Abstract
OBJECTIVES
Primary graft dysfunction (PGD) is a major cause of early morbidity and mortality after cadaveric lung transplantation (CLT). This study examined the incidence, time course and predictive value of PGD after living-donor lobar lung transplantation (LDLLT).
METHODS
We retrospectively investigated 75 patients (42 with LDLLT and 33 with CLT) who underwent lung transplantation from January 2008 to December 2013. Patients were assigned PGD grades at six time points, as defined by the International Society for Heart and Lung Transplantation: immediately after final reperfusion, upon arrival at the intensive care unit (ICU), and 12, 24, 48 and 72 h after ICU admission.
RESULTS
The incidence of severe (Grade 3) PGD at 48 or 72 h after ICU admission was similar for LDLLT and CLT patients (16.7 vs 12.1%; P = 0.581). The majority of the LDLLT patients having severe PGD first developed PGD immediately after reperfusion, whereas more than half of the CLT patients first developed severe PGD upon ICU arrival or later. In LDLLT patients, severe PGD immediately after reperfusion was significantly associated with fewer ventilator-free days during the first 28 postoperative days [median (interquartile range) of 0 (0–10) vs 21 (13–25) days, P = 0.001], prolonged postoperative ICU stay [median (interquartile range) of 20 (16–27) vs 12 (8–14) days, P = 0.005] and increased hospital mortality (27.3 vs 3.2%, P = 0.02). Severe PGD immediately after reperfusion was not associated with ventilator-free days during the first 28 postoperative days, time to discharge from ICU or hospital, or hospital mortality in CLT patients.
CONCLUSIONS
Postoperative incidence of severe PGD was not significantly different between LDLLT and CLT patients. In LDLLT patients, the onset of severe PGD tended to be earlier than that in CLT patients. Severe PGD immediately after reperfusion was a significant predictor of postoperative morbidity and mortality in LDLLT patients but not in CLT patients.
Keywords: Living-donor lobar lung transplantation, Cadaveric lung transplantation, Primary graft dysfunction, Reperfusion injury, Postoperative complications, Hospital mortality
INTRODUCTION
Living-donor lobar lung transplantation (LDLLT) was first performed in the 1990s in response to a mismatch between the supply and demand for donor lungs from brain-dead donors [1]. We recently demonstrated that survival following LDLLT is similar to that observed for cadaveric lung transplantation (CLT), despite the worse preoperative condition of LDLLT patients, and the use of LDLLT as a viable option in patients who may not survive a long waiting period for cadaveric donors [2]. In contrast, our analysis revealed that LDLLT patients require a greater duration of postoperative mechanical ventilation than CLT patients, probably due to the poorer preoperative condition of LDLLT patients. Early identification of patients with a poor postoperative course following LDLLT may help optimize postoperative management strategies, including the alteration of ventilation modalities or pharmacological agents [3–5].
Primary graft dysfunction (PGD), a form of acute lung injury occurring after lung transplantation [3, 6, 7], is known to significantly impact postoperative morbidity and mortality following lung transplantation. Severe PGD is associated with delayed extubation, prolonged stays in both intensive care and hospital, increased early mortality and worsened long-term outcomes in transplant survivors [6, 8–12]. In 2005, the International Society for Heart and Lung Transplantation (ISHLT) proposed a standardized definition of PGD [11], with subsequent studies validating this definition with data regarding clinical outcomes [13, 14]. However, each of these studies involved CLT patients only. We previously reported severe PGD requiring extracorporeal membrane oxygenation (ECMO) in 4 of 14 LDLLT patients receiving grafts from a single donor [15]. However, the incidence of PGD after LDLLT according to ISHLT criteria is currently unknown, and the prognostic value of PGD in LDLLT has yet to be elucidated.
The purpose of this single-centre retrospective study was to examine the incidence and time course of PGD after LDLLT, and to assess the impact of PGD on outcomes in LDLLT patients.
MATERIALS AND METHODS
This retrospective cohort study was approved by the ethics committee of Kyoto University Hospital (approval number: E2080). All patients who underwent lung transplantation (LDLLT or CLT) at Kyoto University Hospital from 1 January 2008 to 31 December 2013 were eligible for this study. The medical records of eligible patients were reviewed with regard to patient characteristics, preoperative examination, and intra- and postoperative clinical course.
In Japan, all patients requiring CLT from brain-dead donors are registered at the Japan Organ Transplantation Network. The organ allocation process for CLT is largely based on the accrued time on the waiting list. The indications and size-matching criteria for LDLLT were described previously [2, 16]. Indication for LDLLT was limited to critically ill patients unlikely to survive waiting for cadaveric lungs. In LDLLT, graft forced vital capacity (FVC) was calculated using the following equation: (graft FVC) = (number of resected segments)/19. We accepted a size disparity when the graft FVC was 45% or more of the predicted FVC of the recipient (calculated according to height, age and gender).
Perioperative management was essentially identical for LDLLT and CLT patients. Although attending anaesthesiologists were responsible for ventilation during general anaesthesia, a positive end-expiratory pressure of 5 cmH2O or greater was used following reperfusion in all cases. Postoperative respiratory management was performed according to a common protocol as previously described [17]. In brief, all patients were transferred to the intensive care unit (ICU) after lung transplantation, intubated and placed on artificial ventilation (Puritan Benett™ 840 Ventilator System: Covidien Japan, Tokyo, Japan) for at least 2 days postoperatively. From postoperative day 2, extubation was considered under the following circumstances: (i) stable vital signs with a ratio ≥200; (ii) stable underlying disease and complications; (iii) minimal artificial ventilation support; and (iv) sufficient spontaneous breathing. Tracheostomy was performed in all cases where prolonged artificial ventilation was necessary. Patients were considered for discharge from the ICU when haemodynamically stable, following extubation or tracheostomy.
Patients were assigned PGD grades at six time points according to the ISHLT definition of PGD: Immediately after final reperfusion (Trep), upon at ICU arrival (T0) and 12 h (T12), 24 h (T24), 48 h (T48) and 72 h (T72) after ICU admission (11). In brief, a ratio >300 was defined as Grade 0–1; a ≥200 and ≤0300 as Grade 2; and a <200 as Grade 3. Although the ISHLT PGD definitions divide patients with a >300 into two groups (Grade 0 and Grade 1) on the basis of the presence or absence of radiographic infiltrates, we classified any patient with a >300 as having Grade 0–1 PGD. Patients who were extubated were also classified as having Grade 0–1 PGD. Patients receiving ECMO were automatically classified as having Grade 3 PGD while receiving support.
When analysing the impact of PGD on outcomes, PGD Grades 0–1 and 2 were combined, and outcome measures were compared between patients with Grades 0–2 PGD and those with Grade 3 PGD. The primary outcome was defined as the number of days of unassisted breathing [ventilator-free days (VFDs)] during the initial 28 postoperative days. Patients who died or underwent retransplantation due to graft dysfunction during the initial 28 postoperative days were assigned 0 VFDs [18]. Unassisted breathing was defined as a period lasting at least 48 consecutive hours beginning with extubation (or removal of ventilatory support for patients with tracheostomies). Secondary outcomes included time to ICU and hospital discharge and hospital mortality.
Statistical analysis
Data were analysed using the statistical program R (http://cran.r-project.org). All data are presented as median (interquartile range) and number (percentage), unless stated otherwise. Differences between groups were compared using the Mann–Whitney U-test for continuous variables. For categorical variables, Pearson's χ2-test or Fisher's exact test were used where appropriate. All statistical tests were two-tailed. Except for multivariate models, statistical significance was set at P < 0.05. Time-to-event analyses were used to compare lengths of ICU and hospital stays. Patient data were censored at the time of death or retransplantation. Medians and interquartile range were obtained using Kaplan–Meier analyses, and the log-rank test was used to assess differences between groups. Multivariate regression analysis was used to evaluate the independent role of Grade 3 PGD at Trep in predicting VFDs following LDLLT. All candidate covariates were included in multivariate analysis with a backward stepwise elimination performed to identify factors independently associated with VFDs. All variables maintaining a P-value <0.1 were included in the final model. Grade 3 PGD at Trep was included in all logistic models regardless of statistical significance as this was the primary variable of interest.
RESULTS
Patient characteristics and operative variables
A total of 75 lung transplantations (42 LDLLTs and 33 CLTs) were performed during the study period. Preoperative patient characteristics and operative variables are listed in Table 1. Although median ages were similar between LDLLT and CLT, 11 children under 15 years old underwent LDLLT, whereas no children underwent CLT. Dyspnoea, determined according to the Hugh-Jones classification, was significantly worse among LDLLT patients (P = 0.005), and the proportion of ventilator-dependent LDLLT patients tended to be higher (11.9 vs 3.0%, P = 0.1597). All patients required cardiopulmonary support during LDLLT; however, more than half (51.5%) of patients underwent CLT without cardiopulmonary support. The total ischaemic time was significantly shorter in LDLLT than that in CLT (P < 0.0001). A median graft FVC of 60.0% was observed in LDLLT patients and was found to be <55% in 15 patients (35.7%).
Table 1:
Preoperative patient characteristics and operative variables
| LDLLT (n = 42) | CLT (n = 33) | P-value | |
|---|---|---|---|
| Age (years) | 44 (12–55) | 40 (29–52) | 0.873 |
| Female gender | 24 (57.1%) | 12 (36.4%) | 0.074 |
| Body mass index (kg/m2) | 16.9 (14.0–19.7) | 18.3 (16.6–21.5) | 0.023 |
| Hugh-Jones classification (III/IV/V) | 1/18/23 | 2/25/6 | 0.005 |
| Pre-existing lung disorder | 0.001 | ||
| Pulmonary fibrosis | 20 (47.6%) | 7 (21.2%) | |
| Bronchiolitis obliterans | 13 (31.0%) | 5 (15.2%) | |
| Pulmonary arterial hypertension | 3 (7.1%) | 2 (6.1%) | |
| Other | 6 (14.3%) | 19 (57.5%) | |
| Preoperative pulmonary hypertension | 8 (19.0%) | 3 (9.1%) | 0.328 |
| Preoperative condition | |||
| NPPV | 2 (4.8%) | 0 (0.0%) | 0.191 |
| Ventilator-dependent | 5 (11.9%) | 1 (3.0%) | 0.160 |
| Type of transplant | 0.001 | ||
| Bilateral | 33 (78.6%) | 14 (42.4%) | |
| Single | 9 (21.4%) | 19 (57.6%) | |
| Use of cardiopulmonary support | 42 (100.0%) | 17 (51.5%) | <0.0001 |
| Blood product volume administered intraoperatively (l) | 5.7 (3.2–7.0) | 2.6 (1.3–4.6) | 0.001 |
| Total ischaemic time (min) | 154 (135–197) | 452 (391–530) | <0.0001 |
| Graft FVC (% of predicted FVC of the recipient) | 60.0 (51.3–74.6) | – | – |
Patients were considered to have pulmonary hypertension when the systolic pulmonary artery pressure (estimated by transthoracic echocardiography) was ≥60 mmHg.
LDLLT: living-donor lobar lung transplantation; CLT: cadaveric lung transplantation; NPPV: non-invasive positive pressure ventilation; FVC: forced vital capacity.
Prevalence and severity of primary graft dysfunction
The distribution of patients according to assigned PGD grades at six study time points is presented in Fig. 1. The incidence of at least one period of Grade 3 PGD between Trep and T72 was 33.3% in LDLLT patients and tended to be lower than that in CLT patients (51.5%; P = 0.113). In LDLLT, 11 (26.2%) patients exhibited Grade 3 PGD at Trep with the incidence of Grade 3 PGD gradually decreasing thereafter. In CLT, 9 (27.2%) patients exhibited Grade 3 PGD at Trep, with the incidence of Grade 3 PGD highest at T0 (36.4%) and decreasing thereafter. The incidence of Grade 3 PGD at T48 or T72 was similar between LDLLT and CLT (16.7 vs 12.1%; P = 0.581) patients. Individual analyses at each time point revealed that the incidence of Grade 3 PGD was similar between LDLLT patients and CLT patients, except at T0, where the incidence of Grade 3 PGD tended to be higher in CLT patients than in LDLLT patients (36.4 vs 16.7%; P = 0.0645).
Figure 1:
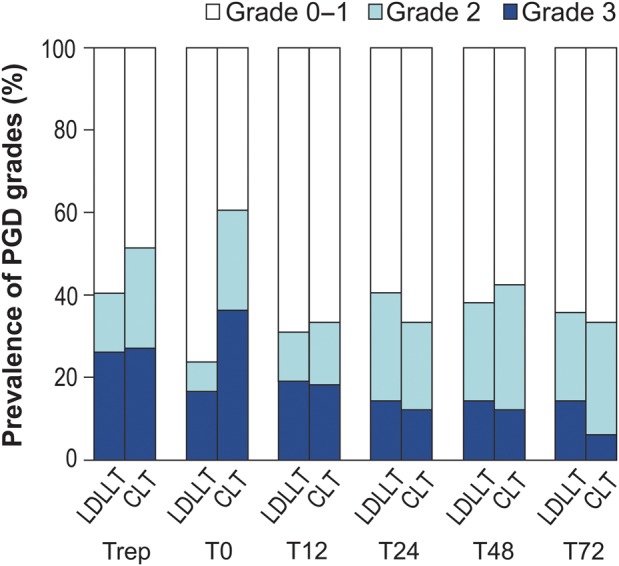
Prevalence and severity of PGD from immediately after reperfusion to 72 h after ICU admission. PGD: primary graft dysfunction; ICU: intensive care unit; CLT: cadaveric lung transplantation; LDLLT: living-donor lobar lung transplantation; Trep: immediately after final reperfusion; T0: at ICU arrival; T12–T72: 12–72 h after ICU admission.
We further examined the timing of Grade 3 PGD onset. Among LDLLT patients, the majority of patients (72.7%) who experienced Grade 3 PGD developed Grade 3 PGD at Trep. Conversely, more than half (61.5%) of the CLT patients developed Grade 3 PGD at T0 or T12 (Fig. 2A). The presence of Grade 3 PGD at Trep was significantly associated with Grade 3 PGD at T48 or T72 in LDLLT patients (P < 0.0001); however, this association was not observed in CLT patients (P = 0.913; Fig. 2B and C).
Figure 2:
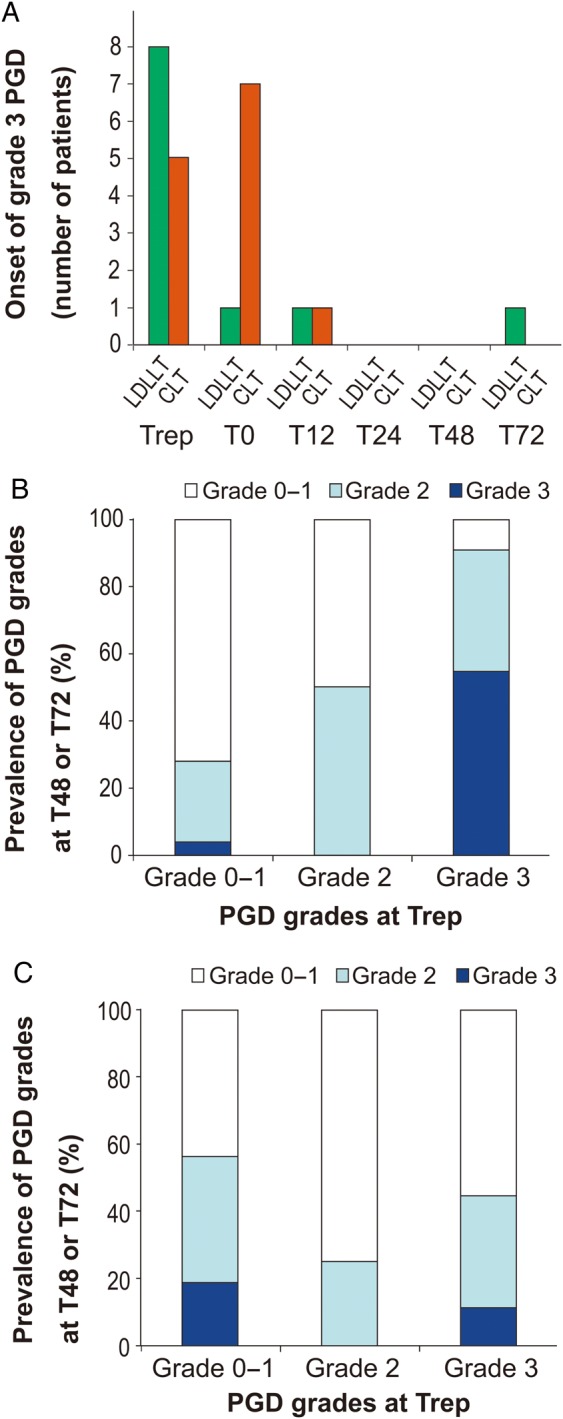
Time series analysis of PGD. (A) Timing of Grade 3 PGD onset; association between Grade 3 PGD at Trep and PGD grades at 48 or 72 h after ICU admission in living-donor lobar (B) and cadaveric (C) lung transplantation. PGD: primary graft dysfunction; ICU: intensive care unit; LDLLT: living-donor lobar lung transplantation; CLT: cadaveric lung transplantation; Trep: immediately after final reperfusion; T0: at ICU arrival; T12–T72: 12–72 h after ICU admission.
Impact of Grade 3 primary graft dysfunction on ventilator-free days
Next, we investigated the impact of Grade 3 PGD at six study time points on VFDs during the initial 28 postoperative days (Table 2). Grade 3 PGD at all time points, including Trep, was strongly associated with decreased VFDs in LDLLT patients. In contrast, in CLT patients, Grade 3 PGD at Trep was not significantly associated with VFDs. Grade 3 PGD at T0 or later was significantly associated with VFDs in CLT patients, and the difference in median VFDs between Grades 0–2 and Grade 3 PGD was the greatest at T48 and T72. Six among 11 (54.6%) LDLLT patients with Grade 3 PGD at Trep had 0 VFDs; in contrast, 7 among 9 CLT patients (77.8%) with Grade 3 PGD at Trep successfully weaned off from ventilator support within 14 postoperative days. As the impact of Grade 3 PGD on VFDs was similar across time points in LDLLT patients, Grade 3 PGD at Trep was used in subsequent analyses.
Table 2:
Impact of severe PGD at each time point on VFDs
| Trep | T0 | T12 | T24 | T48 | T72 | |
|---|---|---|---|---|---|---|
| LDLLT | ||||||
| Grade 0–2 | 21 (13–25) | 21 (10–25) | 22 (9–25) | 21 (6–25) | 21 (6–25) | 21 (6–25) |
| Grade 3 | 0 (0–10) | 0 (0–7) | 0 (0–9) | 0 (0–8) | 0 (0–8) | 0 (0–10) |
| P | 0.001 | 0.002 | 0.002 | 0.004 | 0.004 | 0.007 |
| CLT | ||||||
| Grade 0–2 | 24 (17–26) | 25 (18–26) | 25 (18–26) | 24 (18–26) | 24 (18–26) | 24 (17–25) |
| Grade 3 | 18 (8–25) | 18 (7–24) | 6 (0–17) | 14 (4–18) | 3 (0–15) | 3 (0–5) |
| P | 0.245 | 0.029 | 0.001 | 0.030 | 0.008 | 0.030 |
PGD: primary graft dysfunction; VFDs: ventilator-free days; Trep: immediately after reperfusion; T0: at ICU arrival; T12–T72, 12–72 h after ICU admission; LDLLT: living-donor lobar lung transplantation; CLT: cadaveric lung transplantation.
Impact of Grade 3 primary graft dysfunction on secondary outcome measures
We assessed the effect of Grade 3 PGD on the length of ICU and hospital stays and hospital mortality (Table 3). LDLLT patients with Grade 3 PGD at Trep had significantly longer stays in ICU than those without (P = 0.005). Moreover, hospital mortality was significantly higher in LDLLT patients with Grade 3 PGD at Trep than in those without. In contrast, Grade 3 PGD at Trep was not associated with duration of ICU or hospital stay, or hospital mortality, in CLT patients.
Table 3:
Impact of Grade 3 PGD at Trep on outcomes according to transplantation procedure
| Grade 3 PGD at Trep (n = 11) | Grade 0–2 PGD at Trep (n = 31) | P-value | |
|---|---|---|---|
| LDLLT | |||
| Time to discharge (days) | |||
| From ICU | 20 (16–27) | 12 (8–14) | 0.005 |
| From hospital | 99 (60–114) | 81 (58–91) | 0.093 |
| Hospital mortality | 3 (27.3%) | 1 (3.2%) | 0.020 |
| Grade 3 PGD at Trep (n = 9) | Grade 0–2 PGD at Trep (n = 24) | P-value | |
| CLT | |||
| Time to discharge (days) | |||
| From ICU | 9 (7–13) | 7 (5–11) | 0.741 |
| From hospital | 46 (43–76) | 53 (43–90) | 0.971 |
| Hospital mortality | 1 (11.1%) | 1 (4.2%) | 0.457 |
PGD: primary graft dysfunction; at Trep: immediately after reperfusion; LDLLT: living-donor lobar lung transplantation; CLT: cadaveric lung transplantation; ICU: intensive care unit.
Multivariate analysis of ventilator-free days in patients with living-donor lobar lung transplantation
To determine the predictive value of Grade 3 PGD at Trep in LDLLT patients independently of pretransplant morbidity or transplant procedure type, multiple linear regression was performed, adjusting for the presence of pretransplant morbidity; specifically in age, body mass index, dyspnoea according to Hugh-Jones classifications (3–4 and 5), preoperative pulmonary hypertension, preoperative ventilator dependence; operative variables, specifically single-lung transplant, total donor graft ischaemic time and volume of intraoperatively administered blood products. Following backward elimination, variables that remained in the final model and that were found to be confounders were preoperative pulmonary hypertension, preoperative ventilator dependence and volume of intraoperatively administered blood products. Multivariate linear regression analysis revealed that Grade 3 PGD at Trep was significantly associated with decreased VFDs in LDLLT patients independently of pre- and intraoperative variables (P = 0.01; Table 4).
Table 4:
Multivariate linear regression analysis of VFDs during the initial 28 postoperative days in LDLLT patients
| Variables | Regression coefficient | 95% CI | P-value |
|---|---|---|---|
| Grade 3 PGD at Trep | −8.48 | −14.82 to −2.13 | 0.010 |
| Ventilator-dependent | −13.27 | −22.09 to −4.46 | 0.004 |
| Preoperative pulmonary hypertension | −5.72 | −12.25 to 0.81 | 0.084 |
| Blood volume administered intraoperatively (l) | −0.83 | −1.59 to −0.06 | 0.035 |
VFDs: ventilator-free days; LDLLT: living-donor lobar lung transplantation; PGD: primary graft dysfunction; at Trep: immediately after reperfusion; CI: confidence interval.
Characteristics associated with Grade 3 primary graft dysfunction at Trep in living-donor lobar lung transplantation
Finally, we assessed patient characteristics associated with the development of Grade 3 PGD at Trep in LDLLT patients (Table 5). Patients with Grade 3 PGD at Trep were significantly younger than those without (P = 0.005); of the 11 patients with Grade 3 PGD at Trep, 7 (63.6%) were children under 15 years old. Of note, six of nine single-lobe transplanted recipients developed Grade 3 PGD at Trep. The median graft FVC in the 7 children with PGD Grade 3 at Trep was 73.3% and five had a graft FVC of 55% or more. No significant association was observed between graft FVC and the development of Grade 3 PGD at Trep.
Table 5:
Characteristics associated with Grade 3 PGD at Trep among LDLLT patients
| Grade 0–2 PGD at Trep (n = 31) | Grade 3 PGD at Trep (n = 11) | P-value | |
|---|---|---|---|
| Age (years) | 49 (22–57) | 11 (6–41) | 0.005 |
| Child (<15 years old) | 4 (12.9%) | 7 (63.6%) | 0.001 |
| Single-lobe transplantation | 3 (9.7%) | 6 (54.6%) | 0.002 |
| Blood product volume administered intraoperatively (l) | 6.0 (3.2–7.3) | 5.2 (1.6–7.0) | 0.501 |
| Total ischaemic time (min) | 159 (141–193) | 111 (88–204) | 0.179 |
| Graft FVC (% of predicted FVC of the recipient) | 58.1 (52.7–69.3) | 67.0 (49.1–79.3) | 0.699 |
| Graft FVC <55% | 11 (35.5%) | 4 (36.4%) | 0.958 |
PGD: primary graft dysfunction; at Trep: immediately after reperfusion; LDLLT: living-donor lobar lung transplantation; FVC: forced vital capacity.
DISCUSSION
We investigated the incidence and time course of PGD following LDLLT and assessed the impact of Grade 3 PGD on outcomes in LDLLT patients. Analysis of our cohort revealed the following: (i) the incidence of Grade 3 PGD at 48 or 72 h after ICU admission was similar between LDLLT and CLT patients; (ii) the onset of Grade 3 PGD after LDLLT tended to be earlier than after CLT; and (iii) Grade 3 PGD at Trep was a significant predictor of fewer VFDs during the initial 28 postoperative days, prolonged postoperative ICU stay and increased hospital mortality in LDLLT patients.
Since the publication of the ISHLT standardized definition and grading of PGD, the incidence of Grade 3 PGD at 48 or 72 h after CLT has been reported to be between 10 and 20%, and the incidence rate of Grade 3 PGD at any time point during the initial 72 postoperative hours has been reported as ∼30% [19]. The incidence of Grade 3 PGD after LDLLT observed in our study (33.3% at any time point from Trep to T72 and 16.7% at T48 or T72) was not found to significantly differ from those after CLT and was similar to previously reported incidences in CLT. However, the time course of PGD significantly differed between LDLLT and CLT patients. In CLT, more than half of patients with Grade 3 PGD first developed Grade 3 PGD at T0 or T12, corroborating a previous study that reported a significant variation in the ratio within the first 12 postoperative hours with stabilization thereafter [20]. In contrast, the majority of patients developing Grade 3 PGD following LDLLT developed Grade 3 PGD at Trep, with a few patients found to develop Grade 3 PGD at T0 or later.
The difference in the time course of PGD between LDLLT and CLT patients might be explained by differing mechanisms underlying the development of PGD in these patient populations. PGD is characterized by pulmonary oedema with diffuse alveolar damage associated with ischaemia and reperfusion of lung grafts [3, 11]. Factors thought to contribute to the injury of lung grafts from cadaveric donors during brain death or prolonged mechanical ventilation include pneumonia, aspiration, blood transfusion, haemodynamic instability and ventilation-associated injury. Moreover, graft ischaemic time is much greater in CLT patients than in LDLLT patients. We therefore propose that patients who underwent CLT were at higher risk of severe ischaemia–reperfusion injury. In contrast, grafts from LDLLT donors are not exposed to infection or mechanical ventilation and have a shorter ischaemic time. We discussed the concept of ‘a small but perfect graft’ previously [2], and size mismatch has been shown to be the predominant cause of graft dysfunction in LDLLT [15]. The results of this study may suggest that graft dysfunction due to size mismatch can be assessed at Trep, whereas graft dysfunction due to ischaemia–reperfusion injury cannot be assessed until development of dysfunction at later time points.
Appropriate size matching between donors and recipients is crucial in LDLLT. The use of a graft too small for a recipient may result in poor ventilation (functional size matching), and the use of a graft too large for the recipient hemithorax may result in high airway resistance, atelectasis and haemodynamic instability at chest closure (anatomical size matching) [15]. As is presented in Table 5, paediatric patients receiving single-lobe transplantation were apparently at greater risk of developing severe PGD. This finding corroborates our previous report [15] suggesting that bilateral LDLLT is a better option when two living donors are available.
Our study revealed that Grade 3 PGD at Trep is a useful prognostic marker following LDLLT. As PGD grades at very early time points predict lung transplant outcomes, measurement of this parameter may allow earlier intervention in a specific group of patients. In contrast, Grade 3 PGD at Trep was not found to be a significant prognostic marker following CLT. As the ratio dynamically changes during the early post-transplant period in CLT, the use of later time points may be more appropriate for the assessment of graft function. Previous studies have demonstrated that Grade 3 PGD at T48–T72 has construct validity for determining long-term outcomes and concurrent lung injury markers [14, 21]; therefore, the assessment of PGD on postoperative day 2 or 3 may have greater utility.
In the multivariate analysis of VFDs in LDLLT patients, preoperative mechanical ventilation, preoperative pulmonary hypertension and the volume of intraoperatively administered blood products remained in the final model. Each of these factors has been implicated as a predictive factor in previous studies of lung transplant outcomes in CLT patients [22–25]. These may represent prognostic factors common to both LDLLT and CLT.
The major limitations of this study were its retrospective design and small sample size. Data collected in this study were derived from a single institution. Heterogeneity in procedures (e.g. single and bilateral lung transplantation) and pretransplant status made it difficult to interpret the results; future studies are warranted to confirm our results by taking these variables into account. Ventilator settings potentially influenced . Despite these limitations, our data provide new information regarding the postoperative respiratory management of LDLLT patients.
In conclusion, this study found that the incidence of severe (Grade 3) PGD after LDLLT did not significantly differ from the incidence of severe PGD following CLT. In LDLLT patients, the onset of severe PGD tended to be earlier than that in CLT patients. Severe PGD at Trep was a significant predictor of fewer VFDs, a longer postoperative ICU stay and increased hospital mortality in LDLLT patients.
ACKNOWLEDGEMENTS
The authors thank Enago (www.enago.jp) for the English language review.
Conflict of interest: none declared.
REFERENCES
- 1.Starnes VA, Bowdish ME, Woo MS, Barbers RG, Schenkel FA, Horn MV et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg 2004;127:114–22. [DOI] [PubMed] [Google Scholar]
- 2.Date H, Sato M, Aoyama A, Yamada T, Mizota T, Kinoshita H et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for very ill patients. Eur J Cardiothorac Surg 2015;47:967–72. [DOI] [PubMed] [Google Scholar]
- 3.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 2003;167:490–511. [DOI] [PubMed] [Google Scholar]
- 4.Meade MO, Granton JT, Matte-Martyn A, McRae K, Weaver B, Cripps P et al. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med 2003;167:1483–9. [DOI] [PubMed] [Google Scholar]
- 5.Keshavjee S, Davis RD, Zamora MR, de Perrot M, Patterson GA. A randomized, placebo-controlled trial of complement inhibition in ischemia-reperfusion injury after lung transplantation in human beings. J Thorac Cardiovasc Surg 2005;129:423–8. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med 2005;171:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie JD, Van Raemdonck D, de Perrot M, Barr M, Keshavjee S, Arcasoy S et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: introduction and methods. J Heart Lung Transplant 2005;24:1451–3. [DOI] [PubMed] [Google Scholar]
- 8.King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg 2000;69:1681–5. [DOI] [PubMed] [Google Scholar]
- 9.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg 2002;73:1041–7. [DOI] [PubMed] [Google Scholar]
- 10.Thabut G, Vinatier I, Stern JB, Lesèche G, Loirat P, Fournier M et al. Primary graft failure following lung transplantation: predictive factors of mortality. Chest 2002;121:1876–82. [DOI] [PubMed] [Google Scholar]
- 11.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005;24:1454–9. [DOI] [PubMed] [Google Scholar]
- 12.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant 2005;24:1483–8. [DOI] [PubMed] [Google Scholar]
- 13.Prekker ME, Nath DS, Walker AR, Johnson AC, Hertz MI, Herrington CS et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2006;25:371–8. [DOI] [PubMed] [Google Scholar]
- 14.Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2010;29:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Date H, Shiraishi T, Sugimoto S, Shoji T, Chen F, Hiratsuka M et al. Outcome of living-donor lobar lung transplantation using a single donor. J Thorac Cardiovasc Surg 2012;144:710–5. [DOI] [PubMed] [Google Scholar]
- 16.Date H, Aoe M, Nagahiro I, Sano Y, Andou A, Matsubara H et al. Living-donor lobar lung transplantation for various lung diseases. J Thorac Cardiovasc Surg 2003;126:476–81. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Chin K, Sato M, Aoyama A, Murase K, Azuma M et al. Postoperative respiratory management in living donor lobar lung transplantation. Clin Transplant 2013;27:E383–90. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002;30:1772–7. [DOI] [PubMed] [Google Scholar]
- 19.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oto T, Levvey BJ, Pilcher DV, Bailey MJ, Snell GI. Evaluation of the oxygenation ratio in the definition of early graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg 2005;130:180–6. [DOI] [PubMed] [Google Scholar]
- 21.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2007;175:507–13. [DOI] [PubMed] [Google Scholar]
- 22.Whelan TP, Dunitz JM, Kelly RF, Edwards LB, Herrington CS, Hertz MI et al. Effect of preoperative pulmonary artery pressure on early survival after lung transplantation for idiopathic pulmonary fibrosis. J Heart Lung Transplant 2005;24:1269–74. [DOI] [PubMed] [Google Scholar]
- 23.McIlroy DR, Pilcher DV, Snell GI. Does anesthetic management affect early outcomes after lung transplant? An exploratory analysis. Br J Anaesth 2009;102:506–14. [DOI] [PubMed] [Google Scholar]
- 24.Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg 2010;139:765–73. [DOI] [PubMed] [Google Scholar]
- 25.George TJ, Beaty CA, Kilic A, Shah PD, Merlo CA, Shah AS. Outcomes and temporal trends among high-risk patients after lung transplantation in the United States. J Heart Lung Transplant 2012;31:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


