Abstract
OBJECTIVES
Antibodies targeting angiotensin II type 1 receptor (AT1R) have been associated with malignant hypertension, autoimmune diseases and acute rejection and graft loss in solid organ transplantation. The aim of our study was to assess the impact of anti-AT1R antibodies on survival and incidence of acute cellular rejection (ACR) and pathology antibody-mediated rejection (pAMR) in a population of heart transplant recipients who were bridged to transplantation with a durable mechanical assist device Heart Mate II.
METHODS
Sera of 69 consecutive heart transplant recipients transplanted between October 2008 and August 2014 were tested for the presence of angiotensin II type 1 receptor antibodies before Heart Mate II device implantation and at the time of transplantation. Overall survival and post-transplant rejection-free survival were compared between antibody-negative and antibody-positive recipients using Kaplan–Meier and log-rank tests.
RESULTS
Anti-AT1R antibodies were present in 8 patients (11.6%) before Heart Mate II implantation. During the left ventricular assist device (LVAD) bridging, 44 patients (63.8%) who were initially anti-AT1R antibody-negative became positive, leaving 17 (24.6%) anti-AT1R antibody-negative patients at the time of transplantation for all comparisons. One- and 5-year survival was 88 ± 8 and 76 ± 10% for anti-AT1R antibody-negative and 87 ± 5 and 81 ± 7% for anti-AT1R antibody-positive patients, respectively (P = 0.582). Freedom from ACR at 1 year was 68 ± 12% for anti-AT1R-negative and 75 ± 6% for anti-AT1R-positive recipients (P = 0.218). None of the anti-AT1R-negative patients developed AMR 1 year post-transplantation, whereas freedom from pAMR in anti-AT1R-positive recipients was 98 ± 2% (P = 0.198).
CONCLUSIONS
Our data showed no difference in the overall post-heart transplant survival and freedom from acute cellular and antibody-mediated rejection between anti-AT1R-negative and anti-AT1R-positive recipients. Further research is needed to assess the role of anti-AT1R antibodies in the risk stratification of LVAD-bridged recipients on the post-heart transplantation outcomes.
Keywords: Heart transplantation, Mechanical circulatory support, Angiotensin II type 1 receptor
INTRODUCTION
Left ventricular assist devices (LVADs) have reduced heart transplantation waiting list mortality and improved the quality of life and survival in patients with end-stage heart failure [1, 2]. One of the proposed limitations of mechanical device support therapy is a higher degree of sensitization among LVAD recipients. Apart from antibodies directed against human leucocyte antigen (HLA), several non-HLA antibodies like major histocompatibility class I-related chain, autoantibodies against angiotensin II type 1 receptor (AT1R) and endothelin receptor A as well as antibodies to cardiac self-antigens (myosin and vimentin) have been associated with an LVAD use [3–6]. AT1R differs from all other non-HLA antigenic targets in the mechanism of action. Binding of antibodies to AT1R induces unique physiological effects that mimic those of receptor ligand binding (angiotensin II in the renin–angiotensin system [7]). Anti-AT1R antibodies exert their pathological effects by binding to extracellular loops of vascular receptors, and via intracellular signalling lead to proinflammatory and procoagulatory responses. Anti-AT1R antibodies have also been associated with systemic sclerosis, pre-eclampsia and malignant hypertension [8–10]. There is growing body of evidence of a negative impact of these antibodies on the graft survival in renal transplantation [11–13]. The objective of our study was to compare the survival and freedom from acute cellular- and antibody-mediated rejection in heart transplant recipients bridged with Heart Mate II assist device stratified according to the pretransplant presence of anti-AT1R antibodies.
MATERIALS AND METHODS
Patients
Between October 2008 and August 2014, we prospectively evaluated sera of 69 patients implanted with durable continuous-flow axial mechanical support Heart Mate II who subsequently underwent heart transplantation. A cut-off of 17 U/ml was used to determine anti-AT1R positivity/negativity. Hospital database and medical records were searched for clinical data on the survival and incidence of acute cellular- and antibody-mediated rejection. Identification and classification of rejection episodes was based on histopathology and immunohistochemistry evaluation of endomyocardial biopsy specimens and followed the International Society for Heart and Lung Transplantation guidelines [14, 15]. Patients with acute cellular rejection (ACR) ≥2R and pathology antibody-mediated rejection (pAMR) of any grade were included in the time-to-event analyses. As per our institutional protocol, all heart transplant recipients received induction therapy with antithymocyte globulin (1.5 mg/kg body weight). Maintenance immunosuppression comprised a combination of calcineurin inhibitor with either cyclosporine (trough level 200 mg/dl) or tacrolimus (trough level 3–8 ng/dl), antiproliferative agent (mycophenolate mofetil) and steroids (tapering regimen). The median follow-up was 39 months (24–54 months), was 100% complete, totalled 2587 patient-months and ended on 5 April 2015.
Antibody analysis
The first sample was collected before implanting the device. The second sample was obtained at the time of transplantation. Anti-AT1R antibodies were assayed by sandwich enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Thermo Fisher Scientific—One lambda, Waltham, MA, USA).
Coagulated blood was drawn into sterile 10-ml serum separator tubes. Samples were centrifuged at 1000 g for 15 min; serum was collected and stored at −20°C until the day of measurement. The concentration of anti-AT1R IgG antibody in serum was measured by ELISA according to the manufacturer's instructions. The samples were assayed on angiotensin II type 1-receptor-precoated microtiter plates. Standards and diluted 1:100 samples were added into the wells and incubated for 2 h at 2–8°C. After washing steps, anti-AT1R antibody was detected with POD-labelled anti-human IgG antibody (1:100) followed by colour development with tetramethylbenzidine (TMB) substrate solution and, measured at 450 nm, with the correction wavelength set at 630 nm. Optical densities were then converted into concentration by the use of a standard curve. The detection range of the test was >2, 5 U/ml with positive value set at 17 U/ml and negative value set at ≤17 U/ml.
Statistical analysis
Continuous variables are presented as median with 25th and 75th percentile interval. Categorical variables are shown as the percentage of the sample. Fisher's exact test was used to evaluate the difference between categorical baseline demographic and clinical characteristics. Continuous variable comparisons were performed using Mann–Whitney U-test for two study groups and Kruskal–Wallis one-way analysis of variance test for multiple group analysis. Post-transplant survival and freedom from rejection were assessed by Kaplan–Meier method (with the date of transplant as the time origin for the analysis) and the log-rank test was used for comparison. Univariable analysis was performed to identify risk factors associated with overall post-transplant survival. Variables with P < 0.2 on univariable analysis were entered into multivariable logistic regression with a forward conditional selection model. A P-value of less than 0.05 was considered significant. The statistical analyses were performed with IBM SPSS 18 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Altogether, 69 patients were transplanted with the Heart Mate II device at our institution during the study period. The mean time of mechanical support before heart transplantation was 11 months (range 1–53 months). Anti-AT1R antibodies were present in 8 (11.6%) and anti-HLA antibodies in 3 (4.3%) patients before Heart Mate II implantation. During the support, 44 patients (63.8%) who were initially anti-AT1R-negative became anti-AT1R-positive and 17 (24.6%) remained anti-AT1R antibody-negative until transplantation. Of the 67 patients who were not sensitized against HLA antigens before HM II implantation, 6 (9%) developed anti-HLA antibodies during the support. At the time of transplantation, there were 13 patients who were antibody-negative for both HLA and AT1R antigens (AT1R−HLA−), 3 patients who were anti-AT1R antibody-negative and anti-HLA antibody-positive (AT1R−HLA+), 47 patients who were anti-AT1R antibody-positive and anti-HLA antibody-negative (AT1R+HLA−) and 4 patients who were sensitized against both AT1R and HLA antigens (AT1R+HLA+). Basic demographic and clinical characteristics of patients stratified according to the presence of anti-AT1R antibodies are presented in Table 1.
Table 1:
Basic demographic and clinical characteristics of patients stratified according to the presence of anti-AT1R antibodies before Heart Mate II implantation and throughout the support
| AT1R-positive before HMII implantation (n = 8) | AT1R-positive during HMII support (n = 44) | AT1R-negative (n = 17) | P-value | |
|---|---|---|---|---|
| Age (years) | 49 (41, 58) | 51 (39, 59) | 48 (41, 58) | 0.875* |
| BSA (m2) | 1.81 (1.66, 2.03) | 1.97 (1.81, 2.10) | 2.01 (1.91, 2.13) | 0.118* |
| BMI | 21 (20, 25) | 25 (23, 27) | 27 (24, 29) | 0.054* |
| Female gender (%) | 1 (12.5) | 7 (15.9) | 1 (5.9) | 0.580† |
| Diabetes (%) | 1 (12.5) | 8 (18.2) | 3 (17.6) | 0.926† |
| COPD (%) | 1 (12.5) | 7 (15.9) | 2 (11.8) | 0.905† |
| Previous stroke (%) | 2 (25) | 10 (22.7) | 2 (11.8) | 0.596† |
| INTERMACS I,II (%) | 2 (25) | 27 (61.4) | 10 (58.9) | 0.158† |
| Ischaemic aetiology of HF (%) | 2 (25) | 16 (36.4) | 5 (29.4) | 0.902† |
| HLA sensitized (%) | 0 | 3 (8.6) | 0 | 0.354† |
| Previous sternotomy (%) | 1 (12.5) | 8 (18.2) | 4 (23.5) | 0.792† |
| Previous VA ECMO | 0 | 4 (9.1) | 0 | 0.299† |
| After HMII implantation | ||||
| Concomitant procedure (%) | 14 (31.8) | 2 (11.8) | 0.110† | |
| PRBC (units) | 10 (7, 14) | 9 (7, 17) | 0.863** | |
| Platelets (units) | 4 (3, 6) | 3 (2, 5) | 0.159** | |
| FFP (units) | 24 (18, 35) | 26 (16, 31) | 0.700** | |
| Major bleeding (%) | 6 (13.6) | 0 | 0.173† | |
| Major infection (%) | 11 (25) | 5 (29.4) | 0.725† | |
| Neurological dysfunction (%) | 1 (2.3) | 0 | 0.531† | |
| Device malfunction (%) | 1 (2.3) | 1 (5.9) | 0.478† | |
| ARBs during support (%) | 8 (18.2) | 1 (5.9) | 0.206† | |
| HLA sensitized during support (%) | 13 (30.2) | 7 (41.2) | 0.418† | |
| Mean BP on support (mmHg) | 85 (80, 90) | 90 (81, 99) | 0.048** | |
| Duration of support (months) | 9 (5, 16) | 11 (5, 16) | 0.705** | |
BSA: body surface area; BMI: body mass index; COPD: chronic obstructive pulmonary disease; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; HF: heart failure; HLA: human leucocyte antigen; HMII: Heart Mate II; VA ECMO: veno-arterial extracorporeal membrane oxygenation; PRBC: pure red blood cell; FFP: fresh frozen plasma; ARB: angiotensinogen receptor blocker; BP: blood pressure.
*Kruskal–Wallis test.
**Mann–Whitney U-test.
†Fisher's exact test.
Survival
Of the 69 transplanted patients, 8 did not survive until discharge. Primary graft dysfunction was the leading cause of death, followed by sepsis and neurological complications (Table 2). Four additional patients died after being discharged from the hospital during the follow-up period.
Table 2:
Survival in days and causes of death of individual patients
| Patient | Survival in days | Anti-AT1R antibody at transplantation | Cause of death |
|---|---|---|---|
| Patient 1 | 1 | Negative | PGD |
| Patient 2 | 58 | Positive | Sepsis |
| Patient 3 | 19 | Positive | Ischaemic stroke, Sepsis, MOF |
| Patient 4 | 26 | Positive | PGD, ACR |
| Patient 5 | 6 | Positive | PGD, small bowel ischaemia |
| Patient 6 | 1 | Positive | PGD |
| Patient 7 | 67 | Positive | Sepsis |
| Patient 8 | 16 | Negative | PGD |
| Patient 9 | 672 | Negative | CAV |
| Patient 10 | 830 | Negative | Unknown |
| Patient 11 | 1417 | Positive | Ischaemic stroke |
| Patient 12 | 176 | Positive | Unknown |
AT1R: angiotensin II type 1 receptor; PGD: primary graft dysfunction; MOF: multi-organ failure; ACR: acute cellular rejection; CAV: cardiac allograft vasculopathy.
Of the 42 clinical, demographic, haemodynamic and echocardiographic recipient, donor and perioperative variables, only 11 with P < 0.2 (Table 3) on univariable analysis were entered into multivariable logistic regression model. Serum blood urea nitrogen level at the time of transplantation was identified as a sole predictor for post-transplantation death (odds ratio 1.459, 95% confidence interval: 1.010–2.107, P = 0.044). Survival analysis of recipients stratified according to the presence of anti-AT1R antibodies before transplantation revealed 1- and 5-year survival of 88 ± 8 and 76 ± 10% for anti-AT1R antibody-negative and 87 ± 5 and 81 ± 7% for anti-AT1R antibody-positive patients, respectively (P = 0.582) (Fig. 1).
Table 3:
Univariable analysis for an overall post-heart transplantation survival.
| Variables | Survivors (n = 57) | Non-survivors (n = 12) | P-value |
|---|---|---|---|
| Age (years) | 50 (41, 58) | 55 (42, 61) | 0.103 |
| Creatinine (µmol/l) | 79 (89, 104) | 99 (93, 139) | 0.014 |
| BUN (mmol/l) | 5.8 (4.3, 6.7) | 8.1 (6.1, 9.8) | 0.018 |
| GFR (ml/1.72 m2) | 110 (88, 129) | 107 (67, 112) | 0.053 |
| PASP before HMII implantation (mmHg) | 58 (45, 67) | 66 (53, 69) | 0.002 |
| TPG before HMII implantation (mmHg) | 10 (9, 14) | 14 (11, 19) | 0.023 |
| CVP before HMII implantation (mmHg) | 10 (6, 14) | 15 (10, 19) | 0.026 |
| HMII concomitant procedure (%) | 13 (22.8) | 6 (50) | 0.077 |
| HMII AVR (%) | 3 (5.3) | 5 (41.7) | 0.003 |
| HLA sensitized on HMII (%) | 22 (38.6) | 1 (8.3) | 0.048 |
| AT1R antibody conversion during HMII (%) | 39 (68.4) | 5 (41.7) | 0.149 |
BUN: blood urea nitrogen; GFR: glomerular filtration rate; PASP: pulmonary artery systolic pressure; TPG: trans-pulmonary gradient; CVP: central venous pressure; AVR: aortic valve replacement; HLA: human leucocyte antigen; AT1R: angiotensin II type 1 receptor; HMII: Heart Mate II.
Figure 1:
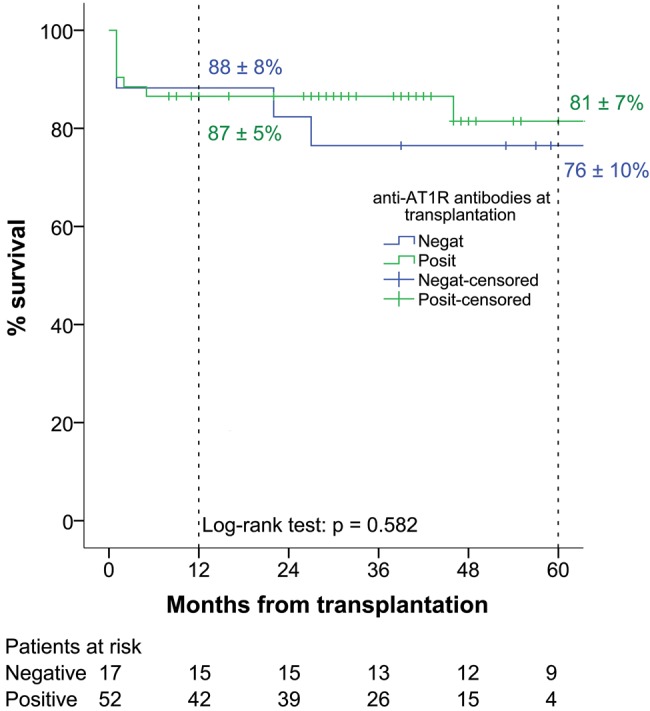
Overall post-heart transplant survival stratified according to the presence of anti-AT1R antibodies before transplantation. AT1R: angiotensin II type 1 receptor.
Acute cellular rejection
Of the 67 heart transplant recipients who had biopsy results available, 14 (20.9%) were diagnosed with ACR with ISHLT grade ≥2R (12 patients 2R and 2 patients 3R). Patient stratification according to the pretransplant presence of antibodies against AT1R and HLA antigens with respect to subsequent post-transplant ACR is depicted in Table 4. Both recipients with grade 3R rejection presented with an associated graft dysfunction. The first patient was successfully treated with 1 g of intravenous solumedrol administered daily for 3 days. The second patient required veno-arterial extracorporeal membrane oxygenation (ECMO) implanted centrally for severe biventricular graft dysfunction on top of pulse steroid therapy. After 12 days of support, the graft function recovered and ECMO was successfully explanted. The median time to ACR episode was 147 days (43 606) in anti-AT1R antibody-negative and 46 days (17 264) in anti-AT1R antibody-positive recipients (P = 0.306). Freedom from ACR at 1 year was 68 ± 12% for anti-AT1R-negative and 75 ± 6% for anti-AT1R-positive recipients (P = 0.218) (Fig. 2).
Table 4:
Acute cellular rejection rate stratified by grade and the presence of anti-AT1R antibodies and anti-HLA antibodies
| ACR ISHLT grade | AT1R−HLA− (n = 13) | AT1R−HLA+ (n = 3) | AT1R+HLA− (n = 47) | AT1R+HLA+ (n = 4) |
|---|---|---|---|---|
| 0 (n = 31) | 7 (53.8%) | 3 (100%) | 19 (40.4%) | 2 (50%) |
| 1R (n = 22) | 3 (23.1%) | 0 | 18 (38.3%) | 1 (25%) |
| 2R (n = 12) | 3 (23.1%) | 0 | 9 (19.1%) | 0 |
| 3R (n = 2) | 0 | 0 | 1 (2.1%) | 1 (25%) |
AT1R: angiotensin II type 1 receptor; HLA: human leucocyte antigen; ACR: acute cellular rejection.
Figure 2:
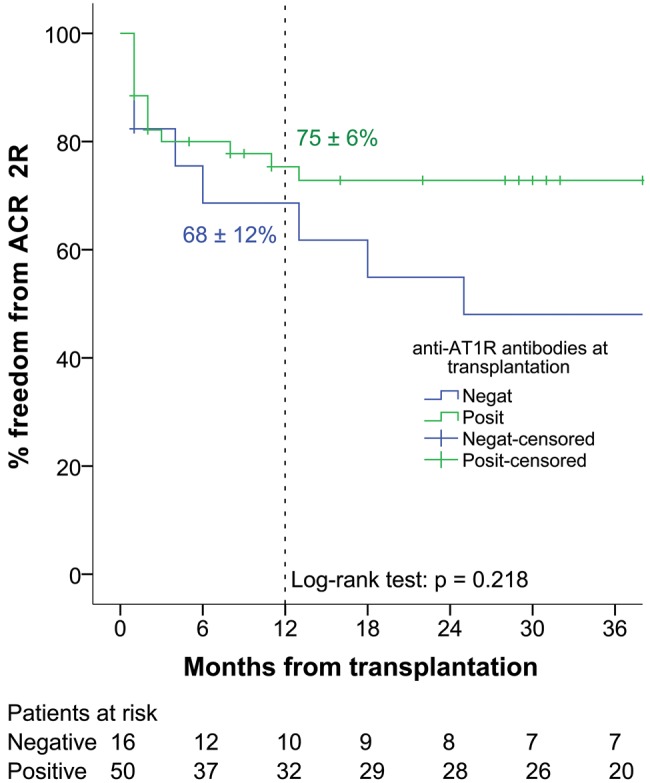
Freedom from ACR ≥2R. AT1R: angiotensin II type 1 receptor; ACR: acute cellular rejection.
Pathologicy antibody-mediated rejection
Four patients' endomyocardial biopsy specimens yielded histology and/or immunohistochemistry signs of antibody-mediated rejection (Table 5). Only the patient with Grade 3 pAMR was positive for donor-specific antibodies against HLA and had concomitant graft dysfunction. Acute rejection was treated with a pulse of steroid that consisted of 1 g of intravenous solumedrol administered for 3 consecutive days, 10 cycles of therapeutic plasma exchange and intravenous immunoglobulins at 100 mg/kg. After multimodality treatment, this patient is now symptom free, showing no signs of rejection in the latest endomyocardial biopsies and the graft function assessed with transthoracic echocardiography is satisfactory. None of the anti-AT1R-negative patients presented with pAMR at 1 year post transplantation, whereas freedom from pAMR in anti-AT1R-positive recipients was 98 ± 2% (P = 0.198) (Fig. 3).
Table 5:
Pathological antibody-mediated rejection rate stratified by grade and the presence of anti-AT1R antibodies and anti-HLA antibodies
| pAMR ISHLT grade | AT1R−HLA− (n = 13) | AT1R−HLA+ (n = 3) | AT1R+HLA− (n = 47) | AT1R+HLA+ (n = 4) |
|---|---|---|---|---|
| 0 (n = 63) | 13 (100%) | 2 (66.7%) | 45 (95.7%) | 3 (75%) |
| 1i (n = 1) | 0 | 0 | 1 (2.1%) | 0 |
| 1 h (n = 0) | 0 | 0 | 0 | 0 |
| 2 (n = 2) | 0 | 0 | 1 (2.1%) | 1 (25%) |
| 3 (n = 1) | 0 | 1 (33.3%) | 0 | 0 |
pAMR: pathology antibody-mediated rejection; AT1R: angiotensin II type 1 receptor; HLA: human leucocyte antigen.
Figure 3:
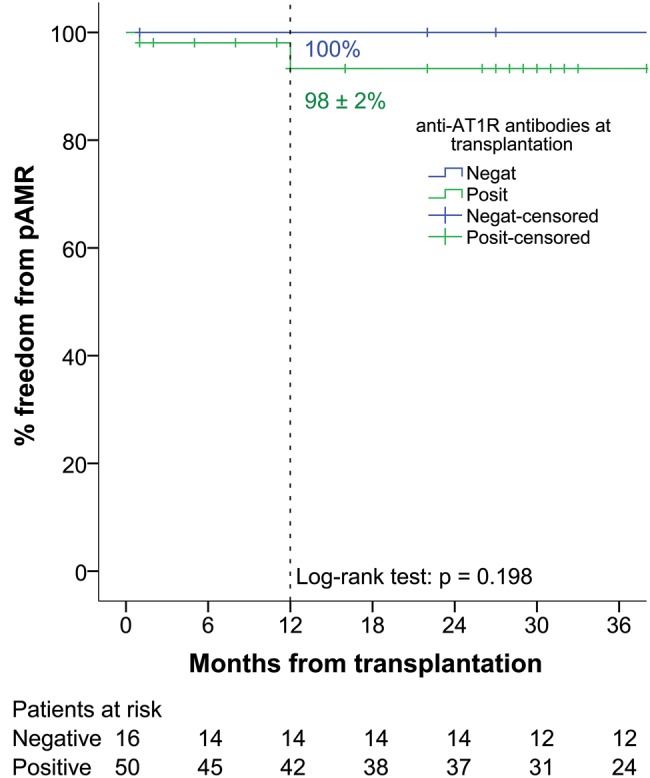
Freedom from pathology antibody-mediated rejection of any grade. AT1R: angiotensin II type 1 receptor.
DISCUSSION
The use of mechanical circulatory support to bridge patients to transplant increased to 41% in 2012, predominantly in the form of LVADs [16]. These patients now constitute a substantial proportion of the heart transplant recipients. They present unique challenges for the healthcare professionals in the perioperative as well as postoperative period. One of the shortcomings of the mechanical assist devices is the overproduction of antibodies. The main finding of our study is that more than 60% of patients with the end-stage heart failure who were bridged to transplantation with Heart Mate II device developed antibodies against AT1R. There are multiple pathways by which these antibodies may appear before transplantation in mechanically supported patients. Protein antigenic determinants may become accessible after injury or surgical stress associated with an LVAD implantation. Inflammatory events might lead to de novo expression of these auto antigens [17, 18]. Anti-AT1R antibodies may also develop through similar pathways as those observed for HLA-specific antibodies: transfusions, pregnancies and previous solid organ transplantations. From our cohort, 12% of patients tested positive for the presence of anti-AT1R antibodies before Heart Mate II implantation. During the support, 64% of the initially negative AT1R patients became positive. We observed no association between preoperative demographics, blood product use or duration of mechanical support and conversion of AT1R-negative to AT1R-positive status. Barten et al. [6] found in their study of 29 VAD recipients that 65.5% were positive for anti-AT1R antibodies. Of note, most of the patients showed extremely high antibody titres up to 1000 U. In contrast to our own observation, they noted higher amount of blood transfusions in AT1R positive compared with AT1R-negative VAD recipients.
Although anti-AT1R antibodies may belong to complement fixing IgG subclasses (IgG1 and IgG3 isotypes), C4d-positive staining was found not to be very frequent in the biopsies of renal transplant recipients with anti-AT1R antibody-mediated rejections [7, 19] implicating complement independent mechanism of injury. This would explain the lack of association between anti-AT1R antibody status and pAMR in our series. We found that pAMR as defined by ISHLT guidelines was an extremely rare event after heart transplantation in our cohort. During the follow-up, we detected only one clinically significant antibody-mediated rejection which was accompanied with graft dysfunction. Our results also showed no statistically significant difference in the freedom from ACR ≥2R between anti-AT1R antibody-negative and -positive recipients. Given the putative mechanism of action of these antibodies that primarily act on vascular endothelium causing non-specific, non-complement-mediated microvascular damage, these results are not surprising. When we stratified the patients by the presence of anti-AT1R antibodies combined with the anti-ALA antibodies status, our results showed that none of the transplant recipients who were both anti-AT1R and anti-HLA antibody negative experienced pAMR or Grade 3R ACR. Conversely, 25% of recipients who were sensitized against both AT1R and HLA antigens presented post-transplantation with high grade ACR with associated graft dysfunction and another 25% with pAMR similarly with graft dysfunction. This leads us to believe that knowing the anti-AT1R antibody status on top of standard evaluation of anti-HLA antibodies pretransplantation adds an incremental value in a risk stratification of post-heart transplantation immunological related adverse events.
Although there is a substantial amount of literature on the deleterious effects of anti-AT1R antibodies on post-renal transplantation outcomes, we were only able to find one manuscript in reference to heart transplantation. Whereas we studied the effect of anti-AT1R antibodies as detected before transplantation, Hiemann et al. [20] evaluated the impact of anti-AT1R antibodies detected immediately post-transplantation and during 1 year of follow-up. The relevant clinical end-points included ACR of any grade, antibody-mediated rejection and microvasculopathy. Evaluating the results of 30 heart transplant recipients, the authors concluded that elevated post-transplantation levels of anti-AT1R antibodies (cut-off >16.5 U/ml) are associated with cellular- and antibody-mediated rejection and early onset of microvasculopathy and should be routinely monitored after heart transplantation. Apart from the difference in the time frame of anti-AT1R antibody evaluation, all our patients were bridged to transplantation with an LVAD and 75% were antibody-positive before transplantation. Also, ISHLT standardization of nomenclature of pAMR [14] was published only 1 year after the study. We believe there are fundamental differences about how the clinical end-points were defined and the results of those two studies are therefore difficult to compare. We nevertheless find the concept of increasing titres of anti-AT1R antibodies after transplantation very intriguing and plan to expand on the results of our study by evaluating the post-transplantation sera of all our patients. Another noteworthy aspect of the study by Hiemann et al. [20] is the suggestion of a potential association between anti-AT1R antibodies and post-transplant microvasculopathy. There is also increasing evidence for the active role of AT1R itself in the pathogenesis of chronic allograft rejection explaining the link between acute rejection and subsequent long-term clinical outcome [21]. Yamani et al. [22] observed an increase in mRNA of AT1R in 14 heart transplant recipients who had recurrent ACR in comparison with controls. In our study cohort, we only had the results of 41 coronary angiograms available and for that reason we did not include cardiac allograft vasculopathy (CAV) among the outcome measures in our study. We nevertheless acknowledge the compelling evidence for the immunoregulatory function of the renin–angiotensin system and its role in the pathogenesis of chronic allograft rejection. Comparing the incidence of CAV between groups of patients stratified by the presence of anti-AT1R antibodies and increased expression of AT1 receptor is a challenge for future studies.
Strengths and limitations
This study is the first of its kind to investigate the impact of anti-AT1R antibodies on post-heart transplantation outcome of LVAD-bridged recipients. It includes a homogenous group of patients supported with the same device. All recipients received identical immunosuppression as per our institutional protocol. The study has several limitations inherent to the retrospective nature of a single-centre observational study. Another limitation is a relatively small number of patients with relatively low event rates increasing the probability of Type II error. The study is meant as a pilot and no power analysis was performed. Another drawback of our study is the fact that all our patients received Heart Mate II device, thus limiting the generalization of our results to other types of mechanical devices. Future studies will need to address the question of whether newer generation of devices would show the same high degree of sensitization against AT1R and assess the role of these antibodies in post-transplantation outcome of mechanically bridged recipients.
In conclusion, although our data failed to demonstrate the association of pretransplant level of anti-AT1R antibodies with overall survival and acute cellular- and antibody-mediated rejection, we believe our study to be a valuable contribution. We consider it to be a first step in further research on the impact of these non-HLA-specific antibodies on post-heart transplantation outcome, especially in the era of mechanical circulatory support devices, improved diagnostic tools and increased awareness of antibody-mediated rejection.
Conflict of interest: none declared.
REFERENCES
- 1.Trivedi JR, Cheng A, Singh R, Williams ML, Slaughter MS. Survival on the heart transplant waiting list: impact of continuous flow left ventricular assist device as bridge to transplant. Ann Thorac Surg 2014;98:830–4. [DOI] [PubMed] [Google Scholar]
- 2.Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail 2014;7:470–8. [DOI] [PubMed] [Google Scholar]
- 3.Askar M, Hsich E, Reville P, Daghstani J, Nowacki A, Zhang A et al. Comparison of HLA & MICA allosensitization patterns among patients supported by ventricular assist devices (VAD) and patients with no devices. J Heart Lung Transplant 2013;32:S74. [DOI] [PubMed] [Google Scholar]
- 4.Askar M, Hsich E, Reville P, Nowacki AS, Baldwin W, Bakdash S et al. HLA and MICA allosensitization patterns among patients supported by ventricular assist devices. J Heart Lung Transplant 2013;32:1241–8. [DOI] [PubMed] [Google Scholar]
- 5.Banan B, Phelan D, Medhat A, Ewald G, Mohanakumar T. (47) - Increased sensitization to HLA and to cardiac self-antigens (myosin and vimentin) in patients waiting for cardiac transplantation with left ventricular assisting device (LVAD). J Heart Lung Transplant 2014;33:S25. [Google Scholar]
- 6.Barten MJ, Dragun D, von Salisch S, Dieterlen MT, Garbade J, Klein S et al. 112 Identification of non-HLA antibodies in ventricular assist device recipients. J Heart Lung Transplant 2012;31:S46. [Google Scholar]
- 7.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kehla M, Dechend R et al. Angiotensin II type 1 receptor activating antibodies in renal-allograft rejection. N Engl J Med 2005;352:558–69. [DOI] [PubMed] [Google Scholar]
- 8.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 1999;103:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riemekasten G, Philippe A, Nather M, Slowinski T, Muller DN, Heidecke H et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis 2011;70:530–6. [DOI] [PubMed] [Google Scholar]
- 10.Fu ML, Herltz H, Schulze W, Wallukat G, Micke P, Eftekhari P et al. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens 2000;18:945–53. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi M, Rebellato LM, Cai J, Hopfield J, Briley KP, Haisch CE et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant 2013;13:2577–89. [DOI] [PubMed] [Google Scholar]
- 12.Giral M, Foucher Y, Dufay A, Van Huyen JP, Renaudin K, Moreau A et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant 2013;13:2567–76. [DOI] [PubMed] [Google Scholar]
- 13.Banasik M, Boratynska M, Koscielska-Kasprzak K, Kaminska D, Bartoszek D, Zabinska M et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl Int 2014;27:1029–38. [DOI] [PubMed] [Google Scholar]
- 14.Berry GJ, Burke MM, Andersen C, Bruneval P, Fedrigo M, Fishbein MC et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2013;32:1147–62. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24:1710–20. [DOI] [PubMed] [Google Scholar]
- 16.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report—2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:996–1008. [DOI] [PubMed] [Google Scholar]
- 17.Dragun D. Humoral responses directed against non-human leucocyte antigens in solid-organ transplantation. Transplantation 2008;86:1019–25. [DOI] [PubMed] [Google Scholar]
- 18.Dragun D, Philippe A, Catar R. Role of non-HLA antibodies in organ transplantation. Transplantation 2012;17:440–5. [DOI] [PubMed] [Google Scholar]
- 19.Reinsmoen NL, Lai CH, Heidecke H, Haas M, Cao K, Ong G et al. Anti-angiotensin type 1 receptor antibodies associated with antibodynmediated rejection in donor HLA antibody negative patients. Transplantation 2010;90:1473–7. [DOI] [PubMed] [Google Scholar]
- 20.Hiemann NE, Meyer R, Wellnhofer E, Schoenemann C, Heidecke H, Lachmann N et al. Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation. Transplantation 2012;94:919–24. [DOI] [PubMed] [Google Scholar]
- 21.Yousufuddin M, Cook DJ, Starling RC, Abdo A, Paul P, Tuzcu EM et al. Angiotensin II receptors from peritransplantation through first-year-post transplantation and the risk of transplant coronary artery disease. J Am Coll Cardiol 2004;43:1565–73. [DOI] [PubMed] [Google Scholar]
- 22.Yamani MH, Cook DJ, Rodrigues ER, Thomas DM, Gupta S, Alster J et al. Incresed expression of Angiotensin II Type 1 Receptor (AGTR1) in heart transplant recipients with acute rejection. J Heart Lung Transplant 2006;25:1283–9. [DOI] [PubMed] [Google Scholar]


