Abstract
Objectives
This study was carried out in order to understand the behavior of dengue viruses through the entomological and laboratory surveillance of outbreaks. The aim of the study was to provide additional research to support current knowledge of epidemiological, clinical, and laboratory diagnosis of dengue virus and ultimately to use this information to forecast dengue as well as to justify intervention measures.
Methods
Data on the presence of Aedes larvae in human dwellings during the entomological surveillance in Cuddalore, Nagapattinam, and Tirunelveli dengue outbreaks were taken to compute indices, namely the House Index (HI), Container index (CI), and the Breteau Index (BI). Standard procedures were followed for nonstructural Protein 1 (NS1) and immunoglobulin M enzyme linked immunosorbent assay for the confirmation of dengue. Serovar confirmation was made in the Kottayam field station of the Vector Control Research Center, Puducherry.
Results
Larval indices HI < 2–3% and BI < 20 contributed to halting the outbreak. Incubation of the dengue viruses in humans was detected at 15 days, NS1 was identified as a tool for the early diagnosis of dengue cases and its presence indicated the need to implement all available interventions. It was also discovered that it is helpful to search for hidden habitats of Aedes when dengue cases have not been reduced even after the sustainable management of the larval indices, HI < 5% and BI < 20. Based on the observed incidences of stopping dengue outbreaks, it was learnt that neighborhood areas of the outbreak villages, around 400 m, should have permissible larval indices < 5% HI and BI < 20. Heterogeneous serovars that led to dengue hemorrhagic fever and Dengue Shock Syndrome (DSS) were identified using reverse transcription polymerase chain reaction and reconfirmed in the field as DEN-1 and DEN-3 viruses and were circulating in Tirunelveli during the outbreak.
Conclusion
The behaviors of dengue viruses experienced in experimental, clinical, epidemiological, entomological, and laboratory surveillance did not deviate from observations in the field during dengue outbreaks in the Cuddalore, Nagapattinam, and Tirunelveli districts of Tamil Nadu, India.
Keywords: behavior of dengue virus, heterogeneous serovar, nonstructural NS1 antigen, reverse transcription polymerase chain reaction
1. Introduction
Since dengue is not currently a vaccine preventable communicable disease, vector control remains the only way to prevent dengue transmission 1, 2, 3.Vector control programs are essentially based on source reduction, eliminating Aedes aegypti larval habitats from the domestic environment with increasing community involvement and inter sectoral action in recent decades 4, 5. Various efforts have been made to detect this disease early to determine transmission risk, define thresholds for dengue epidemic alerts, or set targets for vector control programs 6, 7, 8. Among larval indices, the House Index (HI, percentage of houses positive with Aedes larvae) and the Breteau Index (BI, number of positive containers per hundred houses) have been the most widely used indices 9, 10. Since HI < 1% or BI < 5 were proposed to prevent yellow fever transmission, these values have also been applied to dengue transmission but without much evidence whereas the Pan American Health Organization described three levels of risk for dengue transmission: low (HI ≤ 1%), medium (HI = 0.1–5%), and high (HI > 5%) 11, 12. Pertaining to the determination of the critical threshold levels of HI and BI, it is known that these values were obtained in a specific location and are not applicable to all locations, hence these values need to be verified [13]. The vector density, below which dengue transmission does not occur, continues to be a topic of much debate and conflicting empirical evidence. For example, dengue outbreaks occurred in Singapore when the national overall HI was < 1% [14]. By contrast, researchers from Fortaleza, Brazil found that dengue outbreaks never occurred, when HI was < 1% [15]. However, different geographic levels are used to calculate the indices in various studies and the appropriated level for entomological indices is in itself an issue of debate [16]. Lizet Sanchez et al [17] have attempted to incorporate larval indices for identifying high risk areas for dengue virus transmission. From their study, it was found that the influence of measurements at different geographical levels can establish a threshold for epidemic outbreaks and provide a discussion point for determining their utility for community based Aedes control programs.
Since no protective vaccine or specific treatments are available for dengue fever, accurate diagnosis is critical for the early initiation of specific preventative health measures to curtail epidemic spread and reduce economic losses as well as the timely monitoring of patients to prevent fatalities. Commonly used diagnostic methods for confirming dengue infection involve virus isolation, detection of virus antigens or RNA, and the presence of dengue virus-specific antibodies [18]. Among diagnostic tools available for dengue diagnosis are nonstructural Protein 1 (NS1) and immunoglobulin (Ig)M and IgG enzyme-linked immunosorbent assay (ELISA). NS1 uses the nonstructural protein of the virus and dengue virus specific antibodies respectively. When these tools undertake the detection of dengue infection, it is known that the IgM antibody can be detected by Day 5 of illness, and 93–99% of cases have detectable IgM by Days 6–10 of illness, which may then remain detectable for > 90 days [19]. The NS1 based assays provide a simplified method of diagnosis during the acute stage of dengue infection compared to viral isolation or nucleic acid detection (the detection of viral antigens in the blood stream) [18], its precision in diagnosis of dengue has been enhanced and it may be used for detection in patients in the febrile phase. Other than NS1, acute infection with dengue virus can be confirmed when the virus is isolated from serum or the specific dengue virus genome is identified by reverse transcription polymerase reaction (RT-PCR) from serum or plasma. This diagnostic tool has been widely used to identify more than one serovar of dengue virus circulating in the community and it has been shown to directly elucidate the degree of the transmission of dengue hemorrhagic fever (DHF) from dengue fever (DF) 20, 21.
Even though efforts have been made to stop dengue outbreaks in different parts of the world and particularly in tropical countries such as India and South East Asian countries, with reference to the significance of entomological surveillance, current literature study findings agreeing on the behavior of dengue viruses during outbreak situations is scarce. Hence, the present study was undertaken to reaffirm the facts derived from existing experimental and entomological laboratory surveillances. The current research on the control and prevention of dengue during outbreaks in the field, with reference to entomological and laboratory surveillance alone is unique to this study.
2. Materials and methods
Data on the presence of Aedes larvae in human dwellings in villages and urban areas of, Tirunelveli (Latitude 8°42′ N; Longitude 77°42′ E), Nagapattinam (Latitude 10°46′ N; Longitude 79°50′ E), and Cuddalore (Latitude 11°75′ N; Longitude 79°75′ E) were collected when dengue outbreaks occurred in May 2012, September 2012, and August 2012 to January 2013, in these respective districts. These data were used to compute various indices, namely: the HI, Container index (CI, percentage of wet containers positive for larvae), and the BI. To obtain these data, active searches for Aedes larvae were made by the domestic breeding checkers (DBCs) temporarily placed by the government of Tamil Nadu with political commitment, by going house to house. There were 10 DBCs in each district block and thus, in the current study, 120 DBCs, 130 DBCs, and 110 DBCs were employed in the above districts respectively. Prior to this assignment, full hands on training was given to all DBCs so as to be able to recognize Aedes larvae. Training included identification of wriggling movements in stored water, which is a bottom dwelling property of these larvae and could be seen only with a torch light. A dosage of 2 mL of larvicide temephos, an organophosphorus compound at a 50% emulsifier concentration in 1 L of water was used in the field as 1 mL per 1 L of stored water in wet containers (containers that were not able to drain in a dwelling place etc.). The same intervention was undertaken in neighboring areas of the outbreak villages and in parallel municipal areas to avoid epidemic spread from the affected places.
Standard procedures were followed for NS1 and IgM ELISA for the confirmation of dengue. NS1 kits supplied by Pan Bio (Inverness Medical Innovations, Australia, Pty Ltd., Brisbane, Queensland, Australia) and IgM ELISA kits supplied by the National Institute of Virology, Government of India, were used in this study at the sentinel hospitals with laboratory facilities in Tirunelveli, Nagapattinam, the laboratory of the Zonal Entomological Team (ZET), Cuddalore, and in the Public Health Laboratory, Cuddalore.
Serovars of dengue: DEN-1, DEN-2, DEN-3, and DEN-4, confirmations were made with RT-PCR following the standard procedure of the Vector Control Research Center field station, Kottayam, India, and the premier institute of the Indian Council of Medical Research in New Delhi, India. Statistical analysis for this study was carried out using SPSS version 20 (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was taken to be significant.
3. Results
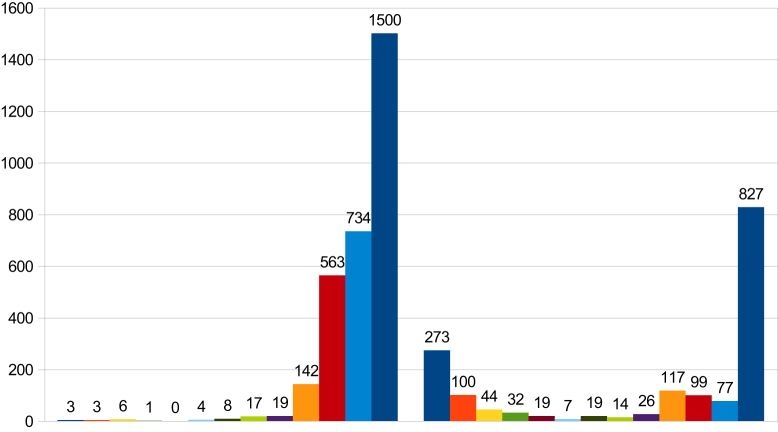
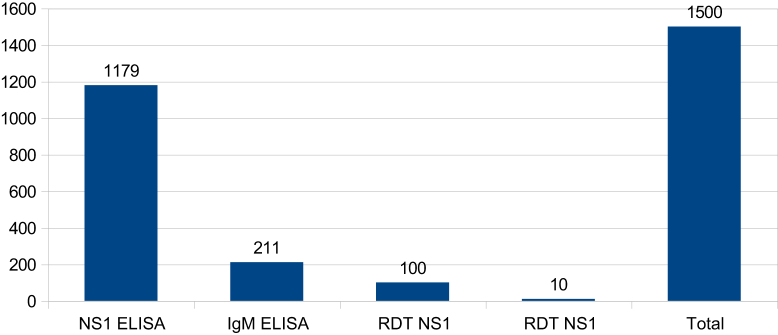
In 2012 there were 1500 dengue cases reported in the Cuddalore district. Among them, 1179 dengue cases were confirmed by NS1 ELISA; 211 cases were confirmed by IgM ELISA; 100 cases were confirmed by the NS1 rapid diagnostic test (RDT); and 10 dengue cases were confirmed by IgM RDT (Table 1, Table 2; Figure 1, Figure 2). These dengue cases were distributed amongst 13 blocks and five areas in urban municipalities. In 2013 the number of cases of dengue was considerably reduced to 827 cases which were confirmed from all these diagnostic tools (Figure 1). To reduce cases in all affected villages and to halt some outbreaks in the district, various interventions had been used with the help of entomological and laboratory surveillance. In order to implement interventions early in a village, fever information had been accumulated every day from different resources, namely the passive surveillance (institutional surveillance) in primary health centers (PHCs), sentinel surveillance hospitals, government hospitals, and medical college hospitals, information from weekly morbidity reports of the Integrated Disease Surveillance Project, malaria fortnightly report (MF9) in PHCs, rumors and news flashes in the media (television, newspapers etc.). Based on this information interventions were implemented as a priority when even one fever case was confirmed as dengue in a village. Simultaneously, any villages that had reported cases in previous years and villages neighboring those experiencing fever outbreaks were also included in the intervention.
Table 1.
The number of dengue cases reported in the Cuddalore district by month in 2012 and 2013.
| Month | 2012 | 2013 | |
|---|---|---|---|
| 1 | Jan | 3 | 273 |
| 2 | Feb | 3 | 100 |
| 3 | Mar | 6 | 44 |
| 4 | Apr | 1 | 32 |
| 5 | May | 0 | 19 |
| 6 | Jun | 4 | 7 |
| 7 | Jul | 8 | 19 |
| 8 | Aug | 17 | 14 |
| 9 | Sep | 19 | 26 |
| 10 | Oct | 142 | 117 |
| 11 | Nov | 563 | 99 |
| 12 | Dec | 734 | 77 |
| Total | 1500 | 827 |
Table 2.
The number of positive cases and the various diagnostic tools used to predict them.
| Diagnostic tools | No. of cases |
|---|---|
| NS1 ELISA | 1179 |
| IgM ELISA | 211 |
| RDT NS1 | 100 |
| RDT NS1 | 10 |
| Total | 1500 |
ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin; NS1 = nonstructural Protein 1; RDT = rapid diagnostic test.
Figure 1.
Dengue cases reported in the Cuddalore district by month for the years 2012 and 2013.
Figure 2.
Diagnostic tools used to confirm dengue cases in the Cuddalore district in 2013. ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin; NS1 = nonstructural Protein 1; RDT = rapid diagnostic test.
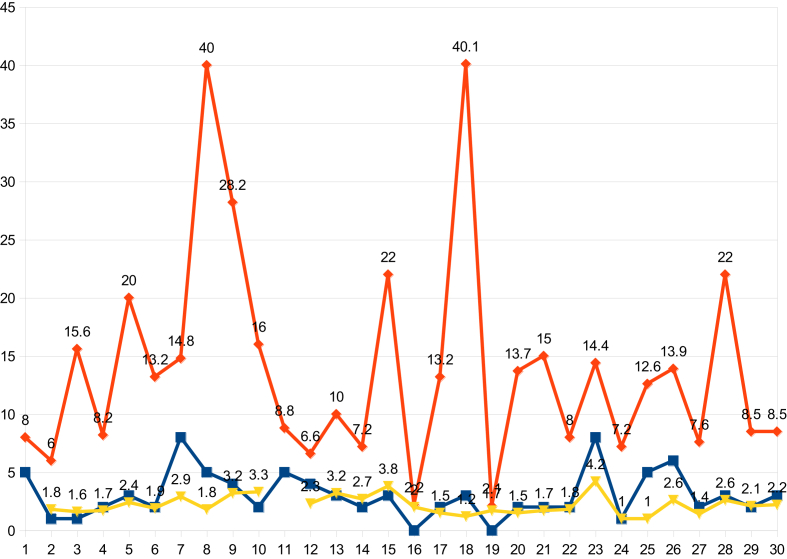
For the present study, selective villages and urban areas of the Cuddalore district had been taken to analyze the above strategy and data collected during the course of outbreaks were depicted in Table 3. Scrutinizing the HI before and after its course from outbreak to the halting of the disease, it was observed through entomological surveillance that the mean HI from 30 outbreaks was 15.3% before the outbreak whereas it was reduced to 2.2% once the intervention was implemented. The associative CI was reduced from 2.5% to 1.7% and BI was reduced from 16.4 to 2.4 (Figure 3).
Table 3.
Aedes larval density monitoring in primary health centers (PHCs) and the Cuddalore Municipality during the dengue outbreak in the Cuddalore district in 2013.
| Name of the block | Name of the PHC | Name of the village | Period of the fever outbreak | No. of dengue positives | Initial House Index (HI) | Container Index (CI) | Breteau Index (BI) if applicable as 100 houses searched | HI | CI | BI | Time taken to halt the outbreak in d | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Annagiramam | Melpattampakkam | Keezharungunam | 01 Aug 2013 to 31 Dec 2013 | 5 | 8 | 1.6 | 10 | 2.1 | 0.49 | 2.82 | 13 |
| 2 | Cuddalore | Naduveerapattu | Nariankuppam | 01 Aug 2013 to 31 Dec 2013 | 1 | 6 | 1.2 | 8 | 1.8 | 0.5 | 1.8 | 12 |
| 3 | Cuddalore | Karaikadu | Manakuppam | 01 Aug 2013 to 31 Dec 2013 | 1 | 15.6 | 3.12 | 17.6 | 1.6 | 0.7 | 1.6 | 11 |
| 4 | Cuddalore | Karaikadu | Sangolikuppam | 01 Aug 2013 to 31 Dec 2013 | 2 | 8.2 | 1.64 | 10.2 | 1.7 | 0.7 | 1.7 | 14 |
| 5 | Cuddalore | Madalapattu | Periyakattupalayam | 01 Aug 2013 to 31 Dec 2013 | 3 | 20 | 4 | 22 | 2.4 | 1.2 | 2.4 | 15 |
| 6 | Cuddalore | Madalapattu | Tazhanguda | 01 Aug 2013 to 31 Dec 2013 | 2 | 13.2 | 2.64 | 15.2 | 1.9 | 0.8 | 1.9 | 14 |
| 7 | Cuddalore | Vellakarai | Vellakarai | 01 Aug 2013 to 31 Dec 2013 | 8 | 14.8 | 2.8 | 16.8 | 2.9 | 1.3 | 3.9 | 10 |
| 8 | Panruti | Kadampuliyur | Malingampattu | 01 Aug 2013 to 31 Dec 2013 | 5 | 40 | 5.88 | 42 | 1.8 | 0.4 | 1.8 | 15 |
| 9 | Panruti | Kadampuliyur | South Melmampattu | 01 Aug 2013 to 31 Dec 2013 | 4 | 28.2 | 3.81 | 30.2 | 3.2 | 1.1 | 3.2 | 13 |
| 10 | Vadalur | Venpettai | Vadakutthu | 01 Aug 2013 to 31 Dec 2013 | 2 | 16 | 3.03 | 18 | 3.3 | 1.5 | 3.3 | 16 |
| 11 | Parangipettai | Gavarapattu | Kovilampoondi | 01 Aug 2013 to 31 Dec 2013 | 5 | 8.8 | 1.62 | 10.8 | 2.5 | 1.5 | 2.5 | 12 |
| 12 | Parangipettai | Killai | Ponnanthittu | 01 Aug 2013 to 31 Dec 2013 | 4 | 6.6 | 1.25 | 8.6 | 2.3 | 0.7 | 2.3 | 14 |
| 13 | Parangipettai | Gavarapattu | Gavarapattu | 01 Aug 2013 to 31 Dec 2013 | 3 | 10 | 1.8 | 12 | 3.2 | 1.13 | 3.2 | 13 |
| 14 | Parangipettai | Ayyepuram | Ayyepuram | 01 Aug 2013 to 31 Dec 2013 | 2 | 7.2 | 1.4 | 9.2 | 2.7 | 0.8 | 2.7 | 11 |
| 15 | Kammapuram | Arasakuzhi | Arasakuzhi | 01 Aug 2013 to 31 Dec 2013 | 3 | 22 | 3.7 | 24 | 3.8 | 1.1 | 6.4 | 14 |
| 16 | Kammapuram | Kammapuram | Kammapuram | 01 Aug 2013 to 31 Dec 2013 | 1 | 22 | 0.44 | 4.2 | 2 | 0.19 | 2 | 13 |
| 17 | Kammapuram | Arasakuzhi | Gangaikondan | 01 Aug 2013 to 31 Dec 2013 | 2 | 13.2 | 0.54 | 15.2 | 1.5 | 0.28 | 1.5 | 12 |
| 18 | Kammapuram | Palakollai | Palakollai | 01 Aug 2013 to 31 Dec 2013 | 3 | 40.1 | 7.7 | 42.1 | 1.2 | 0.8 | 2.3 | 14 |
| 19 | Mangalampettai | Mangalampettai | Mangalampettai | 01 Aug 2013 to 31 Dec 2013 | 1 | 21 | 0.68 | 4.1 | 1.7 | 0.32 | 1.7 | 12 |
| 20 | Nallur | Veppur | Veppur | 01 Aug 2013 to 31 Dec 2013 | 2 | 13.7 | 3.03 | 15.7 | 1.5 | 0.8 | 1.5 | 11 |
| 21 | Nallur | Nallur | Nallur | 01 Aug 2013 to 31 Dec 2013 | 2 | 15 | 3.31 | 17 | 1.7 | 0.8 | 1.7 | 13 |
| 22 | Nallur | Sirumangalam | Elangaiyanur | 01 Aug 2013 to 31 Dec 2013 | 2 | 8 | 1.94 | 10 | 1.8 | 0.8 | 1.8 | 11 |
| 23 | Mangalur | Thozhudur | Thozhudur | 01 Aug 2013 to 31 Dec 2013 | 8 | 14.4 | 2.45 | 16.4 | 4.2 | 0.8 | 4.2 | 14 |
| 24 | Mangalur | Sirupakkam | Sirupakkam | 01 Aug 2013 to 31 Dec 2013 | 1 | 7.2 | 1.51 | 9.2 | 1 | 0.5 | 1 | 12 |
| 25 | Mangalur | E.keeranur | Vasistapuram | 01 Aug 2013 to 31 Dec 2013 | 5 | 12.6 | 2.99 | 14.6 | 1 | 0.4 | 1 | 10 |
| 26 | Cuddalore mty | Devanampattinam | Devanampattinam | 01 Aug 2013 to 31 Dec 2013 | 6 | 13.9 | 2.56 | 15.9 | 2.6 | 0.7 | 2.6 | 18 |
| 27 | Cuddalore mty | Pudupalayam | Pudupalayam | 01 Aug 2013 to 31 Dec 2013 | 2 | 7.6 | 1.4 | 9.6 | 1.4 | 0.7 | 1.4 | 12 |
| 28 | Cuddalore mty | Tsunami nagar | Tsunami nagar | 01 Aug 2013 to 31 Dec 2013 | 3 | 22 | 1.9 | 24 | 2.6 | 0.7 | 2.6 | 15 |
| 29 | Cuddalore mty | Annanagar | Annanagar | 01 Aug 2013 to 31 Dec 2013 | 2 | 8.5 | 2.1 | 10.5 | 2.1 | 0.9 | 1.7 | 14 |
| 30 | Nellikuppam | Vanpakkam | Vanpakkam | 01 Aug 2013 to 31 Dec 2013 | 3 | 8.5 | 1.63 | 10.5 | 2.2 | 1.2 | 2.2 | 16 |
| Total | 15.27 | 2.45 | 16.37 | 2.2 | 1.77 | 2.4 | 13.13 |
Italics denoted the results of intervention as indices reached to its own threshold level to stop dengue.
Figure 3.
The House Index status in some outbreak villages in the Cuddalore district before and after an outbreak.
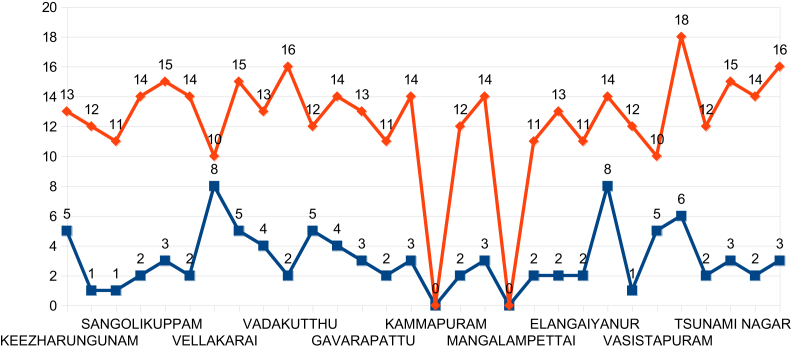
On examination of the time required to halt the disease from the day of intervention, it was found to be 13–15 days. In some places this time extended more than 15 days due to hidden habitats of Aedes. In urban Cuddalore in one area, Devanampattinam, dengue was not stopped even after HI was reduced to < 5% for more than a month. This provoked further searches for Aedes and it was found that refrigerators were the source of breeding, thus a justifiable reason for extending more than one incubation period (4–14 days) was found (Table 4 and Figure 4). Since most outbreaks had ended within 15 days, it was ascertained that the incubation of dengue virus is 4–14 days. This study found that extension over 15 days was due to an insufficiently strong intervention and demonstrates the need for further strengthening of the intervention in these cases.
Table 4.
The habitats of Aedes species in some rural and urban areas of the Cuddalore district that exhibited dengue positives and permissible Aedes indices.
| Name of the block | Name of the villages that took > 15 d to stop dengue | No. of cases reported from NS1 tool | Occult habitats of dengue & its index | NS1 | IgM | Total | |
|---|---|---|---|---|---|---|---|
| 1 | Annagiramam | 25 | 8 | 33 | |||
| 2 | Cuddalore | Kondur | 10 | Refrigerator | 180 | 19 | 199 |
| Koothapakkam | 7 | Refrigerator | |||||
| Pathirikuppam | 8 | Refrigerator | |||||
| Thiruvandipuram | 7 | Refrigerator | |||||
| Knpettai | 9 | Refrigerator | |||||
| Tazhanguda | 9 | Refrigerator | |||||
| Ramapuram | 12 | Refrigerator | |||||
| 3 | Panruti | Silambinathanpettai | 23 | Refrigerator | 103 | 15 | 118 |
| Refrigerator | |||||||
| 4 | Vadalur | Kurinjipadi | 14 | Refrigerator | 112 | 23 | 135 |
| 5 | Parangipettai | 38 | 6 | 44 | |||
| Killai | 4 | Refrigerator | |||||
| Parangipettai | 6 | Refrigerator | |||||
| 6 | Kammapuram | Seplanatham | 6 | Refrigerator | 35 | 9 | 44 |
| Nadiyapattu | 5 | Poclain tyres | |||||
| 7 | Kumaratchi | Annamalainagar | 5 | Refrigerator | 17 | 9 | 23 |
| 8 | Nallur | Veppur | 5 | Refrigerator | |||
| Pennadam | 4 | Refrigerator | |||||
| 9 | Virudhachalam | 28 | 7 | 35 | |||
| Thottikuppam | 3 | Refrigerator | |||||
| 10 | Mangalur | 15 | 4 | 19 | |||
| Alathur | 6 | Refrigerator | |||||
| 11 | Cuddalore urban | 209 | 31 | ||||
| Devanampattinam | 27 | Refrigerator | |||||
| Yandipalayam | 16 | Refrigerator | |||||
| Semmandalam | 10 | Refrigerator | |||||
| Manjakuppam | 19 | Refrigerator | |||||
| Kammiyampettai | 9 | Refrigerator | |||||
| Thirupathiripuliyur | 17 | Refrigerator | |||||
| Pudupalayam | 10 | Refrigerator | |||||
| 12 | Nellikuppam urban | 54 | 19 | 73 | |||
| Nellikuppam | 7 | Refrigerator | |||||
| Thirukandeeswaram | 13 | Refrigerator | |||||
| Vazhapattu | 7 | Refrigerator | |||||
| Jeevanagar | 5 | Refrigerator | |||||
| 13 | Chidambaram urban | Chidambaram | 26 | Refrigerator | 26 | 6 | 32 |
| 14 | Panruti urban | Panruti | 19 | Refrigerator | 26 | 0 | 26 |
| Thiruvadhigai | 4 | Refrigerator | |||||
| Andipalayam | 3 | Refrigerator | |||||
| 15 | Virudhachalam urban | Virudhachalam | 33 | Refrigerator | 33 | 2 | 35 |
| 16 | Neyveli township | Neyveli | 8 | Tree holes | 8 | 0 | 8 |
Ig = immunoglobulin; NS1 = nonstructural Protein 1.
Figure 4.
The time taken in days to halt the dengue outbreaks in the Cuddalore district.
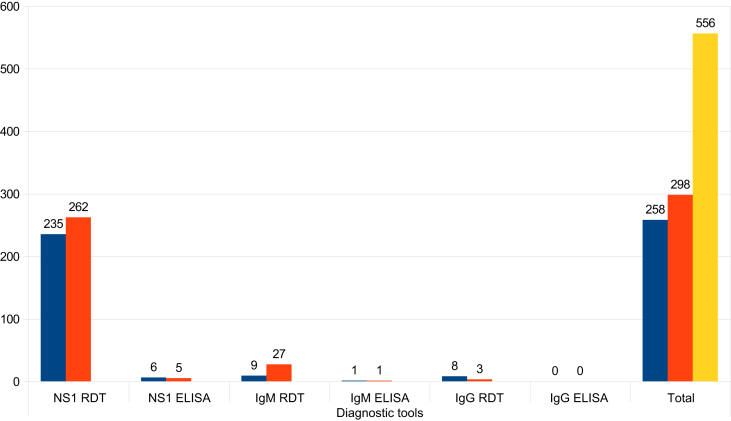
In the Nagapattinam district, dengue outbreaks occurred during the months of August to October 2012. There were 446 dengue cases reported in seven villages (Vadugacherry, Sirkali, Keelaiyur, Vadavoor, Keechankuppam, Akkaraipettai, Thideerkuppam, and Kallar) and in eight areas of Nagapattinam urban (8, 11, 12, 13, 14, 21, 35, and 36). This was a total of 298 and 258 dengue positives from the rural and urban areas respectively (Table 5). The various diagnostic tools used to predict dengue cases in the Nagapattinam district are listed in Table 6.
Table 5.
Basic data describing the dengue situation in the Nagapattinam district in 2013.
| Month | No. of dengue cases in urban areas | No. of dengue cases in Keechankuppam | Other villages | Total | |
|---|---|---|---|---|---|
| 1 | August | 10 | 0 | 0 | 10 |
| 2 | September | 190 | 41 | 81 | 312 |
| 3 | October | 98 | 26 | 110 | 234 |
| Total | 298 | 67 | 191 | 556 |
Table 6.
The number of dengue cases reported in rural and urban areas of the Nagapattinam district and the various diagnostic tools used to confirm them.
| Location | Diagnostic tools |
Total | |||||
|---|---|---|---|---|---|---|---|
| NS1 RDT | NS1 ELISA | IgM RDT | IgM ELISA | IgG RDT | IgG ELISA | ||
| Rural | 235 | 6 | 9 | 1 | 8 | 0 | 258 |
| Urban | 262 | 5 | 27 | 1 | 3 | 0 | 298 |
| Total | 556 | ||||||
ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin; NS1 = nonstructural Protein 1; RDT = rapid diagnostic test.
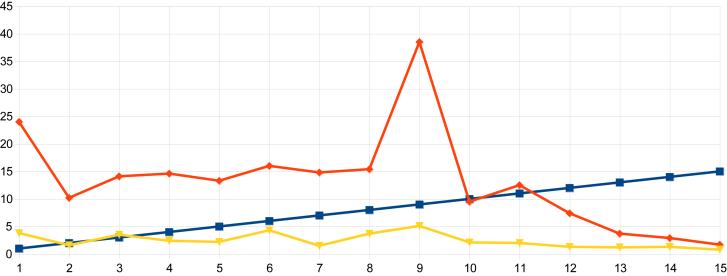
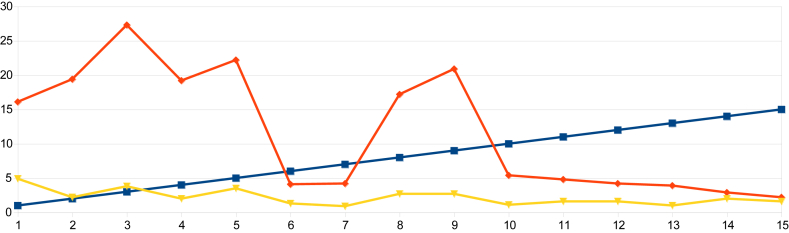
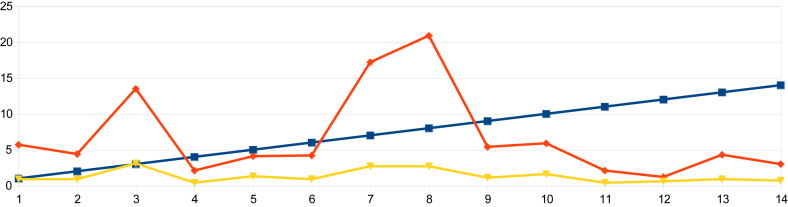
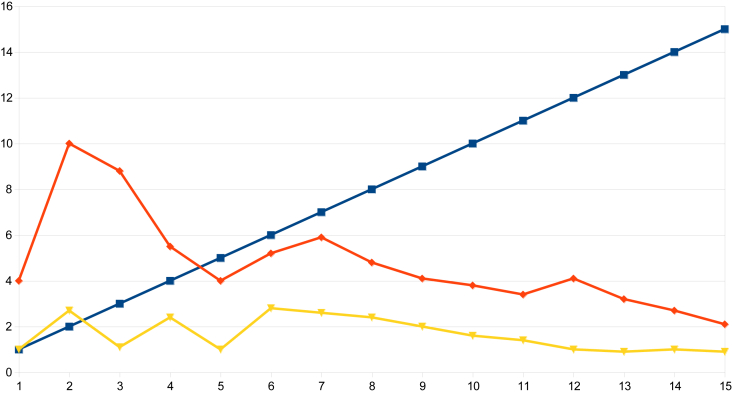
On examination of the data from indices obtained in urban Nagapattinam from the beginning of the outbreak to the end, it was observed that the HI in Sevabharathi was 24% on the 1st day of intervention and it had declined to 1.7% when the outbreak was halted. In Old Nambiyar Nagar the HI was 5.7% initially and declined to 2.6% at the halting of the outbreak. In New Nambiyar Nagar the HI declined to 2.2% from 16.2%, likewise, in Tatanagar, the HI was 4.0% initially and reduced to 2.1% when dengue cases had been stopped (Table 7 and Figure 5, Figure 6, Figure 7, Figure 8). Since all these declining trends of HI occurred on 15th day post intervention, it has been ascertained that the maximum incubation of dengue is 15 days as previously reported by Gubler [22]. The outbreak that began in Tatanagar with a HI of 4.0%, which was less than the permissible level advised by the World Health Organization (i.e., < 5%), was explained by the identification of refrigerators that acted as hidden habitats and provided breeding grounds for Aedes and meant that the HI value determined at the beginning was inaccurate as it was based on observations made in wet containers in and around human dwellings alone.
Table 7.
The declining trends of the House Index (HI) and Container Index (CI) from the intervention days to the halt of the dengue outbreaks in the urban areas of Sevabharathi, Old and New Nambiyar Nagar, and Tatanagar of the Nagapattinam district of Tamil Nadu, India.
| Sevabharathi |
Old Nambiyar Nagar |
New Nambiyar Nagar |
Tatanagar |
|||||
|---|---|---|---|---|---|---|---|---|
| Time from the intervention (d) | HI | CI | HI | CI | HI | CI | HI | CI |
| 1 | 24 | 3.8 | 5.7 | 0.9 | 16.1 | 4.9 | 4 | 1 |
| 2 | 10.2 | 1.6 | 4.4 | 0.9 | 19.4 | 2.2 | 10 | 2.7 |
| 3 | 14.1 | 3.5 | 13.5 | 3.1 | 27.3 | 3.8 | 8.8 | 1.1 |
| 4 | 14.6 | 2.4 | 2.1 | 0.4 | 19.2 | 2 | 5.5 | 2.4 |
| 5 | 13.3 | 2.2 | 4.1 | 1.3 | 22.2 | 3.5 | 4 | 1 |
| 6 | 16 | 4.3 | 4.2 | 0.9 | 4.1 | 1.3 | 5.2 | 2.8 |
| 7 | 14.8 | 1.5 | 17.2 | 2.7 | 4.2 | 0.9 | 5.9 | 2.6 |
| 8 | 15.4 | 3.7 | 20.9 | 2.7 | 17.2 | 2.7 | 4.8 | 2.4 |
| 9 | 38.5 | 5.1 | 5.4 | 1.1 | 20.9 | 2.7 | 4.1 | 2 |
| 10 | 9.5 | 2.1 | 5.9 | 1.6 | 5.4 | 1.1 | 3.8 | 1.6 |
| 11 | 12.5 | 2 | 2.1 | 0.4 | 4.8 | 1.6 | 3.4 | 1.4 |
| 12 | 7.4 | 1.3 | 1.2 | 0.6 | 4.2 | 1.6 | 4.1 | 1 |
| 13 | 3.7 | 1.2 | 4.3 | 0.9 | 3.9 | 1 | 3.2 | 0.9 |
| 14 | 2.9 | 1.3 | 3 | 0.7 | 2.9 | 2 | 2.7 | 1 |
| 15 | 1.7 | 0.8 | 2.6 | 0.6 | 2.2 | 1.6 | 2.1 | 0.9 |
| Average | 13.2 | 2.5 | 6.4 | 1.3 | 11.6 | 2.2 | 4.8 | 1.7 |
Figure 5.
The declining trend of larval indices from the day of intervention to the halting of the dengue outbreak in Sevabharathi of the Nagapattinam district.
Figure 6.
The declining trend of larval indices from the day of intervention to the halting of the dengue outbreak in New Nambiyar Nagar of the Nagapattinam district.
Figure 7.
The declining trend of larval indices from the day of intervention to the halting of the dengue outbreak in Old Nambiyar Nagar of the Nagapattinam district.
Figure 8.
The declining trend of larval indices from the day of intervention to the halting of the dengue outbreak in Tatanagar of the Nagapattinam district.
Prediction of dengue positivity in both the rural and urban areas of the Nagapattinam district with the diagnostic tools NS1 and IgM ELISA were related to the presence of Aedes larvae. Furthermore, it is known that the NS1 diagnostic tool has been proven to detect the presence of dengue in the community as early as the 2nd day after the onset of fever. The early confirmation of dengue leads to early investigation into the location of Aedes habitats in the affected areas. The testing for dengue by IgM ELISA is limited as an affected individual can only be tested after 7 days from the onset of fever; consequently the implementation of an intervention is delayed. Thus the NS1 tool has received attention from public health program managers as it has been shown to be useful for the initiation of early interventions both in entomological and case management dengue control activities (Figure 9).
Figure 9.
The diagnostic tools used to predict dengue cases in rural and urban areas of the Nagapattinam district. ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin; NS1 = nonstructural Protein 1; RDT = rapid diagnostic test.
The degree of involvement amongst DBCs was assessed in a place where the Aedes survey was carried out under different categories of supervision (Health Inspectors, Block level supervisors and district level supervisors) Senior Entomologist based on reports of indices HI, CI and BI arrived. Where it is not significant (<0.1), efforts were made to strengthen to bring down permissible level of indices HI < 1% and BI < 20 (Table 8, Table 9).
Table 8.
The course of larval (AL) indices from the beginning and the end of an outbreak in the urban area of the Nagapattinam district by different supervisory tiers during cross checking of domestic breeding checker (DBC) activities of AL work.
| Name of the urban areas | D | Larval indices from SE cross checks |
Larval indices from junior entomologist cross checks |
Larval indices from health inspectors cross checks |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HI | CI | BIa | HI | CI | BIa | HI | CI | BIa | |||
| 1 | Sevabharthi | 1 | 62.1 | 17 | 0 | 24 | 3.8 | 0 | 6.3 | 1.3 | 0 |
| 2 | 28.1 | 10.4 | 0 | 10.2 | 1.6 | 0 | 4.1 | 1.1 | 0 | ||
| 3 | 9.4 | 3.1 | 0 | 14.1 | 3.5 | 0 | 6.8 | 4.1 | 0 | ||
| 4 | 8 | 3.2 | 0 | 9.5 | 2.1 | 0 | 8.2 | 1.5 | 0 | ||
| 5 | 5.9 | 1.3 | 0 | 3.7 | 1.2 | 0 | 2.5 | 0.4 | 0 | ||
| 6 | 5.4 | 0.9 | 0 | 5.2 | 1 | 0 | 3.1 | 2.3 | 0 | ||
| 7 | 4 | 1 | 0 | 4.4 | 1 | 0 | 2.2 | 0.8 | 0 | ||
| 2 | New Nambiyar Nagar | 1 | 13.3 | 2.7 | 0 | 16.1 | 4.9 | 0 | 4.1 | 1.3 | 0 |
| 2 | 8.8 | 1.3 | 0 | 19.4 | 2.2 | 0 | 4.2 | 0.9 | 0 | ||
| 3 | 23.5 | 3.7 | 0 | 27.3 | 3.8 | 0 | 17.2 | 2.7 | 0 | ||
| 4 | 15.6 | 3.4 | 0 | 19.2 | 2 | 0 | 20.9 | 2.7 | 0 | ||
| 5 | 10 | 1 | 0 | 22.2 | 3.5 | 0 | 7.2 | 1.1 | 0 | ||
| 6 | 7.8 | 0.9 | 0 | 8.2 | 1 | 0 | 4.2 | 0.7 | 0 | ||
| 7 | 4 | 1 | 0 | 5 | 1.2 | 0 | 2.3 | 0.8 | 0 | ||
| 3 | Tatanagar | 1 | 10 | 2.7 | 0 | 9.1 | 2.2 | 0 | 1.8 | 0.4 | 0 |
| 2 | 8.8 | 1.1 | 0 | 8 | 1.2 | 0 | 4.2 | 0.6 | 0 | ||
| 3 | 5.5 | 2.4 | 0 | 4.8 | 1 | 0 | 2.3 | 0.7 | 0 | ||
| 4 | 4 | 1 | 0 | 3.8 | 0.9 | 0 | 1.9 | 0.4 | 0 | ||
| 5 | 4.1 | 1 | 0 | 5 | 1.1 | 0 | 2.6 | 1 | 0 | ||
| 6 | 3.2 | 0.9 | 0 | 3.1 | 1 | 0 | 1.5 | 0.8 | 0 | ||
| 7 | 2.8 | 1 | 0 | 3.1 | 1 | 0 | 0.5 | 0.1 | 0 | ||
| 4 | Old Nambiyar Nagar | 1 | 5 | 1.2 | 0 | 5.7 | 0.9 | 0 | 5.9 | 1.6 | 0 |
| 2 | 4.3 | 1.2 | 0 | 4.4 | 0.9 | 0 | 2.1 | 0.4 | 0 | ||
| 3 | 5 | 1 | 0 | 13.5 | 3.3 | 0 | 1.2 | 0.6 | 0 | ||
| 4 | 4.3 | 1.9 | 0 | 2.1 | 0.4 | 0 | 4.3 | 0.9 | 0 | ||
| 5 | 4 | 0.9 | 0 | 5 | 1 | 0 | 3 | 0.7 | 0 | ||
| 6 | 3.3 | 1.3 | 0 | 3.2 | 1.3 | 0 | 2.6 | 0.6 | 0 | ||
| 7 | 4 | 0.9 | 0 | 4.2 | 1 | 0 | 2.1 | 0.8 | 0 | ||
To compute Breteau Index (BI), minimum hundred houses should be visited to ensure the presence of Aedes larvae in containers whereas BI has not been arrived when searches for Aedes larvae happened to fall <100 houses. BI = Breteau Index; CI = Container Index; HI = House Index; SE = senior entomologist.
Table 9.
Data appraised by the health inspectors of primary health centers (PHCs) and the senior entomologists (SEs).
| Days from the intervention | Sevabharathi |
Old Nambiyar Nagar |
New Nambiyar Nagar |
Tatanagar |
||||
|---|---|---|---|---|---|---|---|---|
| HI of SE | HI of PHC | HI of SE | HI of Hinsp | HI of SE | HI of Hinsp | HI of SE | HI of Hinsp | |
| 1 | 62.1 | 6.3 | 2.8 | 0.5 | 13.3 | 4.1 | 4 | 2.3 |
| 2 | 28.1 | 4.1 | 5 | 5.9 | 8.8 | 4.2 | 10 | 1.8 |
| 3 | 9.4 | 6.8 | 4.3 | 2.1 | 23.5 | 17.2 | 8.8 | 4.2 |
| 4 | 8 | 8.2 | 5 | 1.2 | 15.6 | 20.9 | 5.5 | 2.3 |
| 5 | 5.9 | 2.5 | 4.3 | 4.3 | 10 | 7.2 | 4 | 1.9 |
| 6 | 5.4 | 3.1 | 4 | 3 | 7.8 | 4.2 | 4.1 | 2.6 |
| 7 | 4 | 2.2 | 3.3 | 2.6 | 6.6 | 3.8 | 3.2 | 1.8 |
HI = House Index; Hinsp = health inspector.
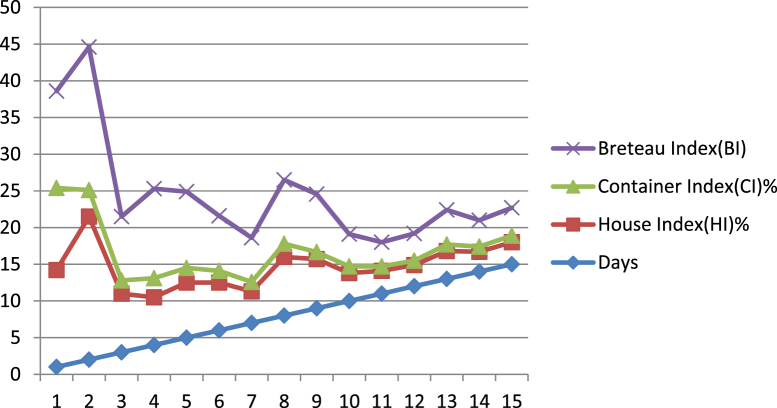
There was a dengue outbreak in the Tirunelveli district in May 2012, data from the PHC in Mukkudal was taken for the present study. In the 1st week of May 2012, there were six IgM ELISA confirmed dengue cases reported from the villages of Mukkudal, Vaddakku Ariyanayagipuram, Kumarasamy Puram, and Arikesavanallur and four deaths were reported in the Maruthamputhur PHC area. All these villages were under entomological surveillance from the day of intervention to 30 days after. Study findings in the form of HI, CI, and BI are reported in Table 10, Table 11 and Figure 10.
Table 10.
The declining trend of Aedes indices within one incubation (15 days) during the dengue outbreak in the Mukkudal primary health center of the Tirunelveli district of Tamil Nadu, India.
| Date | Houses checked | Houses positive with Aedes larvae | Total containers checked | Containers positive | House Index (HI)% | Container Index (CI)% | Breteau Index (BI) | Positive dengue cases confirmed by IgM ELISA |
|---|---|---|---|---|---|---|---|---|
| 12 May 2012 | 715 | 95 | 845 | 95 | 13.2 | 11.2 | 13.2 | 6 |
| 13 May 2012 | 1008 | 197 | 5412 | 197 | 19.5 | 3.6 | 19.5 | 0 |
| 14 May 2012 | 3159 | 250 | 14,542 | 275 | 8 | 1.8 | 8.7 | 0 |
| 15 May 2012 | 3519 | 232 | 16,252 | 429 | 6.5 | 2.6 | 12.2 | 0 |
| 16 May 2012 | 3464 | 261 | 17,607 | 362 | 7.5 | 2 | 10.4 | 0 |
| 17 May 2012 | 4067 | 263 | 18,864 | 305 | 6.5 | 1.6 | 7.5 | 0 |
| 18 May 2012 | 2561 | 112 | 11,773 | 155 | 4.3 | 1.3 | 6 | 0 |
| 19 May 2012 | 3727 | 250 | 14,542 | 275 | 8 | 1.8 | 8.7 | 0 |
| 20 May 2012 | 592 | 40 | 4883 | 47 | 6.7 | 0.96 | 7.9 | 0 |
| 21 May 2012 | 3778 | 144 | 18,838 | 165 | 3.8 | 0.9 | 4.4 | 0 |
| 22 May 2012 | 3277 | 101 | 16,782 | 109 | 3.1 | 0.6 | 3.3 | 0 |
| 23 May 2012 | 2315 | 66 | 13,913 | 85 | 2.9 | 0.6 | 3.7 | 0 |
| 24 May 2012 | 2962 | 113 | 16,394 | 140 | 3.8 | 0.9 | 4.7 | 0 |
| 25 May 2012 | 2691 | 72 | 13,259 | 98 | 2.7 | 0.7 | 3.6 | 0 |
| 26 May 2012 | 2794 | 84 | 11,042 | 108 | 3 | 0.9 | 3.8 | 0 |
| Mean | 6.6 | 2.10 | 7.84 |
ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin.
Table 11.
The declining trend of Aedes indices in the second incubation (15–30 days) during the dengue outbreak in the Mukkudal primary health center of the Tirunelveli district of Tamil Nadu, India.
| Date | Houses checked | Houses positive with Aedes larvae | Total containers checked | Containers positive | House Index(HI)% | Container Index (CI)% | Breteau Index(BI) | Positive dengue cases confirmed by IgM ELISA |
|---|---|---|---|---|---|---|---|---|
| 27 May 2012 | 1111 | 31 | 5719 | 49 | 2.8 | 0.9 | 4.4 | 0 |
| 28 May 2012 | 2929 | 47 | 11,068 | 49 | 1.6 | 0.4 | 1.9 | 0 |
| 29 May 2012 | 3177 | 34 | 14,438 | 39 | 1.1 | 0.3 | 1.2 | 0 |
| 30 May 2012 | 2961 | 32 | 12,759 | 35 | 1.1 | 0.3 | 1.2 | 0 |
| 31 May 2012 | 3018 | 30 | 12,941 | 30 | 0.9 | 0.2 | 0.9 | 0 |
| 01 Jun 2012 | 2686 | 52 | 11,825 | 65 | 1.9 | 0.5 | 2.4 | 0 |
| 02 Jun 2012 | 2404 | 54 | 9632 | 55 | 2.2 | 0.6 | 2.3 | 0 |
| 03 Jun 2012 | 2199 | 39 | 9568 | 40 | 1.8 | 0.4 | 1.8 | 0 |
| 04 Jun 2012 | 2866 | 22 | 9733 | 22 | 0.8 | 0.2 | 0.8 | 0 |
| 05 Jun 2012 | 2836 | 37 | 9723 | 41 | 1.3 | 0.4 | 1.4 | 0 |
| 06 Jun 2012 | 2513 | 19 | 8887 | 19 | 0.8 | 0.2 | 0.8 | 0 |
| 07 Jun 2012 | 2922 | 26 | 12,922 | 26 | 0.9 | 0.2 | 0.9 | 0 |
| 08 Jun 2012 | 2804 | 23 | 11,499 | 23 | 0.8 | 0.2 | 0.8 | 0 |
| 09 Jun 2012 | 2286 | 15 | 8022 | 15 | 0.7 | 0.2 | 0.7 | 0 |
| Mean | 1.34 | 0.36 | 1.54 |
ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin.
Figure 10.
The declining trend of House Index, Container Index, and Breteau Index during the dengue outbreak in the Mukkudal primary health center of the Tirunelveli district of Tamil Nadu, India.
On examination of the daily indices of Aedes, it was shown that the HI was 13.2%, CI 11.2%, and BI 13.2 on the day of intervention, subsequently these indices decreased day by day until the 15th day of intervention when these indices were 3%, 0.9%, and 3.8, respectively. Some cases among the six cases identified in the Mukkudal area were reported after the day of intervention. It has been shown that individuals that were infected before the intervention was implemented expressed symptoms within the incubation period of 4–14 days on different dates; hence the impact of the intervention on the dengue outbreak could best be measured a minimum of 15 days from the day of intervention implementation.
The current intervention was extended beyond the first incubation period in these villages, and findings are reported in Table 11. On the 16th day, the HI was 2.8%, CI was 0.9%, and BI was 4.4, these values decreased to 0.7%, 0.2%, and 0.7 respectively on the 30th day and no new positive cases were reported in these villages. From these observations, it has been concluded that the maximum human incubation period of dengue is 15 days. It was also shown that cases confirmed by the IgM ELISA and NS1 tools are related to the presence of the Aedes larvae. During the dengue outbreaks, cases were reported in most of the blocks in the district, some adjacent blocks such as Nanguneri which did not report any positive cases were analyzed in parallel with reference to the Aedes indices: HI, CI, and BI (Table 12). From this observation, it was shown that all these indices were within permissible levels (HI 1.63%, CI 1.06%, and BI 2.2). These values demonstrate the importance of entomological surveillance and its value in the sustainable management of maintaining a dengue free area. Thus the availability of HI, CI, and BI values play an important role in dengue prevention and control.
Table 12.
The Aedes indices within permissible levels in Nangunery where no dengue positives were reported in May 2012 or June 2012.
| Date | Houses checked | Houses positive with Aedes larvae | Total containers checked | Containers positive | House Index (HI)% | Container Index (CI)% | Breteau Index (BI) | Positive dengue cases confirmed by IgM ELISA |
|---|---|---|---|---|---|---|---|---|
| 25 May 2012 | 5905 | 101 | 11,321 | 244 | 1.7 | 2.2 | 4.1 | 0 |
| 26 May 2012 | 3553 | 78 | 7644 | 114 | 2.1 | 1.5 | 3.2 | 0 |
| 27 May 2012 | 3338 | 48 | 6215 | 56 | 1.6 | 0.9 | 1.7 | 0 |
| 28 May 2012 | 4929 | 89 | 10,162 | 101 | 1.8 | 1.0 | 2.0 | 0 |
| 29 May 2012 | 4523 | 85 | 9487 | 105 | 1.8 | 1.1 | 2.3 | 0 |
| 30 May 2012 | 3945 | 71 | 8326 | 87 | 1.7 | 1.0 | 2.2 | 0 |
| 31 May 2012 | 4024 | 78 | 9280 | 97 | 1.9 | 1.0 | 2.4 | 0 |
| 01 Jun 2012 | 3744 | 70 | 8285 | 106 | 2.1 | 1.3 | 2.8 | 0 |
| 02 Jun 2012 | 3766 | 86 | 8366 | 127 | 2.2 | 1.5 | 3.4 | 0 |
| 03 Jun 2012 | 3797 | 80 | 9374 | 114 | 2.1 | 1.2 | 3.0 | 0 |
| 04 Jun 2012 | 4935 | 71 | 10,931 | 85 | 1.4 | 0.8 | 1.7 | 0 |
| 05 Jun 2012 | 5587 | 65 | 10,645 | 85 | 1.2 | 0.8 | 1.5 | 0 |
| 06 Jun 2012 | 4024 | 56 | 8837 | 86 | 1.4 | 1.0 | 2.1 | 0 |
| 07 Jun 2012 | 4242 | 86 | 10,343 | 118 | 2.0 | 1.1 | 2.8 | 0 |
| 08 Jun 2012 | 4384 | 66 | 10,180 | 91 | 1.5 | 0.9 | 2.1 | 0 |
| 09 Jun 2012 | 4156 | 50 | 9379 | 73 | 1.2 | 0.8 | 1.8 | 0 |
| 10 Jun 2012 | 4290 | 58 | 8437 | 76 | 1.4 | 0.9 | 1.8 | 0 |
| 11 Jun 2012 | 4989 | 64 | 11,531 | 88 | 1.3 | 0.8 | 1.8 | 0 |
| 12 Jun 2012 | 4587 | 65 | 9843 | 65 | 1.4 | 0.7 | 1.4 | 0 |
| 13 Jun 2012 | 3994 | 46 | 8841 | 66 | 1.2 | 0.7 | 1.7 | 0 |
| 14 Jun 2012 | 4279 | 62 | 9710 | 52 | 1.4 | 0.5 | 1.2 | 0 |
| 15 Jun 2012 | 4282 | 60 | 4469 | 73 | 1.4 | 1.6 | 1.7 | 0 |
| Mean | 1.63 | 1.06 | 2.22 |
ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin.
Similar observations were made in the Cheranmahadevi block where the HI was 1.6%; CI 0.5%, and BI 1.6 on the day of intervention. These indices decreased day by day, the values after 23 days were HI 0.5%; CI 0.2%, and BI 0.4. No positive cases of dengue were reported in Cheranmahadevi (Table 13).
Table 13.
The Aedes indices within permissible levels in the Cheranmahadevi district where dengue positives had not been reported in May 2012 or June 2012.
| Date | Houses checked | Houses positive with Aedes larvae | Total containers checked | Containers positive | House Index (HI)% | Container Index (CI)% | Breteau Index (BI) | Positive dengue cases confirmed by IgM ELISA |
|---|---|---|---|---|---|---|---|---|
| 25 May 2012 | 5984 | 93 | 20,593 | 93 | 1.6 | 0.5 | 1.6 | 0 |
| 26 May 2012 | 5409 | 41 | 20,900 | 41 | 0.8 | 0.2 | 0.8 | 0 |
| 27 May 2012 | 60 | 2 | 136 | 2 | 3.3 | 1.5 | 0 | 0 |
| 28 May 2012 | 5589 | 27 | 26,498 | 27 | 0.5 | 0.1 | 0.5 | 0 |
| 29 May 2012 | 6240 | 108 | 27,215 | 108 | 1.7 | 0.4 | 1.7 | 0 |
| 30 May 2012 | 5411 | 21 | 22,569 | 21 | 0.4 | 0.1 | 0.4 | 0 |
| 31 May 2012 | 5911 | 15 | 23,579 | 17 | 0.3 | 0.1 | 0.3 | 0 |
| 01 Jun 2012 | 5139 | 12 | 20,381 | 15 | 0.2 | 0.1 | 0.3 | 0 |
| 02 Jun 2012 | 4626 | 21 | 19,758 | 25 | 0.5 | 0.1 | 0.5 | 0 |
| 03 Jun 2012 | 3094 | 10 | 11,145 | 12 | 0.3 | 0.1 | 0.4 | 0 |
| 04 Jun 2012 | 5314 | 13 | 23,097 | 13 | 0.2 | 0.1 | 0.2 | 0 |
| 05 Jun 2012 | 5312 | 9 | 22,613 | 10 | 0.2 | 0.0 | 0.2 | 0 |
| 06 Jun 2012 | 5524 | 3 | 20,880 | 3 | 0.1 | 0.0 | 0.1 | 0 |
| 07 Jun 2012 | 5569 | 11 | 17,548 | 13 | 0.2 | 0.1 | 0.2 | 0 |
| 08 Jun 2012 | 5073 | 12 | 19,772 | 12 | 0.2 | 0.1 | 0.2 | 0 |
| 09 Jun 2012 | 4310 | 9 | 15,811 | 10 | 0.2 | 0.1 | 0.2 | 0 |
| 10 Jun 2012 | 2947 | 2 | 10,433 | 2 | 0.1 | 0.0 | 0.1 | 0 |
| 11 Jun 2012 | 5577 | 8 | 20,929 | 8 | 0.1 | 0.0 | 0.1 | 0 |
| 12 Jun 2012 | 5546 | 8 | 22,586 | 8 | 0.1 | 0.0 | 0.1 | 0 |
| 13 Jun 2012 | 5381 | 9 | 21,861 | 9 | 0.2 | 0.0 | 0.2 | 0 |
| 14 Jun 2012 | 5233 | 10 | 21,395 | 11 | 0.2 | 0.1 | 0.2 | 0 |
| 15 Jun 2012 | 4897 | 8 | 19,089 | 8 | 0.2 | 0.0 | 0.2 | 0 |
| 16 Jun 2012 | 5215 | 7 | 2066 | 7 | 0.1 | 0.3 | 0.1 | 0 |
| Mean | 0.50 | 0.17 | 0.37 |
ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin.
Apart from these findings, it has also been established that the co-existence of DEN-1 and DEN-3 viruses circulating in the community led to positivity among susceptible groups of all ages, fatalities in both male and female infants of up to 4 years were reported (Table 14). From this observation, a case fatality rate of 10% was determined during the dengue outbreak in the Mukkudal block.
Table 14.
The number of dengue IgM ELISA confirmed cases and deaths by age and sex in the Mukkudal block of the Tirunelveli district of Tamil Nadu, India.
| Age groups (y) | Male | Female | Deaths |
|
|---|---|---|---|---|
| Male | Female | |||
| 0–1 | 1 | 3 | 1 | 1 |
| 1–4 | 2 | 2 | 2 | 0 |
| 5–8 | 3 | 2 | 0 | 0 |
| 9–14 | 10 | 7 | 0 | 0 |
| > 15 | 3 | 13 | 0 | 0 |
ELISA = enzyme linked immunosorbent assay; Ig = immunoglobulin.
4. Discussion
The various findings determined in previous studies on the behavior and association of dengue viruses, Aedes aegypti and Aedes albopictus, both in man and vectors, have been reconfirmed by the current study observations during the course of the dengue epidemic in several parts of three districts, namely Cuddalore, Nagapattinam, and Tirunelveli of Tamil Nadu, India. As these observations were made to forecast the disease as well as to halt the outbreak, the present study has received much attention.
The incubation period of the dengue virus in humans was determined to be a maximum of 15 days and a minimum of 4 days in susceptible individuals among all age groups of both sexes. A similar observation was made by Mungrue [18] who noted that following the bite of an infected mosquito there is an incubation period of 3–14 days but it is usually 5–7 days when symptoms begin to occur.
Of the heterogeneous serovars of dengue in circulation in the community, it was known that 47 patients who travelled to India and Singapore in August 2004 displayed a dual coinfection with two serotypes (Type 1 and 2) of dengue virus (DENV). The first documented case of concurrent infections with more than one serotype of DENV was reported in Puerto Rico in 1982 [23]. Since then, several cases have been reported in New Caledonia, Thailand, Somalia, Mexico, and China 24, 25, 26, 27. The reason for dual infections is explained by the unique feeding behavior of Aedes aegypti mosquitoes. As the female mosquito often feeds on several individuals during a single gonotrophic cycle, there is a chance that it will be dually infected and then in turn transmit multiple viruses to a single individual 27, 28. In the present study, similar observations were made when deaths occurred among children (from 4 months to 4 years) due to DEN 1 and DEN 3 which were identified during the outbreak in the Tirunelveli district.
Furthermore it was evident that when Cassens [29] described the epidemiological concepts with their components as the agents of disease, host factors, and environmental factors, the host factors in which (1) young children are more likely to have subclinical infection, (2) adults are immune to certain diseases because of prior exposure, and (3) children and adults are exposed to different agents of disease, were emphasized. These observations are relevant to the current study as it has been shown that children were more susceptible and fatalities occurred in the heterogeneous strains during the course of the dengue outbreak.
Various studies have been found in literature on the relationships of Stegomyia or Aedes indices namely HI and BI to the transmission of dengue and these indices remain central to the monitoring of the dengue vector population [30]. Little is known about the relationship between the differing proportions of various sampled larval instars and the accuracy of these data as proxy measures of adult mosquito abundance [31]. Despite these doubts, many dengue control authorities worldwide routinely collect vector population data based on these indices although the mathematical relationship between any indices and dengue transmission is far from clear. Thresholds indicating dengue outbreak risks for HI and BI (HI = 1%, BI = 5) have been used for many years 32, 33, even though these values were developed for yellow fever many decades earlier. Simple thresholds may be valid in some situations [8], but a universal critical threshold applicable across many contexts, has never been determined for dengue. These findings support the observations noted in this study as there was a concrete relationship between the recommended HI and BI levels necessary to control dengue outbreaks when their threshold levels fell to between 3% and 5% and < 5, respectively, based on the topography and type of habitat. At the same time, it was found that the impact of dengue in areas where the threshold levels of these indices were sustained for more than an incubation period suggested the existence of hidden habitats such as refrigerators and other unexpected rain dependent materials which accumulate in and around human dwellings. Further, it is also known that some rural villages had not experienced dengue in subsequent years and new villages raised might be based on the proportions of susceptible and immune individuals and in turn the establishment of herd immunity in the group. Epidemics or outbreaks of disease occur when the proportion of susceptible individuals is high, and disappear as the proportion of individuals with immunity increases. This observation was correlated to the present study which noted that a few villages presented dengue cases year after year in the same block. In this situation, maintaining the threshold level of the Aedes indices helped prevent an outbreak.
The above observations agree with the study findings of Tun-Lin et al [8] which showed that the blockwise analysis of Aedes indices to fix threshold levels in areas helped stop dengue transmission. In this context the lowest level of threshold (HI<1%) and BI 0.9) yielded from the average of the both the lowest and highest indices among blocks of the same town. The difference between the real lowest threshold and highest indices (up to 17.9% HI) was 400 meters and 40 days as the spatial and temporal boundaries of maximum dengue transmission in a dengue focus 8, 34. Along with this, a study by Lizet Sanchez [17] showed that BI > 1 and maximum BI ≥ 4 seemed to be a suitable action threshold and target respectively in community based dengue prevention. However, these results are derived from the analysis of epidemic data, and the thresholds identified may not constitute suitable targets in another epidemic or in a location where different ecologic conditions prevail [34]. Similar observation was also made in this research paper which found that some places with low thresholds of Aedes indices yielded cases which were due to different ecological factors and unusual habitats.
Cassens [29] observed that social traits take part in disease transmission as the host goes through life. Marital status, lifestyle, diet, place of residence, and travel are some of the factors that determine disease outcomes and have been shown to be direct factors affecting dengue outbreaks in an area. These factors are further strengthened by many scientists who have reported that DENV infection is a potential risk for travelers to tropical areas where dengue is endemic or epidemic. Furthermore, it is known that the growth of imported cases is increasingly recognized as a serious public health problem in nonendemic countries 30, 31, 32, 33. A study carried out in Cuba by Lizet Sanchez [17] observed that during the inspection cycle, before the outbreak, the overall municipal BI and HI were 0.92 and 0.87% respectively. The mean values of the indices calculated at the health area level were also ∼1 for areas with or without dengue cases during the subsequent epidemic. However, the mean BI and HI were > 1 in neighborhoods reporting cases and substantially < 1 for neighborhoods without cases.
Morrison et al [35] established the degrees of variation of larval prevalence in the Playa Municipality, Cuba, before, during, and after the dengue epidemic. This provided a unique opportunity to analyze entomologic information at different geographic levels. Entomologic data were collected through routine systems; however, this saw some limitations. Firstly, larval prevalence was possibly slightly underestimated in blocks which were inspected by different vector control technicians, procedures used may not have been completely standardized, and few data (randomly) went missing. Secondly, when dengue cases were reported, the control program intensified and more Aedes foci may have been detected. Thirdly, sampling Aedes aegypti can be time sensitive [32].
Furthermore, it is known that the peak incidents of confirmed infection followed the peak larval density by ∼1 month. In Salvador, Brazil, sentinel surveillance in 30 areas detected a significant 1.4× higher serovar incidence when the HI was > 3%. Recently, Scott and Morrison [16] showed that traditional larval indices in Peru are correlated with the prevalence of human dengue infections. The variety of thresholds proposed in these and other studies could be partially explained by different methods and geographic levels of analysis used, but other factors influence the relationship between Aedes density and transmission risk, such as herd immunity, population density, mosquito-human interaction, virus strain, and climate, which affects mosquito biology and most virus interaction 11, 16.
Diagnosis of DENV infection using a commercial test kit alone is not reliable in terms of sensitivity and specificity, and a definitive diagnosis should be made in conjunction with other laboratory findings 27, 34. There is a lacuna on the early implementation of preventive measures and dengue control without complications in an area. Probing its determination with reference to diagnostic tools available to confirm dengue in the laboratory, it is known that NS1 is immensely helpful in the initiation of early entomological surveillance in an outbreak and in bringing down Aedes indices to their permissible level within a fortnight i.e., one incubation period, barring the cases already infected during the intervention. On the contrary, interventions have been delayed about 1 week when the suspected serum sample is confirmed by IgM ELISA after 6 days from the onset of fever [19]. Even though, NS1 specificity and sensitivity differs between manufacturers, cross reaction, etc., confirmation of dengue with this tool relates to the search for Aedes at an immature stage in the place where the case resides and it is known that the serum is eligible for testing the day after the onset of fever. Hence, the NS1 tool contributes to early intervention implementation even before the new complication of dengue cases emerge in an outbreak area. Supporting this view, some studies have shown the significance of the eligibility of serum samples to confirm dengue 18, 19. It is anticipated that clinical diagnosis methods will be strengthened as NS1 plays an important role in the early implementation of entomological interventions.
When discussing the titer of IgM and IgG antibodies during the primary and secondary infection of dengue, it was found that the IgM to dengue virus appears late during the febrile phase of illness, often preceded by IgG. In primary infection, only low levels of IgG to dengue are detected in the febrile or early convalescent phase of infection, whereas levels of IgM are high and greatly exceed IgG levels for 2–4 weeks. In secondary infections, high levels of IgG are detectable even in the acute phase and rise dramatically over the next 2 weeks whereas IgM levels are absent or low, raise little in comparison with IgG and decline quickly. Hence, the detection of IgM is not specific for acute dengue, as IgM may persist for 2–3 months following infection, IgM against other flaviviruses can cross react in some tests 27, 34, and IgG does not distinguish current from past infection, an important issue in endemic areas were secondary infection is common.
To detect early infection of the dengue virus, serum or plasma can be tested using the RT-PCR tool. Based on its cost effectiveness, NS1 based testing should be considered for detection in acute febrile illness of dengue as it allows the implementation of an early intervention in an area even before complications have become apparent [34]. Its use is supported by the current study. Similar to previous study findings, this study also noted the degrees of involvement among DBCs. The first and second tiers of supervision were not statically significant (p > 0.1), whereas the supervision of higher officials is qualitatively significant (p < 0.05). The results of findings on the impact of cases after numerous intervention cycles were made during an outbreak and showed that it is difficult to halt an outbreak within a single span of dengue incubation in humans unless there is a commitment to obtain uniform indices in detecting positive habitats of Stegomyia in an outbreak area irrespective of DBC and the tier of supervision.
In summary, the following study findings have been ascertained: (1) the incubation of DENV in human is 4–14 days; (2) the presence of the heterogeneous serotype DENV leads to increased case fatality rates in an epidemic; and (3) cyclic larval checkups are important as they can detect permissible threshold levels of Aedes indices and in turn help to stop dengue transmission within 15 days from the day of intervention when there is a significant level of commitments in all field staff. Above all, the NS1 ELISA is the tool which can be used for the early implementation of entomological interventions to reduce dengue complications in the community. Other than these findings, a new area of research has to be necessitated to study the cross reaction of dengue virus in places where other flavi viruses are co-existing. Since the Cuddalore district was previously endemic for JE, vaccination with SA 14–14–2 live attenuated vaccine has been administered to children aged from 9.5 months to 1.5 years since 2008 after its inclusion in the routine national immunization program. Hence efforts need to be made to resolve the cross reaction between dengue and other flaviviruses during outbreaks, it is confirmed that this may give a new dimension to dengue diagnosis.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors are very grateful to the Deputy Directors of Health Services, Dr K.R. Jawaharlal and Dr Uma and the Joint Director Dr Selvin for providing much assistance and advice during the outbreak ensuring the effective implementation of the study intervention in the affected villages. The authors are also grateful to the chief entomologists S. Sridharan and M. Kadiresan, the district entomologists assigned from other districts, and the senior entomologist C. Manthiram from the ZET, Tirunelveli, and Mr. Kumar from the ZET, Thanjavur, for their immense help to bring about this research paper. Many thanks to the field and administrative staff from the ZET Cuddalore whose kind cooperation is much appreciated. The assistance from S. Sasikumar who typed this manuscript is appreciated.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Guzman M.G., Kouri G. Dengue: an update. Lancet Infect Dis. 2002 Jan;2(1):33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 2.Deen J.L. The challenge of dengue vaccine development and introduction. Trop Med Int Health. 2004 Jan;9(1):1–3. doi: 10.1046/j.1365-3156.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- 3.Guzman M.G., Mune M., Kouri G. Dengue vaccine: priorities and progress. Expert Rev Anti Infect Ther. 2004;2:895–911. doi: 10.1586/14789072.2.6.895. [DOI] [PubMed] [Google Scholar]
- 4.Guzman D.J., Clark G.G. Community involvement in the control of Aedes aegypti. Acta Trop. 1996 Apr;61(2):169–179. doi: 10.1016/0001-706x(95)00103-l. [DOI] [PubMed] [Google Scholar]
- 5.Guzman M.G., Kouri G. Dengue and dengue hemorrhagic fever in the Americas: Lessons and challenges. J Clin Virol. 2003 May;27(1):1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Reiter P., Gubler D.J. Surveillance and control of urban dengue vectors. In: Gubler D.J., Kuno G., editors. Dengue and dengue hemorrhagic fever. CAB International; New York: 1997. pp. 425–462. [Google Scholar]
- 7.Focks D.A., Brenner R.J., Hayes J., Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg. 2000 Jan;62(1):11–18. [PubMed] [Google Scholar]
- 8.Tun-Lin W., Kay B.H., Barnes A., Forsyth S. Critical examination of Aedes aegypti indices: Correlations with abundance. Am J Trop Med Hyg. 1996 May;54(5):543–547. doi: 10.4269/ajtmh.1996.54.543. [DOI] [PubMed] [Google Scholar]
- 9.Focks D.A. World Health Organization; Geneva: 2003. A review of entomological sampling methods and indicators for dengue vectors. [Google Scholar]
- 10.Getis A., Morrison A.C., Gray K., Scott T.W. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003 Nov;69(5):494–505. [PubMed] [Google Scholar]
- 11.Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17(2):321–335. doi: 10.1093/oxfordjournals.epirev.a036196. [DOI] [PubMed] [Google Scholar]
- 12.Pan American Health Organization . The Organization; Washington: 1994. Dengue and dengue hemorrhagic fever in the Americas: Guidelines for prevention and control. Scientific publication no.548. [Google Scholar]
- 13.Focks D.A., Chadee D.D. Pupal survey: An epidemiologically significant surveillance method for Aedes aegypti: An example using data from Trinidad. Am J Trop Med Hyg. 1997 Feb;56(2):159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- 14.Dengue Seroprevalence of dengue virus infection, Singapore. Wkly Epidemiol Rec. 1992 Apr;67(14):99–101. [PubMed] [Google Scholar]
- 15.Pontes R.J., Freeman J., Oliveira-Lima J.W., Hodgson J.C., Spielman A. Vector densities that potentiate dengue outbreaks in a Brazilian city. Am J Trop Med Hyg. 2000 Mar;62(3):378–383. doi: 10.4269/ajtmh.2000.62.378. [DOI] [PubMed] [Google Scholar]
- 16.Scott T.W., Morrison A.C. Aedes aegypti density and the risk of dengue-virus transmission. In: Takken W., Scott T.W., editors. Ecological aspects for application of genetically modified mosquitoes. Kluwer Academic Publishers; Dordrecht (the Netherlands): 2004. pp. 187–206. [Google Scholar]
- 17.Sanchez L., Vanlerberghe V., Alfonsa L., Marquetti M.C., Guzman M.G., Bissel J. Aedes aegypti larval indices and risk for dengue epidemic. Emerg Infect Dis. 2006 May;12(5):800–806. doi: 10.3201/eid1205.050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mungrue K. The laboratory diagnosis of dengue virus infection, a review. Adv Lab Med Int. 2014;4(1):1–8. [Google Scholar]
- 19.Nogueira R.M.R., Miagostovich M.P., Cavalcanti S.M.B., Mirzicgu K.B.F., Schatzmayr H.G. Levels of IgM antibodies against dengue virus in Rio de Janeiro, Brazil. Res Virol. 1992 Nov–Dec;143(6):423–427. doi: 10.1016/s0923-2516(06)80136-6. [DOI] [PubMed] [Google Scholar]
- 20.Mackenzie J.M., Jones M.K., Young P.R. Immunolocalization of the dengue virus non-structural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996 Jun;220(1):232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 21.Libraty D.H., Young P.R., Pickering D. High circulating levels of the dengue virus non-structural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002 Oct;186(8):1165–1168. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- 22.Gubler D.J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998 Jul;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalille M., Deubel V., Sainte-Marie F.F. Demonstration of concurrent dengue 1 and dengue 3 infection in six patients by the polymerase chain reaction. J Med Virol. 1991 May;34(1):51–54. doi: 10.1002/jmv.1890340109. [DOI] [PubMed] [Google Scholar]
- 24.Kanesa-thasan N., Chang G.J., Smoak B.L. Molecular and epidemiologic analysis of dengue virus isolates from Somalia. Emerg Infect Dis. 1998 Apr–Jun;4(2):299–303. doi: 10.3201/eid0402.980220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorono-Pino M.A., Cropp C.B., Farfan J.A. Common occurrence of concurrent infections by multiple dengue virus serotypes. Am J Trop Med Hyg. 1999 Nov;61(5):725–730. doi: 10.4269/ajtmh.1999.61.725. [DOI] [PubMed] [Google Scholar]
- 26.Wenming P., Man Y., Baochang F. Simultaneous infection with dengue 2 and 3 viruses in a Chinese patient return from Sri Lanka. J Clin Virol. 2005 Mar;32(3):194–198. doi: 10.1016/j.jcv.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Platt K.B., Linthicum K.J., Myint K.S. Impact of dengue virus infection of feeding behaviour of Aedes aegypti. Am J Trop Med Hyg. 1997 Aug;57(2):119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- 28.Scott T.W., Naksathit A., Day J.F. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am J Trop Med Hyg. 1997 Aug;57(2):235–239. doi: 10.4269/ajtmh.1997.57.235. [DOI] [PubMed] [Google Scholar]
- 29.Cassens B.J. 1987. In preventive medicine and Public Health John Wiley & Sons Publication; pp. 1–358. [Google Scholar]
- 30.WHO . 1997. Dengue haemorrhagic fever: Diagnosis, treatment, prevention and control [Internet]http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/ [cited 2005 May 8]. Available from: [Google Scholar]
- 31.World Health Organization Dengue: Guidelines for diagnosis, treatment, prevention and control. Switzerland; WHO, Geneva: 2009. pp. 1–160. [PubMed] [Google Scholar]
- 32.Focks D. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases; 2004. A review of entomological sampling methods and indicators for dengue vectors; pp. 1–40. [Google Scholar]
- 33.Connor M.E., Monroe W.M. Stegomyia indices and their value in yellow fever control. Am J Trop Med Hyg. 1923 Jan;1(1):9–19. [Google Scholar]
- 34.Gubler D.J., Kuno G., Sather G.E. A case of natural concurrent human infection with two dengue viruses. Am J Trop Med Hyg. 1985 Jan;34(1):170–173. doi: 10.4269/ajtmh.1985.34.170. [DOI] [PubMed] [Google Scholar]
- 35.Morrison A.C., Getis A., Santiago M., Rigau-Perez J.G., Reiter P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991–1992. Am J Med Hyg. 1998 Mar;58(3):287–298. doi: 10.4269/ajtmh.1998.58.287. [DOI] [PubMed] [Google Scholar]