Abstract
OBJECTIVES
Residual disease at the bronchial stump (RDBS) is regarded as an important factor possibly resulting in bronchopleural fistula (BPF) after lung cancer surgery, but this has not been confirmed. We conducted this meta-analysis to evaluate the effects of RDBS on BPF formation in patients undergoing lung cancer surgery.
METHODS
PubMed and EMBASE databases were searched for full-text articles that met our eligibility criteria. Odds ratios (ORs) with 95% confidence interval (95% CI) served as the summarized outcomes. Q-test and I2 statistic were used to evaluate the level of heterogeneity, determining the fixed-effect model or random-effect model for quantitative synthesis. Sensitivity analysis was conducted to identify the possible origins of heterogeneity. The publication bias was assessed by Begg's test.
RESULTS
A total of eight retrospective observational studies were included in our meta-analysis. In overall analysis, the pooled outcomes indicated that RDBS was significantly associated with BPF formation after lung cancer surgery (OR: 3.12; 95% CI: 1.72–5.64; P < 0.001). In subgroup analysis, the pooled outcomes revealed a significantly increased risk of post-pneumonectomy BPF in patients with RDBS (OR: 2.78; 95% CI: 1.06–7.28; P = 0.037). The subgroup analysis assessing the effects of RDBS on post-lobectomy BPF was given up due to the scarcity of available data. No heterogeneity was revealed within this meta-analysis. No evidence for publication bias was detected by Begg's test.
CONCLUSIONS
Our meta-analysis indicates that RDBS is positively associated with the increased risk of BPF in patients undergoing lung cancer surgery. The further analysis also reveals an increased risk of post-pneumonectomy BPF in patients with RDBS. More accurate and comprehensive evidence should be collected and summarized in updated meta-analyses.
Keywords: Bronchopleural fistula, Residual disease, Bronchial stump, Lung cancer surgery, Meta-analysis
INTRODUCTION
In recent years, advanced surgical techniques and perioperative managements have largely improved the survival rate and reduced the postoperative complications in patients undergoing lung cancer surgery. However, bronchoplerual fistula (BPF), a devastating complication after pulmonary resections, still troubles thoracic surgeons due to its poor prognoses [1, 2]. The mortality caused by the adverse effects of BPF ranges from 18 to 50%, according to recent investigations [2]. Therefore, determining the risk factors of BPF is an urgent issue.
BPF is generally regarded as a fatal condition closely associated with pulmonary surgical procedures. Some intraoperative factors, including operative modes, bronchial closure and the coverage of stump, may directly lead to the insufficient bronchial stump or anastomosis [2–4]. However, little is known about the effects of residual disease at the bronchial stump (RDBS), an equally important factor possibly resulting in BPF formation during lung cancer surgery. Previous studies mainly focus on evaluating the survival outcomes rather than the risk of BPF in patients with RDBS [5–7]. Although the effects of RDBS on postoperative BPF have been addressed by some studies, there is still a lack of a definitive conclusion with statistical significance.
Since the 1990s, incomplete resection has been largely reduced due to the wide application of neo-adjuvant therapies and early diagnosing [8]. We discover that the small size of enrolled samples in an independent study may cause large negative effects on drawing convincing conclusions. As an important component of evidence-based medicine, a meta-analysis is a well-established statistical method of pooling numbers of homogeneous studies together to settle some controversies in clinical practice. Therefore, we conducted the present meta-analysis, integrating the recent evidence to evaluate the effects of RDBS on BPF formation in patients undergoing lung cancer surgery.
MATERIALS AND METHODS
Systematic reviews and meta-analyses do not require patients' consent or ethical approval. We carried out this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [9]. The additional PRISMA 2009 checklist is given in Supplementary Material.
Searching strategies
Two universal electronic databases, PubMed and EMBASE (via Ovid interface), were selected to identify the full-text literatures published between 1 January 1990 and 23 July 2015. Five searching strings were combined with several key words and the Boolean operators ‘AND’ and ‘OR’. These key words are listed as follows: (i) ‘bronchopleural fistula’ or ‘bronchial fistula’; (ii) ‘risk factor’, ‘incidence’, ‘etiology’; (iii) ‘residual cancer’ or ‘carcinoma’, ‘residual disease’ or ‘tumor’ and (iv) ‘incomplete resection’ or ‘R1 resection’. The complete search details in each database are summarized in Supplementary Material. The reference lists of relevant literatures were also manually screened to identify any qualified study with no duplication.
Inclusion and exclusion criteria
We established the following inclusion and exclusion criteria to determine the eligible literatures into quantitative synthesis.
Inclusion criteria: (i) the target disease was lung cancer, including non-small-cell lung cancer and small-cell lung cancer; (ii) a BPF developing after surgical procedures instead of primary disease progression; (iii) RDBS was independently analysed in original literatures; (iv) demographic or statistical results assessing the association between RDBS and BPF formation were available in the full-text literatures and (v) only manuscripts in the English language were considered for the meta-analysis.
Exclusion criteria: (i) the following article types were excluded: reviews, letters, animal experiments, case reports and conference abstracts; (ii) the occurrence of postoperative BPF was uncertain and (iii) manuscripts in non-English languages were not accepted.
Quality assessment
Newcastle–Ottawa Scale (NOS) was employed to assess the quality of original non-randomized studies [10]. Three fields of parameters, including selection, comparability and exposure, were considered for estimation. The ‘star system’ with a maximum of nine stars was used as the assessment tool. After grading all of the included studies, we regarded 8–9 stars as a good quality, 6–7 stars as a medium quality and lower than 6 stars as a poor quality.
Data collection
We designed a Microsoft Excel sheet to record the following information: (i) publication data including authors, publication years and nations; (ii) experimental data including study design, study period, operative modes, onsets of BPF and the principles of pathological definition; (iii) demographic data including enrolled samples, the number of patients with RDBS and postoperative BPF; (iv) statistical data including any statistic reported in the results of original literatures, including odds ratio (OR), relative risk (RR) and hazard ratio (HR), with corresponding 95% confidence interval (95% CI) and P-value.
Statistical analysis
For the issue to be addressed in this meta-analysis, we discovered that the incidence of postoperative BPF was generally far lower than 20% [2]. Thus, no significant differences between OR and RR were observed, indicating that the bias risk overestimating the effects of RDBS on BPF occurrence could be largely avoided [11]. Finally, ORs with 95% CI were applied as the appropriate summarized outcomes. ORs with 95% CI were directly acquired from published results in original literatures, or from calculating demographic data by SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) if no statistical results were reported. In addition, RRs or HRs conducted from multivariate analysis could be directly regarded as ORs and incorporated into the final quantitative synthesis [11]. If the combined OR with 95% CI was >1, it could suggest a significant association between RDBS and the increased risk of BPF.
Q-test and I2 statistic were used to evaluate the level of heterogeneity within this meta-analysis. Fine heterogeneity was defined as I2 < 50% and P > 0.1, indicating that a standard fixed-effect model (Mantel–Haenszel method) would be applied for the summarized OR. On the contrary, a random-effect model (DerSimonian and Laird method) would be performed if the significant heterogeneity was revealed by I2 ≥ 50% or P ≤ 0.1 [12]. Sensitivity analysis was conducted to further identify the possible origins of heterogeneity. Then, the identified study that contributed to the significant heterogeneity would be removed and a repeated meta-analysis of the remaining studies would be performed for adjustments. The robustness of our meta-analysis would be confirmed when identifying no substantial variations between the adjusted results and primary results [13].
Finally, the publication bias was assessed by Begg's test and visualization of funnel plots in this meta-analysis. Its presence could be suggested by the symmetry of funnel plot conducted by Begg's test, in which log ORs were plotted against their corresponding standard errors (SEs) [14]. The significant bias was confirmed if P < 0.05.
Additionally, all of the above steps of statistical analysis were accomplished by STATA 12.0 (STATA Corporation, College Station, TX, USA).
RESULTS
The selection of included studies
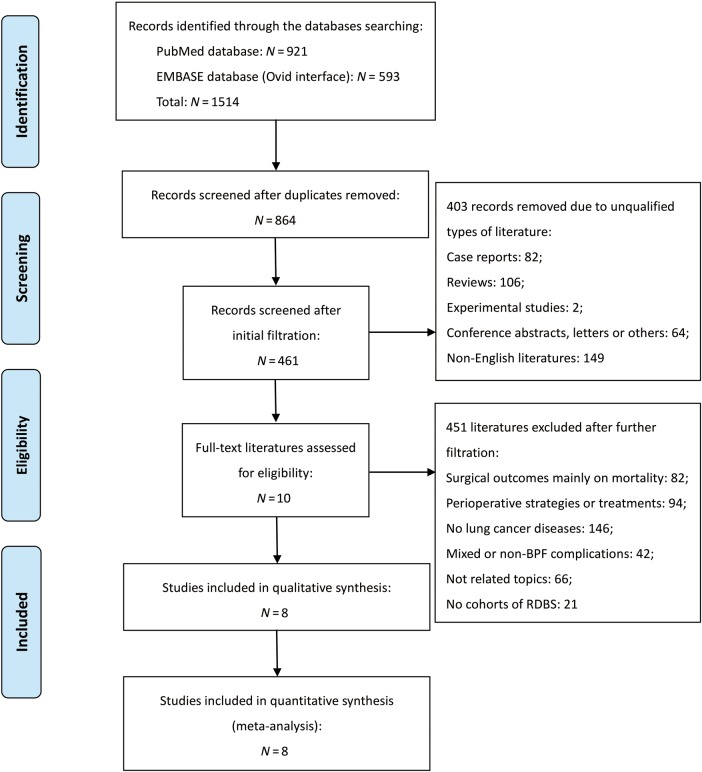
A total of 1514 electronic publications were identified by searching through the selected databases, including 921 citations in PubMed and 593 citations in EMBASE (via Ovid interface). Among them, 864 literatures received the initial filtration after excluding duplicated records. The initial filtration was based on screening titles and abstracts, while further filtration was conducted by reviewing the full text of remaining studies. Then, a total of 10 literatures were identified for possible eligibility of our meta-analysis. The complete procedures of literatures retrieval are given in Fig. 1. Finally, eight eligible literatures were determined into this meta-analysis [15–22]. Details of the two final excluded studies [23, 24], including the reasons for initial inclusion and later exclusion, are briefly described in Supplementary Table 1.
Figure 1:
PRISMA flow diagram of literature retrieval. BPF: bronchopleural fistula; RDBS: residual disease at the bronchial stump; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analysis.
The basic characteristics of included studies
The basic characteristics of the eight included studies are summarized in Table 1. All of them were retrospective observational studies [15–22]. They enrolled a total of 3736 patients undergoing pulmonary resections for lung cancer. RDBS was confirmed by either microscopy or visualization. The incidence of postoperative BPF was calculated as 3.0% (112/3736). Five included studies [17–21] analysed the possible risk factors of BPF in a total of 642 patients undergoing pneumonectomy for lung cancer (642/3736, ratio = 17.2%), including 40 patients with RDBS and 41 patients with post-pneumonectomy BPF. The other three studies [15, 16, 22] reported 3094 lung cancer patients undergoing multiple operations (3094/3736, ratio = 82.8%), but analysed them together. These eight studies were published from 1992 to 2010. The intervals between previous surgical procedures and the occurrence of BPF varied among different studies and their details are summarized in Table 1. Additionally, the mean NOS score was 7.7 (ranged from 7 to 9), suggesting a generally good quality of our included studies. The associated details of evaluation are not given.
Table 1:
Baseline characteristics of the eight included studies
| Authors (year) | Nation | Study design | Study period | NOS | No. of samples |
Onset, days (mean, range) | Operative modes |
Pathological definition | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | RDBS | BPF | PN | LB | BP | |||||||
| Suzuki et al. (2002)[15] | Japan | ROS | 1983–1997 | 8 | 1177 | 39 | 35 | NA | ✓ | ✓ | ✓ | AJCC (1997) |
| Sonobe et al. (2000)[16] | Japan | ROS | 1989–1998 | 8 | 557 | 10 | 10 | 5–481 | ✓ | ✓ | ✗ | ISSLC (1997) |
| Haraguchi et al. (2006)[17] | Japan | ROS | 1983–2005 | 7 | 114 | 5 | 12 | 15.5 (7–45) | ✓ | ✗ | ✗ | UICC (1997) |
| Hubaut et al. (1999)[18] | France | ROS | 1988–1997 | 8 | 199 | 11 | 5 | 43.2 (1–180) | ✓ | ✗ | ✗ | NA |
| Sirbu et al. (2001)[19] | Germany | ROS | 1990–1999 | 7 | 165 | 7 | 12 | 12.7 (2–42) | ✓ | ✗ | ✗ | ISSLC (1997) |
| Matsuoka et al. (2010)[20] | Japan | ROS | 1999–2004 | 8 | 64 | 12 | 5 | 75.4 (36–164) | ✓ | ✗ | ✗ | NA |
| de Perrot et al. (1999)[21] | Switzerland | ROS | 1990–1996 | 7 | 100 | 5 | 7 | 15.0 (7–45) | ✓ | ✗ | ✗ | NA |
| Asamura et al. (1992)[22] | Japan | ROS | 1980–1990 | 9 | 1360 | NA | 26 | 26.2 (3–128) | ✓ | ✓ | ✓ | ISSLC (1986) |
ROS: retrospective observational study; NOS: Newcastle–Ottawa Scale; RDBS: residual disease at the bronchial stump; BPF: bronchopleural fistula; NA: not available; PN: pneumonectomy; LB: lobectomy; BP: bronchoplasty; AJCC: American Joint Committee of Cancer; ISSLC: International System for Staging Lung Cancer; UICC: Union for International Cancer Control.
The statistical characteristics of included studies
Most of the included studies performed both univariate analysis and multivariate analysis to identify the clinically important factors contributing to BPF formation [15, 17, 20–22]. However, only three of them [20–22] reported the statistical results (one was HR, one was OR and one was β-value with SE) conducted from multivariate analysis, to evaluate the effects of RDBS on postoperative BPF (Table 2). In the other five included studies, the respective OR with 95% CI [15–19] could be calculated from published demographic data (Table 2). The 2 × 2 cross-tables of each observational study, the sources of statistics, the types of statistical analysis (univariate analysis or multivariate analysis), statistical significances and authors' attitudes are also listed in Table 2.
Table 2:
Statistical characteristics of the eight included studiesa
| Authors (year) | BPF | RDBS |
Statistical results (OR, 95% CI) | P-value | Sources | Statistical analysis | Attitude | |
|---|---|---|---|---|---|---|---|---|
| RDBS | Non-RDBS | |||||||
| Suzuki et al. (2002) [15] | BPF | 2 (5.1%) | 33 (2.9%) | 1.81 (0.42, 7.83) | 0.32 | DDC | U | Negative |
| Non-BPF | 37 | 1105 | ||||||
| Sonobe et al. (2000) [16] | BPF | 1 (10.0%) | 9 (1.6%) | 6.64 (0.76, 58.08) | 0.17 | DDC | U | Negative |
| Non-BPF | 9 | 538 | ||||||
| Haraguchi et al. (2006) [17] | BPF | 1 (20.0%) | 11 (10.1%) | 2.23 (0.23, 21.74) | 0.48 | DDC | U | Negative |
| Non-BPF | 4 | 98 | ||||||
| Hubaut et al. (1999) [18] | BPF | 0 (0.0%) | 5 (2.7%) | 1.45 (0.08, 27.87) | 1.0 | DDC | U | Negative |
| Non-BPF | 11 | 183 | ||||||
| Sirbu et al. (2001) [19] | BPF | 0 (0.0%) | 12 (7.6%) | 0.78 (0.04, 14.49) | 1.0 | DDC | U | Negative |
| Non-BPF | 7 | 146 | ||||||
| Matsuoka et al. (2010) [20] | BPF | 2 (16.7%) | 3 (5.8%) | 2.81 (0.39, 20.41) | 0.31 | Reported | M | Negative |
| Non-BPF | 10 | 49 | ||||||
| de Perrot et al. (1999) [21] | BPF | 2 (40.0%) | 5 (5.3%) | 5.4 (1.1, 26.0) | 0.038 | Reported | M | Positive |
| Non-BPF | 3 | 90 | ||||||
| Asamura et al. (1992) [22] | Not available | 3.80 (1.46, 9.93) | 0.0064 | Reported | M | Positive | ||
BPF: bronchopleural fistula; RDBS: residual disease at the bronchial stump; OR: odds ratio; 95% CI: 95% confidence interval; DDC: demographic data calculated; U: univariate analysis; M: multivariate analysis.
aNumbers in parentheses indicate the incidence of BPF in patients with RDBS and without RDBS.
Overall analysis
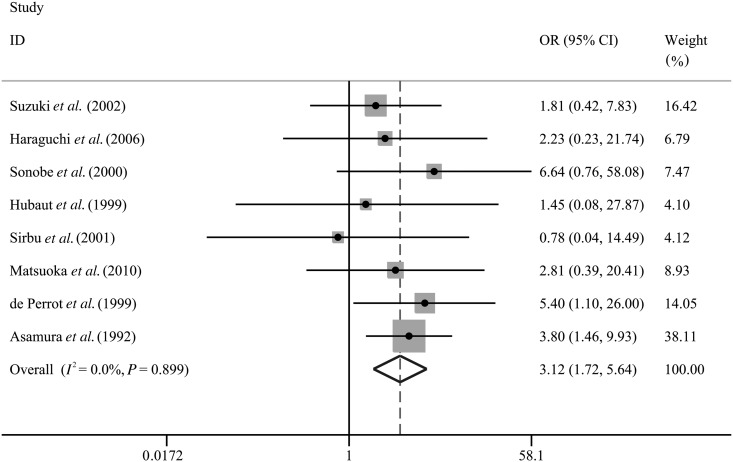
We integrated the patients' outcomes from all of the eight included studies [15–22] to assess the relationship between RDBS and the incidence of BPF in patients undergoing lung cancer surgery. The pooled OR was 3.12 (95% CI: 1.72–5.64; P < 0.001; Table 3 and Fig. 2), without any heterogeneity (I2 = 0.0%; P = 0.90). The summarized outcomes revealed that postoperative BPF appeared more frequently in patients with RDBS compared with those without RDBS.
Table 3:
Meta-analysis of the association between RDBS and BPF formation
| Outcomes | N | No. of patients |
Heterogeneity (I2, P-value) | Model | Publication bias (Begg's test, P-value) | OR (95%CI) | P-value | Conclusion | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | RDBS | BPF | ||||||||
| Overall | 8 | 3736 | NA a | 112 | I2 = 0.0%, P = 0.90 | Fixed | P = 0.27 | 3.12 (1.72,5.64) | <0.001 | Significant |
| Pneumonectomy | 5 | 642 | 40 | 41 | I2 = 0.0%, P = 0.80 | Fixed | P = 0.086 | 2.78 (1.06,7.28) | 0.037 | Significant |
| Lobectomy | Given up due to the scarcity of extractable details | |||||||||
N: reference count; NA: not available; RDBS: residual disease at the bronchial stump; BPF: bronchopleural fistula; OR: odds ratio; 95% CI: 95% confidence interval.
aThe number of patients with RDBS in ref. [22] is not available.
Figure 2:
Overall analysis for association between RDBS and risk of BPF in patients undergoing lung cancer surgery. BPF: bronchopleural fistula; RDBS: residual disease at the bronchial stump; OR: odds ratio; 95% CI: 95% confidence interval.
Subgroup analysis
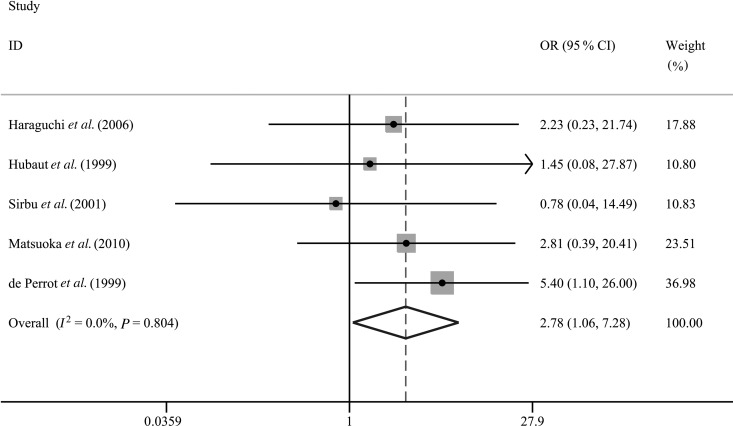
To evaluate the effects of RDBS on post-pneumonectomy BPF, we carried out a subgroup analysis pooling five qualified studies with a total of 642 pneumonectomy cases [17–21]. Finally, the pooled OR was 2.78 (95% CI: 1.06–7.28; P = 0.037; Table 3 and Fig. 3) and a fixed-effect model was applied (I2 = 0.0%; P = 0.80). These summarized results indicated that RDBS was significantly associated with the occurrence of post-pneumonectomy BPF.
Figure 3:
Subgroup analysis for association between RDBS and risk of BPF in patients undergoing pneumonectomy for lung cancer. BPF: bronchopleural fistula; RDBS: residual disease at the bronchial stump; OR: odds ratio; 95% CI: 95% confidence interval.
The demographic or statistical details revealing the association between RDBS and post-lobectomy BPF could not be effectively extracted from the three remaining included studies [15, 16, 22]. Therefore, we gave up the subgroup analysis assessing the effects of RDBS on post-lobectomy BPF (Table 3).
Sensitivity analysis
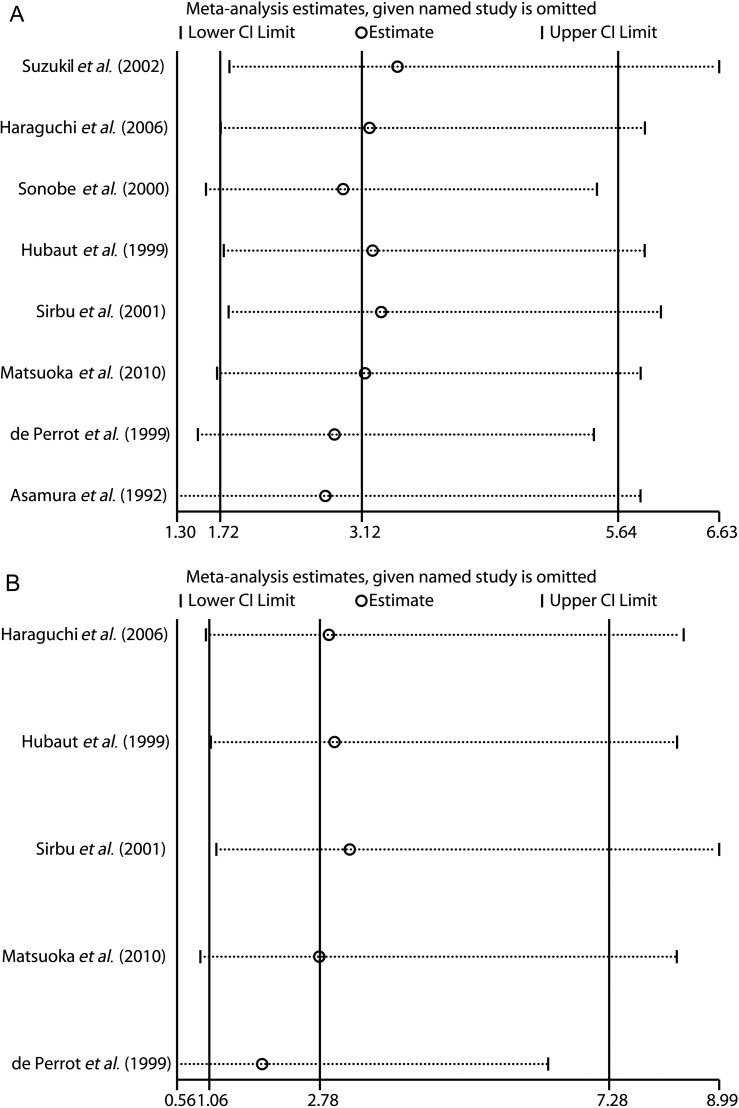
We performed a sensitivity analysis to further identify the possible origins of heterogeneity. The derived forest plots are shown in Fig. 4A and B. By visual inspection, we found that not any of the displayed outcomes from eight included studies was out of the estimated ranges (Fig. 4A and B). Thus, the leave-one-out method and a repeated meta-analysis of remaining studies were no more necessary. The strong stability of our meta-analysis was confirmed.
Figure 4:
Sensitivity analysis of the summarized OR with 95% CI on the association between RDBS and BPF formation in (A) overall analysis of lung cancer surgery and (B) subgroup analysis of pneumonectomy. BPF: bronchopleural fistula; RDBS: residual disease at the bronchial stump; OR: odds ratio; 95% CI: 95% confidence interval.
Publication bias
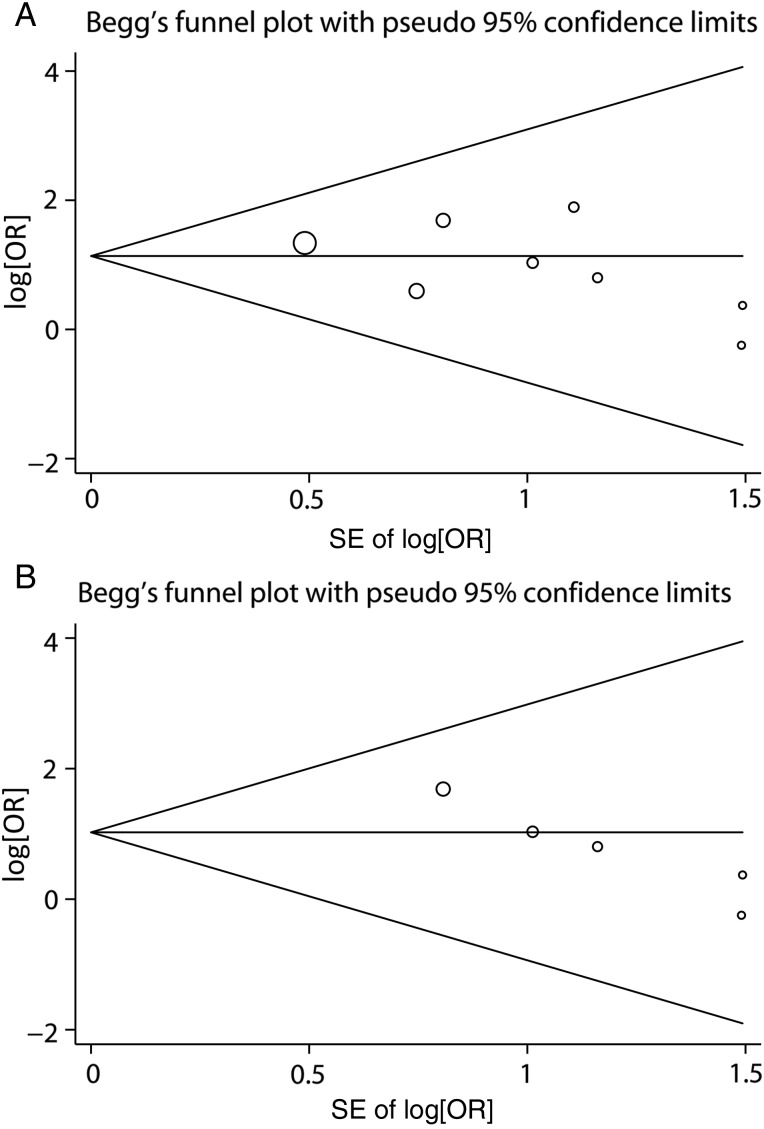
For the overall meta-analysis, there was no evidence of significant publication bias evaluated by visually inspecting the funnel plot conducted by Begg's test (P = 0.27; Table 3 and Fig. 5A). Moreover, no significant publication bias was identified by Begg's test (P = 0.086) in the subgroup assessing the effects of RDBS on post-pneumonectomy BPF (Table 3 and Fig. 5B).
Figure 5:
Begg's funnel plots for publication bias in (A) overall analysis of the association between RDBS and risk of BPF after lung cancer surgery and (B) subgroup analysis of the association between RDBS and post-pneumonectomy BPF. BPF: bronchopleural fistula; RDBS: residual disease at the bronchial stump; OR: odds ratio; SE: standard errors.
DISCUSSION
To the best of our knowledge, this is the first meta-analysis to evaluate the effects of RDBS on postoperative BPF in detail. The overall analysis suggests a significantly increased risk of postoperative BPF in patients with RDBS during lung cancer surgery compared with those without RDBS. In addition, the summarized outcomes obtained from subgroup analysis demonstrate that RDBS is positively associated with the increased incidence of BPF after pneumonectomy for lung cancer. The very low heterogeneity across the included studies can illustrate the rationality of our eligibility criteria and evidence collection, which contributes to accurately demonstrating the relationship between RDBS and BPF formation.
According to the TNM staging system of the Union for International Cancer Control (UICC), residual diseases are characterized by the residual carcinomatous tissues within resection margin either under microscopy or under visible inspection [23]. Incomplete resection at the bronchial stump can cause adverse effects on the prognoses of lung cancer patients as revealed by many reports [5–7]. On the one hand, RDBS increases the risk of lung cancer recurrence, both locally and distantly, which may cause poor prognoses. On the other hand, RDBS may increase the risk of insufficient bronchial stump or anastomosis, which can directly lead to fatal empyema. However, the estimated incidence of RDBS is ∼4–5% in all pulmonary resections [7]. A limited number of enrolled samples in an independent study may cause negative effects on analysing clinical outcomes, and may not be suitable for investigating the differences in BPF frequency between patients with RDBS and without RDBS.
We obtained an initial impression that there was a lack of consensus about the effects of RDBS on BPF development when pooling all the relevant studies together. In this meta-analysis, a total of 8 retrospective observational studies met our eligibility criteria [15–22], including two large studies that enrolled more than 1000 surgical patients and analysed the risk factors of BPF formation after lung cancer surgery. Asamura et al. [22] collected the clinical characteristics of 1360 patients undergoing multiple resections for lung cancer from 1980 to 1990. On the basis of a large number of enrolled samples, a significantly increased risk of BPF formation in patients with RDBS was observed after comprehensive estimations (β = 1.34, SE = 0.49, P = 0.0064). Another large retrospective study enrolling 1177 lung cancer patients was reported by Suzuki et al. [15], but no statistically significant relationship between RDBS and BPF development was revealed (P = 0.32), which was also reported in the other five studies enrolling 64–557 samples [16–20]. These five studies suggested that RDBS was not significantly associated with the increased risk of BPF, but indicated the same trend with no statistical significances [16–18, 20]. Only the study reported by Sirbu et al. [19] indicated a lower BPF incidence in patients with RDBS compared with those without RDBS (0.0 vs 7.6%, P = 1.0). The remaining study was reported by de Perrot et al. [21]. It showed a statistically significant association between RDBS and BPF formation in 100 pneumonectomy cases for lung cancer (P = 0.038), although the small sample size might reduce the validity of the final conclusion.
Given such a review, we proposed that the main issue to be addressed was whether the relationship between RDBS and postoperative BPF was statistically reliable. Thus, a quantitative integration of these included studies using evidence-based methods was performed. It led to the conclusion that RDBS was significantly associated with BPF formation in patients undergoing pulmonary resections for lung cancer. On the basis of applying evidence-based methods to a larger number of pooled samples from previous published studies, the summarized outcomes may help clinicians to clarify the effects of RDBS on BPF development. However, there are two major issues to be addressed judiciously during the interpretation of summarized outcomes in this meta-analysis.
Firstly, these integrated outcomes were mainly based on univariate analysis instead of multivariate analysis. The multivariate analysis using logistic regression or Cox proportional hazards model is an effective method for reducing the bias from some major confounding factors. However, only three included studies published the statistical results from multivariate analysis, which had adequately eliminated other confounders [20–22]. Therefore, we should note that the validity of summarized outcomes in our meta-analysis might be attenuated by the insufficient measurement and elimination of various confounders in the majority of included studies.
Among the possible confounders of the present meta-analysis, the adjuvant treatments of diagnosed RDBS, including chemotherapy, radiotherapy and even reoperation, should not be ignored. The Dutch evidence-based (CBO) guideline indicates that adjuvant chemotherapy or radiotherapy will be necessary when confronting an incomplete resection [7]. However, some investigations have provided evidence revealing the increased risk of BPF in patients receiving adjuvant therapies [2]. The potential mechanisms underlying BPF formation induced by chemotherapy and radiotherapy may be related to the reduction of blood flow in bronchial mucosa or varying degrees of fibrosis at the bronchial stump of surgical patients [25]. Additionally, the secondary attack by reoperation may also increase the risk of postoperative complications including BPF. Therefore, the potential risk caused by adjuvant treatments in patients with RDBS might negatively affect the accuracy of our summarized outcomes. Unfortunately, we were unable to adequately eliminate this major bias in the present meta-analysis, because of the scarcity of available results from multivariate analysis. Thus, clinicians should judiciously evaluate the validity of our summarized outcomes in clinical practice.
Secondly, we concluded that RDBS was positively associated with the increased risk of post-pneumonectomy BPF in subgroup analysis. However, none of the included studies were eligible for the subgroup assessing the relationship between RDBS and post-lobectomy BPF. On the one hand, three included studies enrolled multiple pulmonary resection cases for lung cancer but pooled them together to analyse the significant risk factors of BPF [15, 16, 22]. Specific demographic or statistical details describing the incidence of post-lobectomy BPF in patients with RDBS and without RDBS were not available. On the other hand, we discovered that far fewer patients with early stage or well-differentiated lung cancer had RDBS after lobectomy, compared with those receiving pneumonectomy for advanced lung cancer [5, 6]. The scarcity of included patients might bring huge troubles for analysing the effects of RDBS on post-lobectomy BPF. Thus, we gave up the further subgroup analysis on evaluating the association between RDBS and post-lobectomy BPF, causing slightly negative effects on the integrity of our meta-analysis.
Therefore, we recommend that future studies should better collect more detailed records from multivariate analysis when focusing on analysing BPF formation, to sufficiently eliminate the bias risks from other confounders and thus convincingly demonstrate the validity of identified risk factors. Meanwhile, we hope that more research will separately provide the available data analysing BPF formation in the cohorts of patients undergoing different pulmonary resections in the future. Through these approaches, more accurate and comprehensive results can be summarized in updated meta-analyses.
Limitations
Finally, several limitations should be seriously considered in this meta-analysis. First, integrating the outcomes of 3736 surgical patients from eight retrospective observational studies may not be convincing enough. Second, the assessments of the association between RDBS and BPF formation were mainly based on the outcomes obtained from univariate analysis rather than multivariate analysis. Other possible perioperative confounders, especially the strategies of adjuvant therapies, might reduce the accuracy of summarized outcomes. Third, far fewer than 20 included studies in the present meta-analysis might result in the poor efficacy of Begg's test, although no significant publication bias was detected in the overall meta-analysis. Finally, only literature in the English language were included in this meta-analysis. More additional papers may have been identified if no language limitations had been applied.
CONCLUSIONS
In conclusion, after pooling the recent evidence, our meta-analysis indicates that RDBS is positively associated with the increased risk of BPF in patients undergoing lung cancer surgery. In the further analysis, for patients undergoing pneumonectomy, BPF occurrence appears to be significantly more frequent in patients with RDBS compared with those without RDBS. Some limitations in our study need to be eliminated in the future. More accurate and comprehensive evidence should be collected in updated meta-analyses.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
ACKNOWLEDGEMENTS
We acknowledge the assistance of the staff in the Department of Thoracic Surgery and Lung Cancer Center, West China Hospital, Sichuan University.
Conflict of interest: none declared.
REFERENCES
- 1.Zaheer S, Allen MS, Cassivi SD, Nichols FC III, Johnson CH, Deschamps C et al. Postpneumonectomy empyema: results after the Clagett procedure. Ann Thorac Surg 2006;82:279–87. [DOI] [PubMed] [Google Scholar]
- 2.Shekar K, Foot C, Fraser J, Ziegenfuss M, Hopkins P, Windsor M. Bronchopleural fistula: an update for intensivists. J Crit Care 2010;25:47–55. [DOI] [PubMed] [Google Scholar]
- 3.Zakkar M, Kanagasabay R, Hunt I. No evidence that manual closure of the bronchial stump has a lower failure rate than mechanical stapler closure following anatomical lung resection. Interact CardioVasc Thorac Surg 2014;18:488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Maio M, Perrone F, Deschamps C, Rocco G. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196–200. [DOI] [PubMed] [Google Scholar]
- 5.Pitz CC, Brutel de la Rivière A, Elbers HR, Westermann CJ, van den Bosch JM. Result of resection of T3 non-small cell lung cancer invading the mediastinum or main bronchus. Ann Thorac Surg 1996;62:1016–20. [DOI] [PubMed] [Google Scholar]
- 6.Snijder RJ, Brutel de la Rivière A, Elbers HJ, van den Bosch JM. Survival in resected stage I lung cancer with residual tumor at the bronchial resection margin. Ann Thorac Surg 1998;65:212–6. [DOI] [PubMed] [Google Scholar]
- 7.Wind J, Smit EJ, Senan S, Eerenberg JP. Residual disease at the bronchial stump after curative resection for lung cancer. Eur J Cardiothorac Surg 2007;32:29–34. [DOI] [PubMed] [Google Scholar]
- 8.Gudbjartsson T, Gyllstedt E, Pikwer A, Jönsson P. Early surgical results after pneumonectomy for non-small cell lung cancer are not affected by preoperative radiotherapy and chemotherapy. Ann Thorac Surg 2008;86:376–82. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org. [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 15.Suzuki M, Otsuji M, Baba M, Saitoh Y, Iizasa T, Shibuya K et al. Bronchopleural fistula after lung cancer surgery. Multivariate analysis of risk factors. J Cardiovasc Surg (Torino) 2002;43:263–7. [PubMed] [Google Scholar]
- 16.Sonobe M, Nakagawa M, Ichinose M, Ikegami N, Nagasawa M, Shindo T. Analysis of risk factors in bronchopleural fistula after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2000;18:519–23. [DOI] [PubMed] [Google Scholar]
- 17.Haraguchi S, Koizumi K, Hioki M, Hirata T, Hirai K, Mikami I et al. Analysis of risk factors for postpneumonectomy bronchopleural fistulas in patients with lung cancer. J Nippon Med Sch 2006;73:314–9. [DOI] [PubMed] [Google Scholar]
- 18.Hubaut JJ, Baron O, Al Habash O, Despins P, Duveau D, Michaud JL. Closure of the bronchial stump by manual suture and incidence of bronchopleural fistula in a series of 209 pneumonectomies for lung cancer. Eur J Cardiothorac Surg 1999;16:418–23. [DOI] [PubMed] [Google Scholar]
- 19.Sirbu H, Busch T, Aleksic I, Schreiner W, Oster O, Dalichau H. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330–6. [PubMed] [Google Scholar]
- 20.Matsuoka K, Misaki N, Sumitomo S. Preoperative hypoalbuminemia is a risk factor for late bronchopleural fistula after pneumonectomy. Ann Thorac Cardiovasc Surg 2010;16:401–5. [PubMed] [Google Scholar]
- 21.de Perrot M, Licker M, Robert J, Spiliopoulos A. Incidence, risk factors and management of bronchopleural fistulae after pneumonectomy. Scand Cardiovasc J 1999;33:171–4. [DOI] [PubMed] [Google Scholar]
- 22.Asamura H, Naruke T, Tsuchiya R, Goya T, Kondo H, Suemasu K. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456–64. [PubMed] [Google Scholar]
- 23.Kawaguchi T, Watanabe S, Kawachi R, Suzuki K, Asamura H. The impact of residual tumor morphology on prognosis, recurrence, and fistula formation after lung cancer resection. J Thorac Oncol 2008;3:599–603. [DOI] [PubMed] [Google Scholar]
- 24.Ross P Jr, Grecula J, Bekaii-Saab T, Villalona-Calero M, Otterson G, Magro C. Incorporation of photodynamic therapy as an induction modality in non-small cell lung cancer. Lasers Surg Med 2006;38:881–9. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto R, Tada H, Kishi A, Tojo T. Effects of preoperative chemotherapy and radiation therapy on human bronchial blood flow. J Thorac Cardiovasc Surg 2000;119:939–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.