sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity and cost of care. Ann Surg 1990;49:179–87. [DOI] [PubMed] [Google Scholar]
- 13.Song DH, Wu LC, Lohman RF, Gottlieb LJ, Franczyk M. Vacuum assisted closure for the treatment of sternal wounds: the bridge between debridement and definitive closure. Plast Reconstr Surg 2003;111:92–7. [DOI] [PubMed] [Google Scholar]
- 14.Robicsek F, Fokin A, Cook J, Bhatia D. Sternal instability after midline sternotomy. Thorac Cardiovasc Surg 2000;48:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Wettstein R, Erni D, Berdat P, Rothenfluh D, Banic A. Radical sternectomy and primary musculocutaneous flap reconstruction to control sternal osteitis. J Thorac Cardiovasc Surg 2002;123:1185–90. [DOI] [PubMed] [Google Scholar]
- 16.Shibata T, Hattori K, Hirai H, Fujii H, Aoyama T, Seuhiro S. Rectus abdominis myocutaneous flap after unsuccessful delayed sternal closure. Ann Thorac Surg 2003;76:956–8. [DOI] [PubMed] [Google Scholar]
- 17.Lee AB Jr, Schimert G, Shaktin S, Seigel JH. Total excision of the sternum and thoracic pedicle transposition of the greater omentum; useful strategems in managing severe mediastinal infection following open heart surgery. Surgery 1976;80:433–6. [PubMed] [Google Scholar]
- 18.Nahabedian MY, Riley LH, Greene PS, Yang SC, Van der Kolk CA. Sternal stabilization using allograft fibula following cardiac transplantation. Plast Reconstr Surg 2001;108:1284–8. [DOI] [PubMed] [Google Scholar]
- 19.Chai Y, Zhang G, Shen G. Autogenous rib grafts for reconstruction of sternal defects after partial resection: a new surgical technique. Plast Reconstr Surg 2008;121:353–5. [DOI] [PubMed] [Google Scholar]
- 20.De Feo M, Carozza A, DellaCorte A, Quarto C, Torella M, De Santo LS et al. Achilles tendon for sternal synthesis in the treatment of mediastinitis. Ann Thorac Surg 2005;79:359–60. [DOI] [PubMed] [Google Scholar]
- 21.Demondion P, Mercier O, Kolb F, Fadel E. Sternal replacement with a custom-made titanium plate after resection of a solitary breast cancer metastasis. Interact CardioVasc Thorac Surg 2014;18:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocco G. Overview on current and future materials for chest wall reconstruction. Thorac Surg Clin 2010;20:559–62. [DOI] [PubMed] [Google Scholar]
- 23.Marulli G, Hamad AM, Cogliati E, Breda C, Zuin A, Rea F. Allograft sternochondral replacement after resection of large sternal chondrosarcoma. J Thorac Cardiovasc Surg 2010;139:e69–70. [DOI] [PubMed] [Google Scholar]
- 24.Stella F, Dell'Amore A, Dolci G, Cassanelli N, Caroli G, Zamagni C et al. Allogenic sternal transplant after sternectomy for metastasis of ovarian carcinoma. Ann Thorac Surg 2012;93:e71–2. [DOI] [PubMed] [Google Scholar]
- 25.Dell’Amore A, Cassanelli N, Dolci G, Stella F. An alternative technique for anterior chest wall reconstruction: the sternal allograft transplantation. Interact CardioVasc Thorac Surg 2012;15:944–7. [DOI] [PMC free article] [PubMed] [Google Scholar]