Abstract
Knowledge of how the head moves during locomotion is essential for understanding how locomotion is controlled by sensory systems of the head. We have analyzed head movements of the cat walking along a straight flat pathway in the darkness and light. We found that cats' head left-right translations, and roll and yaw rotations oscillated once per stride, while fore-aft and vertical translations, and pitch rotations oscillated twice. The head reached its highest vertical positions during second half of each forelimb swing, following maxima of the shoulder/trunk by 20–90°. Nose-up rotation followed head upward translation by another 40–90° delay. The peak-to-peak amplitude of vertical translation was ~1.5 cm and amplitude of pitch rotation was ~3°. Amplitudes of lateral translation and roll rotation were ~1 cm and 1.5–3°, respectively. Overall, cats' heads were neutral in roll and 10–30° nose-down, maintaining horizontal semicircular canals and utriculi within 10° of the earth horizontal. The head longitudinal velocity was 0.5–1 m/s, maximal upward and downward linear velocities were ~0.05 and ~0.1 m/s, respectively, and maximal lateral velocity was ~0.05 m/s. Maximal velocities of head pitch rotation were 20–50 °/s. During walking in light, cats stood 0.3–0.5 cm taller and held their head 0.5–2 cm higher than in darkness. Forward acceleration was 25–100% higher and peak-to-peak amplitude of head pitch oscillations was ~20 °/s larger. We concluded that, during walking, the head of the cat is held actively. Reflexes appear to play only a partial role in determining head movement, and vision might further diminish their role.
Keywords: locomotion, posture, vestibular, head fixation point
INTRODUCTION
To navigate successfully through complex natural environments, humans and animals depend on visual, vestibular, and somatosensory information about the orientation of their head and body. Vision is used to locate objects in the environment, determine the route of locomotion, and often to plan individual steps (e.g., Hollands and Marple-Horvat, 1996; Patla, 1997; Sherk and Fowler, 2001; Warren et al., 2001). The vestibular system provides information about the orientation of the head in respect to the vector of gravity, head rotation and acceleration (e.g., Peterson, 2004; Angelaki and Cullen, 2008). Additionally, vestibular and somatosensory mechanisms including vestibulo-collic and cervico-collic reflexes (e.g., Bilotto et al., 1982; Peterson et al., 1985; Wilson et al., 1995; Goldberg and Cullen, 2011), and vestibulo-ocular reflex participate in stabilizing gaze (Donaghy, 1980a,b; rev. in Raphan and Cohen, 2002). Jointly, information from receptors on the head is crucial for successful locomotion, and consequently the knowledge of how the head moves during locomotion is essential for understanding how locomotion is controlled by sensory systems of the head.
Movements of the head during locomotion were intensively studied in humans including during walking overground (Grossman et al., 1988; Pozzo et al., 1990, 1991; Cromwell et al., 2001, 2004; Imai et al., 2001; Kavanagh et al., 2005) and on the treadmill (Crane and Demer, 1997; Hirasaki et al., 1999), walking with different velocities and directions (Cappozzo, 1981; Hirasaki et al., 1999; Nadeau et al., 2003), and walking on irregular and inclined surfaces (Cromwell, 2003; Menz et al., 2003). One focus in these studies was on the relationship between the head vertical displacement and pitch (nose up-down) rotation. These movements are of substantial amplitudes and often are coupled so that the head upward displacement occurs simultaneously with nose-down rotation, while the downward displacement is simultaneous with nose-up rotation. Due to such relationship between these movements, a “head fixation point” is maintained, which is a point in space where the occipito-nasal axes of the head coincide in space for different head positions during the step cycle (Pozzo et al., 1990; Hirasaki et al., 1999; Hirasaki and Kumakura, 2004). Having such a fixation point for the head is believed to assist in processing visual information during locomotion.
Studies of head movements during locomotion in quadrupeds are much fewer. They were conducted in monkeys and horses (Dunbar, 2004; Dunbar et al., 2004, 2008; Xiang et al., 2008), and showed that while movements of the head in quadrupeds and bipeds are similar in many aspects, there are important differences as well. For example, while walking bipeds (humans and non-human primates) typically have a head fixation point, the head of quadrupeds (monkeys and horses) often translates and rotates in the same direction during the stride (nose-up while translating upward and vice versa), resulting in no fixation point in front of the animal. This difference raises the question whether head contribution to gaze behavior is different between bipedal and quadrupedal locomotion.
It is quite surprising that head movements have not been intensely investigated in the cat, which has been the classic subject for studies of visual, vestibular, and motor systems for at least two centuries. Currently, there exist limited data on cat head movement during locomotion (Graf et al., 1995; Carlson-Kuhta et al., 1998; Fowler and Sherk, 2003; Beloozerova et al., 2010). The cat, however, is the animal closest to humans whose unconstrained locomotion behavior can be fully researched in a laboratory setting, including neuronal mechanisms of locomotion and posture (e.g., Beloozerova and Sirota, 1993; Drew, 1993; Matsuyama and Drew, 2000a,b; Karayannidou et al., 2009; Zelenin et al., 2010; Marigold and Drew, 2011; Farrell et al., 2014; Stout et al., 2015). Therefore, researching how cats move their head during walking is of significant interest.
While studying head movements of the walking cat, our first aim was to determine how cats hold the head in respect to the earth horizontal. This orientation is important, because it determines the efficacy of activation of vestibular receptors during locomotion. Studies by Graf and colleagues (1995) showed that, while cats may at times rotate their head substantially, most of the time they keep horizontal semicircular canals oriented within 5–15° of the earth horizontal. Unfortunately, the authors' quantity of data in respect to locomotion was limited. In humans, non-human primates, and horses, however, it was found that during most locomotor tasks the head rotation remains within a 20° range in all planes, providing a relatively stable spatial reference frame to the brain (Pozzo et al., 1990; rev. in Dunbar et al., 2008). We were interested in whether the head orientation of the walking cat complies with this rule.
Our second objective was to estimate the contribution of head mechanical properties and potential contribution of reflexes to head movement during walking. To accomplish this, we analyzed frequencies of head movements, their velocities, and accelerations. It is believed that during locomotion, reflexes participate greatly in establishing orientation of the head with respect to the earth horizontal and support surface, and in governing head movements. For example, rotation of the head around the intra-ural axis (in pitch), which in bipeds stabilizes the head fixation point in space, is considered to be a result of vestibulo-collic reflexes both in bipeds and quadrupeds (Hirasaki et al., 1999; Xiang et al., 2008). Therefore, we investigated whether rotational movements of the head in the cat during walking are a mechanical consequence of head translations, and whether velocities and accelerations of head translations and rotations are in the ranges that activate vestibulo-collic and cervico-collic reflexes.
Finally, we were interested in the role of light in head orientation and movement during locomotion. During walking under normal laboratory illumination there are many potential visual targets in the environment for the animal to look at, ranging from pieces of dirt on the walkway to objects in the laboratory. We previously found that cats look at closer points on the walkway when walking in the light than in darkness (Rivers et al., 2014). This is likely because in the light, proximal areas of the walkway were of interest to the cats. Therefore, to differentiate head movements related to viewing of the environment from those determined by other locomotor mechanisms, we examined head movement during walking in both light and complete darkness.
We found that during walking the head of the cat underwent small translations and rotations along all of its axes: the left-right translation, and roll and yaw rotations oscillated once per step cycle, while the fore-aft and vertical translations, and pitch rotation oscillated twice. During walking, the head was oriented nose-down so that horizontal semicircular canals and utriculi stayed within ±10° of the earth horizontal most of the time. Head velocities and accelerations were well in the activation range for vestibulo-collic and cervico-collic reflexes. Head rotation movements, however, could not be completely explained as reflexes of head translations. Illumination of the room caused cats to hold their head slightly higher and move it more. We concluded that, during walking, the head of the cat is held actively. However, reflexes appear to play only a partial role in determining head movement, and vision might further diminish their role.
A partial account of this study was published in abstract form (Rollando et al., 2012; Zubair et al., 2015).
EXPERIMENTAL PROCEDURES
Three adult cats were used in these studies: two females (Cat 1, 3.7 kg and Cat 3, 3.0 kg) and a male (Cat 2, 4.0 kg). The data were collected during our study of gaze behaviors in freely walking cats, which was reported previously (Rivers et al., 2014). These cats were also used for other studies (Marlinski et al., 2012a,b; Armer et al., 2013; Favorov et al., 2015). All experiments were conducted in accordance with NIH guidelines and with the approval of the Barrow Neurological Institute Animal Care and Use Committee.
Locomotor tasks
Food was used as positive reinforcement to adapt cats to the experimental situation and engage them in locomotor behavior (Skinner, 1938; Pryor, 1975). Cats walked in a chamber divided by a longitudinal wall into two corridors, a test- and return-corridor, each 2.5-m-long and 0.28-m-wide (Fig. 1A). Cats passed through the chamber sequentially and repeatedly in a counter-clockwise direction. The floor in the chamber was covered with rubberized black material. In the test corridor, one wall was constructed of clear acrylic plastic to permit recording of cat movements (see below), while other walls were opaque. The passage of the cat throughout the test corridor was monitored using photo-sensors paired with infrared light-emitting diodes (LEDs). LEDs had emission wavelengths of 850–900 nm, which is outside the visible spectral range of the cat (Guenther and Zrenner, 1993).
Fig. 1.
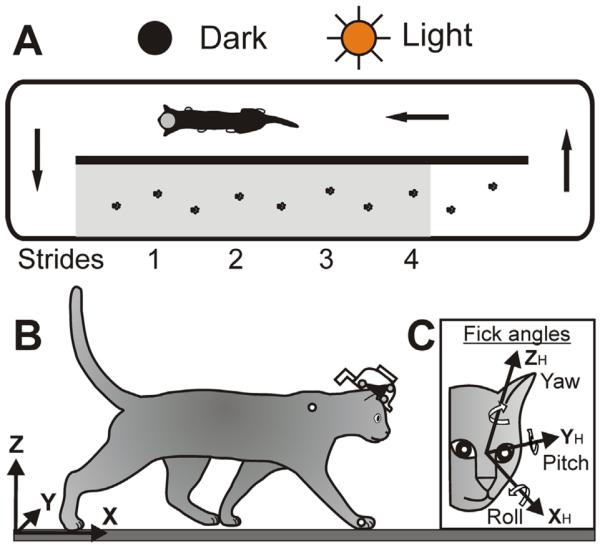
Aerial view of walking in the chamber and coordinate frames. (A) Arrows indicate direction of cat walking. The footprints are placements of right and left forelimbs during a passage along the walkway. The portion of the chamber shaded gray was visible to the VZ recording cameras. (B) The chamber-related global (X,Y,Z) coordinate system was used to describe linear variables of the head in space. (C) The “Fick” coordinate system (XH, YH, ZH) was used to describe rotational variables/the orientation of the head (Fick, 1854). The head was defined as the midpoint between the centers of the left and right eyes. Approximate positions of LEDs on the head implant, right wrist and shoulder are indicated by small circles.
Cats were presented with two locomotor tasks (Fig. 1):
-
(1)
Walking in complete darkness. Recordings during this task provided background information on head movement during locomotion, which was not related to visual sampling of the environment. For this task, all sources of visible light inside the room were either extinguished or covered. The only source of light in the room was a set of computer-controlled standard fluorescent bulbs, which provided approximately 500 lux of illuminance. A photocell was used to record the state of illumination. On randomly selected trials, these lights were turned off, triggered by the cat passing by a photo-sensor at the beginning of the test corridor. Within 17 ms, the level of illumination in the room fell to less than 0.01 lux, as measured by a T-10 illuminance meter (Konica Minolta, Ramsey, NJ, USA). This level of illumination can be considered as complete darkness for the cat (see discussion in Rivers et al., 2014). Lights came back on when the cat passed the photo-sensor near the end of the corridor.
-
(2)
Walking in the light. Recordings during this task provided information on head movements when visual information was available but not necessary for successful passage (since cats could successfully walk in the test corridor in the dark, it is clear that visual information was not required for this task). On trials randomly alternated with those presented in the dark, the lights stayed on and cats walked on the same surface. When the lights were on, there were many potential areas of visual interest for the cats – ranging from proximal areas of the walkway's surface to items in the laboratory far from the cat.
The passage through the return corridor was always accomplished in the light, and the food reward was given in the same location of the return corridor. Cats were trained on the same schedule for at least one month before data collection was initiated. During training, cats were accustomed to wearing a cotton jacket and a light backpack with preamplifiers for signals collected for other studies.
Surgical procedures
After cats were trained, surgery was performed under Isoflurane anesthesia using aseptic procedures. Surgical procedures are described in detail in our previous publications (Beloozerova and Sirota, 1993; Prilutsky et al., 2005). In short, to form a “head base”, the skin and fascia were retracted from the dorsal surface of the skull. At ten points around the circumference of the head, stainless steel screws were inserted into the skull. The screw heads were imbedded into a plastic cast to form the head base. The top of it was made parallel to the stereotaxical horizontal plane. The cats used for this study were also implanted with devices to allow neuronal activity and eye movement recordings during other experiments. The total weight of all implants and a protective cap was approximately 100 g.
Recordings
The three-dimensional coordinates of the head were recorded using a computerized, active-marker real-time motion capture and analysis system Visualeyez (VZ-4000, Phoenix Technologies Inc., Burnaby, BC, Canada). The recording cameras of the Visualeyez apparatus were positioned approximately 2.5 m from the transparent wall of the chamber. Three wide-angle, six-chip infrared LEDs with wavelengths of 755–785 nm, which is outside the visible spectral range of the cat (Guenther and Zrenner, 1993), were attached to the head implant in a non-collinear fashion 3–8 cm apart (Fig. 1B). The “object” function of the VZ software was used to determine coordinates of “the center of mass” of the triangle formed by the LEDs (the triangle is schematically shown in Fig. 1B as a black shape on the cat's head). During data analyses, coordinates of the location of the “object” were transposed to the midpoint between the left and right eyes, which was defined as “the head”. Distances between LEDs and the centers of the eye orbits were obtained from X-ray images.
In addition to LEDs, during recordings cats wore on their heads electromagnetic field emitting antennas for recording of eye movements using a scleral search coil system (Robinson, 1963). These antennas were positioned 5 cm behind the head and weighed 50 g (Fig. 1B, see also Rivers et al., 2014). Cats also wore a preamplifier for signals from the scleral search coil, which was mounted on the top of the head and also weighed 50 g. Data on eye movements obtained from the eye coil system are not included in this report. Cats did not appear to be disturbed by the added weight of head implants and electronics.
A “head-in-space” or “global” coordinate frame (X, Y, Z) was defined in relation to the walkway (Fig. 1B). Coordinates of this frame originated in the left corner of the walkway's surface closest to the VZ cameras. The X-axis was parallel to the longitudinal axis of the test corridor, the Y-axis was orthogonal to the X-axis and run along the width of the walkway (an increase in lateral displacement indicated that the cat head traveled further away from the VZ), and the Z-axis was orthogonal to the XY-plane and was directed upward.
To determine rotation of the head in space and angular velocities, a `rigid body' was created based on the LEDs using the “rigid body” function of VZ software. An adopted “Fick” coordinate system (XH, YH, ZH) was used (Fig. 1C; Fick, 1854). It was defined in stereotaxical planes as follows: the XH (roll) axis was the rostro-caudal line (an increase in roll indicated the head clockwise rotation from the animal's point of view, a rotation when the right ear was going downward), the YH (pitch) axis was orthogonal to the rostro-caudal line and parallel to the inter-aural line (positive rotation was upward), and the ZH (yaw) axis was orthogonal to those two and was directed upward (positive rotation was to the cat's left).
To record swing and stance phases of the stride, an LED was placed on the base of the fifth metacarpal of the right paw (the metacarpophalangeal joint, MCP; Fig. 1B). In addition, in Cats 1 and 2 an LED was placed on the skin projection of the greater tubercle (shoulder joint) of the right forelimb (Fig. 1B), and in Cat 3 an LED was positioned on the backpack, which will be termed “trunk” below.
Calibration of the VZ system was performed according to manufacturer specifications; error for recording an LED position did not exceed 0.5 mm. Signals from all LEDs were sampled at a frequency of 200 Hz (Cats 1 and 3) or 340 Hz (Cat 2) and saved to a computer hard disk.
Before testing during locomotion on each experimental day, a reference for recordings was obtained in the stationary cat. The cat was seated next to the chamber in a comfortable position and its head was fixed to an external frame with zero degrees rotation in yaw and roll, and −17° in pitch (nose down), which was close to the natural orientation of head in the sitting cat. Once placed in the experimental chamber, the cat continuously walked through corridors, stopping only briefly after each trip for a food reward. We refer to the full locomotor cycle of the limb (beginning of swing to beginning of next swing of the same limb) as a “step cycle” or “stride” (Fig. 1A), and use them interchangeably. We call one half of such cycle a “step”. Cats generally completed four strides in the corridor. However, only the first three strides were analyzed in this study, because the LEDs on the head and right forelimb during the fourth stride were outside of the optimal view for the recording cameras.
Data analysis
For the analysis we only included the trials in which walking in the test corridor began with the swing of the right forelimb. Data on the head linear displacement, displacement velocity, rotation and angular velocity, as well as on linear displacement of cat's paw and shoulder/trunk were exported to Matlab (MathWorks, Natick, MA, USA) and smoothed with the medfilt1 function (median filter size 31) (Tukey, 1977; Moore and Jorgenson, 1993; Stone, 1995). Linear accelerations of the head were calculated by differentiating smoothed velocity data.
The swing and stance phases of the right forelimb were determined based on velocity of LED on the right foot: the first frame in which the longitudinal velocity of this LED exceeded 0.3 m/s was taken as the beginning of the swing phase, and the first frame in which it was zero was taken as the beginning of the stance phase of that limb. The duration of each stride was divided into 20 equal bins, and head movement data in each bin were averaged across all selected trials.
To analyze phase relationships between the vertical displacement of the shoulder/trunk, vertical displacement of the head, and the head pitch rotation, these variables were fitted with sine functions using Igor Pro software (WaveMetrics, Portland, OR, USA), and the phase difference between the functions was determined. To increase accuracy of the fit, the data were corrected for any steady changes before fitting. For that, the data were approximated with a linear regression and then rectified by subtracting the change estimated by the regression. A Fast Fourier Transform (FFT) was computed on stretches of rectified raw data to produce a power spectrum.
The head resonant frequencies during walking were calculated based on dimensional similarity between species. The following equation was used: t ∝ Mn, where M is mass, t is the time taken to accomplish a movement, 1/12<n<1/3, and frequency α=1/t (Jones and Spells, 1963; Dunbar et al., 2004).
Descriptive statistics, mean, and standard deviation (mean±SD) were used to quantify data samples. Differences between categories were determined using independent two-tailed t-test. A One-Way ANOVA was used to compare averaged variables among cats and among sequential strides along the test corridor. Fisher's exact test was used to compare proportions. Significance level of all tests was set at 0.05.
RESULTS
The data analyzed in this study were collected during two experimental days with each animal that were 5–8 months after the surgery and were separated by 1–2 days. These were the same experiments which produced data reported in our previous publication on gaze behaviors during walking (Rivers et al., 2014). Data were averaged across all selected trials from both experimental days. For each cat, 36±9 passages through the test corridor during each locomotor task were included. During these passages, 96±34 strides were analyzed for each locomotor task in each cat, including 36±9 strides for each of Strides 1, 2, and 3 (Fig. 1A).
General characteristics of walking
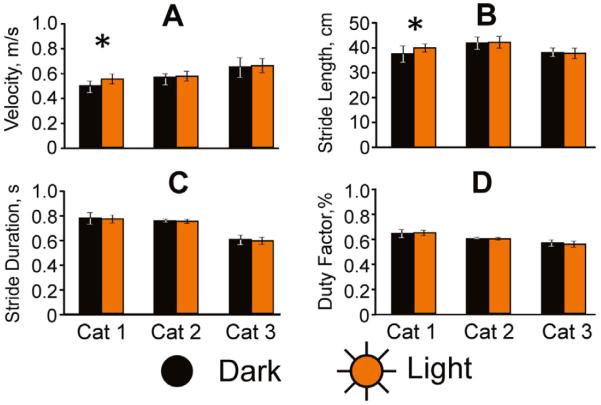
General characteristics of walking in the darkness and light are shown in Fig. 2. Velocity of walking in the dark slightly varied among cats, and was 0.49±0.1, 0.57±0.04, and 0.65±0.16 m/s in Cat 1, 2, and 3, respectively (p<0.05). Cat 1 walked faster in the light than in darkness (p<0.01), but Cats 2 and 3 walked with similar velocity in both conditions (p>0.05, Fig. 2A). Length of strides varied, but across cats 90% of strides measured between 36 and 47 cm. Cat 1 took larger strides in the light than in the darkness (p<0.01), but the stride length of Cats 2 and 3 did not vary between light and darkness (p>0.05, Fig. 2B). Stride durations were similar during walking in the darkness in Cats 1 and 2 (768±100 ms and 746±29 ms, respectively), but were shorter in Cat 3 (594±75 ms; p<0.01). All three cats, however, had strides of a similar duration during the two illumination conditions (Fig. 2C). Duty factor, which is the ratio of stance duration to overall stride duration, was about 60% in all three cats, and did not differ between the conditions (Fig. 2D).
Fig. 2.
General characteristics of walking. Velocity (A), stride length (B), stride duration (C) and duty factor (the ratio of stance duration to stride duration) (D) are shown for both dark (black) and light (orange/gray) conditions for each of the three cats. Asterisks indicate a statistically significant difference. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Resonant frequencies of the head
The head of the cats used in this study was implanted with a head stage and devices for neuronal activity recording. The total weight of implants and a protective cap was ~100 g. In addition, during recordings cats wore on their heads LEDs, connectors, electromagnetic field-emitting antennas for recording eye movements using a scleral search coil system, and a preamplifier for signals from the scleral search coil (Rivers et al., 2014). The preamplifier was mounted on the top of the head and weighed 50 g, while the antennas were positioned about 5 cm behind the head and weighed an additional 50 g (Fig. 1B, see also Rivers et al., 2014). Collectively, all head implants and recording equipment weighed about 200 g, which is equivalent to approximately 50% of the weight of an intact head (the weight of Cat 1 head was 375 g). The weight of the apparatus shifted the head's center of mass 1.0 cm caudally and 1.7 cm dorsally in relation to the center of mass of an intact head.
Peterson and Goldberg (1981) estimated that in the intact awake cat, inertia only begins to play a role in stabilization of the head when it moves at a frequency higher than 5 Hz; they further estimated that inertia only becomes a dominant factor at frequencies of 10 Hz and higher. In order to account for the added weight of recording devices to the cat's head, we used a correction previously employed by other researchers to estimate resonant frequencies for the head when the mass of the head is different (Jones and Spells, 1963; Dunbar et al., 2004). According to this correction, the same frequency threshold for the effect of inertia in our cats is between 4.3 and 4.8 Hz rather than 5 Hz in a cat with an intact head.
Translations and rotations of the head during walking
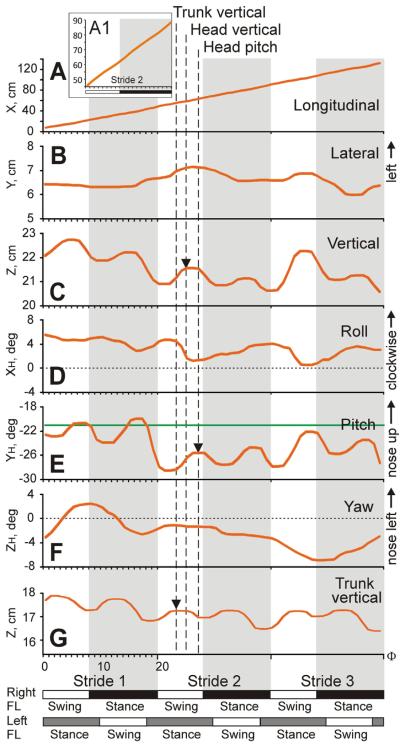
The six first-order variables of head movement during the first three strides of an example passage through the test corridor are depicted in Fig. 3. Three of these variables involved displacement of the head in space: longitudinal (fore-aft) displacement along X axis, lateral displacement along Y axis, and vertical displacement along Z axis (Fig. 1B). In this passage, the cat (Cat 3) walked steadily along the corridor (Fig. 3A) with two very minor oscillations per step cycle (Fig. 3A1). With every stride, the cat's head oscillated once in the lateral direction, moving to the left during swing and to the right during stance phase of the right foot (Fig. 3B, oscillations are most visible during Strides 2 and 3). The position of the head oscillated twice during each stride in the vertical direction, reaching the first maximum during the swing and the second maximum during stance phase of the right foot (Fig. 3C). Vertical position of the trunk oscillated at the same frequency with peaks occurring slightly earlier in the cycle (Fig. 3G).
Fig. 3.
Head movements during an example trial. Six first order head movement variables (A–F) are shown for one passage by Cat 3 along with vertical displacement of the cat's trunk (G). Strides 1, 2, and 3 are shown; they were time-normalized and divided into 20 bins each. Durations of stride phases of the right forelimb (FL) are shown on the bottom: white rectangles depict the swing and black rectangles depict the stance phases of the strides. Estimated durations of swing and stance phases of the strides of the left forelimb are shown as white and gray rectangles, respectively. (D, F) Horizontal dotted lines indicate zero degree in roll (D) and yaw rotation (F). (E) Horizontal green line indicates −21° in pitch rotation, at which horizontal semicircular canals and utriculi are parallel to the earth horizontal (Curthoys et al., 1977a,b). Vertical dashed lines and arrows highlight positive peaks in the head vertical position (C), head upward pitch rotation (E), and vertical trunk position (G). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Three other variables of the head movement characterized rotation of the head: roll around the XH axis, pitch around the YH axis, and yaw around the ZH axis (Fig. 1C). During each stride, the cat rolled its head to the left (left ear down) during swing and to the right (right ear down) during stance phase of the right foot (Fig. 3D). In addition, there were two pitch oscillations of the head during every stride. They reached the first maximum at the end of swing and the second maximum at the end of stance phase of the right foot, slightly later in the cycle than the head vertical displacement (Fig. 3E). The head yaw rotation was not related to phases of the stride during this trial (Fig. 3F).
Head movement data for one cat
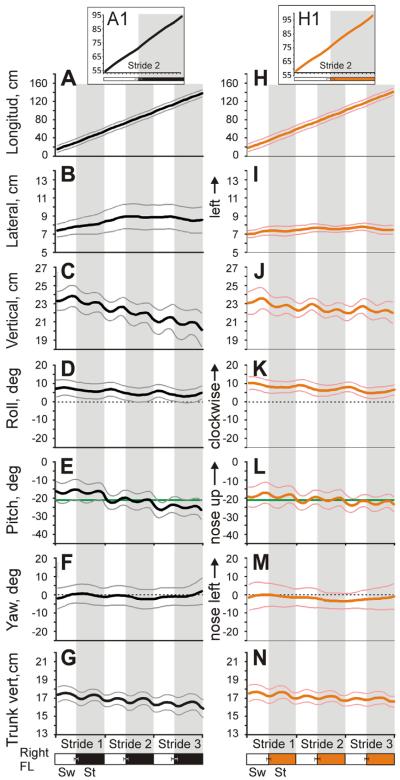
Fig. 4 shows first-order displacement and angular variables of the head movement of the same cat (Cat 3) averaged over all selected trials of walking in the darkness (Fig. 4A–G) and light (Fig. 4H–N). In both conditions, the cat's head moved steadily along the corridor at a similar velocity (Fig. 4A, H) with only very minor oscillations (Fig. 4A1, H1). The head lateral displacement (Fig. 4B, I), roll (Fig. 4D, K), and yaw (Fig. 4F, M) rotations oscillated once per stride, while the longitudinal (Fig. 4A1, H1), vertical displacements (Fig. 4C, J), and pitch rotation (Fig. 4E, L) oscillated twice. There were no stride phase-related differences between the illumination conditions.
Fig. 4.
Head movement of Cat 3 (mean±SD) during three strides of walking in the darkness (A–G) and light (H–N). Thick black and orange/gray lines show the mean for all selected trials of walking in each illumination condition. Thin gray and pink/light gray lines show one standard deviation for dark and light conditions, respectively. Phases of time-normalized strides of the right forelimb are shown at the bottom; zero percent corresponds to swing onset of this limb. White rectangles depict swing (Sw) and black or orange/gray rectangles depict stance (St) phases of the strides during walking in the darkness and light, respectively; standard deviations of the means are shown. (D, F, K, M) Horizontal dotted lines indicate zero degree in roll (D, K) and yaw (F, M) rotations. (E, L) Horizontal green lines indicate −21° in pitch rotation, at which horizontal semicircular canals and utriculi are parallel to the earth horizontal. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
There were differences between darkness and light, however, in how the head movement developed during walking along the corridor. At the beginning of Stride 1 in both conditions, the cat's head was about 23 cm above the ground. The cat steadily lowered it as it walked, with an overall decrease of 3 cm over the course of the three strides in the dark but only half as much (1.5 cm) in the light (p<0.01; Fig. 4C, J). Also, at the beginning of Stride 1 in both conditions, the head was tilted in pitch about 17° nose-down and, with every stride, rotated further down. This rotation was about 4° in the dark but only 2° in the light (p<0.01; Fig. 4E, L). Over the course of three strides, the cat typically wandered slightly closer to the left wall of the corridor; this displacement tended to be greater in the darkness than in the light (Fig. 4B, I). In both conditions, at the beginning of Stride 1 the cat rotated its head around the vertical axis (yaw) about 3° to the right, which was toward the transparent side of the corridor (Fig. 4F, M); however, by the end of the third stride in both conditions, the cat's head was neutral regarding yaw.
Head movement data across all cats
Graphical summaries of the head translational and rotational variables across all selected trials of walking in the darkness and light for each of the three cats are shown in Figs. 5 and 6. Cats were slightly different in size, general walking characteristics (Fig. 2), and in how they moved their head; therefore, we present data separately by the cat. Differences between illumination conditions were typically small in all cats, however; and, to simplify presentation, the numerical data in this section and in sections describing head velocities and accelerations below are given for walking in the darkness. Illumination conditions are compared in a subsequent section.
Fig. 5.
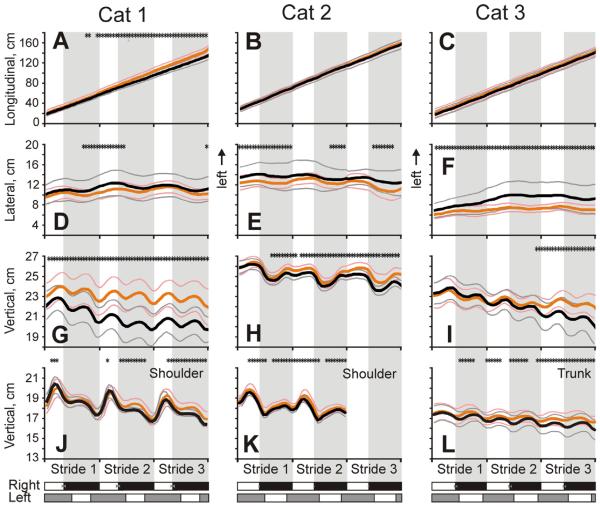
Linear head and shoulder/trunk translations (mean±SD) in each cat during three strides of walking in the darkness and light. Head displacement in the longitudinal (A–C), lateral (D–F), and vertical (G–I) directions. J–L: Shoulder and trunk displacements along the vertical axis. On all graphs, data for the two illumination conditions are superimposed; and stars indicate stride phases, in which a variable was statistically significantly different (p<0.05) between the conditions. Other designations as in Figs. 3 and 4.
Fig. 6.
Head rotations (mean±SD) in each cat during three strides of walking in the darkness and light. Yaw (A–C), roll (D–F), and pitch (G–I) rotations are shown. Designations as in Figs. 4 and 5.
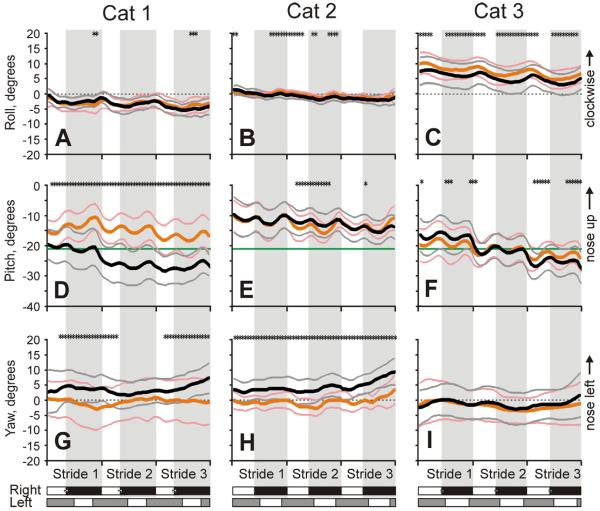
All cats walked smoothly along the corridor in both darkness and light (Fig. 5A–C). During each stride, the head translated laterally once during the stride (Fig. 5D–F). It started its leftward movement at the end of the stance phase of the right forelimb and continued this movement during swing phase of that limb, while the rightward movement started at the end of the stance phase of the left forelimb and continued during swing phase of that limb. Average peak-to-peak lateral translations were similar between cats: 1.2±0.6, 1.0±0.5, and 1.3±0.5 cm in Cat 1, 2, and 3, respectively (p>0.05). Additionally, the head had two cycles of translation along the vertical axis during the stride, reaching positive peaks in the second half of the swing of each forelimb, and negative peaks in the first half of the stance phase of each limb (Fig. 5G–I). The amplitude of peak-to-peak vertical linear translations was also similar between cats: 1.7±1.5, 1.6±0.9, and 1.4±1.2 cm in Cat 1, 2, and 3, respectively (p>0.05). In Cat 2, the vertical translations were larger than the lateral translations (p<0.001); this trend was true in Cat 1 but the difference was not statistically significant (p=0.07); and there was no difference between these two values in Cat 3 (p>0.05). Vertical movement of the right shoulder had one positive peak during the stride (Fig. 5J, K), while that of the trunk had two positive peaks: one in the middle of the swing phase of each forelimb (Fig. 5L). Ranges of vertical displacements of the shoulder in Cats 1 and 2 and the trunk in cat 3 were 2.9±1.4, 2.2±1.5, and 0.8±1.4 cm, respectively. In addition to the stride-related oscillations, over the course of three strides, vertical position of the head of all cats, as well as that of the shoulder of Cats 1 and 2 and trunk of Cat 3 decreased (p<0.05). This decrease was larger in the darkness (p<0.05).
Simultaneously with linear translations, the head underwent rotary movements (Fig. 6). It moved around the longitudinal axis (in roll) once every step cycle rolling to the left during swing and to the right during stance phase of the right foot. The range was similar among cats: 2.9±2.1°, 1.5±1.3°, and 2.9±3.9° in Cat 1, 2, and 3, respectively (p>0.05; Fig. 6A–C). In respect to pitch, Cats 1 and 3 started walking along the corridor with their heads tilted nose-down 17–19° in relation to the earth horizontal, while Cat 2 had it tilted ~10° down. During walking, the head of all cats oscillated twice in pitch during each step cycle, reaching the first maximum at the end of swing and the second maximum at the end of stance phase of the right foot. The peak-to-peak amplitudes were also similar among cats: 3.4±5.4°, 3.0±4.2°, and 2.7±4.7° in Cat 1, 2, and 3, respectively (p>0.05; Fig. 6D–F). In Cat 2, the amplitude of pitch rotation was larger than that of roll (p<0.05), but was similar to that in Cats 1 and 3 (p>0.05). Over the course of three strides, all cats tended to tilt their head still more nose-down, with Cats 1 and 3 tilting it to 25–30° below the earth horizontal. This gradual tilting reached statistically significant values only during walking in the darkness, however.
The head of Cat 1 had no stride-related oscillations in yaw, while heads of Cats 2 and 3 had small oscillations, once per step cycle, but they were phased differently in these cats (Fig. 6G–I).
Phase relationships between vertical movements, the head fixation point
Frequency of movements
Stride-related changes in the head vertical position, pitch rotation, and vertical position of the trunk were of the same frequency, which was double the frequency of the step cycle (Figs. 5G–I, L; 6D–F). To explicitly demonstrate this and also to look for any high frequency components in the head rotary movement, we conducted Fourier analyses. Graphical summaries of results across selected trials of walking in the darkness for each of the cats are shown in Fig. 7. According to the duration of strides that ranged between 600 and 800 ms across the cats (Fig. 2C), the power spectrum for the forelimb movement peaked at the stride frequency between 1.25 and 1.75 Hz, and had a smaller peak at the step frequency around 3 Hz (Fig. 7A–C). The dominant frequency of the head vertical translation was also around 3 Hz for Cats 1 and 3, and the power spectrum for Cat 2 had a prominent peak at this frequency (Fig. 7D–F). The dominant frequencies of head pitch rotations were also around 3 Hz in all cats (Fig. 7G–I). The broad low frequencies observed in head translations and rotations reflected irregularities in head movement during walking. In Cat 2, the peak in the power spectrum for the head translation at the stride frequency reflected non-symmetry of head movement in this cat in respect to steps of the right and left limb (Fig. 5H). None of the movements had any appreciable frequencies higher than 4 Hz, including in the 4.3–4.8 Hz range (highlighted by gray vertical bars in Fig. 7G–I), where pitch rotations would be expected based on the resonant frequency of the head.
Fig. 7.
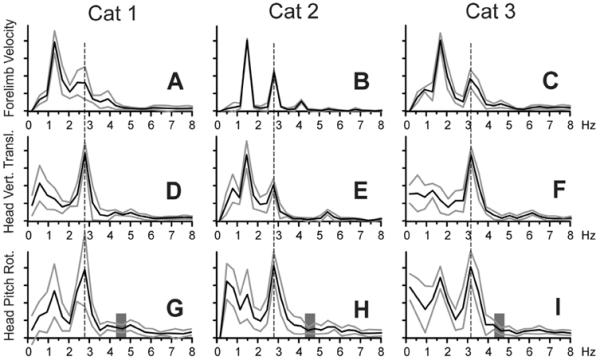
Fourier power spectra (mean±SD) of the forelimb forward velocity (A–C), the head vertical translation (D–F), and pitch rotation (G–I) for each cat. The dominant frequency of head pitch rotation is indicated with a vertical dashed line. Values for frequencies above 8 Hz were very small and are not shown. (G–I) Vertical gray bars highlight the range of resonant frequencies for the head. Other designations as in Fig. 4.
Phase relationships between vertical movements
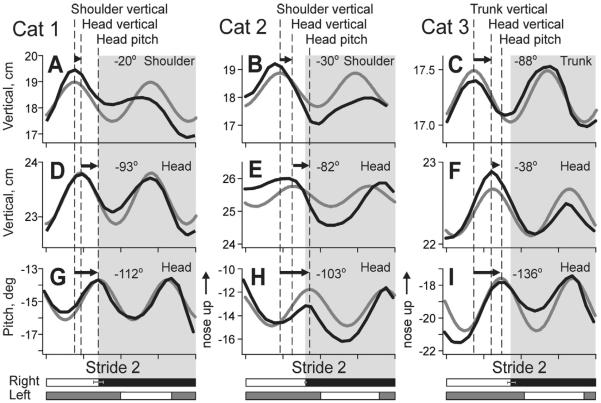
To quantify stride phase relationships between the head vertical position, pitch rotation, and vertical position of the shoulder/trunk, we have fitted the averaged data shown in Figs. 5G–L and 6G–I with sinusoids (Fig. 8). We found that peaks in the vertical head position followed peaks in shoulder/trunk position by 20±17°, 30±4°, and 88±9° in Cat 1, 2, and 3, respectively (Fig. 8G–I). At the same time, peaks in the head pitch rotation followed peaks in the head vertical position with phase lags of 93±14°, 82±25°, and 38±8° in Cat 1, 2, and 3, respectively (Fig. 8D–F). In Cats 1 and 2, a smaller phase lag between peaks in the head and shoulder vertical positions (Fig. 8G, H) was followed by a larger lag between the head vertical position and pitch upward rotation (Fig. 8D, E), while this was the opposite in Cat 3 (Fig. 8F, I). In result, the most upward pitch position of the head followed the maximal vertical shoulder/trunk displacement with a similar phase delay in all three cats: 112±6°, 103±30°, and 136±15° in Cat 1, 2, and 3, respectively (p>0.05; Fig. 8A–C).
Fig. 8.
Phase relationships between vertical shoulder/trunk translation, head vertical translation, and head pitch rotation. Black lines show averaged data for Stride 2, and gray lines show sinusoidal fits for the data. Positive peaks in the shoulder/trunk and head vertical translations, and head pitch rotation are indicated with dashed lines and arrows. Mean average phase differences between them for walking in the darkness are indicated on top of the graphs.
Location of head fixation point in space
We calculated the “Head Fixation Point”, a point where the head occipito-nasal (XH) axes coincide in space for the highest and lowest head vertical positions during the step cycle (Fig. 9A1; Pozzo et al., 1990; Hirasaki et al., 1999). We found that this point was behind the head during 59–63% of Strides 1 and 3 and 86% of Strides 2. This means that, although during nearly a half of the step cycle, the head was rotating downward while translating upward and rotating upward while translating downward (Fig. 8), pitch rotations were most often non-compensatory, meaning that they were insufficient at opposing disturbances caused by the head vertical translations and maintaining the head's fixation point in front of the subject. During the strides when the head fixation point was in front of the cat (39% of strides in dark and 37% in light), the point was between 5 and 100 cm ahead of the cat, and was between 10 and 40 cm in ~40% of these strides (Fig. 9). At distances shorter than the length of the average stride (5–30 cm), it was 5–25 cm above the walking surface, and was within 5 cm of the surface at stride length and longer distances. It was higher during walking in the light than in darkness (12.6±8.6 cm vs. 6.7±6.0 cm above the ground; p<0.001). In 11% and 7% of the strides in the darkness and light, respectively (not statistically different), the head fixation point was 20–200 cm or occasionally more below the walking surface.
Fig. 9.
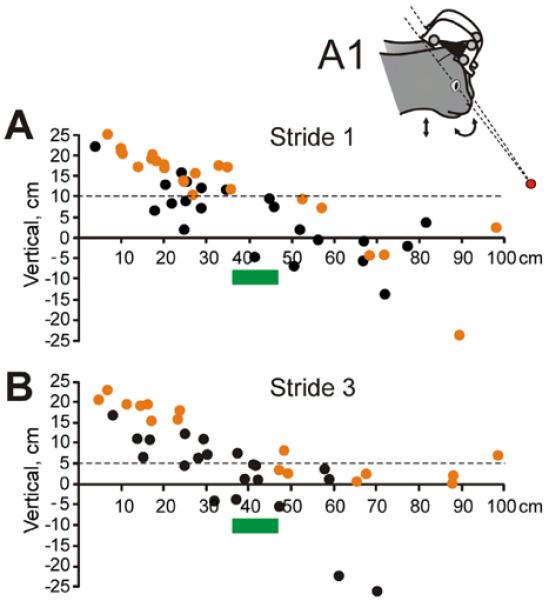
Locations of the head fixation point in front of the cat. (A) Positions of head fixation points, where the head occipito-nasal (XH) axes coincided in space for the head's highest and lowest positions during the step cycle, during Stride 1 of different trials of walking in the darkness (black symbols) and light (orange symbols). Intersecting axes are schematically shown in the insert A1. (B) Positions of head fixation points during Stride 3 of different trials. (A, B) Green bars highlight the stretch of the supporting surface where the forefoot will be placed during the stride. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Velocities of head movements
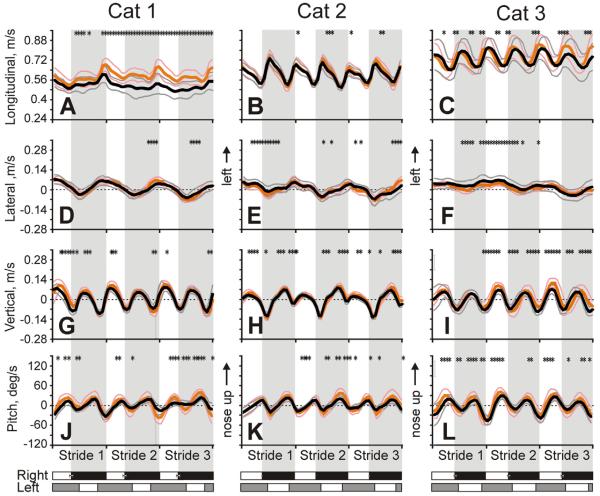
Head linear velocities and velocity of pitch rotation averaged across all selected trials for each cat are shown in Fig. 10. Velocity of head movement along the longitudinal axis had two distinct components (Fig. 10A–C). One component was the basic steady velocity of the cat progressing along the corridor, which among cats ranged between 0.5 and 0.7 m/s. The second component was stride-related oscillations in the velocity that were superposed on this basic progression velocity. During these oscillations, the head velocity was the highest at the beginning of the swing phase and the smallest at the beginning of the stance phase of each forelimb. The peak-to-peak amplitudes of the oscillations were slightly different among cats at 0.09±0.06, 0.19±0.04, and 0.15±0.09 m/s for Cat 1, 2 and 3, respectively (p<0.05; Fig. 10A–C).
Fig. 10.
Velocities of head linear translations and pitch rotations (mean±SD) in each cat during three strides of walking in the darkness and light. Velocity in the longitudinal (A–C), lateral (D–F), and vertical (G–I) directions. J–L: Velocity of head pitch rotations. Designations as in Fig. 5.
Velocity of the head lateral translations oscillated once per stride with positive and negative peaks at the end of stance of the right and left forelimb, respectively (Fig. 10D–F). Maximal velocities were similar among cats at 0.05±0.04, 0.04±0.03, and 0.05±0.03 m/s for Cat 1, 2, and 3, respectively, as were peak-to-peak velocity amplitudes at 0.10±0.06, 0.09±0.06, and 0.06±0.07 m/s for Cat 1, 2, and 3, respectively (p>0.05).
Vertical velocity of the head oscillated twice per stride reaching the maximum in the middle of the swing phase and the minimum in the beginning of the stance phase of each forelimb (Fig. 10G–I). Maximal velocities of the head moving upward were similar between cats: 0.07±0.03, 0.06±0.02, and 0.06±0.04 m/s in Cat 1, 2, and 3, correspondingly. Maximal velocity of the head moving downward was higher than upward velocity (p<0.01 for each cat), but also did not vary among cats: −0.10±0.03 m/s, −0.13±0.02 m/s, and −0.09±0.04 m/s in Cat 1, 2, and 3, respectively.
Angular velocity of the head rotation in pitch oscillated twice per stride (Fig. 10J–L). Velocity of the nose-up head rotation reached the maximum at the end of the swing phase of each forelimb. Maximal upward velocities were: 17.4±5.3, 17.1±9.0, and 22.4±11.1 °/s for Cat 1, 2, and 3, respectively. Velocity of the head downward rotation was maximal in the first half of the stance phase of each forelimb. Maximal downward velocities were: 30.2±14.4, 19.9±9.9, and 45.4±7.5 °/s for Cat 1, 2, and 3, respectively. For Cats 1 and 3 they were significantly higher than upward velocities (p<0.01). The amplitude of the peak-to-peak velocity oscillations was 47.6±19.7, 37.0±19.1, and 67.9±18.7 °/s in Cat 1, 2, and 3, respectively.
There was no clear relationship between velocities of the head roll and yaw rotations and the step cycle.
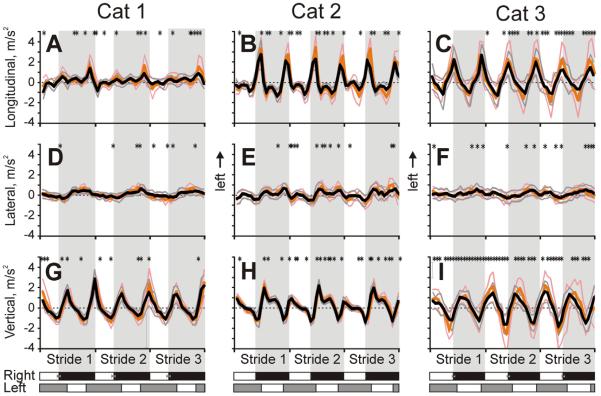
Accelerations of head linear translations
Linear accelerations averaged across all selected trials for each cat are shown in Fig. 11. The head accelerated forward twice per step cycle, at the beginnings of the stance phases of each forelimb (Fig. 11A–C). The maxima were 2.1±0.4; 1.9±0.5 and 1.3±1.0 m/s2 in Cat 1, 2, and 3, respectively. The maxima of aft acceleration were 1.2±0.4, 1.5±0.5, and 1.7±1.0 m/s2, respectively.
Fig. 11.
Accelerations of head linear translations (mean±SD) in each cat during three strides of walking in the darkness and light. Accelerations in the longitudinal (A–C), lateral (D–F), and vertical (G–I) directions. Designations as in Fig. 5.
Accelerations of head translations in the lateral direction were smaller (Fig. 11D–F). Maximal lateral accelerations were: 0.4±0.2, 0.6±0.2, and 0.4±0.2 m/s2 for Cat 1, 2, and 3, respectively. Acceleration to the left increased during the stance phase of the right forelimb, and acceleration to the right increased during the stance phase of the left forelimb. The amplitudes of their peak-to-peak changes were 0.8±0.4, 1.1±0.5, and 0.8±0.5 m/s2 in Cat 1, 2, and 3, respectively.
The head also accelerated upward twice per step cycle: in the initial part of stance phase of each forelimb (Fig. 11G–I). Maximal upward accelerations were 2.1±0.6, 1.9±0.4, and 1.3±0.8 m/s2 in Cat 1, 2, and 3, respectively. Downward accelerations lasted during the swing phase of each forelimb and had maxima of −1.2±0.6, −1.5±0.4, and −1.7±0.8 m/s2 in Cat 1, 2, and 3, respectively.
Data on rotational accelerations were too noisy to draw any conclusions.
Relationship between head movements and velocity of walking
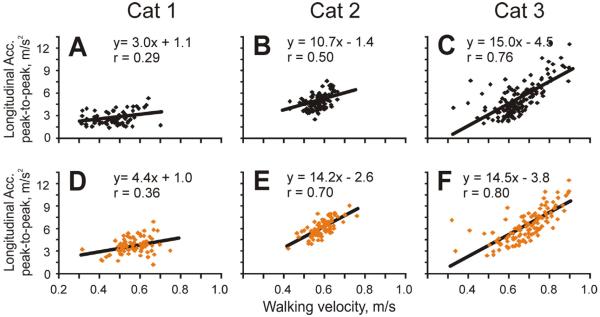
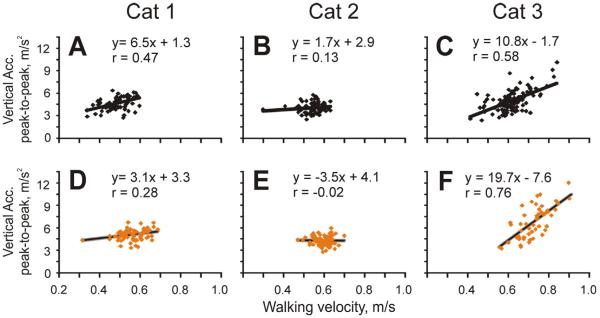
We have analyzed relationships between the minimum/maximum/range values of head movement variables including longitudinal, lateral, and vertical displacements; roll, pitch, and yaw angles; longitudinal, lateral, and vertical velocities; roll, pitch, and yaw velocities; and longitudinal, lateral, and vertical accelerations on one hand and the velocity of strides and length of strides on the other hand. Of these combinations, the strongest relationship found was between the longitudinal acceleration of the head and the velocity of the stride. In particular, there was a positive correlation between the maximal longitudinal acceleration of the head during the stride and stride velocity (r=0.66), and a negative correlation between the minimal longitudinal acceleration during the stride and this variable (r=−0.7). As a result, in two cats there was a good positive correlation between the range of head accelerations in the longitudinal direction and walking velocity in both illumination conditions (Fig. 12B, C, E, F). In addition, there was a negative correlation between the minimal vertical acceleration of the head during the stride and velocity of the stride (r=−0.75), and in Cat 3 there was a positive correlation between the range of vertical head acceleration and stride velocity, especially in the light (Fig. 13C, F). There was no correlation between walking velocity and the head's lateral accelerations in any of the cats.
Fig. 12.
Relationship between head longitudinal acceleration and walking velocity in the darkness (A–C) and light (D–F). Black and orange/gray symbols indicate walking in the darkness and light, respectively. Regression line, equation, and coefficient of correlation of linear fit of the data are shown on each plot. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 13.
Relationship between head vertical acceleration and walking velocity in the darkness (A–C) and light (D–F). Designations as in Fig. 12.
Relationships between the velocity of head rotations and velocity of the stride were rather weak. The range of pitch rotation velocity had a tendency to positively correlate with the velocity of Cat 1 walking in the illuminated corridor. The range of roll and yaw rotation velocities during the stride did not correlate with the velocity of walking.
We did not find any clear correlations between any of the head movement variables and the length of the stride. The only relationships worth mentioning were tendencies for positive correlations between the minimal and maximal vertical displacements of the head during the stride and the length of the stride (r=0.46 and 0.39, respectively), and similar tendencies for correlations between the head's minimal and maximal pitch rotations and the stride length (r=0.39 and 0.36, respectively).
Evolution of head movements over three strides of straight walking
Separate analysis of strides that were made first, second, and third along the walkway showed that they were different in respect to the head translation and orientation. As we have mentioned above in the “Translations and Rotations of the Head during Walking” section, over the course of the three strides, vertical position of the head of all cats, as well as those of the shoulder of Cats 1 and 2 and trunk of Cat 3 were progressively diminishing (p<0.05): by 2.5/1.0 cm for the head, 1.5/1.1 cm for the shoulder, and 1.5/0.9 cm for the trunk during walking in the darkness/light, respectively (Fig. 5). The decrease was statistically significantly larger during walking in the darkness (p<0.05). In addition, in the darkness, Cats 1 and 3 rotated their head more nose-down during Strides 2 and 3 than during Stride 1 (one-way ANOVA, p<0.001; Fig. 6D, F). They had a tendency to do so also during walking in the light but the values did not reach the level of statistical significance. Pitch orientation of the head of Cat 2 did not change during walking in either illumination condition.
Strides 2 and 3 were also different from Stride 1 in that Cats 1 and 3 had the head further away from the right transparent side of the chamber during these strides compared to Stride 1. For Cat 1 this was true for walking in the light only (Fig. 5D), but for Cat 3 it was true for both illumination conditions (one-way ANOVA, p<0.001; Fig. 5F).
Comparison of head movements during walking in the darkness and light
General characteristics of walking were fairly similar between illumination conditions. Only Cat 1 walked somewhat faster in the light than darkness, while two other cats walked with the same pace (Figs. 2A, B; 5A–C). In the light, all cats had a tendency to walk closer to the right transparent wall of the chamber (Figs. 1A; 5D–F), the amplitude of left-right oscillations of the head, however, did not vary between illumination conditions (Fig. 5D–F).
When walking in the light, cats held their head higher than in the darkness. The difference was most conspicuous in Cat 1 at about 2 cm (p<0.01; Fig. 5G). It was smaller, about 0.5 cm, but also statistically significant in Cat 2 (p<0.01; Fig. 5H). In Cat 3, the difference became statistically significant only during Stride 3 (1–1.5 cm; p<0.01; Fig. 5I). All cats also stood taller in shoulders/trunk during walking in the light (p<0.01; Fig. 5J–L), but the difference was only 0.3–0.5 cm.
Orientation of the head was rather similar between illumination conditions. Only for Cat 3 was there a difference in the head's roll rotation, as the cat rolled its head 7.5° more clockwise during walking in the light (Fig. 6C). Similarly, only for Cat 1 was there any prominent difference in the head pitch rotation, as in the light this cat steadily held the head 5–10° nose-up compared to in darkness (Fig. 6D). The peak-to-peak amplitudes of roll and pitch oscillations did not differ between illumination conditions in any of the cats (p>0.05; Fig. 6A–F). For the pitch, the most noticeable difference was that in the light the head pitch did not change as the cat walked along the corridor, while the head of Cats 1 and 3 rotated nose-down during walking in the darkness. All cats held their head neutral in yaw during walking in the light, while in the darkness two of them rotated it left by ~5° toward the opal wall of the chamber (Fig. 6G–I).
For Cat 1 the phase lag between head vertical displacement and pitch rotation during walking in the light was smaller than in darkness (47±8° vs. 93±14°; p<0.01), while for other two cats the phase differences were similar between conditions. Across all cats, the head fixation point during walking in the light was about 6 cm higher than in the darkness (p<0.001), and came to the ground further away from the cat (p<0.01; Fig. 9).
There were a couple of differences in head velocities and accelerations between the illumination conditions. For Cats 1 and 2, the peak-to-peak amplitudes of head longitudinal velocity oscillations and pitch angular velocity oscillations during walking in the light were by ~0.04 m/s and ~20 °/s, respectively, larger than in the darkness (Fig. 10). Additionally, while fore and aft accelerations were similar in the darkness, they differed in the light, with the forward acceleration being 25–100% higher (p<0.05; Fig. 11).
DISCUSSION
This study is the first to describe in detail 3-D movement of the head in the walking cat. We found that during walking, the head of the cat rhythmically displaces and rotates in all directions in synchrony with strides. Overall, it is oriented 10–30° nose-down. Oscillations in pitch follow vertical displacements by 40–90°. In about 40% of the strides, the head fixation point is in front of the cat, and comes to the ground at a distance of 1–2 strides. While all displacements and rotations of the head (except the overall forward progression) are rather small, their maximal linear and angular velocities are in the 0.05–0.15 m/s and 20–50 °/s ranges, respectively, and maximal linear accelerations are 0.5–2.0 m/s2. Illumination of the walkway causes cats to hold their head slightly higher, increases head forward acceleration, and may increase longitudinal and pitch velocity oscillations.
Orientation of the head in respect to the earth horizontal
The first goal of this study was to examine how cats hold their head in respect to the earth horizontal when walking. This was of interest because head orientation is an important factor that determines the efficacy of activation of vestibular receptors during locomotion. We found that two of the cats held their head tilted nose-down by 15–30° while the other cat had it 9–16° nose-down (Fig. 6D–F). The range of stride-related oscillations in pitch was ~3°. In the cat, the horizontal semicircular canals and utriculi are parallel with the earth horizontal and thus most sensitive to stimulation when the head is tilted 21° nose-down (Curthoys et al., 1977b). Roll oscillations were of similar amplitude to pitch oscillations and typically stayed within 0±10° (Fig. 6A–C). Thus, during walking, for the majority of the time the cat kept its head ±10° of the most sensitive orientation for the horizontal semicircular canal and utriculi. This finding agrees with the report of Graf and colleagues (1995) who found that while cats may sometimes rotate their head substantially, most of the time they keep horizontal semicircular canals oriented within 5–15° of the earth horizontal, including during walking. In humans, non-human primates, and horses, it was also shown that during most locomotor tasks the head rotation remains within a 20° range in all planes, thereby providing a relatively stable spatial reference frame to the brain (Pozzo et al., 1990; rev. in Dunbar et al., 2008).
In our study we used cats who wore on their heads apparatuses for neuronal activity and eye movement recordings. The total weight of these devices was approximately 200 g, which is about 50% of the weight of the cat's head. In addition, this weight was placed on the top of and behind the head, thus shifting the head's center of mass roughly 20% upward and 10% backward. It is remarkable that although mechanical properties of the head of our animals were substantially altered, during walking they oriented it similarly to intact cats from our previous study (Beloozerova et al., 2010) and the study of Fowler and Sherk (2003): nose down so that the head was directed at the ground between 40 and 80 cm or 1–2 strides ahead of the cat (compare Fig. 9 of Fowler and Sherk (2003) and our Fig. 9). This suggests that this orientation was very important to the cats and was maintained despite the disturbance to head mechanical properties. We believe that the optimal orientation of horizontal semicircular canals and utriculi was the main reason. The other potential reason could be that this orientation assures that the gaze intersected the walking surface ~1 s ahead of foot placement of the walking cat, which appears to be optimal for processing of visual information, as we have discussed in our recent publication (Rivers et al., 2014).
Contribution of reflexes to head movement during walking
The second goal of our study was to estimate contributions of head mechanical properties and reflexes to head movement during walking. The fact that our implanted cats held their much heavier heads similarly to intact cats of Fowler and Sherk (2003) already suggests that the head is actively controlled. Results of Fourier analysis further supported this assertion by showing that the frequency of the head rotary oscillations in pitch peaks at around 3 Hz, the frequency of steps (Fig. 7), and thus is distinct from the 4.3–4.8-Hz range where, according to our estimate, inertia would become a significant contributor to the movement (Peterson and Goldberg, 1981; Dunbar et al., 2004). The head roll and yaw rotations were of a still lower frequency (Fig. 6) and therefore could not be determined by mechanical properties of the head. If not passive, what active mechanisms contribute to the head movement during walking?
We found that amplitudes of head rotational velocities peaked at 20–50 °/s (Fig. 10J–L) and linear accelerations peaked at ~0.2 G (Fig. 11). These, according to results of previous studies, were both optimal for transduction of motion signals to firing activity of afferents from semicircular canals and otolith organs (Fernandez and Goldberg, 1971, 1976; Blanks et al., 1975; Dutia and Price, 1987). Interestingly, despite differences in the amplitude of head vertical translations during walking between cats and monkeys on one hand (~1.5 cm; our data for cats and Xiang and colleagues (2008) data for monkeys) and humans on the other hand (5–9 cm; Pozzo et al., 1990), accelerations of vertical head translation are almost identical in quadrupeds and humans (~2 m/s2, our data; Hirasaki et al., 1999; Menz et al., 2003; Xiang et al., 2008). The amplitude of pitch rotation appears to vary with the size of the animal, however, being 2–4° in cats (our data), 5–6° in rhesus and cynomolgus monkeys (Xiang et al., 2008), and 8–9° in humans (Pozzo et al., 1990). Although smaller in amplitude, velocities of head pitch rotations were as high in the cat (20–50 °/s) as in rhesus and cynomolgus monkeys (40–50 °/s; Xiang et al., 2008), and somewhat higher than in humans (~30 °/s; Pozzo et al., 1990).
Head-motion signals detected by vestibular endorgans are further processed in the vestibular nuclei. There they are integrated with signals from neck and limb proprioceptors, visual and oculomotor information, as well as with locomotion-related signals from the spinal cord and efference copies of cortical motor commands (e.g., Orlovsky, 1972; Keller and Precht, 1979; Marlinsky, 1992; Gdowski and McCrea, 1999; McCrea et al., 1999; Roy and Cullen, 2001; Marlinski and McCrea, 2009; rev. in Cullen, 2012). Vestibulo-spinal signals that descend to cervical segments of the spinal cord give rise to vestibulo-collic reflexes that stabilize head position during movements (Wilson et al., 1995; Goldberg and Cullen, 2011). It was suggested that during locomotion these reflexes produce head rotations that in humans and bipedal monkeys keep the head fixation point at a certain distance ahead of the walking subject (Pozzo et al., 1990; Hirasaki et al., 1999; Mamoto et al., 2002; Hirasaki and Kumakura, 2004). Since pitch rotations were the most robust rotational movements of the head that we have observed (Fig. 6), we have considered whether they can be explained as a reflex of the head vertical translation.
Vertical displacements of the head appeared to mainly result from vertical displacements of the trunk, which was cyclically elevated and lowered during locomotor cycle (Fig. 5). The trunk reached its highest vertical position in the middle of the swing phase of each forelimb. The head was the highest soon after, in the middle of the second half of the swing phase, a lag of 20–90° (Fig. 8). Pitch oscillations followed vertical head translations, as the head was rotated maximally nose-up at the end of the swing phase of each forelimb when the head has already started moving downward (Fig. 8). The phase difference between pitch rotation and vertical translation was 40–90°. This shows that, during a half of the cycle or more, pitch movement of the head cannot be explained as a reflexive response of the head vertical translation. This finding is consistent with data from quadrupedal non-human primates and horses where pitch rotation of the head typically do not compensate for disturbance created by the head vertical displacement (Dunbar, 2004; Dunbar et al., 2004, 2008; Xiang et al., 2008).
In addition to the fact that head pitch rotations were only 40–90° out of phase with its vertical translations, there are several other indications that head rotations during walking in the cat are not produced by reflexes to head linear movements. Across all cats, head lateral translations were very consistent with the step cycle, while yaw rotations were not (Figs. 6G–I, 5D–F). Had yaw rotations been a reflex of head lateral translations, we would expect them to be modulated with the step cycle and consistently follow lateral translations. Also, while head vertical translations in Cat 2 were asymmetrical between strides of the right and left forelimb (Fig. 5H), no such imbalance existed for head pitch rotations in this cat (Fig. 6E). Furthermore, as the velocity of walking increased, longitudinal and vertical accelerations of the head increased in some subjects (Figs. 12 and 13). We did not find, however, any concurrent changes in the head roll or pitch rotations in these animals. The only cat, in which the range of pitch rotational velocity had a tendency to positively correlate with the velocity of walking, was Cat 1, which showed only a weak relationship between its head vertical acceleration and walking pace (Fig. 13A, D). Finally, it is worth noting that when we compared phase shifts between head vertical translation and pitch rotation we found a wide range of phase differences across cats (38–93°, Fig. 8D–F). The difference between the head pitch rotation and shoulder/trunk vertical displacement was only half as large (112–136°, Fig. 8G–I). This lower range suggests that head pitch rotation may be more related with movement of the shoulder/trunk than the head vertical translation, and instead of being a reflex to the latter is a part of the whole body's centrally programed locomotor synergy. This would be similar to the organization of eye movement in the swimming tadpole, where it was shown that efference copies of intrinsic signals produced by the locomotor central pattern generator supersede, rather than supplement, reactive vestibulo-ocular reflexes (Combes et al., 2008; Lambert et al., 2012).
It can be advocated that vestibulo-collic reflexes, while not generating head movements during walking, are serving to neutralize inertial head rotations caused by displacements of the head. In the decerebrate cat, it was shown that nose-down rotations of the head increased, and nose-up rotations decreased electromyographic activity in the neck extensor muscle biventer cervicis (Dutia and Hunter, 1985). This sagittal vestibulo-collic reflex would oppose inertial rotation of the head resulting from its vertical translation, and reduce amplitude of pitch oscillatons. Indeed, in patients with vestibular deficiency, amplitudes of head pitch rotations during walking were found to be larger than in normal subjects even though vertical translations of the head were similar (Mamoto et al., 2002). Amplitudes of the stride cycle-related pitch and roll rotations of the head also increased in a cynomolgus monkey after all six semicircular canals were “plugged”, limiting their sensitivity to ranges 3–20 Hz (Cohen et al., 2009).
The dynamics of signal processing in the vestibular nuclei appears to be well-suited for enabling vestibulo-spinal signals to contribute effectively to the control of locomotor activity in limb muscles. It was found that the peak activity of vestibular nuclei neurons sensitive to translations lags behind changes in linear acceleration of the head by ~60° (Schor et al., 1998; Angelaki and Dickman, 2000). The firing activity of vestibular nuclei neurons sensitive to rotations leads changes in the angular velocity of the head by ~20° (Buettner et al., 1978; Dickman and Angelaki, 2004). We found that during walking the head positive vertical acceleration reached the peak in the initial part of stance phase of each forelimb (Fig. 11G–I). At the same time, the angular velocity of the head pitch rotation peaked at the end of the swing phase (Fig. 10J–L). As a result of phase delays in signal processing in the vestibular nuclei, both the phase lag of the activity of otolith-related neurons, and the phase lead of the activity of semicircular canal-related neurons set the maxima of descending vestibulo-spinal signals to the very beginning of the stance phase of the forelimb. During locomotion, the firing activity of neurons in the vestibular nuclei is modulated in the rhythm of strides (Orlovsky, 1972; Marlinsky, 1992; Matsuyama and Drew, 2000a,b); and for hindlimbs it was shown that the peak discharge of vestibulo-spinal neurons in the decerebrate cat usually occurs at the beginning of the stance phase of the ipsilateral hindlimb (Orlovsky, 1972; Marlinsky, 1992). In intact cats 46% of these neurons discharge two activity peaks, which are time-locked to the activity of extensor muscles of each of the hindlimbs (Matsuyama and Drew, 2000a, b). Thus, the step cycle phase of vestibulo-spinal signals is well-fit for these signals to increase the activity of anti-gravitational muscles when the foot contacts the walking surface and the limb is loading with the weight of the body.
In the decerebrate cat, it was found, however, that during locomotion most vestibulo-spinal neurons projecting to lumbar segments of the spinal cord greatly reduce their responses to stimulation produced by roll tilts or even become entirely insensitive to it (Orlovsky and Pavlova, 1971; Arshavsky et al., 1986). In addition, most neurons of reticulo-spinal and rubro-spinal tracts that respond to roll tilts in the quiescent animal behave similarly. This indicates that locomotion-related signals from the spinal cord can block, or possibly, nullify signals arriving from the vestibular endorgans much like other signals can (Roy and Cullen, 2004; Marlinski and McCrea, 2009; rev. in Cullen, 2012). If this is also true for neurons projecting to cervical segments of the spinal cord in the intact cat, then vestibulo-collic reflexes with their greatly diminished inputs should be weak during locomotion. Cervico-collic reflexes may be similarly modulated. As discussed above, there are several indications that in the walking cat head rotational movements are not, or at least not primary, caused by reflexive responses to head linear movements. If this is so, then signals from the centrally generated locomotor synergy must be the main drivers for head movements during walking.
Head fixation point
In accordance with a relatively small phase shift between head vertical translation and pitch rotation (Fig. 8), the head fixation point (Pozzo et al., 1990; Hirasaki et al., 1999) was in front of the cat only during 37–39% of the strides. This is consistent with the finding of Xiang and colleagues (2008) that quadrupedally walking Rhesus and Cynomolgus monkeys do not have a forward head fixation point. Five of seven animals in their study had the head fixation point behind the head most of the time, and the remaining two had it in front of the head only half of the time. This finding is also consistent with data on the head of Vervet monkeys (Dunbar, 2004), and hanuman langurs and bonnet macaques (Dunbar et al., 2004), which during locomotion often pitches up while displacing up, resulting in no head fixation point in front of the animal. In contrast, Hirasaki and Kumakura (2004) found that two Japanese macaques had the head fixation point in front of them during both quadrupedal and bipedal walking, as did a bipedally walking gibbon. Xiang and colleagues (2008) hypothesized that the difference could be because animals had a visual target in front of them in the Hirasaki and Kumakura (2004) study, which could have altered their head movements.
During the strides when cats had the head fixation point in front of the head, it was located between 5 and 100 cm ahead, and was between 10 and 40 cm ahead in about half of them (Fig. 9). This distance is similar to the head fixation distance in bipedal primates. The two macaques and gibbon of Hirasaki and Kumakura (2004) during bipedal walking had head fixation points at distances of 20–30 cm in front of them, and Hirasaki and colleagues (1999) reported that this point was 40–100 cm in front of walking humans. Having a head fixation point during locomotion may simplify visual fixation of objects and assist in processing visual information. The retina of cats and primates has many features in common, including a blood vessel-free area with a high concentration of cones and ganglion cells (Rapaport and Stone, 1984). This region is referred to as the fovea in primates and area centralis in cats. In both primates and cats, cells in this area have small receptive fields, high spatial resolution, and high representation in upper levels of the visual system. However, there also are notable differences between retinas of primates and cats. One of these is a large number of rods in the cat area centralis compared to nearly no rods in the primate fovea. For this reason, the area centralis of cats might be considered less specialized than the fovea of primates (Pasternak et al., 1983). Therefore, maintaining a strict head fixation point during locomotion may be less important for cats than for primates.
The role of light in orientation and movement of the head during walking
When walking in the light, cats held their head 1–2 cm higher than in the darkness. Their taller standing in the shoulders contributed about 0.5 cm, with the remaining 0.5–1.5 cm apparently due to the tilted up or stretched neck. The most prominent difference in respect to orientation of the head was that in the light its pitch angle did not change as the cat progressed along the corridor, while the head progressively rotated nose-down over the three strides in the darkness. Because in the light the head was held higher and pitched less nose-down, the head fixation point was higher than in the darkness, by about 6 cm, and came to the ground further away from the animal (Fig. 9). This would be useful for detection of any possible obstacles at a distance.
While walking in the light, cats tended to walk closer to the right transparent wall of the chamber (Figs. 1A; 5D–F), probably due to their preference for wider turns. In contrast, while walking in the darkness cats moved or rotated their heads toward the left opal wall (Figs. 5D–F and 6G, H). This behavior was likely due to the asymmetry between the right and left sides of the experimental corridor, the right wall of which was transparent whereas the left was not. Cats certainly noticed that the walking corridor was elevated on a ~1 m high platform, which may have rendered the right side more ominous to the animals.
Although most variables of head movement were similar between illumination conditions, there were several noteworthy differences as well. In particular, during walking in the light, forward acceleration was 25–100% higher in all subjects and peak-to-peak amplitude of head oscillations in pitch was ~20 °/s larger in two of the cats. This suggests that vision could have reduced effectiveness of any head stabilizing reflexes and/or altered the locomotion-related coupling between the head and the rest of the body. The fact that for one of the cats the phase lag between head vertical displacement and pitch rotation was smaller during walking in the light than darkness also suggests such reduction/alteration. This finding is consistent with data from Pozzo and colleagues (1991) who found that amplitudes and velocities of head pitch rotations in humans are larger during running in the light than darkness.
CONCLUSION
Results of this study show that orientation and movement of the head in the walking cat are active processes. Velocities and accelerations of the head are well within activation ranges for vestibulo-collic and cervico-collic reflexes, however, reflexes appear to play only a partial role in determining head movement during walking, and vision may further diminish their role. We hypothesize that the whole body locomotor synergy is principally responsible for head movement during walking.
Acknowledgments
The study was supported by NIH Grant R01 NS-058659 to INB and by Barrow Neurological Foundation. HS was supported by the Christopher Getch Fellowship granted by the Congress of Neurological Surgeons (CNS). The authors are indebted to Dr. Trevor Rivers and Mr. Neet Shah for collection of the data and to Mr. Peter Wettenstein for outstanding engineering assistance. Additionally, Mr. Mohammadhassan Izady and Mr. Neet Shah were of great assistance with processing of recordings and data analysis.
REFERENCES
- Arshavsky YuI, Gelfand IM, Orlovsky GN. Cerebellum and rhythmical movements. Springer; Berlin: 1986. [Google Scholar]
- Armer MC, Nilaweera WU, Rivers TJ, Dasgupta NM, Beloozerova IN. Effect of light on the activity of motor cortex neurons during locomotion. Behav Brain Res. 2013;250:238–250. doi: 10.1016/j.bbr.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol. 2000;84:2113–2132. doi: 10.1152/jn.2000.84.4.2113. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG. The role of the motor cortex in the control of accuracy of locomotor movements in the cat. J Physiol. 1993;461:1–25. doi: 10.1113/jphysiol.1993.sp019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Farrell BJ, Sirota MG, Prilutsky BI. Differences in movement mechanics, electromyographic, and motor cortex activity between accurate and nonaccurate stepping. J Neurophysiol. 2010;103(4):2285–2300. doi: 10.1152/jn.00360.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotto G, Schor RH, Uchino Y, Wilson V. Localization of proprioceptive reflexes in the splenius muscle of the cat. Brain Res. 1982;238:217–221. doi: 10.1016/0006-8993(82)90786-7. [DOI] [PubMed] [Google Scholar]
- Blanks RH, Estes MS, Markham CH. Physiologic characteristics of vestibular first order canal neurons in the cat: II. Response to constant angular acceleration. J Neurophysiol. 1975;38:1250–1268. doi: 10.1152/jn.1975.38.5.1250. [DOI] [PubMed] [Google Scholar]
- Buettner UW, Büttner U, Henn V. Transfer characteristics of neurons in vestibular nuclei of the alert monkey. J Neurophysiol. 1978;41:1614–1625. doi: 10.1152/jn.1978.41.6.1614. [DOI] [PubMed] [Google Scholar]
- Cappozzo A. Analysis of the linear displacement of the head and trunk during walking at different speeds. J Biomech. 1981;14:411–425. doi: 10.1016/0021-9290(81)90059-2. [DOI] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol. 1998;79(4):1687–1701. doi: 10.1152/jn.1998.79.4.1687. [DOI] [PubMed] [Google Scholar]
- Cohen B, Xiang Y, Yakushin SB, Kunin M, Raphan T, Minor L, Della Santina CC. Effect of canal plugging on quadrupedal locomotion in monkey. Ann NY Acad Sci. 2009;1164:89–96. doi: 10.1111/j.1749-6632.2009.03845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes D, Le Ray D, Lambert FM, Simmers J, Straka H. An intrinsic feed-forward mechanism for vertebrate gaze stabilization. Curr Biol. 2008;18(6):R241–R243. doi: 10.1016/j.cub.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Crane BT, Demer JL. Human gaze stabilization during natural activities: translation, rotation, magnification, and target distance effects. J Neurophysiol. 1997;78:2129–2144. doi: 10.1152/jn.1997.78.4.2129. [DOI] [PubMed] [Google Scholar]
- Cromwell RL. Movement strategies for head stabilization during incline walking. Gait Posture. 2003;17:246–253. doi: 10.1016/s0966-6362(02)00094-2. [DOI] [PubMed] [Google Scholar]
- Cromwell RL, Aadland-Monahan TK, Nelson AT, Stern-Sylvestre SM, Seder B. Sagittal plane analysis of head, neck, and trunk kinematics and electromyographic activity during locomotion. J Orthop Sports Phys Ther. 2001;31:255–262. doi: 10.2519/jospt.2001.31.5.255. [DOI] [PubMed] [Google Scholar]
- Cromwell RL, Pidcoe PE, Griffin LA, Sotillo T, Ganninger D, Feagin M. Adaptations in horizontal head stabilization in response to altered vision and gaze during natural walking. J Vestib Res. 2004;14:367–373. [PubMed] [Google Scholar]
- Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35(3):185–196. doi: 10.1016/j.tins.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS, Markham CH, Curthoys EJ. Semicircular duct and ampulla dimensions in cat, guinea pig and man. J Morphol. 1977;151:17–34. doi: 10.1002/jmor.1051510103. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Blanks RH, Markham CH. Semicircular canal functional anatomy in cat, guinea pig and man. Acta Otolaryngol. 1977;83(3–4):258–265. doi: 10.3109/00016487709128843. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Angelaki DE. Dynamics of vestibular neurons during rotational motion in alert rhesus monkeys. Exp Brain Res. 2004;155:91–101. doi: 10.1007/s00221-003-1692-1. [DOI] [PubMed] [Google Scholar]
- Donaghy M. The cat's vestibulo-ocular reflex. J Physiol. 1980;300:337–351. doi: 10.1113/jphysiol.1980.sp013165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy M. The contrast sensitivity, spatial resolution and velocity tuning of the cat's optokinetic reflex. J Physiol. 1980;300:353–365. doi: 10.1113/jphysiol.1980.sp013166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol. 1993;70:179–199. doi: 10.1152/jn.1993.70.1.179. [DOI] [PubMed] [Google Scholar]
- Dutia MB, Hunter MJ. The sagittal vestibulocollic reflex and its interaction with neck proprioceptive afferents in the decerebrate cat. J Physiol. 1985;359:17–29. doi: 10.1113/jphysiol.1985.sp015572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutia MB, Price RF. Interaction between the vestibulo-collic reflex and the cervico-collic stretch reflex in the decerebrate cat. J Physiol. 1987;387:19–30. doi: 10.1113/jphysiol.1987.sp016559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar DC. Stabilization and mobility of the head and trunk in vervet monkeys (Cercopithecus aethiops) during treadmill walks and gallops. J Exp Biol. 2004;207:4427–4438. doi: 10.1242/jeb.01282. [DOI] [PubMed] [Google Scholar]
- Dunbar DC, Badam GL, Hallgrímsson B, Vieilledent S. Stabilization and mobility of the head and trunk in wild monkeys during terrestrial and flat-surface walks and gallops. J Exp Biol. 2004;207:1027–1042. doi: 10.1242/jeb.00863. [DOI] [PubMed] [Google Scholar]
- Dunbar DC, Macpherson JM, Simmons RW, Zarcades A. Stabilization and mobility of the head, neck and trunk in horses during overground locomotion: comparisons with humans and other primates. J Exp Biol. 2008;211:3889–3907. doi: 10.1242/jeb.020578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell BJ, Bulgakova MA, Beloozerova IN, Sirota MG, Prilutsky BI. Body stability and muscle and motor cortex activity during walking with wide stance. J Neurophysiol. 2014;112(3):504–524. doi: 10.1152/jn.00064.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorov OV, Nilaweera WU, Miasnikov AA, Beloozerova IN. Activity of somatosensory-responsive neurons in high subdivisions of SI cortex during locomotion. J Neurosci. 2015;35(20):7763–7776. doi: 10.1523/JNEUROSCI.3545-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971;34:661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fernández C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol. 1976;39:996–1008. doi: 10.1152/jn.1976.39.5.996. [DOI] [PubMed] [Google Scholar]
- Fick AE. Die bewegungen des menschlichen augapfels. Zeitschrift fur rationale Medizin. 1854;4:109–128. [Google Scholar]
- Fowler GA, Sherk H. Gaze during visually-guided locomotion in cats. Behav Brain Res. 2003;139(1–2):83–96. doi: 10.1016/s0166-4328(02)00096-7. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, McCrea RA. Integration of vestibular and head movement signals in the vestibular nuclei during whole-body rotation. J Neurophysiol. 1999;82:436–449. doi: 10.1152/jn.1999.82.1.436. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Cullen KE. Vestibular control of the head: possible functions of the vestibulocollic reflex. Exp Brain Res. 2011;210(3–4):331–435. doi: 10.1007/s00221-011-2611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf W, de Waele C, Vidal PP, Wang DH, Evinger C. The orientation of the cervical vertebral column in unrestrained awake animals. II. Movement strategies. Brain Behav Evol. 1995;45:209–231. doi: 10.1159/000113551. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Guenther E, Zrenner E. The spectral sensitivity of dark- and light-adapted cat retinal ganglion cells. J Neurosci. 1993;13:1543–1550. doi: 10.1523/JNEUROSCI.13-04-01543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasaki E, Kumakura H. Head movements during locomotion in a gibbon and Japanese macaques. NeuroReport. 2004;15:643–647. doi: 10.1097/00001756-200403220-00014. [DOI] [PubMed] [Google Scholar]
- Hirasaki E, Moore ST, Raphan T, Cohen B. Effects of walking velocity on vertical head and body movements during locomotion. Exp Brain Res. 1999;127:117–130. doi: 10.1007/s002210050781. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Marple-Horvat DE. Visually guided stepping under conditions of step cycle-related denial of visual information. Exp Brain Res. 1996;109(2)::343–356. doi: 10.1007/BF00231792. [DOI] [PubMed] [Google Scholar]
- Imai T, Moore ST, Raphan T, Cohen B. Interaction of the body, head, and eyes during walking and turning. Exp Brain Res. 2001;136(1):1–18. doi: 10.1007/s002210000533. [DOI] [PubMed] [Google Scholar]
- Jones GM, Spells KE. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc R Soc Lond Biol. 1963;157:403–419. doi: 10.1098/rspb.1963.0019. [DOI] [PubMed] [Google Scholar]
- Karayannidou A, Beloozerova IN, Zelenin PV, Stout EE, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during standing and walking on an inclined plane. J Physiol (L.) 2009;587(Pt 15):3795–3811. doi: 10.1113/jphysiol.2009.170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh JJ, Morrison S, Barrett RS. Coordination of head and trunk accelerations during walking. Eur J Appl Physiol. 2005;94:468–475. doi: 10.1007/s00421-005-1328-1. [DOI] [PubMed] [Google Scholar]
- Keller EL, Precht W. Modification of central vestibular neuron response by conflicting visual-vestibular stimulation. Prog Brain Res. 1979;50:763–770. doi: 10.1016/S0079-6123(08)60873-0. [DOI] [PubMed] [Google Scholar]
- Lambert FM, Combes D, Simmers J, Straka H. Gaze stabilization by efference copy signaling without sensory feedback during vertebrate locomotion. Curr Biol. 2012;22(18):1649–1658. doi: 10.1016/j.cub.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Mamoto Y, Yamamoto K, Imai T, Tamura M, Kubo T. Three-dimensional analysis of human locomotion in normal subjects and patients with vestibular deficiency. Acta Otolaryngol. 2002;122(5):495–500. doi: 10.1080/00016480260092282. [DOI] [PubMed] [Google Scholar]
- Marigold DS, Drew T. Contribution of cells in the posterior parietal cortex to the planning of visually guided locomotion in the cat: effects of temporary visual interruption. J Neurophysiol. 2011;105(5):2457–2470. doi: 10.1152/jn.00992.2010. [DOI] [PubMed] [Google Scholar]
- Marlinsky VV. Activity of lateral vestibular nucleus neurons during locomotion in the decerebrate guinea pig. Exp Brain Res. 1992;90:583–588. doi: 10.1007/BF00230942. [DOI] [PubMed] [Google Scholar]
- Marlinski V, McCrea RA. Self-motion signals in vestibular nuclei neurons projecting to the thalamus in the alert squirrel monkey. J Neurophysiol. 2009;101:1730–1741. doi: 10.1152/jn.90904.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V, Sirota MG, Beloozerova IN. Differential gating of thalamocortical signals by reticular nucleus of thalamus during locomotion. J Neurosci. 2012;32(45):15823–15836. doi: 10.1523/JNEUROSCI.0782-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V, Nilaweera WU, Zelenin PV, Sirota MG, Beloozerova IN. Signals from the ventrolateral thalamus to the motor cortex during locomotion. J Neurophysiol. 2012;107(1):455–472. doi: 10.1152/jn.01113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. I. Walking on a level surface. J Neurophysiol. 2000;84(5):2237–2256. doi: 10.1152/jn.2000.84.5.2237. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. II. Walking on an inclined plane. J Neurophysiol. 2000;84(5):2257–2276. doi: 10.1152/jn.2000.84.5.2257. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol. 1999;82:416–428. doi: 10.1152/jn.1999.82.1.416. [DOI] [PubMed] [Google Scholar]
- Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture. 2003;18:35–46. doi: 10.1016/s0966-6362(02)00159-5. [DOI] [PubMed] [Google Scholar]
- Moore AW, Jorgenson JW. Median filtering for removal of low frequency background drift. Anal Chem. 1993;65:188–191. doi: 10.1021/ac00050a018. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Amblard B, Mesure S, Bourbonnais D. Head and trunk stabilization strategies during forward and backward walking in healthy adults. Gait Posture. 2003;18:134–142. doi: 10.1016/s0966-6362(02)00070-x. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. Activity of vestibulospinal neurons during locomotion. Brain Res. 1972;46:85–98. doi: 10.1016/0006-8993(72)90007-8. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Pavlova GA. Vestibular responses in neurons of descending pathways during locomotion. Neurophysiologia. 1971;4(3):311–316. In Russian. [Google Scholar]
- Pasternak T, Merigan WH, Flood DG, Zehl D. The role of area centralis in the spatial vision of the cat. Vision Res. 1983;23(12):1409–1416. doi: 10.1016/0042-6989(83)90152-9. [DOI] [PubMed] [Google Scholar]
- Patla AE. Understanding the roles of vision in the control of human locomotion. Gait Posture. 1997;5:54–69. [Google Scholar]
- Peterson BW. Current approaches and future directions to understanding control of head movement. Prog Brain Res. 2004;143:369–381. doi: 10.1016/s0079-6123(03)43035-5. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Goldberg J. Role of vestibular and neck reflexes in controlling eye and head position. In: Roucoux A, Crommelinck M, editors. Physiological and pathological aspects of eye movements. Dr W. Junk, Publishers; The Hague: 1981. pp. 352–364. [Google Scholar]
- Peterson BW, Goldberg J, Bilotto G, Fuller JH. Cervicocollic reflex: its dynamic properties and interaction with vestibular reflexes. J Neurophysiol. 1985;54:90–109. doi: 10.1152/jn.1985.54.1.90. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans. I. Normal subjects. Exp Brain Res. 1990;82(1):97–106. doi: 10.1007/BF00230842. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Berthoz A, Lefort L, Vitte E. Head stabilization during various locomotor tasks in humans. II. Patients with bilateral peripheral vestibular deficits. Exp Brain Res. 1991;85(1):208–217. doi: 10.1007/BF00230002. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol. 2005;94:2959–2969. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Pryor K. Lads before the wind: adventures in porpoise training. Harper & Row; New York: 1975. [Google Scholar]
- Rapaport DH, Stone J. The area centralis of the retina in the cat and other mammals: focal point for function and development of the visual system. Neuroscience. 1984;11(2):289–301. doi: 10.1016/0306-4522(84)90024-1. [DOI] [PubMed] [Google Scholar]
- Raphan T, Cohen B. The vestibulo-ocular reflex in three dimensions. Exp Brain Res. 2002;145(1):1–27. doi: 10.1007/s00221-002-1067-z. [DOI] [PubMed] [Google Scholar]
- Rivers TJ, Sirota MG, Guttentag AI, Ogorodnikov DA, Shah NA, Beloozerova IN. Gaze shifts and fixations dominate gaze behavior of walking cats. Neuroscience. 2014;275:477–499. doi: 10.1016/j.neuroscience.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Rollando AK, Rivers TJ, Beloozerova IN. A biomechanical description of the head movement during locomotion in the cat. 2012 biomedical engineering society annual meeting; Atlanta. Oct 24–27, 2012. [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor RH, Steinbacher BC, Jr, Yates BJ. Horizontal linear and angular responses of neurons in the medial vestibular nucleus of the decerebrate cat. J Vestib Res. 1998;8:107–116. [PubMed] [Google Scholar]
- Sherk H, Fowler GA. Visual analysis and image motion in locomoting cats. Eur J Neurosci. 2001;13(6):1239–1248. doi: 10.1046/j.0953-816x.2001.01493.x. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The behavior of organisms: an experimental analysis. Appleton-Century-Crofts Inc; New York: 1938. [Google Scholar]
- Stone DC. Application of median filtering to noisy data. Can J Chem. 1995;73:1573–1581. [Google Scholar]
- Stout EE, Sirota MG, Beloozerova IN. Known and unexpected constraints evoke different kinematic, muscle, and motor cortical neuron responses during locomotion. Eur J Neurosci. 2015;42(9):2666–2677. doi: 10.1111/ejn.13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Addison-Wesley; Reading, Mass: 1977. [Google Scholar]
- Warren WH, Jr, Kay BA, Zosh WD, Duchon AP, Sahuc S. Optic flow is used to control human walking. Nat Neurosci. 2001;4(2):213–216. doi: 10.1038/84054. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Boyle R, Fukushima K, Rose PK, Shinoda Y, Sugiuchi Y, Uchino Y. The vestibulocollic reflex. J Vestib Res. 1995;5:147–170. [PubMed] [Google Scholar]
- Xiang Y, Yakushin SB, Kunin M, Raphan T, Cohen B. Head stabilization by vestibulocollic reflexes during quadrupedal locomotion in monkey. J Neurophysiol. 2008;100:763–780. doi: 10.1152/jn.90256.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV, Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG. Activity of red nucleus neurons in the cat during postural corrections. J Neurosci. 2010;30(43):14533–14542. doi: 10.1523/JNEUROSCI.2991-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair HN, Izady MY, Sun H, Marlinski V, Beloozerova IN. Feline head movement during walking. Program No 335.14. Neuroscience meeting planner; Chicago, IL: Society for Neuroscience; 2015. Online. [Google Scholar]