Abstract
To systematically review early surgery and the optimal timing of surgery in patients with infective endocarditis (IE), a search for foreign and domestic articles on cohort studies about the association between early surgery and infective endocarditis published from inception to January 2015 was conducted in the PubMed, EMBASE, Chinese Biomedical Literature (CBM), Wanfang and Chinese National Knowledge Infrastructure (CNKI) databases. The studies were screened according to the inclusion and exclusion criteria, the data were extracted and the quality of the method of the included studies was assessed. Then, the meta-analysis was performed using the Stata 12.0 software. Sixteen cohort studies, including 8141 participants were finally included. The results of the meta-analysis revealed that, compared with non-early surgery, early surgery in IE lowers the incidence of in-hospital mortality [odds ratio (OR) = 0.57, 95% confidence interval (CI) (0.42, 0.77); P = 0.000, I2 = 73.1%] and long-term mortality [OR = 0.57, 95% CI (0.43, 0.77); P = 0.001, I2 = 67.4%]. Further, performing operation within 2 weeks had a more favourable effect on long-term mortality [OR = 0.63, 95% CI (0.41, 0.97); P = 0.192, I2 = 39.4%] than non-early surgery. In different kinds of IE, we found that early surgery for native valve endocarditis (NVE) had a lower in-hospital [OR = 0.46, 95% CI (0.31, 0.69); P = 0.001, I2 = 73.0%] and long-term [OR = 0.57, 95% CI (0.40, 0.81); P = 0.001, I2 = 68.9%] mortality than the non-early surgery group. However, for prosthetic valve endocarditis (PVE), in-hospital mortality did not differ significantly [OR = 0.83, 95% CI (0.65, 1.06); P = 0.413, I2 = 0.0%] between early and non-early surgery. We concluded that early surgery was associated with lower in-hospital and long-term mortality compared with non-early surgical treatment for IE, especially in NVE. However, the optimal timing of surgery remains unclear. Additional larger prospective clinical trials will be required to clarify the optimal timing for surgical intervention and determine its efficacy in PVE.
Keywords: Early surgery, Infective endocarditis, Systematic review, Meta-analysis, Cohort study
INTRODUCTION
Infective endocarditis (IE) is an inflammation of the inner tissues of the heart or endocardium, which if untreated, can lead to life-threatening complications and is associated with a high risk of morbidity and mortality. Epidemiological research has revealed that the annual incidence of IE is 3–10 cases per 100 000 persons [1], and the mortality rate is as high as 15–30% [2]. In addition, although the diagnosis and treatment strategies continually improve, the mortality does not decrease significantly [3].
At present, early surgery has been the treatment of choice for IE, but its effectiveness has not been systematically and comprehensively assessed. The recommendations on early surgery from current guidelines [4–6] vary in indications, the choices of operation time and their strength, and most were supported by expert opinions or clinical experience. Additionally, prosthetic valve endocarditis (PVE) occurs in 3–6% of patients within 5 years of the valve implantation procedure and is linked with significant morbidity and mortality [7]. The effectiveness of early surgery for PVE is unclear. Due to its low incidence, and ethical and financial constraints, there is no large-sample, multicentre randomized, controlled trials (RCTs) to draw a definitive conclusion about its efficacy. To date, only one RCT [8] has explored the effectiveness of early surgery for IE, and because of the trial setting (South Korea), small sample size (76 patients) and short follow-up period (6 months), we remain uncertain about the reliability and external validity of the results.
Recently, several cohort studies with large sample size [2, 7, 9–22] explored the effectiveness of early surgery for IE, but the results were not consistent. The objectives of this meta-analysis were (i) to assess whether early surgery therapy is associated with lower in-hospital and long-term mortality; (ii) to assess the prognostic outcomes of the optimal timing for surgery; (iii) to evaluate the difference in effectiveness of early surgery in patients with native valve endocarditis (NVE) and PVE.
METHODS
Data sources and inclusion criteria
We conducted a search in the MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Chinese Biomedical Literature (CBM), Wanfang and Chinese National Knowledge Infrastructure (CNKI) databases to identify relevant articles published from inception to January 2015. Medical Subject Headings terms were added in all searches for MEDLINE, EMBASE and the Cochrane Library. Search terms included ‘Infective Endocarditis’, ‘Endocarditides’, ‘Heart Surgery’, ‘Cardiac Surgery’ and ‘early surgery’. The search strategy was developed by two reviewers and peer-reviewed by a third. We checked the reference lists of relevant review articles, meta-analyses and original studies. There was no language restriction.
Eligible trials had to fulfil the following criteria for inclusion: (i) cohort study, either prospective or retrospective, (ii) patients received a definite diagnosis of IE according to the modified Duke criteria [23], (iii) the study compared early surgical intervention versus non-early surgical intervention, including medical therapy or late surgery. Early surgery was defined during initial hospitalization before the completion of a full therapeutic course of antibiotics [6], (iv) the study investigated the following outcomes: short-term efficacy, which was measured by in-hospital mortality; long-term efficacy, which was assessed by mortality with a follow-up of more than 6 months. The following studies were excluded: the outcomes and parameters of patients were not clearly described; it was not possible to extract reliable data from the reported results; and an overlap between authors or centres in the published literature existed.
Study selection and data extraction
Study selection and data extraction were conducted independently by two investigators, who screened the titles and abstracts. Full articles were retrieved if a decision could not be reached. Data were extracted into a form that included a prespecified set of variables (study quality, study characteristics, patient characteristics, interventions and outcome data). Disagreements in study selection and data extraction were resolved by consensus.
Risk of bias assessment
Two authors independently assessed risk of bias for each study using the Newcastle–Ottawa Scale [24]. Cohort studies were accessed by three kinds of categories, namely selection, exposure and comparability. Studies were rated using an ordinal star grading scale where studies of higher quality receive higher scores or more stars. A study can receive a maximum of one star for each listed item within the selection and exposure categories. A maximum of two stars can be awarded for comparability. Studies that achieve six or more stars were deemed to be of better quality. In our analysis, studies of low, intermediate and high quality were defined with NOS scores of 1–3, 4–6 and 7–9, respectively.
Statistical analysis
The meta-analysis was performed using the Stata software, version 12.0 (Stata Corporation, College Station, TX, USA). We analysed in-hospital and long-term mortality using estimation of odds ratios (ORs) with a 95% confidence interval (95% CI). The Z test was used to estimate the statistical significance of the pooled data. Subgroup analysis was performed to determine (i) the optimal timing of early surgery in IE based on different time periods before completion of a full therapeutic course of antibiotics, (ii) the efficacy of early surgery in NVE and PVE.
Heterogeneity between studies in the subgroups and the at large heterogeneity for all studies included were assessed by the Cochran's Q statistic and I2 test. When the presence of significant heterogeneity was indicated by a Q-test value of P < 0.05 or an I2 test rate of >50%, the random-effects model was employed [25]; otherwise, the fixed-effects model was used [26]. To evaluate whether publication bias may have influenced the statistical results, visual inspection of funnel plots and the Egger's test were performed, and publication bias was considered to be statistically significant when P < 0.10 [27].
RESULTS
Study selection
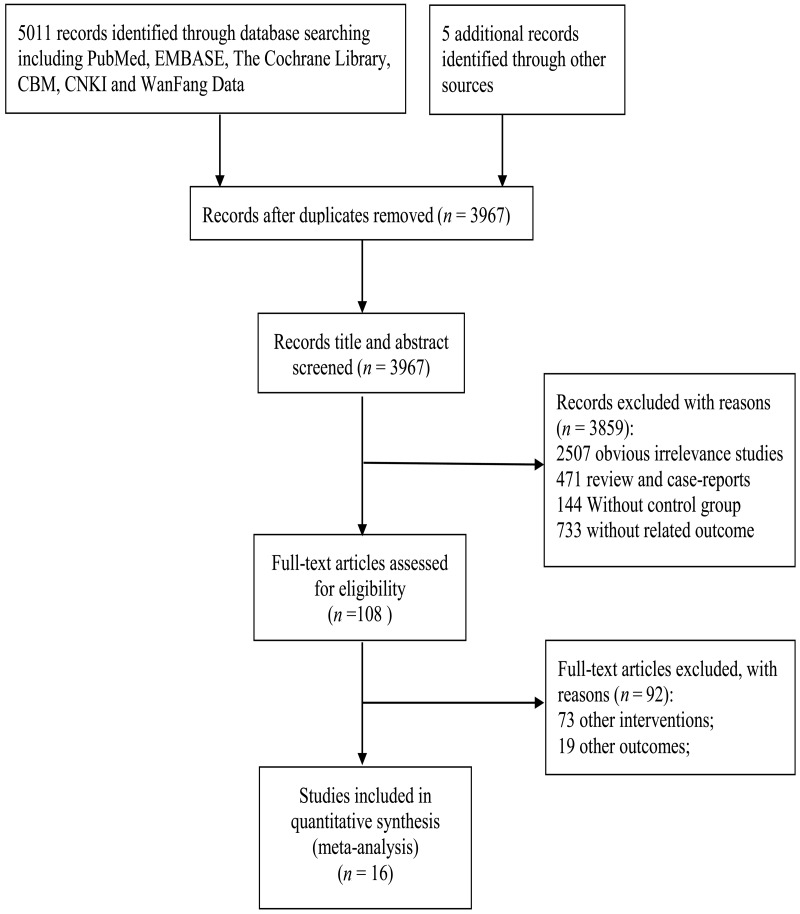
After screening titles and abstracts, we identified 108 studies. Of these, 92 citations were excluded, specifically 73 other interventions and 19 other outcomes. Finally, 16 [2, 7, 9–22] cohort studies were included in the meta-analysis (Fig. 1).
Figure 1:
Literature search and study selection.
Baseline characteristics
The baseline characteristics are presented in Table 1. Sixteen cohort studies [2, 7, 9–22] encompassing a total of 8141 enrolled patients who matched the selection criteria. The area distributions of the 16 studies were: 4 [2, 7, 11, 20] multinational, 3 [9, 18, 19] in the USA, 3 [10, 14, 17] in France, 2 in China, 2 [12, 15] in Japan, 1 [13] in Belgium and 1 [16] in Australia. Fourteen studies compared early surgery with medical therapy, and 2 studies [13, 17] compared early surgery with late surgery.
Table 1:
The characteristics of the studies included in the meta-analysis
| Authors | Study region | Number |
Male (%) |
Age (mean ± SD) |
Definition of early surgery | Intervention |
Primary end-point | Study period | Included valve | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | C | E | C | E | C | E | C | ||||||
| Aksoy et al. [9] | USA | 78 | 255 | 52 (66.7) | 134 (52.6) | 54.2 ± 20.6 | 58.4 ± 26.8 | During initial hospitalization | Early surgery | Medical therapy | All-cause mortality | In-hospital and 5 years | Left-sided NVE and PVE |
| Bannay et al. [10] | France | 240 | 209 | 188 (78.3) | 146 (69.9) | 57.6 ± 13.5 | 64.4 ± 15.6 | A median of 20 days | Early surgery | Medical therapy | All-cause mortality | 5 years | Left-sided NVE and PVE |
| Cabell et al. [11] | Multinational | 610 | 906 | 451 (73.9%) | 577 (63.7%) | 54.7 ± 15.2 | 61.1 ± 17.4 | During initial hospitalization | Early surgery | Medical therapy | All-cause mortality | In-hospital | Left- and right- sided NVE |
| Funakoshi et al. [12] | Japan | 73 | 139 | 44 (60) | 85 (61) | 55 ± 18 | 53 ± 17 | 2 weeks after diagnosis | Early surgery | Medical therapy | All-cause mortality | In-hospital and 5.5 years | Left-sided NVE |
| Hill et al. [13] | Belgium | 58 | 37 | 66 | 65 | First 7 days after diagnosis | Early surgery | Late surgery | All-cause mortality | 6 months | Left-sided NVE and PVE | ||
| Lalani et al. (2010) [2] | Multinational | 720 | 832 | 525 (72.9) | 545 (66.1) | 53 | 61 | Median time was 7 days | Early surgery | Medical therapy | Mortality | In-hospital | Left- or right-sided NVE |
| Lalani et al. (2013) [7] | Multinational | 490 | 535 | 343 (70) | 335 (63) | 59.4 (0.5–88) | 63.8 (0.3–91) | Median time was 8 days | Early surgery | Medical therapy | All-cause mortality | In-hospital and 1 year | Right- or left-sided PVE |
| Mourvillier et al. [14] | France | 104 | 124 | N | N | N | N | Median time was 17 days | Early surgery | Medical therapy | All-cause mortality | In-hospital | Left and right-sided NVE and PVE |
| Ohara et al. [15] | Japan | 237 (NVE) 335 (PVE) |
111 (NVE) 46 (PVE) |
168 (71) (NVE) 21 (60) (PVE) |
58 (52) (NVE) 22 (48) (PVE) |
55 ± 16 (NVE) 64 ± 16 (PVE) |
63 ± 19 (NVE) 69 ± 15 (PVE) |
During initial hospitalization | Early surgery | Medical therapy | All-cause mortality | In -hospital | Left- and right-sided PVE and NVE |
| Sy et al. [16] | Australia | 62 | 161 | 41 (66) | 114 (71) | 46.9 (15.1) | 57.9 (19.2) | From admission to surgery 18 days | Early surgery | Medical therapy | All-cause mortality | 5.2 years | Left-sided NVE and PVE |
| Thuny et al. [17] | France | 95 | 196 | 69 (73) | 155 (79) | 53 + 16 | 58 + 15 | <1st week | Early surgery | Late surgery | Mortality | 6 months | Left-sided NVE and PVE |
| Wang et al. [20] | Multinational | 148 | 207 | 106 (71.6) | 124 (59.9) | 62.0 (50–71) | 70.0 (56–76) | Median time was 12 days | Early surgery | Medical therapy | All-cause mortality | In-hospital | PVE |
| Wang et al. [21] | China | 70 | 169 | 47 (67.1) | 106 (62.7) | 41.6 ± 12.0 | 45.6 ± 17.2 | <2nd week | Early surgery | Medical therapy | All-cause mortality | In-hospital and 2 years | Left-sided NVE |
| Jia et al. [22] | China | 74 | 61 | 52 (71.6) | 42 (68.9) | 47.7 ± 15.9 | 45.4 ± 13.8 | <4th week | Early surgery | Medical therapy | All-cause mortality | 6–42 months | Left-sided NVE |
| Vikram et al. [19] | USA | 230 | 283 | 156 (68) | 175 (62) | 53 ± 16.3 | 56.6 ± 18.6 | Not reported | Early surgery | Medical therapy | All-cause mortality | 6 months | Left-sided NVE |
| Tleyjeh et al. [18] | USA | 129 | 417 | 86 (66.67) | 273 (65.47) | 56.72 ± 17.40 | 64.03 ± 15.58 | Within 30 days | Early surgery | Medical therapy | All-cause mortality | 6 months | Left-sided NVE and PVE |
E: early surgery group; C: non-early surgery group; N: not reported; NVE: native valve endocarditis; PVE: prosthetic valve endocarditis; SD: standard deviation.
The quality assessment of the included studies is presented in Table 2. The quality scores of the 16 studies ranged from 4 to 8, with 7 [2, 7, 9, 10, 17, 19, 20] studies considered to be of high quality and 9 [11–16, 18, 21, 22] of intermediate quality. The average score of the included studies was 6.3 point.
Table 2:
Newcastle–Ottawa Scale checklist
| Study ID | Selection |
Comparability |
Outcome |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort? | Selection of non-exposed cohort? | Ascertainment of exposure? | Demonstration that outcome of interest was not present at start of study? | Study controls for age and sex? | Study controls for additional risk factors? | Assessment of outcome? | Was follow-up long enough for outcome to occur? | Adequacy of follow-up of cohorts? | |
| Aksoy et al. [9] | √ | √ | √ | – | √ | √ | – | √ | √ |
| Bannay et al. [10] | √ | √ | √ | – | √ | √ | √ | √ | √ |
| Cabell et al. [11] | – | √ | √ | √ | √ | √ | – | – | √ |
| Funakoshi et al. [12] | – | √ | √ | – | √ | √ | – | √ | √ |
| Hill et al. [13] | – | √ | √ | – | √ | √ | √ | – | √ |
| Lalani et al. (2010) [2] | √ | √ | √ | √ | √ | √ | √ | – | √ |
| Lalani et al. (2013) [7] | √ | √ | √ | √ | √ | √ | √ | – | √ |
| Mourvillier et al. [14] | – | √ | √ | – | √ | √ | – | – | √ |
| Ohara et al. [15] | √ | √ | √ | – | √ | √ | – | – | √ |
| Sy et al. [16] | – | √ | √ | – | √ | √ | – | √ | √ |
| Thuny et al. [17] | √ | √ | √ | – | √ | √ | √ | – | √ |
| Wang et al. [20] | √ | √ | √ | – | √ | √ | √ | – | √ |
| Wang et al. [21] | – | √ | √ | – | √ | √ | √ | – | – |
| Jia et al. [22] | – | √ | √ | – | √ | √ | – | – | – |
| Vikram et al. [19] | √ | √ | √ | √ | √ | √ | √ | – | √ |
| Tleyjeh et al. [18] | – | √ | √ | – | √ | √ | – | – | √ |
Meta-analysis
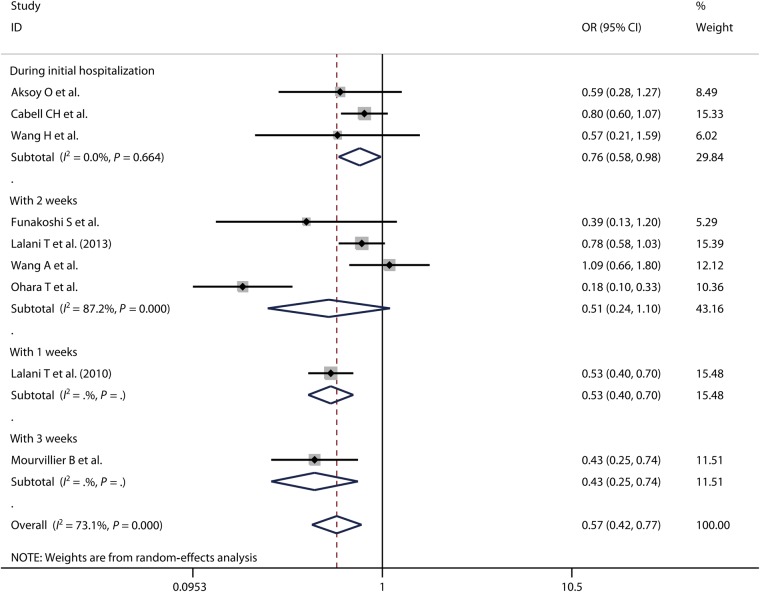
There were 9 [2, 7, 9, 11, 12, 14, 15, 20, 21] and 11 [7, 9, 10, 12, 13, 16–19, 21, 22] studies which reported in-hospital and long-term mortality, respectively. Because significant heterogeneity existed among these studies (P = 0.000, I2 = 73.1%; P = 0.001, I2 = 67.4%), the random-effects model was used. According to our meta-analysis, both in-hospital mortality [OR = 0.57, 95% CI (0.42, 0.77)] and long-term mortality [OR = 0.57, 95% CI (0.43, 0.77)] were significantly lower for the early surgery group than for the non-early surgery group (Figs 2 and 3).
Figure 2:
In-hospital mortality in patients with IE, comparing early surgery versus non-early surgery, including subgroup analysis for different operation time periods. IE: infective endocarditis; OR: odds ratio; CI: confidence interval.
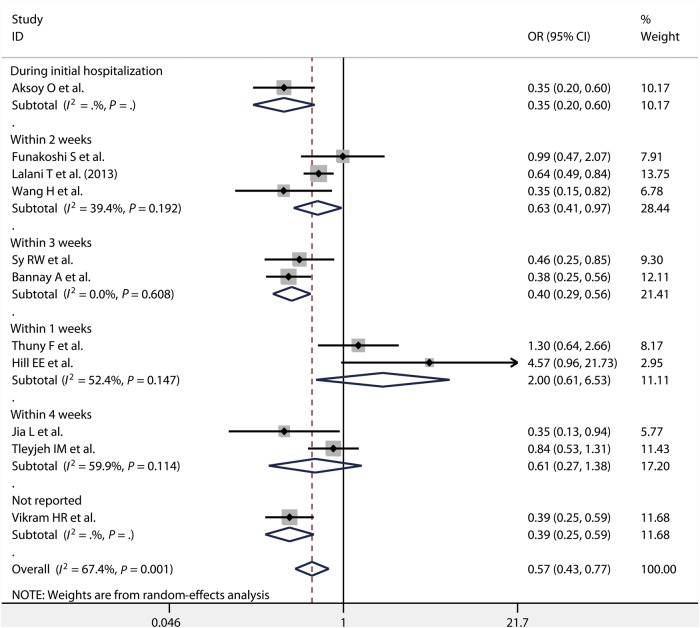
Figure 3:
Long-term mortality in patients with IE, comparing early surgery versus non-early surgery, including subgroup analysis for different operation time periods. IE: infective endocarditis; OR: odds ratio; CI: confidence interval.
For different time periods in which surgeries were performed, we observed in-hospital mortality was significantly lower for operation performed during initial hospitalization [OR = 0.76, 95% CI (0.58, 0.98); P = 0.664, I2 = 0.0%], within 1 week [OR = 0.53, 95% CI (0.40, 0.70)] and within 3 weeks [OR = 0.43, 95% CI (0.25, 0.74)] compared with for non-early surgery. In-hospital mortality did not differ significantly between operation performed within 2 weeks [OR = 0.51, 95% CI (0.24, 1.10); P = 0.000, I2 = 87.2%] and non-early surgery (Fig. 2). Performing operation during the initial hospitalization, within 2 weeks [OR = 0.63, 95% CI (0.41, 0.97); P = 0.192, I2 = 39.4%] and within 3 weeks [OR = 0.40, 95% CI (0.29, 0.56); P = 0.608, I2 = 0.0%] can significantly prolong the patients' long-term survival time compared with the non-early surgery group. There was no difference on long-term mortality between surgeries performed within 1 week [OR = 2.00, 95% CI (0.61, 6.53); P = 0.147, I2 = 52.4%] and 4 weeks [OR = 0.61, 95% CI (0.27, 1.38); P = 0.114, I2 = 59.9%] compared with the non-surgery group (Fig. 3).
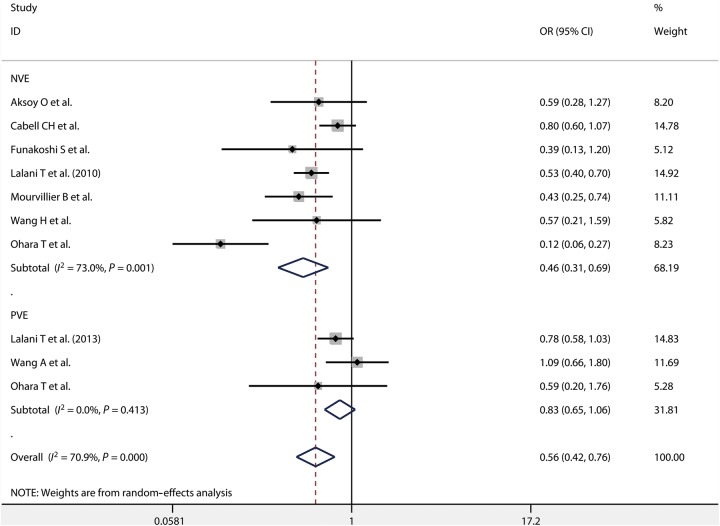
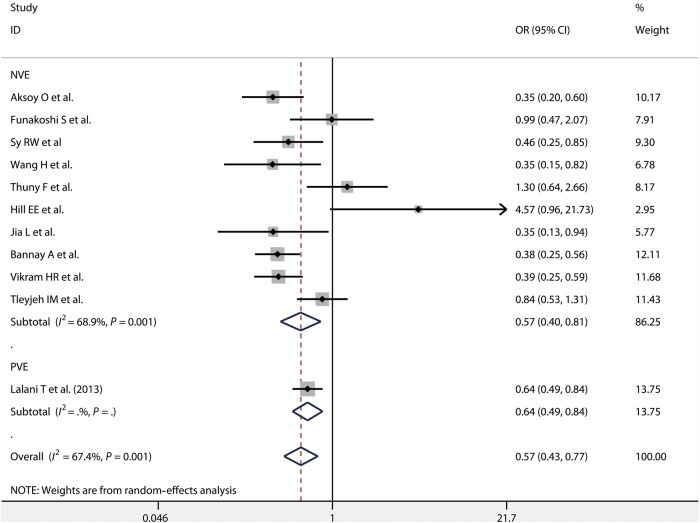
In-hospital mortality was reported for patients with NVE and PVE by 7 [2, 9, 11, 12, 14, 15, 21] and 3 [7, 15, 20] studies, respectively. We found that early surgery for NVE had a lower in-hospital mortality than the non-early surgery group [OR = 0.46, 95% CI (0.31, 0.69); P = 0.001, I2 = 73.0%], whereas no difference was found between early surgery and non-early surgery group for PVE [OR = 0.83, 95% CI (0.65, 1.06); P = 0.413, I2 = 0.0%] (Fig. 4). Ten studies [9, 10, 12, 13, 16–19, 21, 22] provided information on long-term mortality of patients who underwent early surgery for NVE. We observed long-term mortality was significantly lower for early surgery than for non-early surgery for NVE [OR = 0.57, 95% CI (0.40, 0.81); P = 0.001, I2 = 68.9%]. Among the reported results, only one study [7] showed that long-term mortality was significantly lower for early surgery than for non-early surgery for PVE [OR = 0.64, 95% CI (0.49, 0.84)] (Fig. 5).
Figure 4:
In-hospital mortality in patients with NVE and PVE. NVE: native valve endocarditis; PVE: prosthetic valve endocarditis.
Figure 5:
Long-term mortality in patients with NVE and PVE. NVE: native valve endocarditis; PVE: prosthetic valve endocarditis; OR: odds ratio; CI: confidence interval.
Publication bias
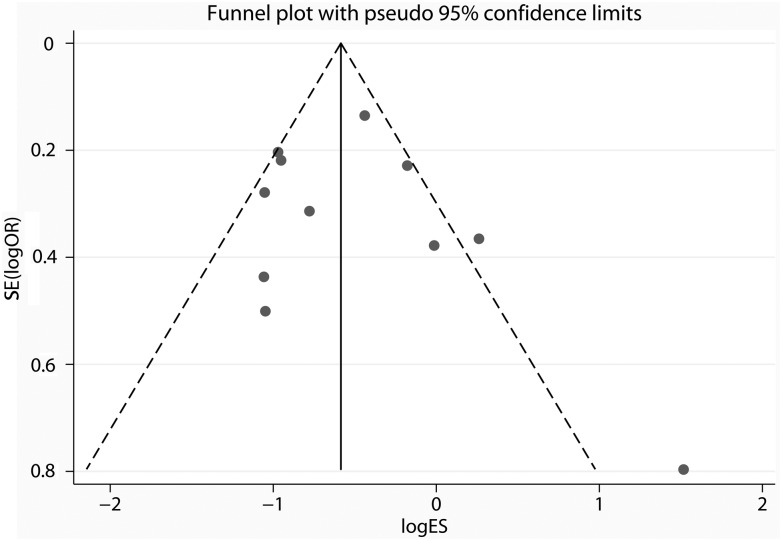
Inspection of the funnel plots indicated that the studies were nearly similarly distributed on both sides of the vertical line (Fig. 6), which reveals no evidence of the existence of significant publication bias among these studies. Besides, the P-value for Egger's test was 1.40, which further suggests a low probability of publication bias.
Figure 6:
Funnel plots of included studies.
Sensitivity analysis
To confirm reliability of the pooled estimates, we performed a sensitivity analysis by eliminating one study in each turn and the result was consistent with the primary outcome, manifesting the stability of the meta-analysis (Supplementary Figs S1 and S2).
DISCUSSION
Summary of findings
It is well known that IE has poor prognosis, and mortality is the most intuitive and important outcome reflecting the treatment effect [5]. In terms of short-term efficacy, our study found that early surgery in patients with IE had significantly lower in-hospital mortality [OR = 0.57, 95% CI (0.42, 0.77); P = 0.000, I2 = 73.1%] than non-early surgery. A small RCT, which was conducted by Kang et al. [8], reported a 3% in-hospital mortality in patients treated by early surgery, which was significantly lower than the 23% observed in the drug therapy group patients. In fact, such a difference was statistically significant [hazard ratio = 0.10, 95% CI (0.01, 0.82); P = 0.03], which is consistent with our finding. Regarding the long-term efficacy, our study showed that patients in the early surgery group survived longer than those in the non-early surgery group [OR = 0.57, 95% CI (0.43, 0.77); P = 0.001, I2 = 67.4%]. Propensity analysis was used for most of the included studies to control for the intrinsic biases of treatment selection and adjust the baseline prognostic heterogeneity. Some studies [19, 28, 29] have demonstrated that when these intrinsic biases are rigorously controlled, the observational studies can arrive at estimates of the impacts of therapeutic interventions which are actually quite similar to RCTs. Chatterjee et al. [30] conducted a meta-analysis with data from only propensity-matched patients and their findings are consistent with ours [OR 0.41, 95% CI (0.20, 0.83); P = 0.01, I2 = 0%].
Although short- and long-term benefits have been found in this meta-analysis, it does not mean that early surgery is beneficial and must be performed in all patients with IE. The two most influential sets of consensus guidelines [5, 6] for the performance of early surgery on the basis of surgical indication are basically identical. The revised 2009 European Society of Cardiology guideline [5] recommend heart failure, uncontrolled infection and prevention of embolism as main indications for early surgery, whereas the 2014 American College of Cardiology–American Heart Association guidelines [6] recommend valve dysfunction causing heart failure, antibiotic resistant organism, heart block or abscess, persistent infection as important indication for early surgery. Indeed, the predictors revealed in most of the included studies are established as well-recognized indications for early surgery and reflect practices in concert with the guidelines. Accordingly, except for patients with an obvious indication, deciding whether to perform surgery is usually a difficult and complicated clinical decision.
The optimal timing of valve surgery in patients with IE still remains unclear. In fact, the definition of early surgery varied among the included studies, from ‘operation during initial hospitalization’ to ‘operation within 30 days after diagnosis of IE.’ Our study showed that an operation performed within a week after diagnosis of IE could reduce the patients' in-hospital mortality, but could not improve long-term survival. These results are consistent with those of Kang et al. [8], whose study enrolled patients who underwent valve surgery within 48 h after randomization and found that early surgery could significantly reduce in-hospital mortality, but it did not reduce 6-month mortality. Additionally, performing surgery within 2 weeks of diagnosis could prolong the long-time survival time of patients and tended to reduce in-hospital mortality, which makes this choice seemingly worth considering. Due to lack of studies including operations in the 3- and 4-week periods, we could not determine whether performing operation at such time periods can improve the prognosis of the patients. However, a dilemma always exists as to when to perform surgery: should we operate early to reduce the risk of thrombosis and progressive deterioration of cardiac function or should we perform the surgery after the effective control of infection to reduce the surgical risks and complications. On the one hand, early surgical intervention in the acute phase of IE, involving uncontrolled sepsis, shock and organ failure, causes concerns regarding high operative mortality and risk of deterioration. On the other hand, delaying surgery to finish a course of antimicrobial therapy might raise the risk of embolism and produce widespread cardiac tissue damage, which would result in more challenging repair, progressive cardiogenic shock and organ failure and, ultimately, increased mortality [10, 12]. Furthermore, due to different surgical indications, such as vegetation size, embolic events, expansion of infection, relapsing IE, the timing of surgery should be dependent on the specific condition of the patient. In summary, our study could not draw definitive conclusions on the optimal timing of surgery for IE patients.
Compared with NVE, PVE is a more serious disease with a higher mortality, which ranges from 20 to 40% of in-hospital mortality rate [5]. Surgical indications for PVE recommendation by guidelines [5, 6] are similar to those for NVE. Moreover, surgical intervention is generally considered the best option for PVE. Our results indicated that early surgery could not provide a definite short-term survival benefit for PVE, when compared with NVE. However, a positive effect of early surgery for PVE was found in long-term mortality, albeit there was only one study in the analysed results. One reason may be that patients with PVE were older and more often affected by resistant microorganisms and health care-associated infections, and complications with paravalvular and chronic illness [7]. Other possible reasons are the small number of studies, small sample size and baseline heterogeneity. To date, no randomized studies of early surgery for PVE have been reported. Further study is needed to confirm the efficacy of early surgery in PVE.
Currently, there is an ongoing, large sample-size, multicentre RCT, namely ENDOVAL 1 [31], which is designed to compare 30-day mortality between early surgery and drug therapy, incidence of complications and other important outcomes for IE, which should provide us more reliable evidence on the efficacy and safety of early surgical intervention in IE.
Strength and limitations
Our study has several strengths. Our meta-analysis is the first one to perform a systematic and detailed evaluation of early surgery in patients with IE. This meta-analysis included 16 cohort studies with a total of 8141 participants addressing whether and when the performed surgery could provide short- and long- term benefits for patients with IE. We also performed subgroup analysis to distinguish the between the effects of early surgery in patients with NVE or PVE. Furthermore, all the included studies were of moderate or high quality (average score above 6) according to the Newcastle–Ottawa Scale. Some researchers [32] have reported that meta-analysis of well-designed non-RCTs of surgical procedures is probably as accurate as that of RCTs. Nevertheless, our study still has several limitations. Firstly, a common limitation encountered in all meta-analyses is the heterogeneity of the data. Although the meta-analysis possibly provides the best methodology, it is usually limited by clinical heterogeneity. The heterogeneity may be because of the diverse patient populations, surgical techniques, surgeons with unequal skills, operative durations and follow-up time. However, the amount of information was not sufficient for stratifying or regression analysis. Secondly, the studies included in the meta-analysis were all non-randomized, cohort studies. Although most of the included studies used propensity analyses to control for bias in treatment selection, some other intrinsic biases, which may influence the interpretation of their results, still exist. Thirdly, due to limited number of included studies and published data, we could not conduct analysis of other important outcomes, such as recurrence rate and heart failure rate. Besides, as we just searched English and Chinese medical databases, this means that papers published in another language were not likely to be found.
CONCLUSION
In conclusion, we found that early surgery was associated with lower in-hospital and long-term mortality compared with non-early surgery therapy in patients with IE, especially for NVE. However, the optimal timing of surgery is still unclear. Additional larger prospective clinical trials will be required to clarify the optimal timing for surgical intervention and identify the efficacy of surgical intervention for PVE.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at ICVTS online.
Conflict of interest: none declared.
APPENDIX. CONFERERNCE DISCUSSION
Dr M. Wyler Von Ballmoos (Milwaukee, WI, USA): Dr Song and his team have sought to address the important controversial question as to whether early or late timing of surgery is more beneficial in patients with infective endocarditis. They include 16 studies with over 8000 patients in a meta-analysis and suggest that patients have significantly reduced odds of in-hospital mortality and long-term mortality with an early surgery strategy, defined as surgery occurring prior to conclusion of a full therapeutic course of antibiotics, and that these effects are more pronounced in native valve endocarditis patients compared with prosthetic valve endocarditis patients.
The authors therefore conclude that early surgery is the preferred treatment modality for patients with infective endocarditis. Of note is that only two studies included in your meta-analysis actually compared early versus late surgery, while all other studies compared early surgery versus medical management only. The study is therefore probably more reflective of the controversy between going to the OR or just using antibiotics for treatment. Also, only three studies looked at in-hospital mortality and only one study at the long-term mortality in patients with prosthetic valve endocarditis, somewhat limiting the conclusion we can draw, I think, from the pooled effect estimates.
I do have two questions for you and a comment that I will save for last.
Firstly, did you look at the indications for surgery in the individual studies, if they differed between the different studies and whether they were equally distributed in both treatment arms?
Dr Song: Yes, that is a good question. This table shows the surgical indications of all included studies. We found that heart failure, antibiotic-resistant organisms and large vegetations, and the last one is persistent infection, will appear in almost all these 16 studies. We used these indications to perform the comparisons. So we think the indications were equally present in both groups of the comparison.
Dr Wyler Von Ballmoos: My second question would be: There was a large degree of heterogeneity both qualitatively and quantitatively in your meta-analysis. How do you explain this and how would that change the interpretation of your results?
Dr Song: As you said, a lot of degree of heterogeneity was found in our studies, both the clinical and the statistical heterogeneity. In our study, because we have rigorous inclusion criteria and a high quality of assessment, so we don't think it is from clinical heterogeneity. And to assess the statistical heterogeneity, we did subgroup analysis based on the different surgical time and different patients; but, however, the heterogeneity still exists. I think someone argued that since clinical and methodological diversity always occur in a meta-analysis the statistical heterogeneity is inevitable.
Dr Wyler Von Ballmoos: As far as my comment is concerned I'll just give you my two cents on what problems you probably ran into. I think conducting the meta-analysis and you really ran into three problems probably: For one, that of non-positivity because only two of your studies really looked at early surgery versus late surgery. Furthermore, I think most of these studies used propensity score matching, which, selected individuals in the early surgery group that at baseline would be predicted to have very similar outcomes of those that only had medical treatment.
Secondly, I think the differences in the treatment arms may not have been equalized completely between the groups as one might like to think even after propensity score matching.
Thirdly, and most importantly, I think, you probably run into time-to-treatment bias problems with your study and the underlying studies you used. Only patients that survived the early treatment phase were eligible to go into the surgery arms in these studies and were therefore selected. These are obviously patients that are less sick than those who didn't make it and would therefore be expected to have better outcomes.
Dr M. Mokhles (Rotterdam, Netherlands): I have only a short question. Your study period covered almost 30 years and there have been, of course, some improvements in the management of patients with infective endocarditis. So did you perform a sensitivity analysis to explore whether there is a time effect?
Dr Song: I am unable to answer your question.
Dr Mokhles: I will repeat the question, the studies that you have included in your meta-analysis, some of them were published in the 1980s. Was there a time effect? Did you perform a sensitivity analysis?
Dr Song: Our other articles are from inception to January 2015.
REFERENCES
- 1.Hoen B, Alla F, Selton-Suty C, Beguinot I, Bouvet A, Briancon S et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 2002;288:75–81. [DOI] [PubMed] [Google Scholar]
- 2.Lalani T, Cabell CH, Benjamin DK, Lasca O, Naber C, Fowler VG Jr et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010;121:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001;345:1318–30. [DOI] [PubMed] [Google Scholar]
- 4.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394–434. [DOI] [PubMed] [Google Scholar]
- 5.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009;30:2369–413. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–88. [DOI] [PubMed] [Google Scholar]
- 7.Lalani T, Chu VH, Park LP, Cecchi E, Corey GR, Durante-Mangoni E et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013;173:1495–504. [DOI] [PubMed] [Google Scholar]
- 8.Kang DH, Kim YJ, Kim SH, Sun BJ, Kim DH, Yun SC et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012;366:2466–73. [DOI] [PubMed] [Google Scholar]
- 9.Aksoy O, Sexton DJ, Wang A, Pappas PA, Kourany W, Chu V et al. Early surgery in patients with infective endocarditis: a propensity score analysis. Clin Infect Dis 2007;44:364–72. [DOI] [PubMed] [Google Scholar]
- 10.Bannay A, Hoen B, Duval X, Obadia JF, Selton-Suty C, Le Moing V et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: do differences in methodological approaches explain previous conflicting results? Eur Heart J 2011;32:2003–15. [DOI] [PubMed] [Google Scholar]
- 11.Cabell CH, Abrutyn E, Fowler VG Jr., Hoen B, Miro JM, Corey GR et al. Use of surgery in patients with native valve infective endocarditis: results from the International Collaboration on Endocarditis Merged Database. Am Heart J 2005;150:1092–8. [DOI] [PubMed] [Google Scholar]
- 12.Funakoshi S, Kaji S, Yamamuro A, Tani T, Kinoshita M, Okada Y et al. Impact of early surgery in the active phase on long-term outcomes in left-sided native valve infective endocarditis. J Thorac Cardiovasc Surg 2011;142:836–842 e831. [DOI] [PubMed] [Google Scholar]
- 13.Hill EE, Herregods MC, Vanderschueren S, Claus P, Peetermans WE, Herijgers P. Outcome of patients requiring valve surgery during active infective endocarditis. Ann Thorac Surg 2008;85:1564–9. [DOI] [PubMed] [Google Scholar]
- 14.Mourvillier B, Trouillet JL, Timsit JF, Baudot J, Chastre J, Regnier B et al. Infective endocarditis in the intensive care unit: clinical spectrum and prognostic factors in 228 consecutive patients. Intensive Care Med 2004;30:2046–52. [DOI] [PubMed] [Google Scholar]
- 15.Ohara T, Nakatani S, Kokubo Y, Yamamoto H, Mitsutake K, Hanai S. Clinical predictors of in-hospital death and early surgery for infective endocarditis: results of CArdiac Disease REgistration (CADRE), a nation-wide survey in Japan. Int J Cardiol 2013;167:2688–94. [DOI] [PubMed] [Google Scholar]
- 16.Sy RW, Bannon PG, Bayfield MS, Brown C, Kritharides L. Survivor treatment selection bias and outcomes research: a case study of surgery in infective endocarditis. Circ Cardiovasc Qual Outcomes 2009;2:469–74. [DOI] [PubMed] [Google Scholar]
- 17.Thuny F, Beurtheret S, Mancini J, Gariboldi V, Casalta JP, Riberi A et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J 2011;32:2027–33. [DOI] [PubMed] [Google Scholar]
- 18.Tleyjeh IM, Ghomrawi HM, Steckelberg JM, Hoskin TL, Mirzoyev Z, Anavekar NS et al. The impact of valve surgery on 6-month mortality in left-sided infective endocarditis. Circulation 2007;115:1721–8. [DOI] [PubMed] [Google Scholar]
- 19.Vikram HR, Buenconsejo J, Hasbun R, Quagliarello VJ. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. JAMA 2003;290:3207–14. [DOI] [PubMed] [Google Scholar]
- 20.Wang A, Pappas P, Anstrom KJ, Abrutyn E, Fowler VG Jr., Hoen B et al. The use and effect of surgical therapy for prosthetic valve infective endocarditis: a propensity analysis of a multicenter, international cohort. Am Heart J 2005;150:1086–91. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Zhang S, Tian Z, Guo L. [The impact of early surgery on long-term outcome of patients with left-sided infective endocarditis]. Zhonghua Nei Ke Za Zhi 2014;53:450–4. [PubMed] [Google Scholar]
- 22.Jia L, Fu Q, Yang S, Liang D, Lv X, Wei M. Clinical analysis of early surgical operation in infective endocarditis. Chin J Thorac Cardiovasc Surg 2012;28:464–6. [Google Scholar]
- 23.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr., Ryan T et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 26.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000;342:1878–86. [DOI] [PubMed] [Google Scholar]
- 29.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S, Sardar P. Early surgery reduces mortality in patients with infective endocarditis: insight from a meta-analysis. Int J Cardiol 2013;168:3094–7. [DOI] [PubMed] [Google Scholar]
- 31.San Roman JA, Lopez J, Revilla A, Vilacosta I, Tornos P, Almirante B et al. Rationale, design, and methods for the early surgery in infective endocarditis study (ENDOVAL 1): a multicenter, prospective, randomized trial comparing the state-of-the-art therapeutic strategy versus early surgery strategy in infective endocarditis. Am Heart J 2008;156:431–6. [DOI] [PubMed] [Google Scholar]
- 32.Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol 2010;63:238–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.