Abstract
OBJECTIVE
This study aims to compare mid-long-term clinical outcomes between patients younger than 60 years of age undergoing bioprosthetic and mechanical aortic valve replacement.
METHODS
From January 2002 to December 2009, patients younger than 60 years of age who received Medtronic Hancock II porcine bioprostheses were selected and compared with those who received mechanical bi-leaflet valves in the aortic position. A stepwise logistic regression propensity score identified a subset of 112 evenly matched patient-pairs. Mid-long-term outcomes of survival, valve-related reoperations, thromboembolic events and bleeding events were assessed.
RESULTS
The follow-up was only 95.1% complete. Fourteen measurable variables were statistically similar for the matched cohort. Postoperative in-hospital mortality was 3.6% (bioprosthetic valves) and 2.7% (mechanical valves) (P = 0.700). Survival at 5 and 10 years was 96.3 and 88.7% for patients receiving bioprosthetic valve replacement versus 96.3 and 87.9% for patients receiving mechanical valve replacement (P = 0.860), respectively. At 5 and 10 years after operations, freedom from valve-related reoperation was 97.2 and 94.8% for patients receiving mechanical valve replacement, and 96.3 and 90.2% for patients receiving bioprosthetic valve replacement (P = 0.296), respectively. There was no difference between freedom from thromboembolic events (P = 0.528) and bleeding events (P = 0.128) between the matched groups during the postoperative 10 years.
CONCLUSIONS
In patients younger than 60 years of age undergoing aortic valve replacement, mid-long-term survival rate was similar for patients receiving bioprosthetic versus mechanical valve replacement. Bioprosthetic valves were associated with a trend for a lower risk of anticoagulation treatment and did not have significantly greater likelihood of a reoperation. These findings suggest that a bioprosthetic valve may be a reasonable choice for AVR in patients younger than 60 years of age.
Keywords: Aortic valve replacement, Heart valve prosthesis, Younger patients, Outcome, Clinical trials
INTRODUCTION
Aortic valve replacement (AVR) is commonly performed in patients younger than 60 years of age [1]. The 2012 EACTS guidelines for AVR recommend mechanical valves in patients younger than 60 years of age and bioprosthetic valves in patients older than 60, also in patients of any ages who are reluctant to receive lifelong anticoagulant therapy [2]. Structural valve deterioration (SVD) is the major disadvantage of bioprosthetic valves. However, bioprosthetic valves are associated with the improved durability and haemodynamic performance based on valve design and tissue processing, so many clinicians are considering bioprosthetic rather than mechanical valves for patients younger than 60 years of age undergoing AVR [3]. Bioprosthetic valves are implanted in ∼80% patients performed AVR in North America [4].
Various studies have reported on the mortality and reoperations after implanting different types of prosthetic aortic valves, but few series that compared bioprosthetic and mechanical valves have evaluated the long-term outcomes, such as survival and freedom from reoperation in patients younger than 60 years of age. However, conflicting results have been reported [5–7]. Nevertheless, bioprosthetic valves for AVR in patients younger than 60 years of age remain controversial, and expected survival and freedom from reoperation have not been clearly determined for this subset of patients.
Medtronic Hancock II porcine bioprostheses have been used in our department since 2002. The objective of the retrospective propensity-matched study was to evaluate the mid-long-term clinical outcomes in young individuals treated with bioprosthetic versus mechanical aortic valves, and to see if the current trend towards implanting bioprosthetic valves into younger patients is justifiable.
PATIENTS AND METHODS
This retrospective cohort study reviewed patients who underwent AVR with Medtronic Hancock II porcine bioprostheses from January 2002 to December 2009 in our department. The inclusion criteria were as follows: age was less than 60 years old, isolated AVR or with concomitant procedures such as coronary artery bypass grafting (CABG) and mitral or tricuspid valve repair. The exclusion criteria were additional valve replacements and ascending or aortic root surgery. The control group included patients who underwent AVR with mechanical valves (St Jude Medical, ATS Medical) during the same period. In the setting of the study, we used propensity-matching to improve comparability and reduce selection bias.
Unless warfarin therapy was indicated for other reasons (atrial fibrillation), patients receiving a bioprosthetic valve received warfarin therapy for 3–6 months after valve replacements, whereas patients receiving a mechanical valve received warfarin therapy interminably. Warfarin therapy was managed to maintain an international normalizing ratio between 1.8 and 2.4.
Patient preference was the primary determinant of valve selection. Reasons for the selection of bioprosthetic valves were contraindication to anticoagulation (27.3%), non-compliance to anticoagulation (14.8%), desire for pregnancy (17.5%) and unknown (40.4%).
All patients were operated under general anaesthesia with cardiopulmonary bypass under moderate hypothermia. The 4 : 1 blood cardioplegia was used for myocardial protection from the left and right coronary artery perfusion. The diameters of prosthesis implanted are presented in Table 1.
Table 1:
Diameters size of prosthesis implanted
| Prosthesis size (mm) | Overall cohort |
Propensity-matched |
||
|---|---|---|---|---|
| Bioprosthetic | Mechanical | Bioprosthetic | Mechanical | |
| 17 | 0 (0) | 11 (1.2) | 0 (0) | 2 (1.8) |
| 19 | 0 (0) | 42 (4.5) | 0 (0) | 7 (6.3) |
| 21 | 33 (21.6) | 290 (31.4) | 26 (23.2) | 35 (31.2) |
| 23 | 75 (49.0) | 278 (30.1) | 61 (54.5) | 46 (41.0) |
| 25 | 38 (24.8) | 243 (26.3) | 21 (18.7) | 17 (15.2) |
| 27 | 7 (4.6) | 61 (6.6) | 4 (3.6) | 5 (4.5) |
Data presented as mean ± standard deviation, % (n).
Adverse events were classified according to the standardized definitions from the Society of Thoracic Surgeons/American Association for Thoracic Surgery ‘Guidelines for Reporting Morbidity and Cardiac Valvular Operations’ [8]. The primary study end-points included overall survival and cardiac-related mortality. The secondary study end-points were valve-related reoperation, thromboembolic events and bleeding events. Thromboembolic events were diagnosed by clinical examinations, including stroke, transient ischaemic attack and non-cerebral embolic events. Bleeding events refer to major bleeding episodes requiring hospitalization or blood transfusion. End-points were classified as occurring early (in-hospital after implanting) and late (mid-long term after implanting).
Preoperative clinical characteristics, operation variables and postoperative in-hospital complications were acquired according to the medical records. Follow-up data were acquired through clinic re-examination and telephone interview. The follow-up was only 95.1% complete; the mean and median follow-up period was 104 ± 29 and 106 months (range: 54–153 months), respectively.
Propensity-matched cohort
We conducted a matched group analysis using propensity-matched cases (bioprosthetic valve) and controls (mechanical valve). Propensity was evaluated using logistic regression for bioprosthetic valves. Variables were selected based on literature review, differences between the two groups (Table 2) and clinical judgement. Separate forward-stepwise regression analyses were conducted for each variable, including examinations for interaction effects. Any variable with a P < 0.15 was entered into the final model, which was an enter-method logistic regression. The final model consisted of 10 variables: age, gender, hypertension, diabetes, ejection fraction, type of aortic valve lesion (insufficiency, stenosis or mixed lesion), concomitant CABG, concomitant mitral valvuloplasty (MVP) or tricuspid valvuloplasty (TVP), New York Heart Association (NYHA) Class III or IV and cross-clamp time (min). The resulting adjusted predicted probability score for each patient was then used to select matched pairs based on probability scores <0.01.
Table 2:
Basic characteristics and operative characteristics of the propensity-matched and overall cohort
| Variable | Overall cohort |
P-value | Propensity-matched |
P-value | ||
|---|---|---|---|---|---|---|
| Bioprosthetic | Mechanical | Bioprosthetic | Mechanical | |||
| Cases | 153 | 925 | 112 | 112 | ||
| Patient characteristics | ||||||
| Age (years) | 52.5 ± 7.3 | 48.2 ± 8.7 | 0.002 | 50.3 ± 9.1 | 49.2 ± 10.5 | 0.647 |
| Male | 94 (61.4) | 612 (66.2) | 0.681 | 67 (59.8) | 70 (62.5) | 0.681 |
| NYHA Class III–IV | 32 (20.9) | 216 (23.4) | 0.507 | 23 (20.5) | 19 (17.0) | 0.492 |
| Hypertension | 27 (17.6) | 254 (27.4) | 0.01 | 21 (18.8) | 15 (13.4) | 0.281 |
| Diabetes | 20 (13.1) | 74 (8.0) | 0.039 | 11 (9.8) | 13 (11.6) | 0.674 |
| Lung disease | 17 (11.1) | 97 (10.5) | 0.816 | 11 (9.8) | 12 (10.8) | 0.826 |
| Hypohepatia | 11 (7.2) | 35 (3.8) | 0.049 | 7 (6.3) | 7 (6.3) | 1 |
| Renal insufficiency | 5 (3.7) | 21 (2.2) | 0.443 | 3 (2.7) | 4 (3.6) | 0.701 |
| Ejection fraction (%) | 58 ± 11 | 51 ± 9 | <0.001 | 58 ± 10 | 57 ± 13 | 0.632 |
| Aortic valve insufficiency | 89 (58.1) | 387 (41.7) | <0.001 | 58 (51.8) | 57 (50.9) | 0.894 |
| Mixed lesion | 36 (23.5) | 272 (29.1) | 0.157 | 32 (28.5) | 33 (29.5) | 0.883 |
| Aortic valve stenosis | 28 (18.3) | 276 (29.5) | 0.004 | 22 (19.6) | 22 (19.6) | 1 |
| Urgent or emergency status | 9 (5.9) | 69 (7.5) | 0.485 | 6 (5.4) | 10 (8.9) | 0.299 |
| Endocarditis | 8 (5.2) | 72 (7.8) | 0.264 | 7 (6.3) | 6 (5.4) | 0.775 |
| Atrial fibrillation | 11 (7.2) | 99 (10.7) | 0.184 | 6 (5.4) | 11 (9.8) | 0.207 |
| Operative characteristics | ||||||
| Concomitant CABG | 18 (11.7) | 182 (19.5) | 0.023 | 12 (10.8) | 12 (10.8) | 1 |
| Concomitant MVP | 11 (7.2) | 88 (9.5) | 0.357 | 6 (5.4) | 7 (6.3) | 0.776 |
| Concomitant TVP | 6 (3.9) | 72 (7.8) | 0.087 | 3 (2.7) | 5 (4.5) | 0.472 |
| Perfusion time (min) | 81.6 ± 42.5 | 95.7 ± 39.2 | <0.001 | 85.6 ± 40.3 | 88.7 ± 36.1 | 0.536 |
| Cross-clamp time (min) | 55.3 ± 18.1 | 61.2 ± 19.7 | 0.023 | 53.5 ± 21.7 | 51.2 ± 23.7 | 0.278 |
| Mechanical ventilation time (h) | 36.1 ± 13.3 | 35.6 ± 9.2 | 0.463 | 32.7 ± 14.3 | 34.6 ± 11.2 | 0.563 |
Data presented as mean ± standard deviation, % (n), or median.
AVRm, aortic valve replacement with mechanical valve; AVRb: AVR with bioprosthetic valve; NYHA: New York Heart Association; CABG: coronary artery bypass grafting.
Statistical analysis
The Statistical Package for Social Sciences, version 19.0(SPSS) was used for data storage and analysis with P < 0.05 as the criterion for significance. Continuous variables are reported as the mean standard deviation and categorical variables as percentages. An intergroup comparison was conducted using the χ2 test and Fisher's exact test for categorical and continuous variables, respectively. For the time-to-event analysis, survival, freedom from valve-related reoperation, anticoagulation-related bleeding events and thromboembolic events were analysed using the Kaplan–Meier analysis.
RESULTS
From January 2002 to December 2009, a total of 1972 patients underwent AVR in our centre, of which 1078 patients were younger than 60 years of age (bioprosthetic valves for 153 and mechanical valves for 925 patients). The base characteristics and operative characteristics of the overall cohort are presented in Table 2. Patients receiving a bioprosthetic valve were older and had more hypertension, hypohepatia and diabetes. Patients receiving a mechanical prosthesis had a lower ejection fraction, longer perfusion time and longer cross-clamp time. There was a significant difference in the types of aortic valves more aortic valve insufficiency and less stenosis in patients receiving a bioprosthetic valve. More concomitant CABG was performed in the patients receiving a mechanical prosthesis.
The final propensity-matched group of this study consisted of 112 patients who received Medtronic Hancock II porcine bioprostheses and 112 patients who received mechanical aortic valves. The base characteristics and operative characteristics are listed in Table 2.
After propensity-matching, no statistically significant differences were observed in the matched parameters between propensity-matched groups. The preoperative characteristics were substantially similar regarding the proportion of age, gender, NYHA Class and ejection fraction. Both groups had similar disorder histories (hypertension, diabetes, lung disease, hypohepatia, renal insufficiency).
The indications for surgery were not different between propensity-matched groups, aortic valve insufficiency patients [AVR with bioprosthetic valve (AVRb) versus AVR with mechanical valve (AVRm), 58 of 112 vs 57 of 112, respectively; P = 0.894] and aortic valve stenosis patients (AVRb versus AVRm, 22 of 112 vs 22 of 112, respectively; P = 1) in the AVRb group. Meanwhile, no difference was observed in the two groups between patients of mixed lesion (AVRb vs AVRm, 32 of 112 vs 33 of 112, respectively; P = 0.883).
The operative data showed no statistically significant differences in the perfusion time and cross-clamp time between propensity-matched groups. Twelve of 112 patients of AVRb group (10.8%) and 12 of 112 patients of AVRm group (10.8%) had undergone concomitant CABG (P = 1).
Early-term outcomes
Although there were some differences in early-term outcomes between Hancock II bioprostheses and mechanical valves groups for the overall cohort (Table 3), the early outcomes did not significantly differ between propensity-matched groups (Table 3). The hospital mortality was 3.6% (4 of 112) in AVRb group and 2.7% (3 of 112) in AVRm group (P = 0.700). Major complications also appeared as not statistically significant different: perivalvular peak (P = 0.316), reoperation for bleeding (P = 0.651), ventricular arrhythmias (P = 0.207), endocarditis (P = 0.316), multisystem organ failure (P = 0.651) and thromboembolism (P = 0.651).
Table 3:
Early-term outcomes for both the propensity-matched and overall cohort
| Variable | Overall cohort |
P-value | Propensity-matched |
P-value | ||
|---|---|---|---|---|---|---|
| Bioprosthetic | Mechanical | Bioprosthetic | Mechanical | |||
| Cases | 153 | 925 | 112 | 112 | ||
| Length of stay (days) | 12.7 ± 4.7 | 16.1 ± 3.8 | 0.002 | 12.2 ± 5.7 | 11.1 ± 5.3 | 0.871 |
| Perivalvular leak | 1 (0.7) | 9 (0.9) | 0.710 | 1 (0.9) | 0 (0) | 0.316 |
| Reoperation for bleeding | 2 (1.3) | 33 (2.6) | 0.149 | 2 (1.8) | 3 (2.7) | 0.651 |
| Ventricular arrhythmias | 8 (5.2) | 134 (14.3) | 0.001 | 6 (5.4) | 11 (9.8) | 0.207 |
| Endocarditis | 1 (0.6) | 9 (0.9) | 0.710 | 0 (0) | 1 (0.9) | 0.316 |
| Multisystem organ failure | 4 (2.6) | 49 (5.2) | 0.170 | 3 (2.7) | 2 (1.8) | 0.651 |
| Thromboembolism | 2 (1.3) | 19 (2.0) | 0.546 | 2 (1.8) | 3 (2.7) | 0.651 |
| Mortality | 4 (2.6) | 17 (1.8) | 0.492 | 4 (3.6) | 3 (2.7) | 0.700 |
Data presented as mean ± standard deviation, % (n), or median.
Mid-long-term clinical outcomes: matched cohort
Thromboembolic events
A total of 12 thromboembolic events occurred during the follow-up period, of which 5 were in AVRb group and 7 were in AVRm group. By Kaplan–Meier analysis, the estimated freedom from thromboembolic events at 5 and 10 years was 98.1 and 94.3% in AVRb group, 98.0 and 91.0% in AVRm group, respectively (Fig. 1). There was no statistically significant difference between propensity-matched groups (P = 0.528).
Figure 1:
Kaplan–Meier curve of freedom from thromboembolic events for propensity-matched groups. AVRm, aortic valve replacement with mechanical valve; AVRb: AVR with bioprosthetic valve.
Bleeding events
A total of 11 major bleeding events occurred during the follow-up period, of which 3 were in AVRb group and 8 were in AVRm group. By Kaplan–Meier analysis, the estimated freedom from major bleeding event at 5 and 10 years was 98.1 and 96.9% in AVRb group, and the lower cumulative proportions were observed in AVRm group, which was 95.4 and 91.5%, respectively (Fig. 2). However, there was no statistically significant difference between propensity-matched groups (P = 0.128).
Figure 2:
Kaplan–Meier curve of freedom from bleeding events for propensity-matched groups. AVRm, aortic valve replacement with mechanical valve; AVRb: AVR with bioprosthetic valve.
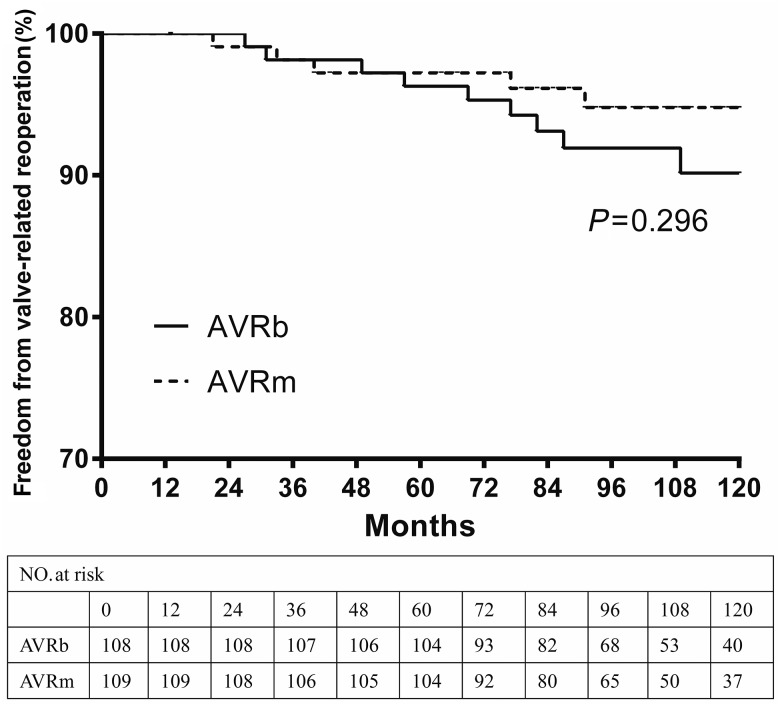
Valve-related reoperations
There were 14 valve-related reoperations during the follow-up period, of which 9 were in AVRb group (6 SVD, 1 perivalvular leak and 2 endocarditis), 5 were in AVRm group (2 perivalvular leak, 2 endocarditis and 1 mechanical prosthetic valve dysfunction). By Kaplan–Meier analysis, the estimated freedom from valve-related reoperations at 5 and 10 years was 96.3 and 90.2% in AVRb group and 97.2 and 94.8% in AVRm group, respectively (Fig. 3). Although, lower reoperation rate was observed at postoperative 10 years in AVRm group, there was no statistically significant difference between propensity-matched groups (P = 0.296).
Figure 3:
Kaplan–Meier curve of freedom from valve-related reoperation for propensity-matched groups. AVRm, aortic valve replacement with mechanical valve; AVRb: AVR with bioprosthetic valve.
Survival
A total of 21 deaths occurred during the follow-up period. There were 11 deaths in the bioprosthetic group of which 8 were cardiac-related. In the mechanical group, there were 10 deaths of which 8 were cardiac-related. The influence of cardiac-related causes on late survival is most certainly higher for both groups (Table 4). By Kaplan–Meier analysis, the estimated survival at 5 and 10 years was 96.3 and 88.7% in AVRb group and 96.3 and 87.9% in AVRm group, respectively (Fig. 4). There was no statistically difference between propensity-matched groups (P = 0.860).
Table 4:
Causes of late death
| Causes of death | Overall cohort |
P-value | Propensity-matched |
P-value | ||
|---|---|---|---|---|---|---|
| Bioprosthetic | Mechanical | Bioprosthetic | Mechanical | |||
| Cardiac-related | 8 (5.3) | 38 (4.1) | 0.525 | 8 (7.1) | 8 (7.1) | 1 |
| Cardiac arrest/myocardial infarction | 6 (3.9) | 21 (2.3) | 0.226 | 6 (5.4) | 5 (4.5) | 0.757 |
| Endocarditis | 1 (0.7) | 5 (0.5) | 0.862 | 1 (0.9) | 0 (0) | 0.316 |
| Stroke | 1 (0.7) | 5 (0.5) | 0.862 | 1 (0.9) | 2 (1.8) | 0.561 |
| Haemorrhage | 0 (0) | 7 (0.8) | 0.280 | 0 (0) | 1 (0.9) | 0.316 |
| Non-cardiac-related | 4 (2.6) | 15 (1.6) | 0.387 | 3 (2.7) | 2 (1.8) | 0.722 |
| Unknown | 0 (0) | 19 (2.1) | 0.074 | 0 (0) | 0 (0) | 1 |
Data presented as mean ± standard deviation, % (n).
Figure 4:
Kaplan–Meier curve of survival for propensity-matched groups. AVRm, aortic valve replacement with mechanical valve; AVRb: AVR with bioprosthetic valve.
Mid-long-term clinical outcomes: overall cohort
The late outcomes for the overall cohort echoed those found in the matched cohort. A total of 84 deaths occurred during the follow-up period. There were 12 deaths in the bioprosthetic group of which 8 were cardiac-related. In the mechanical group there were 72 deaths; however, we failed to acquire causes of death in 19 patients (Table 4). There was no difference in 10-year survival for the mechanical and bioprosthetic groups in the overall cohort (P = 0.611), despite the fact that the mechanical group in the overall cohort had poorer early outcomes, such as longer hospital stays and higher incidence rate of ventricular arrhythmias. Although there was a trend towards increased bleeding events at follow-up in the mechanical group from the overall cohort, this did not reach statistical significance (P = 0.073), just as it had in the matched cohort comparison. However, 10-year freedom from reoperations favoured mechanical valves, but did not reach statistical significance (P = 0.132).
COMMENT
To minimize information and selection biases, we performed a propensity-matched study involving a large, contemporary, single-centre cohort that underwent AVR with either the Hancock II bioprostheses or the mechanical valves (St Jude Medical, ATS Medical) in our department from January 2002 to December 2009. The present propensity-matched study included 224 patients younger than 60 years of age who underwent AVR. We compared patients with respect to early morality, complications, long-term survival, valve-related reoperations, anticoagulation-related bleeding and thromboembolic events.
The present study provides a contemporary opinion of early and mid-long-term outcomes with bioprosthetic versus mechanical valves for AVR in individuals younger than 60 years of age from a large cardiovascular surgery centre in China. Several important findings stem from these results. Overall mortality was similar between patients who received bioprosthetic and mechanical valves. Both the groups had a high long-term overall survival with no difference. Incidence of valve-related reoperations was not significantly different between patients with bioprosthetic valves and those with mechanical valves in 10 years after implanting. Finally, there was a higher trend of anticoagulation-related bleeding events for patients with mechanical valve implanting.
Motives for implanting bioprosthetic valves
The current guidelines of AVR do not emphasize age as an absolute selection criterion for the decision regarding what type of valve prosthesis should be recommended for AVR. Sixty years is the threshold age where it is difficult to balance the risk of the anticoagulation therapy and the need for reoperations. However, because of excellent long-term durability of current bioprosthetic valves or a desire for a better quality of life or pregnancy, younger patients are willing to choose the bioprosthetic valves for AVR. In our study cohort, around one-fifth of patients chose bioprosthetic valves due to their desire for pregnancy and around one-third had contraindication to anticoagulation treatment. Both of these are the major reasons for Chinese patients to choose bioprosthetic valves and can remind the cardiovascular surgeons that once Chinese patients acquire more knowledge about the advantages of current bioprosthetic valves and recognize the risk of reoperations, more and more bioprosthetic valves will be utilized in China.
Mid-long-term survival and freedom from valve-related reoperations
Various studies have reported on the mortality and reoperations after implanting different types of prosthetic aortic valve, but only few were primarily concerned with the outcomes in younger patients. However, conflicting results have been reported. Several retrospective studies could not demonstrate differences in late survival when mechanical versus bioprosthetic valves were compared. A retrospective observational study from Weber [5] showed in a 10-year follow-up for a population of patients younger than 60 years of age undergoing AVR, those with mechanical valves had a survival advantage compared with matched patients who received stented pericardial tissue valves. However, a study by Ruel et al. [6] showed no difference in 10-year survival after mechanical AVR (80%) and bioprosthetic AVR (80%) in patients with the mean age of 47.6 years old. Oxenham et al. [7] reported an advantage of freedom from reoperation at 10-year follow-up for mechanical valves compared with bioprosthetic valves, but no advantage of survival.
On the basis of the results of the previous studies, Medtronic Hancock II offers 10- and 15-year survival ranging from 63 to 67 and 35 to 47%, respectively [9–13]. Valfrè et al. [9] reported outcomes after Hancock II stented bioprosthetic AVR in 517 patients younger than 60 years of age between 1983 and 1993, the 10-year survival was 69.4% and freedom from reoperations was 87.4%. Une et al. [14] focused on patients with the mean age of 49 years old undergoing Hancock II stented bioprosthetic AVR, the 10-year survival rate was 80.7% and freedom from reoperations was 91.4%. A study by Chan et al. [15] showed a lower 10-year survival rate (59%) and higher rate of freedom from reoperations (97%) in patients with the mean age of 73 years after implanting Hancock II stented bioprosthetic aortic valves. The result of our research has showed a higher 10-year survival (88.7%) than most of the other studies. We think the better outcomes may be due to the better preoperative conditions of our cohort, such as good cardiac function measured by ejection fraction (53 ± 17%). On the other hand, the mean age in our entire study cohort that chose bioprosthetic AVR was younger (50.3 ± 9.1 years), when age and cardiac function have been shown to be very important factors for life expectancy after AVR.
All patients in our department who underwent bioprosthetic AVR have received Medtronic Hancock II porcine bioprostheses over the past 15 years. Compared with other types of bioprostheses, the results of our data showed Hancock II had a similar result of 10-year freedom from reoperations (90.2%). Chirst et al. [16] reported excellent clinical outcomes and durability of Edwards Prima Plus stentless bioprostheses in patients younger than 60 years of age with an overall actuarial survival rate of 71.8% and freedom from reoperation rate of 85.6% at 10 years after implanting. Forcillo et al. [17] recently reported outcomes after AVR with Carpentier-Edwards pericardial valves in 144 patients younger than 60 years of age. In these patients, 10-year survival rate was also 79% and freedom from reoperations was 84%.
Anticoagulation-related bleeding and thromboembolic events
The results of our study were consistent with prior reports [7, 18, 19] as we have shown a similar incidence of thromboembolic events between mechanical and bioprosthetic valves. However, thromboembolic events occurred less frequently in the overall cohort of our study (4.6%). This lower rate of freedom from thromboembolic events may be due to the fact that some risk factors of thromboembolic events are less common in patients of 60 years old or younger, such as coronary artery disease and atrial fibrillation.
Bleeding event is considered a major problem for mechanical valves. Previous randomized control studies [7, 18] and a recent retrospective study [19] showed a greater rate of bleeding events with the mechanical valve. In the study by Brown et al. [20], the incidence of bleeding events at 10 years after valve replacements was significantly lower in patients who received bioprosthetic valves (6 vs 14%), and freedom from bleeding events was 98% at 10 years. Kulik et al.' research [19] also presented a higher 10-year freedom from bleeding events in middle-aged patients undergoing bioprosthetic AVRs, which was similar to our result (96.9%). However, Badhwar et al. [21] reported no difference of bleeding events between mechanical and bioprosthetic valves in their propensity-matched analysis for patients <65 years who had undergone AVR. Stassano et al. [22] performed a randomized control study of patients aged 55–70 years underwent AVR, which showed there was a similar incidence of bleeding events between mechanical and bioprosthetic valves group. We infer that the higher incidence of bleeding events occurred in these two studies may be related to older age of the patients in their research cohort as older patients were more likely to suffer from atrial fibrillation and transient ischaemic attack, which leads to constant anticoagulation treatment, even if they received bioprosthetic valves. But the exact number of such patients was not reported in their publications.
Study limitations
The present research was a retrospective, single-centre observational study as such is subject to limitations. Selection bias is a potential confounding factor. We tried to compensate for the bias in patient selection by propensity-matching patients with similar preoperative and operative conditions. Despite these efforts, as with any observational analysis, unmeasured confounders may have influenced the accuracy of the reported comparisons.
The follow-up was only 95.1% complete, which may result in underreporting of long-term complications. The follow-up time was limited, which may be suboptimal for the analysis of outcomes in a young patient population. Underestimations of late events (valve-related reoperations, anticoagulation-related bleeding and thromboembolic events) were possible. Specific to the questionnaire responses, recall bias may result in some degree of underestimation. In addition, we failed to obtain the data of proportion of the patients who underwent bioprosthetic valves replacement and received anticoagulant therapy all the time.
What makes China different from Europe or North America are the unique cultural, social and financial issues in China, it may impact the likelihood of Chinese patients to pursue a reoperation. Our study just reflected the feature of China.
Finally, there was no ideal and complete echocardiographic data to analyse the patient–prosthesis mismatch (PPM) during the follow-up period. However, longer, more complete and exact follow-up multicentre research is necessary to confirm our findings.
CONCLUSIONS
Among propensity-matched patients younger than 60 years of age who underwent AVRbs compared with mechanical valves, Hancock II bioprosthetic valve provided satisfactory mid-long-term clinical outcomes after implantation. There was no significant difference in mid-long-term survival. Bioprosthetic valves were associated with a lower risk of anticoagulation treatment and did not have a significantly greater likelihood of reoperation. These findings suggest that bioprosthetic valves may be a reasonable choice for AVR in patients younger than 60 years of age.
Funding
This study was supported by Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period of China ‘Research of clinical characteristics and surgical treatment of valve disease in china’ (2011BAI11B19).
Conflict of interest: none declared.
REFERENCES
- 1.Rahimtoola SH. Choice of prosthetic heart valve in adults: an update. J Am Coll Cardiol 2010;55:2413–26. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1–44. [DOI] [PubMed] [Google Scholar]
- 3.Cunanan CM, Cabiling CM, Dinh TT, Shen SH, Tran-Hata P, Rutledge JH et al. Tissue characterization and calcification potential of commercial bioprosthetic heart valves. Ann Thorac Surg 2001;71:417–21. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82–90. [DOI] [PubMed] [Google Scholar]
- 5.Weber A, Noureddine H, Englberger L, Dick F, Gahl B, Aymard T et al. Ten-year comparison of pericardial tissue valves versus mechanical prostheses for aortic valve replacement in patients younger than 60 years of age. J Thorac Cardiovasc Surg 2012;144:1075–83. [DOI] [PubMed] [Google Scholar]
- 6.Ruel M, Chan V, Bédard P, Kulik A, Ressler L, Lam BK et al. Very long term survival implications of heart valve replacement with tissue versus mechanical prostheses in adults <60 years of age. Circulation 2007;116(11 Suppl):I294–300. [DOI] [PubMed] [Google Scholar]
- 7.Oxenham H, Bloomfield P, Wheatley DJ, Lee RJ, Cunningham J, Prescott RJ et al. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart 2003;89:715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;85:1490–5. [DOI] [PubMed] [Google Scholar]
- 9.Valfrè C, Ius P, Minniti G, Salvador L, Bottio T, Cesari F et al. The fate of Hancock II porcine valve recipients 25 years after implant. Eur J Cardiothorac Surg 2010;38:141–6. [DOI] [PubMed] [Google Scholar]
- 10.Rizzoli G, Mirone S, Ius P, Polesel E, Bottio T, Salvador L et al. Fifteen-year results with the Hancock II valve: a multicenter experience. J Thorac Cardiovasc Surg. 2006;132:602–9. [DOI] [PubMed] [Google Scholar]
- 11.Rizzoli G, Bottio T, Thiene G, Toscano G, Casarotto D. Long-term durability of the Hancock II porcine bioprosthesis. J Thorac Cardiovasc Surg 2003;126:66–74. [DOI] [PubMed] [Google Scholar]
- 12.Masters RG, Haddad M, Pipe AL, Veinot JP, Mesana T. Clinical outcomes with the Hancock II bioprosthetic valve. Ann Thorac Surg 2004;78:832–6. [DOI] [PubMed] [Google Scholar]
- 13.David TE, Armstrong S, Sun Z. The Hancock II bioprosthesis at ten years. Ann Thorac Surg 1995;60(2 Suppl):S229–34. [DOI] [PubMed] [Google Scholar]
- 14.Une D, Ruel M, David TE. Twenty-year durability of the aortic Hancock II bioprosthesis in young patients: is it durable enough? Eur J Cardiothorac Surg 2014;46:825–30. [DOI] [PubMed] [Google Scholar]
- 15.Chan V, Kulik A, Tran A, Hendry P, Masters R, Mesana TG et al. Long-term clinical and hemodynamic performance of the Hancock II versus the Perimount aortic bioprostheses. Circulation 2010;122(11 Suppl):S10–6. [DOI] [PubMed] [Google Scholar]
- 16.Christ T, Grubitzsch H, Claus B, Konertz W. Long-term follow-up after aortic valve replacement with Edwards Prima Plus stentless bioprostheses in patients younger than 60 years of age. J Thorac Cardiovasc Surg 2014;147:264–9. [DOI] [PubMed] [Google Scholar]
- 17.Forcillo J, El Hamamsy I, Stevens LM, Badrudin D, Pellerin M, Perrault LP et al. The Perimount valve in the aortic position: twenty-year experience with patients under 60 years old. Ann Thorac Surg 2014;97:1526–32. [DOI] [PubMed] [Google Scholar]
- 18.Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol 2000;36:1152–8. [DOI] [PubMed] [Google Scholar]
- 19.Kulik A, Bédard P, Lam BK, Rubens FD, Hendry PJ, Masters RG et al. Mechanical versus bioprosthetic valve replacements in middle-aged patients. Eur J Cardiothorac Surg 2006;30:485–91. [DOI] [PubMed] [Google Scholar]
- 20.Brown ML, Schaff HV, Lahr BD, Mullany CJ, Sundt TM, Dearani JA et al. Aortic valve replacement in patients aged 50 to 70 years: improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg 2008;135:878–84. [DOI] [PubMed] [Google Scholar]
- 21.Badhwar V, Ofenloch JC, Rovin JD, van Gelder HM, Jacobs JP. Noninferiority of closely monitored mechanical valves to bioprostheses overshadowed by early mortality benefit in younger patients. Ann Thorac Surg 2012;93:748–53. [DOI] [PubMed] [Google Scholar]
- 22.Stassano P, Di Tommaso L, Monaco M, Iorio F, Pepino P, Spampinato N et al. Aortic valve replacement: a prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J Am Coll Cardiol 2009;54:1862–8. [DOI] [PubMed] [Google Scholar]