Abstract
OBJECTIVES
The totally thoracoscopic left atrial Maze (TT-Maze) is a relatively new surgical solution for the treatment of atrial fibrillation (AF). The procedure consists of a complete left atrial Maze, which is performed by video-assisted thoracoscopy with the use of radiofrequency ablation. We describe our rhythm results as well as our learning curve experience of the TT-Maze.
METHODS
To evaluate the learning curve, all consecutive patients who underwent a TT-Maze and were operated by one surgeon (Bart P. Van Putte) were included in the study. The endpoint of surgery was sinus rhythm with a bidirectional block of the box and pulmonary veins.
RESULTS
A total of 83 patients were included. Fifty percent of the patients had paroxysmal AF. The mean indexed left atrial volume was 44 ± 15 ml/m2 and 38% of the patients had a previous catheter ablation for AF. During a mean follow-up of 10.9 ± 4.9 months, there were no major events. At latest follow-up, 82% of the patients did not have a single registration of AF or other atrial tachyarrhythmias longer than 30 s. Patients without AF were also free from anti-arrhythmic drugs in 90% of the cases, free from coumadins or direct oral anticoagulants in 63% of the cases and free from both in 58% of the cases.
CONCLUSIONS
After almost 1-year follow-up, the TT-Maze is proved to be a successful, safe and reproducible strategy for the treatment of all types of AF including patients with enlarged left atria and previously failed catheter ablation.
Keywords: Atrial fibrillation, Mini-Maze, Totally thoracoscopic Maze, Pulmonary vein isolation, Thoracoscopic ablation, Maze
INTRODUCTION
Cardiac surgery was the first non-medical treatment that could cure patients with atrial fibrillation (AF) [1]. Already in 1991, J. Cox and his colleagues reported about the Maze I operation that was able to cure patients with AF [2]. The Maze I operation evolved from the Maze II into the Maze III operation, which is currently still seen as a very successful procedure in terminating AF [3]. The procedure is, however, time-consuming, complex, invasive and extracorporeal circulation is a necessity.
Just before the introduction of the Maze operation, it was recognized that the left atrial isolation operation, which was initially performed to exclude left-sided atrial tachyarrhythmias, was able to restore atrio-ventricular co-ordination in patients with AF [1]. This finding showed the important role of the left atrium in the pathogenesis of AF. This became even clearer by the work of Haïssaguerre and co-workers. They showed that ablating ectopic foci from the pulmonary veins could cure paroxysmal AF [4].
Due to a better understanding of the pathophysiology of AF and with the introduction of new (ablation) techniques, minimally invasive procedures for AF became possible [5]. In the early 2000s, the first minimally invasive procedure was described with excellent results and limited morbidity [6]. This procedure is known as the ‘Wolf Maze’ and includes a bilateral pulmonary vein isolation that is performed through a double-sided mini-thoracotomy. Nowadays, this procedure can be performed in a complete thoracoscopic setting [7].
Although surgery cures patients with AF for decades now, it has only a class IIB recommendation in the latest ESC guidelines as a stand-alone procedure for patients with symptomatic AF and previously failed catheter ablation [8].
This report describes the results and the learning curve of a totally thoracoscopic left atrial Maze (TT-Maze) performed by one surgeon with no previous experience in rhythm surgery at all. We investigated if a ‘one size fits them all’ approach is feasible, successful and reproducible for cardiac surgeons with only basic endoscopic skills.
MATERIALS AND METHODS
Patient selection
All consecutive patients who underwent a TT-Maze, which consisted of a pulmonary vein isolation (PVI) with a box, left atrial appendage and trigonum lesion, and operated by one surgeon (Bart P. Van Putte) between January 2012 and February 2013 were included in the study.
All patients who were proposed for surgery were accepted, except patients who had a history of heart or lung surgery. Neither the type of AF, nor the duration of AF or the left atrial size were exclusion criteria. One patient was excluded because of a severe unforeseen pleural adhesion and we were unable to perform a TT-Maze. The first 15 totally thoracoscopic procedures included various and incomplete lesion sets and were excluded from this study. None of these patients had to be converted to a sternotomy, died or had other major complications.
The study was approved by the local ethical committee who waived the need for a fully approved protocol.
Operation technique
Our technique is similar to what others have described [7,9,10]. In brief, the patient is placed on supine position and a left atrial Maze procedure is performed by a bilateral totally thoracoscopic approach. Both sets of pulmonary veins are isolated by the AtriCure Isolator Synergy ablation clamp (AtriCure, Inc., West Chester, OH, USA). On the right side, the Waterstone groove is dissected for a better positioning of the clamp. Due to the lack of evidence regarding the effectiveness of ganglionic plexus (GP) ablation, we have not performed this in our series. We perform at least 10 overlapping lesions with the clamp on the antrum of the left atrium. An entry block was confirmed in all pulmonary veins in all patients. Since many patients cannot be converted into sinus rhythm after PVI on the right side, we only confirm the entry block of the pulmonary veins. Conduction block is confirmed with the AtriCure Isolator Transpolar Pen (AtriCure, Inc.).
After isolating the right pulmonary veins, a trigonum line is made. The trigonum line is performed in every patient and is made by so-called stamping lesions with the AtriCure Isolator Transpolar Pen. For every ‘stamp’, we take at least 5 s to ablate the tissue. In our institute, the trigonum line cannot be checked for transmurality and therefore making this lesion is time consuming. From the trigonum line, a separate lesion is made to the left atrial appendage. Both a roof line and a floor line are created with the Multifunctional Linear Pen (AtriCure, Inc.), making up the so-called box lesion. The box lesion is checked for conduction block by epicardial sensing and pacing with the AtriCure Isolator Transpolar Pen (AtriCure, Inc.). Whenever a patient is in AF, first an electrical cardioversion is performed. We always pace with an output of 25 mA. If conduction over the box is observed, additional ablation of the roof and floor lines will be performed until a bidirectional block of the box has been achieved.
The primary endpoint of the operation is sinus rhythm and a bidirectional block of the box. This was achieved in all patients, except for 4.
The last step is amputating the left auricle. This is performed with the Endo-Gia stapler (Tyco Healthcare Group, North Haven, CT, USA). Routine transoesophageal echocardiography is performed to reveal any remnant of the left appendix. If needed, such a remnant is removed with a second or third stapler.
In-hospital rhythm management
Postoperatively, patients received the same rhythm and anticoagulation medication as preoperatively, except for amiodarone which was always stopped. The dosage of rhythm medication was adjusted in function of the heart rate and rhythm type. All patients stayed on continuous rhythm monitoring until discharge.
Follow-up
Referring cardiologists from other hospitals were contacted to provide follow-up information concerning rhythm and medication use. The method of follow-up and prescribed medication were left to the discretion of the referring cardiologists. Patients who were referred by our local cardiologists received outpatient clinic controls at 3, 6 and 12 months which included 24-h Holter monitoring. The number of patients who had a follow-up at 3, 6 and 12 months was 60, 73 and 45, respectively.
In line with the ESC AF guidelines, a blanking period of 3 months was taken into account [8]. Any episode of AF or other atrial tachyarrhythmias for more than 30 s after the blanking period was noted as failure. Normal β-blocker (class II anti-arrhythmic drug) was not noted as anti-arrhythmic medication, because it is often used to treat hypertension.
Data analysis
Descriptive statistics are given as a percentage for count data, mean with standard deviation for normally distributed variables and median with interquartile range for non-normally distributed variables (based on visual inspection of the histograms); comparisons between success and failure were performed by means of the Fisher's exact, Student's t-test or Mann–Whitney U-test, respectively. Multivariable modelling of the success rate at the end of the follow-up was performed with logistic regression; we allowed for only two variables based on clinical relevance and univariable P-value. For the learning curve effect, we split the dataset in the first 37 patients versus the last 37 patients where success rate was assessed and regarding operation duration we used general linear modelling, with smoothing using the running mean with a window width of 10 observations. All statistical analyses were performed in R (www.r-project.org).
RESULTS
Baseline characteristics
In total, 82 patients (mean age 59.9 ± 8.6 years) were included in this study. The time since first diagnosis of AF was 5.3 (IQR 3.2–8.4) years. Half of the patients had paroxysmal AF, 38% had persistent AF and 12% had longstanding persistent AF. The mean left atrial diameter and indexed volume were 45.2 ± 5.9 mm and 44 ± 15 mm3/m2, respectively. The complete baseline characteristics of the study population are further presented in Table 1.
Table 1:
Patient characteristics
| Total (N = 82) | Failure (N = 16) | Success (N = 66) | P-value | |
|---|---|---|---|---|
| Age (years) | 59.9 ± 8.6 | 60.3 ± 5.6 | 59.8 ± 9.2 | 0.798 |
| Female gender | 24 (29%) | 6 (38%) | 18 (27%) | 0.541 |
| BMI (kg/m2) | 29.2 ± 4.8 | 28.9 ± 4.9 | 29.3 ± 4.8 | 0.814 |
| Duration of AF (years) | 5.3 [3.2–8.4] | 5.1 [3.5–5.8] | 5.6 [3.2–9.9] | 0.114 |
| Type of AF | 0.781 | |||
| Paroxysmal | 41 (50%) | 7 (44%) | 34 (52%) | |
| Persistent | 31 (38%) | 5 (31%) | 26 (39%) | |
| Longstanding persistent | 10 (12%) | 4 (25%) | 6 (9%) | |
| LVEF mean (%) | 56 ± 9 | 54 ± 11 | 57 ± 9 | 0.369 |
| >50 | 79 (96%) | 15 (94%) | 64 (97%) | 0.483 |
| 30–50 | 1 (1%) | 0 (0%) | 1 (2%) | |
| <30 | 2 (2%) | 1 (6%) | 1 (2%) | |
| Mitral valve incompetence | 0.268 | |||
| None | 53 (70%) | 9 (56%) | 44 (73%) | |
| Grade I | 18 (24%) | 5 (31%) | 13 (22%) | |
| Grade II | 5 (7%) | 2 (13%) | 3 (5%) | |
| Atrial dimensions | ||||
| LA diameter (mm)a | 45 ± 6 | 48 ± 7 | 44 ± 5 | 0.052 |
| LA volume (ml) | 93 ± 34 | 108 ± 43 | 89 ± 30 | 0.102 |
| LA indexed volume (ml/m2) | 44 ± 15 | 51 ± 18 | 42 ± 14 | 0.078 |
| RA diameter (mm)b | 44 ± 7 | 45 ± 6 | 44 ± 7 | 0.633 |
| Previous failed catheter ablation | 31 (38%) | 4 (25%) | 27 (41%) | 0.373 |
| Of which >1 failed ablation | 12 (39%) | 2 (50%) | 10 (37%) | 0.630 |
| Preoperative ischaemic CVA | 5 (6%) | 0 (0%) | 5 (8%) | 0.577 |
| Preoperative TIA | 5 (6%) | 1 (6%) | 4 (6%) | 1.000 |
| Preoperative pacemaker | 3 (4%) | 0 (0%) | 3 (5%) | 1.000 |
Values in square brackets depict interquartile range.
BMI: body mass index; LVEF: left ventricular ejection fraction; LA: left atrium; RA: right atrium; CVA: cerebrovascular accident; TIA: transient ischaemic attack; AF: atrial fibrillation.
aLong axis in parasternal view.
bShort axis in four-chamber view.
Baseline characteristics of patients with or without success did not differ significantly (Table 1).
In-hospital results
The perioperative results are summarized in Table 2. No major in-hospital complications, such as death, stroke, oesophagus perforation or conversion to sternotomy, occurred. The mean operation time was 160 ± 38 (range 79–260) min.
Table 2:
Perioperative data
| Total (N = 82) | Failure (N = 15) | Success (N = 67) | P-value | |
|---|---|---|---|---|
| Mortality | 0 (0%) | 0 (0%) | 0 (0%) | |
| Sternotomy | 0 (0%) | 0 (0%) | 0 (0%) | |
| Stroke | 0 (0%) | 0 (0%) | 0 (0%) | |
| Duration of operation (min) | 160 ± 38 | 159 ± 32 | 161 ± 39 | 0.818 |
| In-hospital AF | 18 (23%) | 9 (60%) | 9 (14%) | 0.001 |
| Discharged with AF | 5 (6%) | 1 (7%) | 4 (6%) | 1.000 |
| In-hospital pacemaker | 1 (1.2%) | 1 (6.7%) | 0 (0%) | 0.036 |
| Duration of hospital stay (days) | 4 [3–6] | 5 [4–8] | 4 [3–5] | 0.036 |
Values in square brackets depict interquartile range.
Twenty-three percent of the patients had an episode of AF postoperatively. After a median of 4 (IQR 3–6) days, the patients were discharged from the hospital.
The following complications were noted: high-standing hemidiaphragm in 4 (4.8%), permanent pacemaker implantation in 1 because of a sick sinus syndrome (1.2%), thoracostomy drain because of a pneumothorax in 1 (1.2%), late pericardial tamponade necessitating percutaneous drainage in 1 (1.2%), reintubation because of respiratory insufficiency in 1 (1.2%) and readmission because of pleural fluid which was treated conservatively in 1 (1.2%). The 4 patients with a high-standing diaphragm postoperatively were consulted by a pulmonologist of the referring hospital and during the follow-up the function of the phrenic nerve fully recovered within 1 year in all 4 patients.
Comparing the two groups (failure versus success), there was a significant difference in in-hospital AF. The success group had significantly less in-hospital AF compared with the failure group (14 vs 60%, respectively, P = 0.001).
Rhythm and follow-up results
There were no major events such as death, cerebrovascular accidents or transient ischaemic attacks during the follow-up of 10.9 ± 4.9 (range 2.5–26.0) months. At the latest follow-up, 80% [95% confidence limit (CL) 70–88%] of the patients did not have a single registration of AF (or other atrial tachyarrhythmias) longer than 30 s. This was confirmed by at least one 24-h Holter monitoring in 91% of the cases. Patients who were free from AF (and other atrial tachyarrhythmias) were also free from anti-arrhythmic drugs in 90% of the cases, free from coumadins or direct oral anticoagulants in 63% of the cases and free from both in 58% of the cases.
Freedom from AF (and other atrial tachyarrhythmias) was 83, 84 and 60% for paroxysmal, persistent and longstanding persistent AF, respectively (P = 0.299). Patients who had a relapse of AF in the hospital had more often AF at the end of the follow-up than those who did not have an in-hospital relapse of AF (90 vs 50%, respectively, P < 0.001).
A subgroup of patients (n = 31), with a history of one or more failed catheter ablations, were now free from AF (and other atrial tachyarrhythmias) in 87% of the cases after a mean follow-up of 11.3 ± 5.6 months.
The mean follow-up was almost 11 months and may raise concerns about the effectiveness at 1 year or later. Therefore, a subanalysis of the follow-up at 1 year (range 9–15 months) and longer than 1 year was made. At 1-year follow-up, 78% of the patients were free from AF (and other atrial tachyarrhythmias; n = 45). Eighty-five percent of them were free from anti-arrhythmic drugs. The follow-up of 1 year or longer showed that 74% of the patients were free from AF (and other atrial tachyarrhythmias; n = 38, mean follow-up of 14.9 ± 4.1 months). Eighty-five percent of them were free from anti-arrhythmic drugs.
Four patients received a pacemaker implantation after hospital discharge. Three of them received a His-ablation because of symptomatic refractory AF and 1 because of atrio-ventricular conduction block after a mitral valve repair.
Multivariable analyses showed the grade of mitral valve insufficiency and the indexed left atrial volume as independent factors for success after a TT-Maze. With every grade increase of the mitral valve regurgitation, there is a relative decrease in the success rate of 59% (P = 0.060, OR 0.41, CL 0.16–1.04) and for every ml/m2 increase of the indexed left atrial volume, the success rate decreased relatively with 4% (P = 0.024, OR 0.96, CL 0.92–0.99).
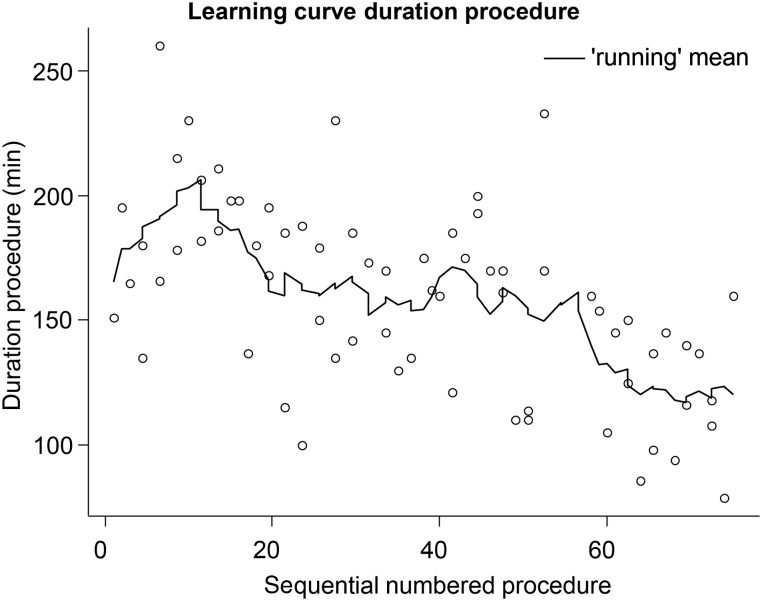
To assess the learning effect, we analysed both the success rate and operation duration over time. There was no significant difference in success rate, but there was a significant effect on operation duration. The operation duration decreased by a mean of 0.96 min with every operation (P < 0.001, 95% CL 0.63–1.30, n = 74; Figure 1).
Figure 1:
Operation time, learning curve. The figure represents the consecutive operation times. The line represents the mean of 10 procedures. The operation duration decreased on average 0.96 min per consecutive procedure, P < 0.001, 95% CLs 0.63–1.30.
DISCUSSION
Recent comparative studies have shown that minimally invasive surgery is superior to percutaneous intervention in terms of freedom from AF [11, 12]. The current study shows excellent efficacy of the TT-Maze, even in patients with previously failed percutaneous catheter ablation(s) and/or longstanding persistent AF.
During a mean follow-up of almost a year, 80% of the patients were free from AF (and other atrial tachyarrhythmias) and 90% of them were also free from anti-arrhythmic drugs. For patients with longstanding persistent AF and for patients with previously failed catheter ablation, this was 60 and 87%, respectively. These results are in line with previously published results, but were however accomplished by one surgeon without any previous experience in anti-arrhythmic surgery [10–12]. The most reasonable explanation for the reproducibility of these results is most likely based on the hard endpoint of this operation, namely sinus rhythm plus a bidirectional block of the box lesion. Theoretically, and apparently practically too, the level of experience of the surgeon does not influence the results significantly.
Although the surgeon had no previous experience with rhythm surgery, no major complications occurred. None of the 82 patients died, needed a sternotomy or had a stroke perioperatively or during follow-up. The absence of stroke needs to be emphasized, because a stroke rate of ∼1% is reported during and/or directly after percutaneous catheter ablation [13, 14]. This is probably because catheters are foreign bodies and thus are potentially highly thrombogenic. In contrast, during the surgery, all lesions are made from the epicardial side without entering the left atrium and therefore avoiding the risk of thrombus formation and thus stroke. Furthermore, percutaneous catheter ablation causes most tissue damage on the endocardial side, because the energy is delivered from the endocardial to the epicardial side. The damaged tissue could be on the side of thrombus formation and thus could cause emboli and stroke.
Postoperatively, 4 patients had a unilateral high-standing diaphragm, but all fully recovered within a year. Due to the presence of a high-standing hemidiaphragm, we slightly changed our operating technique. In this series, we opened the pericardium on the right side ∼1 cm anterior to the phrenic nerve followed by placing two stay sutures in order to improve the exposure of the pulmonary veins. We speculate that the diathermy and/or tension on these stay sutures may have caused some reversible damage of the phrenic nerve. Therefore, we nowadays open the pericardium at least 3 cm away from the nerve and since this change in technique, we never faced this complication anymore.
In terms of freedom from AF (success), there was no significant learning curve. However, there was a learning curve in operation time. This study shows that the TT-Maze, which is a standardized method, can be performed by a cardiac surgeon with limited or no previous experience in the field of AF surgery.
Predictors for success
The multivariable analysis showed that the indexed left atrial volume was a predictor for success. With every ml/m2 increase of the volume, there was a relative decrease in the success rate of 4%. The relation between success after ablation for AF and the size of the left atrium has already been recognized [15].
In this series, there was also a trend towards a lower success rate when mitral valve incompetence was present. Despite the absence of severe mitral valve incompetence, we observed a relation between the grade of incompetence and the success after the TT-Maze. With every grade increase of the mitral valve incompetence, there is a relative decrease in the success rate of 59%. It is known that surgery for AF in combination with mitral valve surgery is less successful. This evidence is derived from patients who were operated for at least moderate mitral regurgitation. Our data revealed that even grade 1 or 2 mitral valve regurgitation decreases the success rate for stand-alone AF surgery. Structural changes in the atrial wall are probably the reason for a lower success rate [15].
Ganglionic plexus ablation
From experimental data, it is clear that the autonomic nervous system directly influences the electrical properties of the atria (‘substrate’) and can lead to premature depolarizations (‘triggers’) [16]. This interaction between initiating factors (or triggers), substrate and modulating factors (i.e. autonomic nervous system) is well known as the ‘Triangle of Coumel’ [17]. This concept of a triangle emphasizes the important role of the autonomic nervous system in AF. There is, however, no clear evidence that localizing GPs and ablating them will improve success. It could even lead to a pro-arrhythmic effect due to the formed scar tissue. For these reasons, we do not perform systematical GP ablation.
Trigonum lesion
Currently, the trigonum line is widely being discussed, because of the potential benefits and hazards. The rationale behind the trigonum line is based on the idea that it could interrupt macro-reentry circuits around the mitral valve. Therefore, this line is meant to mimic the left atrial isthmus line (including the coronary sinus lesion), which is part of the Maze III and IV lesion set. On the other hand, conduction block cannot be confirmed in our setting. An incomplete trigonum line could potentially result in an atypical flutter. Indeed, we had 3 patients with an atypical left-sided flutter. These patients were noted as failure. Furthermore, this lesion is crossing Bachmann's bundle that might result in some degree of atrial asynchrony. Electrical activity from the right to left atrium now has to be conducted through the left atrial isthmus. To reduce the effect of atrial asynchrony, the trigonum line was made starting from the left superior pulmonary vein (rather than starting from the mid-part or the right side of the roof line) over the base of the left appendage towards the aortic-mitral continuity.
Minimally invasive rhythm surgery versus percutaneous intervention
Comparative studies show the superiority of minimally invasive rhythm surgery over percutaneous catheter ablation in terms of rhythm success [11, 12]. There are a few explanations for this. First, in contrast to catheter ablation, surgical thoracoscopic ablation is performed under direct vision. This enables precise position of the ablation devices with appropriate overlap of the ablation lines. Second, the introduction of bidirectional devices enables clamping of the pulmonary veins, which prevents heat sink and thus enables optimal ablation. The clamp also creates linear lesions instead of dots. Despite all of these, we often need multiple clamp ablation applications to fully isolate the pulmonary veins. This emphasized how difficult it is to completely isolate the pulmonary veins.
Another advantage of performing the ablation under direct vision is that we can prevent severe or even life-threatening complication such as atrio-oesophageal fistulas and pulmonary vein stenosis. Before we start to ablate the floor line, we retrieve the TEE probe a few centimetres and we then identify the left inferior pulmonary vein and the coronary sinus. Once we have recognized these structures, we start ablation while lifting the left atrium away from the posterior pericardium and oesophagus. This approach minimizes and probably excludes the risk on atrio-oesophageal fistula. Furthermore, before we start to ablate the roof line, we identify the base of the left appendix and the right superior pulmonary vein in order to make sure that we ablate on the atrial side of the appendix avoiding potential coronary damage.
In contrast to the catheter ablation, pulmonary vein isolation is exclusively performed by clamping and ablating the antrum under direct vision. This prevents pulmonary vein stenosis and allows isolation of not only triggers that are located inside the pulmonary veins, but also triggers that are located at the base of the pulmonary veins.
Taken together with the possibility of amputating the left atrial appendage, the above method describes several clear advantages of an epicardial, thoracoscopic approach. This comes, however, with a cost. It remains an operation that has its own disadvantages compared with a percutaneous treatment. Patients are, for example, longer admitted to the hospital and conversion to sternotomy does happen. This article shows, however, that the procedure can be performed with excellent result and without major complications by a cardiac surgeon who has no experience in rhythm surgery.
Limitations
Because we were largely dependent on external referring cardiologists for the follow-up, not all patients received a 24-h Holter monitoring at 3, 6 and 12 months. However, 90% of the patients received at least 1 Holter monitoring after the blanking period and every documented episode of AF longer than 30 s after the blanking period was seen as a failure. Taken together with the retrospective nature of this study, the success could have been overestimated.
CONCLUSION
In conclusion, the TT-Maze is an effective and safe therapy for AF in terms of short- and mid-term outcome regarding freedom from AF and complications. This is also true for surgeons without any previous experience in rhythm surgery.
Conflict of interest: Bart P. Van Putte is consultant for AtriCure, Inc.
REFERENCES
- 1.Williams JM, Ungerleider RM, Lofland GK, Cox JL. Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg 1980;80:373–80. [PubMed] [Google Scholar]
- 2.Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg 1991;101:584–92. [PubMed] [Google Scholar]
- 3.Cox JL, Jaquiss RD, Schuessler RB, Boineau JP. Modification of the maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the maze III procedure. J Thorac Cardiovasc Surg 1995;110:485–95. [DOI] [PubMed] [Google Scholar]
- 4.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 5.Manasse E, Infante M, Ghiselli S, Cariboni U, Alloisio M, Barbone A et al. A video-assisted thoracoscopic technique to encircle the four pulmonary veins: a new surgical intervention for atrial fibrillation ablation. Heart Surg Forum 2002;5:337–9. [PubMed] [Google Scholar]
- 6.Wolf RK, Schneeberger EW, Osterday R, Miller D, Merrill W, Flege JB Jr et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:797–802. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz A, Van Putte BP, Van Boven WJ. Completely thoracoscopic bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2008;136:521–2. [DOI] [PubMed] [Google Scholar]
- 8.European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz A, Geuzebroek GS, Van Putte BP, Boersma LV, Sonker U, De Bakker JM et al. Completely thoracoscopic pulmonary vein isolation with ganglionic plexus ablation and left atrial appendage amputation for treatment of atrial fibrillation. Eur J Cardiothorac Surg 2010;38:356–60. [DOI] [PubMed] [Google Scholar]
- 10.Krul SP, Driessen AH, van Boven WJ, Linnenbank AC, Geuzebroek GS, Jackman WM et al. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:262–70. [DOI] [PubMed] [Google Scholar]
- 11.Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23–30. [DOI] [PubMed] [Google Scholar]
- 12.De Maat GE, Van Gelder IC, Rienstra M, Quast AF, Tan ES, Wiesfeld AC et al. Surgical vs. transcatheter pulmonary vein isolation as first invasive treatment in patients with atrial fibrillation: a matched group comparison. Europace 2014;16:33–9. [DOI] [PubMed] [Google Scholar]
- 13.Patel D, Bailey SM, Furlan AJ, Ching M, Zachaib J, Di Biase L et al. Long-term functional and neurocognitive recovery in patients who had an acute cerebrovascular event secondary to catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2010;21:412–7. [DOI] [PubMed] [Google Scholar]
- 14.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. [DOI] [PubMed] [Google Scholar]
- 15.Ballaux PK, Geuzebroek GS, van Hemel NM, Kelder JC, Dossche KM, Ernst JM et al. Freedom from atrial arrhythmias after classic maze III surgery: a 10-year experience. J Thorac Cardiovasc Surg 2006;132:1433–40. [DOI] [PubMed] [Google Scholar]
- 16.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J 1994;15(Suppl A):9–16. [DOI] [PubMed] [Google Scholar]
- 17.Coumel P. The management of clinical arrhythmias: an overview on invasive versus non-invasive electrophysiology. Eur Heart J 1987;8:92–9. [DOI] [PubMed] [Google Scholar]