Abstract
Purpose
Prior studies suggest that fungal keratitis is more common in hot, humid climates while bacterial keratitis is independent of seasonal variation. This study analyzes seasonal trends in the incidence of fungal and bacterial keratitis at the Aravind Eye Hospital in southeast India.
Methods
Using microbiology records from August 2006 to July 2009, retrospective analyses of infectious keratitis were performed. Bacterial and fungal keratitis incidence data were analyzed for seasonal patterns.
Results
Among the 6,967 infectious keratitis cases, cultures were performed in 5,221 (74.9%) and positive in 3,028 (58%). Of the positive cultures cases, 1,908 (63%) and 1,081 (35.7%) were of fungal and bacterial etiology, respectively. The predominant fungal organism was Fusarium spp (42.3%) and the predominant bacterial organisms were Streptococcus pneumoniae (35.1%), Pseudomonas aeruginosa (24.3%), and Nocardia spp (8.1%). Analyses revealed an uneven distribution of fungal keratitis throughout the year (p<0.001) with peaks in July and January. No significant seasonal trend was observed for the combined bacterial keratitis group.
Conclusion
A higher incidence of fungal keratitis occurs during the months corresponding to the windy and harvest seasons, during which time infection from vegetative corneal injury may be more likely. Robust screening efforts during these periods may mitigate visually debilitating sequelae from infectious keratitis.
Keywords: infectious keratitis, season, India
Introduction
Globally, 6-8 million people are bilaterally blind due to corneal disease, the majority due to sequelae from infectious keratitis.1 The reported incidence varies widely, from 11 per 100,000 person-years in Olmsted County, Minnesota2 to 113 per 100,000 person-years in Madurai, southeast India.3
While contact lens associated bacterial keratitis and agricultural trauma related fungal keratitis account for the majority of cases in developed and developing nations, respectively, the epidemiology of infectious keratitis remains incompletely understood. Previous studies have reported an association between climate and keratitis. Bharathi et al reported that fungal keratitis predominated in the hot and windy environment of southeast India.4 A longitudinal study of keratitis in Australia found hot summer months conducive to infection by Pseudomonas aeruginosa while winter months favored Streptococcus pneumoniae.5 Acanthamoeba keratitis has been reported to occur more frequently in the summer and autumn seasons.6
A better understanding of the seasonal patterns of infectious keratitis may lead to improved treatment or prevention for two reasons. First, in areas with limited access to ophthalmologic care, practitioners often start empiric treatment for keratitis without the benefit of culture data. Tailoring empiric treatment to cover the most common pathogens may improve clinical outcomes. Second, intensifying screening efforts during periods of higher infectious keratitis incidence supports front-line health care providers who often are not ophthalmologists and have minimal training in eye disease.
We present the results of an investigation of seasonal trends in infectious keratitis in southeast India, an area in which a high incidence has been previously reported. This study analyzed data from the microbiology laboratory of the Aravind Eye Hospital, a large volume referral center with a catchment area covering much of southeast India.
Materials and Methods
A retrospective chart review of the microbiological laboratory database at the Aravind Eye Hospital in Madurai, India, was conducted on all patients seen from August 1, 2006 to July 31, 2009. The study included cases with a clinical diagnosis of a corneal ulcer, defined as an epithelial defect with stromal infiltration and suppuration associated with signs of inflammation. For inclusion, cases must have had a smear and/or culture performed. Marginal ulcers, Mooren's ulcers, interstitial keratitis, sterile neurotrophic ulcers, and peripheral ulcerative keratitis were excluded. The study excluded duplicates, so that when repeat cultures from the same patient were recorded within a two month period, only the first sample was included for analysis.
A detailed description of smear and culture technique at the Aravind microbiology laboratory has been reported previously.7 In brief, after instillation of topical preservative-free lidocaine, a flame-sterilized Kimura spatula was used to perform a corneal scraping, the material of which was inoculated onto sheep's blood agar, chocolate agar, potato dextrose agar, and brain heart infusion broth without gentamicin. Material from the scrapings was also smeared onto three separate glass slides for Gram stain, Giemsa stain, and KOH wet mount. When KOH smears were positive for amoebic cysts, a further corneal scraping was performed and the material was inoculated onto non-nutrient agar overlaid with Escherichia coli to isolate Acanthamoeba spp. Microbial cultures were considered positive only if growth of the same organism was demonstrated on two or more solid media or there was semiconfluent growth at the site of inoculation on one solid medium associated with the identification of the organism on Gram or Giemsa stained corneal smears. Staphylococcus epidermidis and diphtheroids were considered positive only if there was moderate growth on at least two solid media.
Based on prior research from the Aravind Eye Hospital,3 the number of incident cases of the most common organisms (Fusarium spp, Streptococcus pneumoniae, Nocardia spp, Pseudomonas aeruginosa, and Acanthamoeba spp) were analyzed. The primary outcome variable was the number of culture-positive cases occurring each two week period for each of the organisms investigated. However, secondary analyses involved the biweekly fraction of cases due to each type of organism (bacterial, fungal, protozoan), or to specific genera (fractions due to Fusarium, Streptococcus pneumoniae, Nocardia, and Pseudomonas aeruginosa).
Seasonality of culture counts was tested using the Edwards test for an annual cycle as well as for a twice-yearly cycle.8,9 In addition, power spectra were estimated based on the smoothed periodogram for both the biweekly case counts and the biweekly fractions indicated above. Analyses were performed specifically for an annual cycle and a twice-yearly cycle. Hypothesis tests for the significance of annual and biannual peaks in the power spectrum were conducted using a permutation test.10 Seasonal lowess plots (locally weighted regression) were constructed for summarization and description of the data for Acanthamoeba spp, Fusarium spp, Pseudomonas aeruginosa, Nocardia spp, Streptococcus pneumoniae, total fungi cases, and total bacteria cases. A secular trend in the number of case counts was tested using least squares regression of the number of cases for each two-week period with time as the regressor. Because of possible autocorrelation, time series bootstrap with a fixed window was used in hypothesis testing.11 All computations were done in R version 2.10 for MacIntosh.
We received ethical approval for this study from the University of California, San Francisco Committee on Human Research, and the Aravind Eye Care System.
Results
Over the three year time period, there were 6,967 patients with infectious keratitis, of which 5,221 had corneal cultures performed (Table 1). Among the 3,028 positive corneal cultures, 1,908 (63%) had fungal infections, 1,081 (35.7%) had bacterial infections, and 39 cases (1.3%) grew Acanthamoeba spp (Table 1). No evidence of secular trends in case counts was seen over the three year period (Table 1). Smoothed plots of the number of culture-confirmed cases due to fungal, bacterial, and Acanthamoeba spp for each day of the year suggested the presence of seasonal patterns (Fig. 1).
Table 1.
Number of patients with fungal keratitis due to Acanthamoeba, bacteria, and fungal causes at Aravind Eye Hospital, August 2006–July 2009. The total count of unique individuals is shown by study year. The total number of cases for which cultures were performed is labeled “Number of cultures, all”. The three right columns report, respectively, the result of a test for overall temporal trend, the Edwards test for a 12-month (annual) cycle, and the Edwards test for a 6-month (biannual) cycle (see text for details).
| Culture | Number of patients | Overall Trend (P-value) | Annual Cycle | Biannual Cycle | |||||
|---|---|---|---|---|---|---|---|---|---|
| 8/06 to 7/07 | 8/07 to 7/08 | 8/08 to 7/09 | |||||||
| Number of cultures, all | 1620 | 1814 | 1787 | 0.17 | 0.17 | < 0.001 | |||
| Number of negative cultures | 653 | 808 | 732 | - | - | - | |||
| Number of positive cultures | All organisms | 967 | 1006 | 1055 | 0.38 | 0.002 | < 0.001 | ||
| Fungi | Total fungal positive | 572 | 644 | 692 | 0.15 | 0.007 | < 0.001 | ||
| Fusarium | 262 | 253 | 292 | 0.34 | 0.83 | < 0.001 | |||
| Other | 310 | 391 | 400 | - | - | - | |||
| Bacteria | Total bacterial positive | 378 | 348 | 355 | 0.47 | 0.30 | 0.31 | ||
| Streptococcus pneumoniae | 140 | 113 | 126 | 0.60 | 0.58 | 0.04 | |||
| Nocardia | 29 | 30 | 29 | 0.89 | 0.12 | 0.92 | |||
| Pseudomonas | 82 | 94 | 87 | 0.89 | < 0.001 | 0.26 | |||
| Mycobacteria | 0 | 5 | 3 | 0.18 | 0.04 | 0.49 | |||
| Other | 127 | 106 | 110 | - | - | - | |||
| Acanthamoeba | 17 | 14 | 8 | 0.56 | < 0.001 | 0.014 | |||
Figure 1.
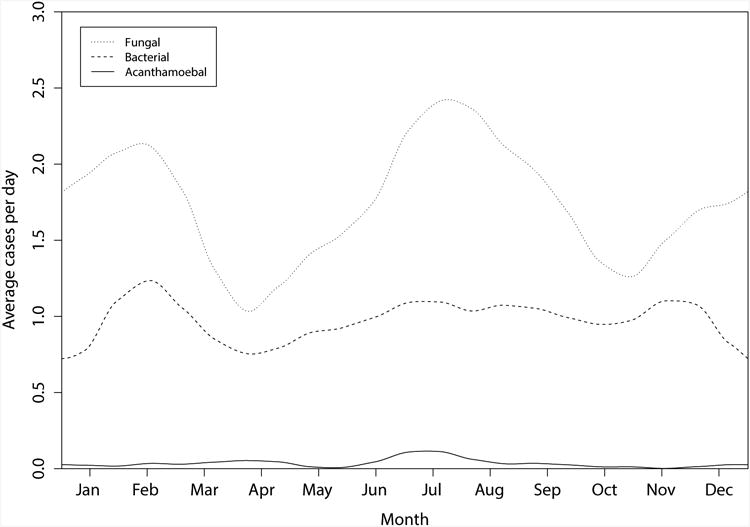
Smoothed plot of the number of culture-confirmed infectious keratitis cases due to fungal, bacterial, and Acanthamoeba spp for each day of the year between August 1, 2006 and July 31, 2009, Aravind Eye Hospital.
The biweekly total numbers of cases of fungal keratitis exhibited a major peak in July and a minor peak in January (Fig. 2, P=0.007, Edwards test for 12 month cycle; P<0.001, Edwards test for six month cycle). The proportion of culture-positive cases due to fungus exhibited no annual pattern (P=0.72, periodogram), but displayed a prominent six-month cycle (P=0.03, periodogram).
Figure 2.
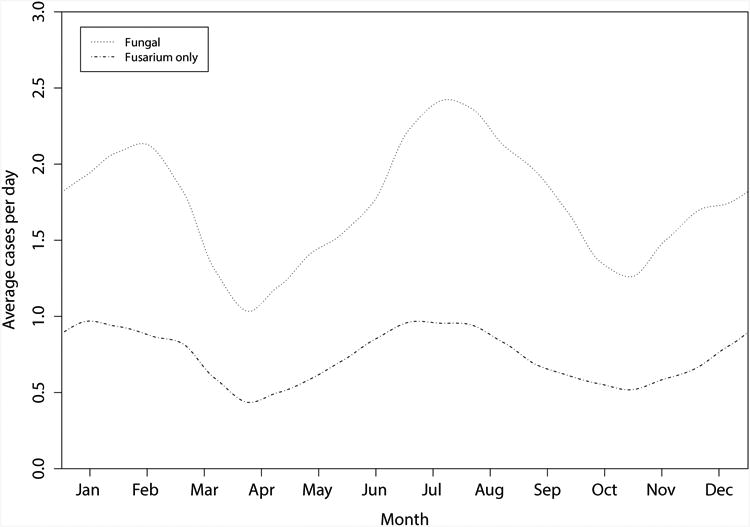
Smoothed plot of the number of culture-confirmed infectious keratitis cases due to all fungal and Fusarium spp between August 1, 2006 and July 31, 2009, Aravind Eye Hospital.
For the predominant fungal organism, Fusarium spp (Fig 2), two nearly identical peaks positioned approximately six months apart were observed. Therefore, as shown in Table 1, no evidence of a one-period annual cycle was seen in the Fusarium counts (P=0.83, Edwards test for 12 month cycle), but the pattern is clearly revealed when testing for the six-month cycle (P<0.001, Edwards test). The incidence of Fusarium spp was approximately 120% higher at its peak compared to its trough. Periodogram analyses likewise revealed evidence of oscillations in the fraction of culture positive case counts due to Fusarium case counts at an approximate six-month cycle (P=0.048).
Smoothed plots of the number of culture-confirmed cases due to all bacteria, Streptococcus pneumoniae, and Nocardia spp are shown in Figure 3. Analyses of total bacterial keratitis, Streptococcus pneumoniae, and Nocardia spp revealed no evidence of periodicity using either the Edwards test or the periodogram (Table 1). There appeared to be a suggestion of biannual periodicity in the cases of Streptococcus pneumoniae (p=0.04), but this was not significant when accounting for the Bonferroni correction. In contrast, the majority of cases of Pseudomonas spp occurred from July to December (Fig. 3), with the smoothed peak incidence rate being 216% of the incidence at the lowest point of the cycle (P<0.001, Edwards test for 12 month cycle) (Table 1). Repeating the analysis using the fraction of total positive cultures attributable to Pseudomonas spp yielded the same result (periodogram 12 month cycle, P=0.011). While only eight cases of culture-confirmed mycobacteria were seen, all such cases occurred between November and April (P=0.04, Edwards test for 12 month cycle).
Figure 3.
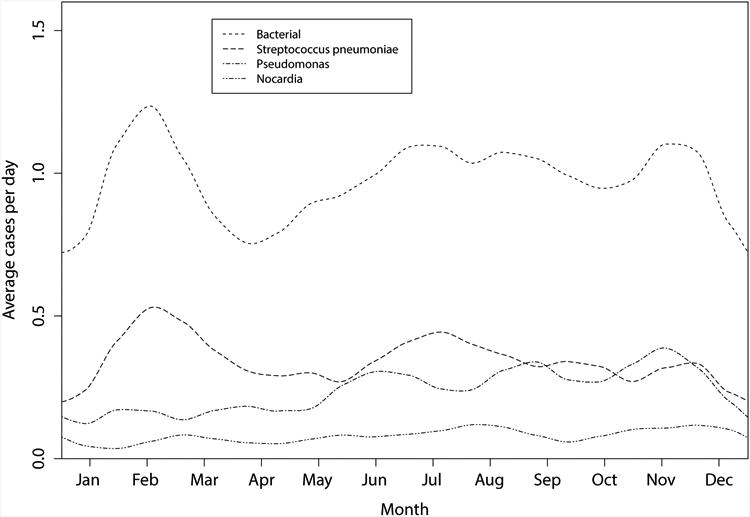
Smoothed plot of the number of culture-confirmed infectious keratitis cases due to all bacteria, Streptococcus pneumoniae, Nocardia spp, and Pseudomonas spp between August 1, 2006 and July 31, 2009, Aravind Eye Hospital.
Of the 39 cases of Acanthamoeba keratitis, twelve of these occurred in July, a pattern suggestive of seasonal fluctuations (Table 1, P<0.001, Edwards test for 12 month cycle; P=0.014, Edwards test for 6 months). Similar results were obtained from the periodogram analysis of the fraction of culture positive cases due to Acanthamoeba.
Discussion
This paper identifies several seasonal trends in infectious keratitis in the southeast Indian population served by the Aravind Eye Hospital. Notably, overall cases of infectious keratitis peak in July with a smaller spike in February. This may largely reflect the seasonal pattern of fungal keratitis, which makes up the majority of positive culture cases.
What can explain the two seasonal peaks in fungal keratitis and its subset Fusarium spp keratitis observed in this study? Prior studies have reported an increased incidence of fungal keratitis with higher temperatures.5, 7 In southeast India however, a hot climate persists perennially, and therefore other factors must account for the observed trends. Due to the proclivity of fungi to moist environments, one may expect an increase in cases during the wet season and a corresponding decrease during the dry season. However, this was not seen in our analyses, suggesting that environmental humidity may not have been a significant factor in the seasonal patterns of fungal keratitis. Seasonal fluctuations in agriculture-related ocular trauma, known to be a major risk factor for the development of fungal keratitis, provide a plausible explanation. The windy season in Tamil Nadu, which peaks in June, may predispose to the development of infectious keratitis as pathogens invade corneal stroma following trauma by airborne dust particles dispersed in high wind conditions. More ocular surface trauma may also occur in January during the main harvest season in Tamil Nadu. During this time, farmers may be particularly susceptible to fungal keratitis as suggested by Chang et al,12 which reported higher airborne fungal concentrations during the harvest season in Taiwan.
In contrast, bacterial keratitis did not follow a statistically significant seasonal pattern with the exception of Pseudomonas aeruginosa, which exhibited a broad peak from July to December. In Australia, higher temperatures have previously been associated with Pseudomonas aeruginosa keratitis5 and the organism appears to favor tropical, humid environs compared to temperate climates.13-17 Extra-ocular Pseudomonas aeruginosa infections such as otitis externa and folliculitis are associated with exposure to contaminated water, suggesting a similar risk factor for keratitis. Indeed, the months from July to December correspond with the heavy rainfall season for southeast India, raising a possible association between the monsoon season and the seasonal fluctuation of Pseudomonas aeruginosa keratitis.
Streptococcus pneumoniae keratitis has been reported to occur more commonly in winter months5 but the present study did not find any significant seasonal trend for this pathogen. The lack of a true winter season in the tropical environment of southeast India may account for this discrepancy. This example underscores the importance of understanding local climate patterns in extrapolating seasonal infectious keratitis patterns.
As for Acanthamoeba spp, the number of cases peaked in July, a trend reproduced in two out of the three years analyzed. While this result should be interpreted with some caution given the limited number of cases analyzed, prior research sheds some light on this observed trend. Contact lens wear is the most common risk factor for Acanthamoeba spp keratitis but is relatively rare in this study population. Other purported risk factors include exposure to a contaminated water supply, ocular surface trauma, and higher temperatures.6 In southeast India, the perfect storm may be created for Acanthamoeba spp keratitis in June, when higher temperatures coincide with an increase in ocular surface trauma during the windy season. The delay in diagnosing Acanthamoeba spp keratitis would appear as a peak in cases several weeks later in July.
Our paper has the following limitations. First, this study examined data from only one center with a limited catchment area in southeast India. Although the demographics of patients seen at the Aravind Eye Hospital likely represent much of southeast India, selection bias inherent to a referral center may result in a disproportionate number of recalcitrant cases. Also, geography plays a crucial role in creating unique microclimates in south India. For example, while much of India, including Tamil Nadu's neighboring western state of Kerala, receives its heaviest rainfalls from July to August, southeast India is sheltered by the Western Ghat mountain range and thus experiences its primary wet season from October to December.
Despite the large number of cases examined in this study, the short time series of the dataset poses another limitation. Future investigations based on longer time series may not only allow testing of the cyclic patterns detected in this study, but also permit a more precise estimate of the infectious keratitis burden attributable to seasonal changes. Such studies may also provide greater power to detect smaller cyclic changes over the course of the year and thus permit detection of seasonal trends in incidence of Streptococcus pneumoniae and Nocardia spp, which the current study was not powered to find. Of note, identification of cyclic patterns in short-term time series does not permit us to conclude that seasonal fluctuations in climate, rainfall, or other factors are causally linked to changes in the incidence of infectious keratitis. While we suspect that environmental factors contribute to these seasonal trends, it is possible that other factors play a role as well, such as human-to-human transmission of infectious organisms. Subsequent studies analyzing a larger number of bacterial keratitis cases caused by environmental bacteria may be able to test the hypothesis that seasonal trends differ between environmental and endogenous organisms.
Despite these limitations, the results of this study have important implications for the public health efforts of the Aravind Eye Hospital and its environs. First, knowledge of these seasonal trends can help tailor empiric treatment. In rural southeast India, frontline health care providers treating corneal ulcers are often not ophthalmologists nor do they have extensive ocular disease training. As fungal keratitis is more common in the months corresponding with the windy and harvest seasons, additional vigilance may be warranted during these times, especially in the presence of antecedent agricultural trauma. Rather than beginning initial treatment with a sole antibacterial agent, it may be prudent to cover the infection with additional anti-fungal treatment and refer earlier to a facility with an adequate microbiological laboratory facility. For these providers, increased awareness and education regarding corneal ulcers would equip them with more astute diagnostic skills and allow them to more effectively counsel patients to seek appropriate care earlier if expected improvement does not occur.
Second, these study results can support preventive efforts for infectious keratitis, such as screening and public health education. The Aravind Eye Hospital operates an extensive outreach campaign to surrounding villages, comprised of daily eye camps and telemedicine videoconferencing. Increasing the number and frequency of these eye camps during peak periods of infectious keratitis could prevent more severe visual morbidity among affected patients. In addition, raising public health awareness regarding the use of eye protection and avoidance of using contaminated water to irrigate the eye would be beneficial.
Acknowledgments
Support: This study was supported in part by an unrestricted grant from That Man May See, Inc (San Francisco, CA), an unrestricted grant from Research to Prevent Blindness (New York, NY), the Heed Ophthalmic Foundation (CCL), and NIH-NEI 1U10EY018573.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Erie JC, Nevitt MP, Hodge DO, et al. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993;111:1665–1671. doi: 10.1001/archopht.1993.01090120087027. [DOI] [PubMed] [Google Scholar]
- 3.Gonzales CA, Srinivasan M, Whitcher JP, et al. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3:159–66. doi: 10.3109/09286589609080122. [DOI] [PubMed] [Google Scholar]
- 4.Bharathi MJ, Ramakrishnan R, Meenakshi R, et al. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–69. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 5.Green M, Apel A, Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea. 2008;27:33–39. doi: 10.1097/ICO.0b013e318156cb1f. [DOI] [PubMed] [Google Scholar]
- 6.McAllum P, Bahar I, Kaiserman I, et al. Temporal and seasonal trends in Acanthamoeba keratitis. Cornea. 2009;28:7–10. doi: 10.1097/ICO.0b013e318181a863. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards JH. The recognition and estimation of cyclic trends. Annals of Human Genetics. 1961;25:83–87. doi: 10.1111/j.1469-1809.1961.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 9.Cave DR, Freedman LS. Seasonal variations in the clinical presentation of Crohn's disease and ulcerative colitis. Int J Epidemiol. 1975;4:317–320. doi: 10.1093/ije/4.4.317. [DOI] [PubMed] [Google Scholar]
- 10.Pardo-Igúzquiza E, Rodriguez-Tovar FJ. The permutation test as a non-parametric method for testing the statistical significance of power spectrum estimation in cyclostratigraphic research. Earth and Planetary Science Letters. 2000;181:175–189. [Google Scholar]
- 11.Efron B, Tibshirani R. An introduction to the bootstrap. Boca Raton, FL: Chapman & Hall; 1993. [Google Scholar]
- 12.Chang CW, Ho CK, Chen ZC, et al. Fungi genus and concentration in the air of onion fields and their opportunistic action related to mycotic keratitis. Arch Environ Health. 2002;57:349–354. doi: 10.1080/00039890209601420. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop AA, Wright ED, Howlader SA, et al. Suppurative corneal ulceration in Bangladesh. A study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust N Z J Ophthalmol. 1994;22:105–110. doi: 10.1111/j.1442-9071.1994.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 14.Fong CF, Tseng CH, Hu FR, et al. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am J Ophthalmol. 2004;137:329–336. doi: 10.1016/j.ajo.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Houang E, Lam D, Fan D, et al. Microbial keratitis in Hong Kong: relationship to climate, environment and contact-lens disinfection. Trans R Soc Trop Med Hyg. 2001;95:361–367. doi: 10.1016/s0035-9203(01)90180-4. [DOI] [PubMed] [Google Scholar]
- 16.Leck AK, Thomas PA, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ormerod LD. Causation and management of microbial keratitis in subtropical Africa. Ophthalmology. 1987;94:1662–1668. doi: 10.1016/s0161-6420(87)33235-x. [DOI] [PubMed] [Google Scholar]


