Abstract
BACKGROUND
Inherited mutations in DNA-repair genes such as BRCA2 are associated with increased risks of lethal prostate cancer. Although the prevalence of germline mutations in DNA-repair genes among men with localized prostate cancer who are unselected for family predisposition is insufficient to warrant routine testing, the frequency of such mutations in patients with metastatic prostate cancer has not been established.
METHODS
We recruited 692 men with documented metastatic prostate cancer who were unselected for family history of cancer or age at diagnosis. We isolated germline DNA and used multiplex sequencing assays to assess mutations in 20 DNA-repair genes associated with autosomal dominant cancer-predisposition syndromes.
RESULTS
A total of 84 germline DNA-repair gene mutations that were presumed to be deleterious were identified in 82 men (11.8%); mutations were found in 16 genes, including BRCA2 (37 men [5.3%]), ATM (11 [1.6%]), CHEK2 (10 [1.9% of 534 men with data]), BRCA1 (6 [0.9%]), RAD51D (3 [0.4%]), and PALB2 (3 [0.4%]). Mutation frequencies did not differ according to whether a family history of prostate cancer was present or according to age at diagnosis. Overall, the frequency of germline mutations in DNA-repair genes among men with metastatic prostate cancer significantly exceeded the prevalence of 4.6% among 499 men with localized prostate cancer (P<0.001), including men with high-risk disease, and the prevalence of 2.7% in the Exome Aggregation Consortium, which includes 53,105 persons without a known cancer diagnosis (P<0.001).
CONCLUSIONS
In our multicenter study, the incidence of germline mutations in genes mediating DNA-repair processes among men with metastatic prostate cancer was 11.8%, which was significantly higher than the incidence among men with localized prostate cancer. The frequencies of germline mutations in DNA-repair genes among men with metastatic disease did not differ significantly according to age at diagnosis or family history of prostate cancer. (Funded by Stand Up To Cancer and others.)
Carcinoma of the prostate is a common cancer with a wide spectrum of clinical behavior that ranges from decades of indolence to rapid metastatic progression and lethality.1,2 Prostate cancer is also among the most heritable of human cancers, with 57% (95% confidence interval [CI], 51 to 63) of the interindividual variation in risk attributed to genetic factors.3 Thus far, genomewide association studies have identified more than 100 common variants that account for approximately 33% of the excess familial prostate cancer risk.4–7 Mutations in other genes, including BRCA1, BRCA2, MSH2,8–10 and HOXB13,11 account for a small proportion of familial cases, with BRCA2 mutations associated with 1.2 to 1.8% of prostate cancer overall.9,12
Thus far, only mutations that disrupt the function of genes involved in repairing DNA damage through homologous recombination have been shown to be associated with the aggressive clinical behavior of localized prostate cancer and with cancer-specific mortality.9,12–14 The need for genetic prognostic markers is critical, because the clinicopathological diversity of prostate cancer has confounded efforts to develop effective screening strategies that avoid overdetection and overtreatment yet capture cancers that are destined to affect survival.15 Persons who are shown to have cancer-predisposition mutations in the germline may serve as sentinels for the identification of families at high risk. It should be noted that men with metastatic prostate cancer and DNA-repair gene mutations have been reported to have sustained responses to poly-ADP ribose polymerase (PARP) inhibitors and platinum-based chemotherapy.16,17
Although the prevalence of germline DNA-repair gene mutations is low among men with localized prostate cancer who are unselected for family predisposition, the frequency of such mutations among men with metastatic prostate cancer has not been established. We recently reported an analysis of the spectrum of somatic aberrations that occur in metastatic prostate cancer, using whole-exome sequencing of metastatic tumors.18 For comparison purposes, we also sequenced germline DNA exomes from these men and unexpectedly found that 8% carried pathogenic germline mutations in DNA-repair genes. This finding suggested that men with metastatic prostate cancer represent a population that is enriched for heritable defects in DNA repair. To confirm this finding and to further ascertain the spectrum and prevalence of germline DNA-repair gene mutations in metastatic prostate cancer, we recruited 542 additional men with a confirmed prostate cancer metastasis and used next-generation sequencing to analyze DNA-repair genes associated with autosomal dominant cancer-predisposition syndromes.
METHODS
STUDY POPULATIONS
Seven case series of men with metastatic prostate cancer across multiple institutions in the United States and United Kingdom, including a total of 692 patients, were analyzed. All the patients had a diagnosis of metastatic prostate cancer and were not selected on the basis of family history, age, or any knowledge of genetic background. The demographic characteristics of the men in each series are summarized in Table 1. Detailed information on the specific germline mutations and on clinical features of mutation carriers in each series is provided in Tables S1, S2, and S3 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Table 1.
Demographic Characteristics of the Patients with Prostate Cancer.*
| Characteristic | Case Series 1 (N = 150) |
Case Series 2 (N = 84) |
Case Series 3 (N = 131) |
Case Series 4 (N = 91) |
Case Series 5 (N = 69) |
Case Series 6 (N = 43) |
Case Series 7 (N = 124) |
All Case Series (N = 692) |
TCGA Cohort with Primary Prostate Cancer (N = 499)† |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-I Risk | High Risk | |||||||||||||||||||
| M | NM | M | NM | M | NM | M | NM | M | NM | M | NM | M | NM | M | NM | M | NM | M | NM | |
| number of patients | ||||||||||||||||||||
| Total | 15 | 135 | 9 | 75 | 16 | 115 | 8 | 83 | 7 | 62 | 4 | 39 | 23 | 101 | 82 | 610 | 4 | 158 | 19 | 318 |
| Age | ||||||||||||||||||||
| <50 yr | 2 | 16 | 0 | 2 | 1 | 6 | 1 | 5 | 1 | 2 | 0 | 3 | 2 | 9 | 7 | 43 | 0 | 15 | 1 | 11 |
| 50–59 yr | 6 | 40 | 4 | 30 | 3 | 37 | 2 | 18 | 2 | 15 | 0 | 6 | 9 | 47 | 26 | 193 | 3 | 63 | 8 | 103 |
| 60–69 yr | 6 | 63 | 3 | 24 | 11 | 56 | 3 | 38 | 3 | 20 | 2 | 25 | 11 | 36 | 39 | 262 | 1 | 67 | 8 | 167 |
| 70–79 yr | 1 | 15 | 1 | 11 | 1 | 15 | 2 | 19 | 1 | 11 | 2 | 5 | 1 | 9 | 9 | 85 | 0 | 13 | 2 | 37 |
| ≥80 yr | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 |
| Unknown | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 2 | 0 | 6 | 0 | 0 | 0 | 0 | 1 | 14 | 0 | 0 | 0 | 0 |
| Race or ethnic background‡ | ||||||||||||||||||||
| Non-Hispanic white | 12 | 116 | 8 | 60 | 16 | 103 | 5 | 70 | 3 | 37 | 4 | 30 | 22 | 90 | 70 | 506 | 2 | 118 | 16 | 270 |
| Hispanic | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 7 | 0 | 0 | 0 | 0 | 3 | 8 | 0 | 1 | 1 | 5 |
| Non-Hispanic black | 1 | 8 | 0 | 4 | 0 | 12 | 2 | 0 | 1 | 5 | 0 | 2 | 0 | 5 | 4 | 36 | 2 | 27 | 2 | 27 |
| Asian or Pacific Islander | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 1 | 0 | 1 | 1 | 11 | 0 | 3 | 0 | 9 |
| Other or unknown | 1 | 8 | 0 | 9 | 0 | 0 | 1 | 13 | 1 | 8 | 0 | 6 | 1 | 5 | 4 | 49 | 0 | 9 | 0 | 7 |
| History of cancer in a first- degree relative |
||||||||||||||||||||
| Prostate cancer | 4 | 31 | 3 | 11 | 2 | 16 | 0 | 14 | 2 | 12 | 0 | 4 | 5 | 29 | 16 | 117 | NA | NA | NA | NA |
| Other cancer | 10 | 55 | 4 | 35 | 8 | 53 | 6 | 30 | 3 | 14 | 1 | 19 | 19 | 64 | 51 | 270 | NA | NA | NA | NA |
| No cancer | 2 | 46 | 2 | 27 | 5 | 35 | 1 | 25 | 3 | 29 | 1 | 12 | 4 | 25 | 18 | 199 | NA | NA | NA | NA |
| Unknown | 2 | 15 | 1 | 5 | 2 | 17 | 1 | 21 | 1 | 9 | 2 | 6 | 0 | 0 | 9 | 73 | NA | NA | NA | NA |
| Gleason score§ | ||||||||||||||||||||
| ≤6 | 0 | 8 | 0 | 3 | 0 | 11 | 1 | 9 | 1 | 3 | 0 | 2 | 0 | 4 | 2 | 40 | 1 | 32 | 1 | 11 |
| 3+4 | 0 | 19 | 1 | 9 | 0 | 10 | 1 | 6 | 1 | 3 | 0 | 2 | 2 | 11 | 5 | 60 | 3 | 94 | 1 | 50 |
| 4+3 | 1 | 18 | 2 | 7 | 1 | 11 | 0 | 15 | 2 | 7 | 0 | 4 | 4 | 20 | 10 | 82 | 0 | 32 | 5 | 64 |
| 8–10 | 11 | 70 | 3 | 44 | 12 | 65 | 6 | 39 | 3 | 28 | 4 | 16 | 17 | 64 | 56 | 326 | 0 | 0 | 12 | 193 |
| Unknown | 3 | 20 | 3 | 12 | 3 | 18 | 0 | 14 | 0 | 21 | 0 | 15 | 0 | 2 | 9 | 102 | 0 | 0 | 0 | 0 |
The studies included in each case series were as follows: 1, Stand Up To Cancer–Prostate Cancer Foundation discovery series; 2, Stand Up To Cancer–Prostate Cancer Foundation validation series; 3, Royal Marsden Hospital; 4, University of Washington; 5, Weill Cornell Medical College; 6, University of Michigan; and 7, Memorial Sloan Kettering Cancer Center. L-I denotes low to intermediate, M mutation present, NA not applicable, and NM no mutation present.
The Cancer Genome Atlas (TCGA) cohort includes a series of patients with localized prostate cancer.
Race and ethnic background were self-reported.
The Gleason score is a measure of the differentiation state of prostate cancer; scores range from 2 to 10, with higher scores associated with worse clinical outcomes. When two grades are given (e.g., 3+4), the first indicates the more common grade found in the tumor.
Case Series 1, the Stand Up to Cancer–Prostate Cancer Foundation (SU2C-PCF) International Prostate Cancer Dream Team discovery series, was made up of 150 patients for whom data were previously reported in the SU2C-PCF study of molecular stratification of metastatic prostate cancer.18 Case Series 2, the SU2C-PCF validation series, was made up of 84 patients who were newly enrolled in the SU2C-PCF study and for whom data had not been reported previously. Case Series 3, Royal Marsden Prostate Cancer Genomics series, included 131 patients who were considered for enrollment in clinical trials at the Royal Marsden Hospital from January 2013 through July 2015. Case Series 4 consisted of 91 consecutive patients included in the University of Washington rapid autopsy program from 1997 through 2013. Case Series 5 included 69 consecutive patients who were enrolled in the Weill Cornell Medical College precision medicine program. Case Series 6 was made up of 43 consecutive patients from the University of Michigan rapid autopsy program. Case Series 7, from the Memorial Sloan Kettering Cancer Center, included 124 consecutive patients who were enrolled through the Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) study.
The protocols for these case series were approved by the local institutional review boards, and written informed consent was obtained from all patients at the local sites before enrollment. Correlative clinical data were collected at each site with the use of electronic patient records and were entered into deidentified databases. The study was designed by the Stand Up To Cancer–Prostate Cancer Foundation International Prostate Cancer Dream Team investigators. The study sponsors had no role in the design of the study, the collection or analysis of the data, or the preparation of the manuscript. The manuscript was written by four of the authors. All authors reviewed the manuscript, agreed to submit the manuscript for publication, and vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
SEQUENCING AND BIOINFORMATICS ANALYSIS
For the analysis involving Case Series 1, 2, and 6, whole-exome sequencing of germline and tumor DNA was performed as described previously.18 Germline DNA from buccal swabs, buffy coats, or whole blood was isolated with the use of the QIAGEN DNeasy Blood and Tissue Kit. Whole-exome sequencing was performed on the Illumina HiSeq 2500 in paired-end mode.
For the analysis of Case Series 3, germline DNA was extracted from saliva or buccal swab samples with the use of the Oragene kit (DNA Genotek). Libraries for targeted sequencing were constructed with a customized GeneRead DNaseq Panel (Qiagen) covering 53 genes and run on the Illumina MiSeq sequencer, as described previously.16
For the analyses of Case Series 4 and 5, germline DNA was extracted from peripheral blood or nontumor tissue and from matched tumor DNA, as described previously.19 Targeted deep sequencing was performed with the BROCA panel of 53 DNA-repair pathway genes. The bioinformatics pipeline has been described previously.20,21 For tumors from Case Series 5, analyses were performed by means of exome sequencing, as described previously.22 For Case Series 7, tumor and germline genomic sequencing was performed as described previously, with the use of the MSK-IMPACT hybrid capture-based next-generation sequencing assay.23,24
The mean sequencing depth of coverage was more than 100× for all case series, with the exception of sequencing of BAP1, BARD1, BRIP1, and FAM175A, which were not included on the Royal Marsden Hospital panel, and GEN1, which was not included on the Royal Marsden Hospital or Memorial Sloan Kettering panel. Data from the Royal Marsden Hospital and Memorial Sloan Kettering cases were censored for analyses of these genes. In addition, data were censored for CHEK2 in 158 cases for which exon sequencing coverage was incomplete. The depth of coverage for each gene according to site is provided in Table S4 in the Supplementary Appendix.
To compare our results with data from a large series of patients with localized prostate cancer, we analyzed public data from the Cancer Genome Atlas prostate cancer study.25 Paired-end reads (100 bp) were aligned to the hg19 reference human genome with the use of the Burrows–Wheeler Aligner. Annotations were defined with ANNOVAR (http://annovar.openbioinformatics.org/en/latest). Population allele frequencies were extracted from the Exome Aggregation Consortium ExAC Browser (http://exac.broadinstitute.org/), 1000 Genomes (www.1000genomes.org), and the single-nucleotide polymorphism database of the National Center for Biotechnology Information (dbSNP), version 138 (www.ncbi.nlm.nih.gov/projects/SNP).
INTERPRETATION OF VARIANTS
Our analysis focused on variants identified among 20 genes associated with autosomal dominant cancer-predisposition syndromes that involve maintenance of DNA integrity (Table 2). The pathogenicity of germline variants was determined according to established American College of Medical Genetics and Genomics and Association for Molecular Pathology consensus criteria and International Agency for Research on Cancer guidelines.24,26 At least two independent expert reviewers evaluated all variants against published literature and public databases, including ClinVar and variant-specific databases, in addition to population frequency databases, including 1000 Genomes and the Exome Aggregation Consortium. Expected high-penetrance or moderate-penetrance variants classified as mutations that are pathogenic or likely to be pathogenic are reported here. Low-penetrance variants, such as CHEK2 p.I157T, were excluded.
Table 2.
Germline Mutations in Metastatic Cases as Compared with the General Population and Primary Cases.
| Gene | Metastatic Prostate Cancer (N = 692)* |
Exome Aggregation Consortium (N = 53,105)† |
TCGA Cohort with Primary Prostate Cancer (N = 499) |
Metastatic Prostate Cancer vs. Exome Aggregation Consortium |
Metastatic Prostate Cancer vs. TCGA Cohort |
||
|---|---|---|---|---|---|---|---|
| No. of Mutations (% of Men) | Relative Risk (95% CI) |
P Value | Relative Risk (95% CI) |
P Value | |||
| ATM | 11 (1.59) | 133 (0.25) | 5 (1.00) | 6.3 (3.2–11.3) | <0.001 | 1.6 (0.8–2.8) | 0.12 |
| ATR | 2 (0.29) | 43 (0.08) | 0 | 3.6 (0.4–12.8) | 0.11 | — | — |
| BAP1‡ | 0 | 1 | 0 | — | — | — | — |
| BARD1‡ | 0 | 38 (0.07) | 1 (0.20) | — | — | — | — |
| BRCA1 | 6 (0.87) | 104 (0.22) | 3 (0.60) | 3.9 (1.4–8.5) | 0.005 | 1.4 (0.5–3.1) | 0.32 |
| BRCA2 | 37 (5.35) | 153 (0.29) | 1 (0.20) | 18.6 (13.2–25.3) | <0.001 | 26.7 (18.9–36.4) | <0.001 |
| BRIP1‡ | 1 (0.18) | 100 (0.19) | 1 (0.20) | 0.9 (0.02–5.3) | 1.0 | 0.9 (0.0–4.9) | 1.0 |
| CHEK2‡ | 10 (1.87) | 314 (0.61) | 2 (0.40) | 3.1 (1.5–5.6) | 0.002 | 4.7 (2.2–8.5) | <0.001 |
| FAM175A‡ | 1 (0.18) | 52 (0.10) | 0 | 1.8 (0.05–10.1) | 0.42 | — | — |
| GEN1‡ | 2 (0.46) | 42 (0.08) | 0 | 5.8 (0.7–20.8) | 0.048 | — | — |
| MLH1 | 0 | 11 (0.02) | 0 | — | — | — | — |
| MRE11A | 1 (0.14) | 36 (0.07) | 1 (0.20) | 2.1 (0.1–11.8) | 0.38 | 0.7 (0.0–4.0) | 1.0 |
| MSH2 | 1 (0.14) | 23 (0.04) | 1 (0.20) | 3.3 (0.1–18.5) | 0.26 | 0.7 (0.0–4.0) | 1.0 |
| MSH6 | 1 (0.14) | 41 (0.08) | 1 (0.20) | 1.9 (0.05–10.4) | 0.41 | 0.7 (0.0–4.0) | 1.0 |
| NBN | 2 (0.29) | 61 (0.11) | 1 (0.20) | 2.5 (0.3–9.1) | 0.19 | 1.4 (0.2–5.2) | 0.40 |
| PALB2 | 3 (0.43) | 65 (0.12) | 2 (0.40) | 3.5 (0.7–10.3) | 0.05 | 1.1 (0.2–3.1) | 0.76 |
| PMS2 | 2 (0.29) | 56 (0.11) | 1 (0.20) | 2.7 (0.3–9.8) | 0.17 | 1.4 (0.2–5.2) | 0.40 |
| RAD51C | 1 (0.14) | 59 (0.11) | 2 (0.40) | 1.3 (0.03–7.2) | 0.54 | 0.4 (0.0–2.0) | 0.54 |
| RAD51D | 3 (0.43) | 40 (0.08) | 1 (0.20) | 5.7 (1.2–16.7) | 0.02 | 2.2 (0.4–6.3) | 0.16 |
| XRCC2 | 0 | 23 (0.04) | 0 | — | — | — | — |
The denominators for genes for which data were censored were 561 (BAP1, BARD1, BRIP1, and FAM175A), 437 (GEN1), and 534 (CHEK2).
Data are for the persons in the Exome Aggregation Consortium, minus the patients included in the TCGA studies. The percent with a mutation was calculated on the basis of the total number of persons for whom sequence coverage was adequate for the given allele, which differed slightly from the total of 53,105 persons, depending on the specific mutation.
Data for metastatic cases with inadequate sequencing for this gene were censored.
STATISTICAL ANALYSIS
Associations between DNA-repair gene mutation status and age, race, or Gleason score strata were evaluated with the use of two-sided Fisher’s exact tests. The frequencies of DNA-repair gene mutations among the 692 patients with metastatic prostate cancer were evaluated relative to the expected frequencies from the Exome Aggregation Consortium (53,105 persons) or the Cancer Genome Atlas cohort (499 persons) with the use of two-sided exact binomial tests. We also performed analyses in which the 150 men from the previously reported Case Series 1 were excluded18 (Table S5 in the Supplementary Appendix). No adjustments were made for multiple comparisons; P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
PATIENT CHARACTERISTICS
All 692 men in our analysis had documented metastatic prostate cancer, as determined by histologic evaluation of a tumor-biopsy specimen or surgical-resection specimen. The demographic characteristics of the men from each case series are shown in Table 1.
GERMLINE DNA-REPAIR GENE MUTATIONS
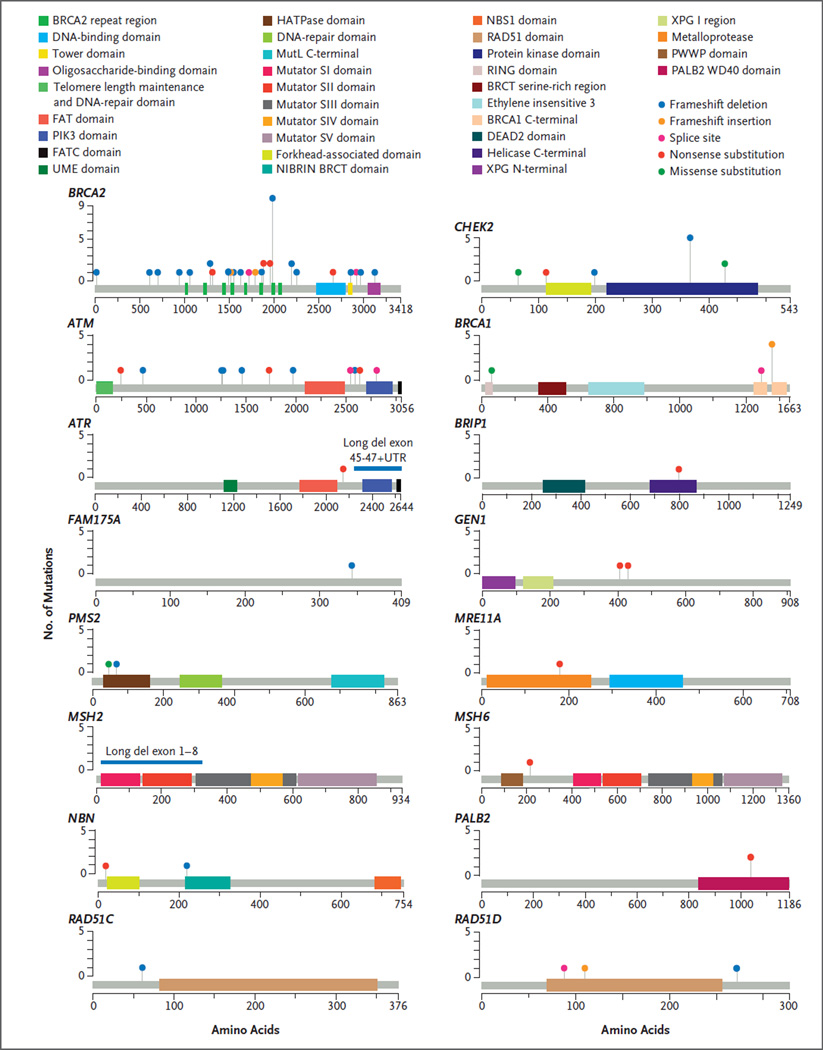
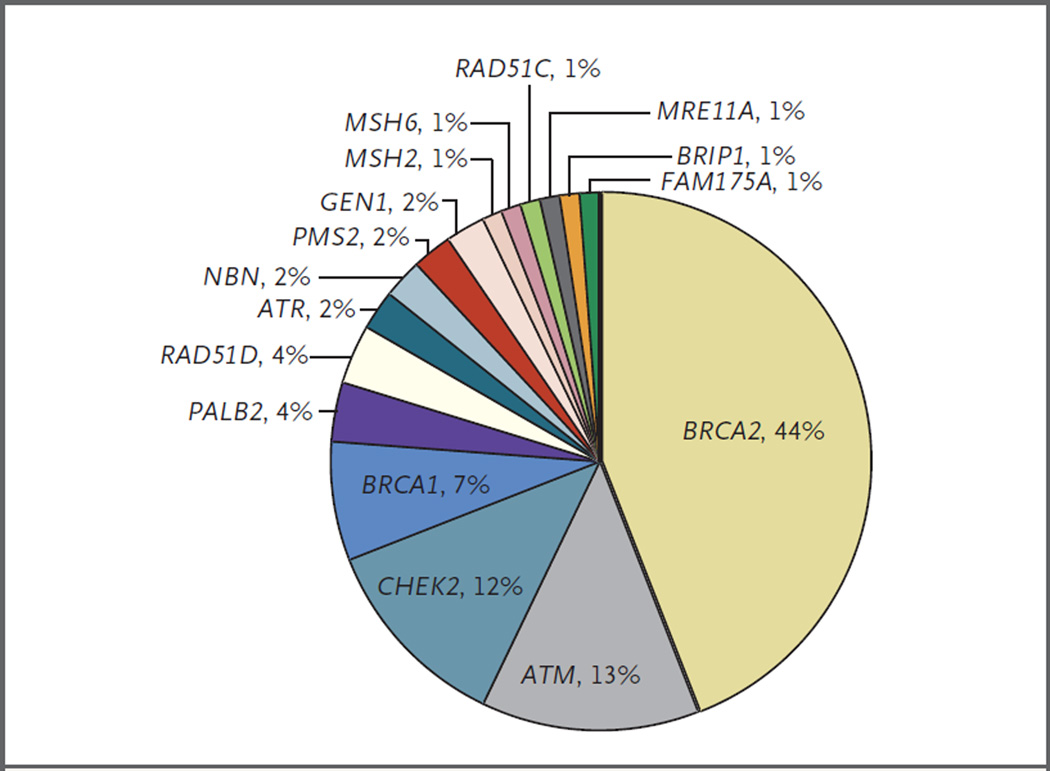
We assessed 20 genes that maintain DNA integrity and have been associated with autosomal dominant cancer-predisposition syndromes (Table 2), using whole-exome sequencing or targeted next-generation sequencing assays designed to interrogate the status of DNA-repair genes.27 Of the 692 men evaluated, 82 (11.8%) had at least one presumed pathogenic germline mutation in a gene involved in DNA-repair processes (Table 2). Mutation frequencies were similar across independent case series (Table 3). The 84 germline mutations that were presumed to be pathogenic (2 men had mutations in 2 genes) included 79 truncating mutations and 5 known deleterious missense mutations (Fig. 1, and Table S1 in the Supplementary Appendix). Mutations were identified in 16 different genes, including BRCA2 (37 mutations [44% of total mutations]), ATM (11 [13%]), CHEK2 (10 [12%]), BRCA1 (6 [7%]), RAD51D (3 [4%]), and PALB2 (3 [4%]) (Fig. 2). Four genes had no clearly detrimental aberrations. One man had mutations in ATM and CHEK2, and one man had mutations in BRCA2 and CHEK2. The majority of men with DNA-repair gene mutations for whom the Gleason score was available (73 men) had primary tumors with high scores (Gleason scores range from 2 to 10, with higher scores associated with worse clinical outcomes): 56 men (77%) had a Gleason score of 8 through 10, 15 men (21%) had a score of 7, and 2 men (3%) had a score of 6. We found no association between the presence of a germline DNA-repair gene mutation and an age at diagnosis of younger than 60 years versus 60 years or older (P = 0.90) or non-Hispanic white versus other race (P = 0.84). There was marginal evidence that the presence of a germline DNA-repair gene mutation was associated with a Gleason score of 8 through 10 versus 7 or lower (odds ratio, 1.8; 95% confidence interval [CI], 1.0 to 3.5; P = 0.04).
Table 3.
Germline DNA-Repair Gene Mutations in Seven Metastatic Prostate Cancer Case Series.
| Case Series |
Description | Patients | Patients with Mutations |
|---|---|---|---|
| no. | no. (%) | ||
| 1 | Stand Up To Cancer–Prostate Cancer Foundation discovery series |
150 | 15 (10.0) |
| 2 | Stand Up To Cancer–Prostate Cancer Foundation validation series |
84 | 9 (10.7) |
| 3 | Royal Marsden Hospital | 131 | 16 (12.2) |
| 4 | University of Washington | 91 | 8 (8.8) |
| 5 | Weill Cornell Medical College | 69 | 7 (10.1) |
| 6 | University of Michigan | 43 | 4 (9.3) |
| 7 | Memorial Sloan Kettering Cancer Center |
124 | 23 (18.5) |
| Total | 692 | 82 (11.8) | |
Figure 1. Presumed Pathogenic Germline Mutations.
Locations of mutations and domains in proteins encoded by 16 predisposition genes are shown by lollipop structures, with the mutation type indicated by color. Protein domains are also distinguished by color. On the graph of each gene, the x axis reflects the number of amino acid residues, and the y axis represents the total number of mutations identified. Of the 20 genes analyzed, 4 (BAP1, BARD1, MLH1, and XRCC2) had no presumed pathogenic germline mutations.
Figure 2. Distribution of Presumed Pathogenic Germline Mutations.
Shown are mutations involving 16 DNA-repair genes. Four genes did not have any pathogenic mutations identified and are not included in the distribution.
FAMILY CANCER HISTORY
Information regarding family history was available for 72 of 82 men (88%) with presumed pathogenic mutations in DNA-repair genes and for 537 of 610 men (88%) without DNA-repair gene mutations. In both groups, 22% of the men (16 of 72 men with DNA-repair gene mutations and 117 of 537 men without such mutations) had a first-degree relative with prostate cancer (P = 1.0). However, 51 of the 72 patients with DNA-repair gene mutations (71%) had a first-degree relative with cancer other than prostate cancer, whereas 270 of the 537 patients without DNA-repair gene mutations (50%) had a first-degree relative with cancer other than prostate cancer (odds ratio, 2.4; 95% CI, 1.4 to 4.3; P = 0.001). Inspection of extended pedigree information of probands with DNA-repair gene mutations revealed affected relatives with breast cancer (24 probands), ovarian cancer (10), leukemia and lymphoma (6), pancreatic cancer (7), or other gastrointestinal cancers (18).
SOMATIC MUTATIONS IN DNA-REPAIR GENES
Tumor sequencing data were available for 61 of the men with germline DNA-repair gene mutations. For 36 (59%) of these men, the second allele was clearly aberrant, in that either a second loss-of-function mutation or a gene-copy loss was present (Table S1 in the Supplementary Appendix). A study of cancer-predisposition genes in children with cancer showed that 66% of children with a presumed pathogenic gene mutation had a second “hit” somatic aberration within the tumor genome,28 and a study involving patients with advanced cancer showed that 21.4% of patients with a presumed pathogenic gene mutation had a somatic second-allele aberration.23 Although a subset of germline loss-of-function mutations may not represent the causal event in the genesis of a given tumor, inactivation of the remaining allele may occur through epigenetic mechanisms or other processes.29
GERMLINE MUTATIONS IN DNA-REPAIR GENES IN LOCALIZED PROSTATE CARCINOMAS
We compared the frequency of germline DNA-repair gene mutations among men with metastatic prostate cancer with the frequency of such mutations among men with localized prostate cancer. In the Cancer Genome Atlas prostate cancer study,25 which included 499 men for whom germline whole-exome sequencing data were available, 23 men (4.6%) had germline mutations in DNA-repair genes (P<0.001 for the comparison with metastatic disease). In addition, 6 men harbored the BRCA2 K3326⋆ polymorphism, a C-terminal truncating variant that is unlikely to be associated with a predisposition to prostate cancer.30 It should be noted that to accommodate Cancer Genome Atlas requirements, the majority of tumors had high-risk characteristics: 90% were clinical stage T2c or greater, and 91% of the carcinomas had a Gleason score higher than 6, which far exceeds the approximately 30% of cancers with a Gleason score higher than 6 that was reported among men whose cancer was diagnosed by screening.31–33 Presumed pathogenic mutations in DNA-repair genes were identified in 2 of 45 men (4%) who had cancer with a Gleason score of 6, in 9 of 249 men (4%) who had cancer with a Gleason score of 7, and in 12 of 205 men (6%) who had cancer with a Gleason score of 8, 9, or 10 (P = 0.37 for trend). Four of 162 men (2%) with localized low-to-intermediate–risk tumors and 19 of 337 men (6%) with localized high-risk tumors, as categorized according to National Comprehensive Cancer Network risk criteria,34 had germline DNA-repair gene mutations (Table 1). The odds of DNA-repair gene mutations being present among men with metastatic prostate cancer differed significantly from the odds among men with localized low-to-intermediate–risk tumors (odds ratio, 5.3; 95% CI, 1.9 to 20.2; P<0.001) or among those with high-risk tumors (odds ratio, 2.2; 95% CI, 1.3 to 4.0; P = 0.002) (Table S6 in the Supplementary Appendix). As observed in men with metastatic prostate cancer, there was no association between the presence of a germline mutation in a DNA-repair gene and an age at diagnosis of younger than 60 versus 60 years of age or older (P = 0.28) or non-Hispanic white versus other race (P = 0.39).
GERMLINE MUTATIONS IN DNA-REPAIR GENES IN THE POPULATION
To estimate the population frequencies of germline mutations in DNA-repair genes, we analyzed exome data compiled from 53,105 persons included in the Exome Aggregation Consortium. We excluded data from persons with cancer who had been included in the Cancer Genome Atlas studies, the inclusion of which could have biased the comparisons with men with prostate cancer. The odds of any deleterious DNA-repair gene mutation being present in men with metastatic prostate cancer differed significantly from the odds in the Exome Aggregation Consortium population (odds ratio, 5.0; 95% CI, 3.9 to 6.3; P<0.001); a similar result was obtained when men from the previously reported Case Series 1 were excluded (odds ratio, 5.2; 95% CI, 4.0 to 6.8; P<0.001) (Table S5 in the Supplementary Appendix). The relative risk of mutations in individual DNA-repair genes among men with metastatic prostate cancer, as compared with men in the Exome Aggregation Consortium population, was substantial, ranging from 18.6 (95% CI, 13.2 to 25.3; P<0.001) for BRCA2 to 3.1 (95% CI, 1.5 to 5.6; P = 0.002) for CHEK2 (Table 2).
DISCUSSION
Inherited and acquired defects in DNA damage repair are key mechanisms in the genesis of malignant tumors. The detection of mutations in DNA-repair genes identifies persons and families who have a predisposition to cancer and defines cancer subtypes that have distinct vulnerabilities to specific therapeutics.35 The ascertainment of germline mutations in DNA-repair genes in men with prostate cancer has several important clinical implications. First, the recent finding that pharmacologic inhibitors of PARP1 induce substantial objective responses in patients with metastatic prostate cancer expressing homologous recombination DNA-repair defects provides a clear treatment pathway in accordance with precision medicine strategies.16 These tumors also appear to be responsive to platinumbased chemotherapy,17 as has been documented for cancers of the ovary and breast in carriers of BRCA1 and BRCA2 mutations.36,37 Second, the identification of a germline mutation in a DNA-repair gene provides information that is key to relatives, both male and female, and that can prompt “cascade” counseling to identify cancer predisposition and deploy risk-reduction strategies. Prospective studies assessing the prognostic and predictive significance of mutations in DNA-repair genes with regard to clinical outcomes are now needed to inform personalized care.
The significant family history of nonprostate cancers among men with mutations in DNA-repair genes was largely accounted for by breast, ovarian, and pancreatic cancers, in which mutations in DNA-repair pathways are known. The possible association between mutations in DNA-repair genes and familial hematologic and gastrointestinal cancers requires further analysis of cosegregation in affected kindreds. As observed for BRCA1 and BRCA2 in breast cancer, mutations may be found in persons who do not have a known syndromic history.38,39 Thus, broader testing of patients with metastatic prostate cancer without regard to family history will increase the yield of actionable mutations identified, in a manner parallel to the recent inclusion of all patients with epithelial ovarian cancers for germline testing regardless of family history.40
This study has several limitations. First, although efforts were made to standardize DNA-sequencing analyses, direct comparability across institutions and with public data is not guaranteed. Second, we focused on clearly deleterious mutations in a selected set of DNA-repair genes; consequently, our findings may underestimate the true frequency of pathogenic events that influence the development of metastatic prostate cancer. Third, although patients across institutions and in the control populations were unselected for family history, possible bias cannot be ruled out. Finally, our case series and the Cancer Genome Atlas study include few persons who were older than 70 years of age at diagnosis, and the incidence of germline DNA-repair gene mutations may differ in this older age group.
In conclusion, the 11.8% overall frequency of germline aberrations in genes responsible for maintaining DNA integrity in men with metastatic prostate cancer is substantially higher than the 1.2 to 1.8% incidence of BRCA2 mutations alone in localized prostate cancer9,12 or the 7.3% incidence of mutations in 22 tumor-suppressor genes in familial prostate cancer.14 Because the high frequency of DNA-repair gene mutations is not exclusive to an early-onset phenotype and is associated with clinically and histologically aggressive disease, with compelling evidence for therapeutic relevance, it may be of interest to routinely examine all men with metastatic prostate cancer for the presence of germline mutations in DNA-repair genes.
Supplementary Material
Acknowledgments
Supported by a Stand Up To Cancer–Prostate Cancer Foundation (SU2C-PCF) International Prostate Cancer Dream Team Translational Cancer Research Grant. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research (SU2C-AACR-DT0712). The project was also supported by the following National Institutes of Health and Department of Defense awards: Prostate SPORE (grants P50CA097186 and P50CA92629, P30CA008748, PC131820, PC140799, R01CA116337, and 1K08CA188615) and the Prostate Cancer Foundation (Movember Challenge Awards). Drs. Beltran and Van Allen are supported by Damon Runyon Clinical Investigator Awards. Drs. Pritchard, Abida, Cheng, Schultz, and Van Allen are supported by Prostate Cancer Foundation Young Investigator Awards. Drs. Chinnaiyan and Sawyers are supported by the Howard Hughes Medical Research Institute. We also acknowledge funding from the Richard M. Lucas Foundation, the Institute for Prostate Cancer Research, Prostate Cancer UK and Movember to the London Prostate Cancer Centre of Excellence, the Starr Cancer Consortium (support to Drs. Beltran and Rubin), a Medical Research Council-Prostate Cancer UK Fellowship (to Dr. Mateo), an Experimental Cancer Medical Centre grant, a Biomedical Research Centre grant to the Institute of Cancer Research–Royal Marsden, the Andrew Sabin Family Foundation, the Marie-Josée and Henry R. Kravis Center for Molecular Oncology, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the men who participated in this study for helping us to gain a better understand the role of genetic predisposition in advanced prostate cancer, the investigators and staff participating in the Stand Up to Cancer–Prostate Cancer Foundation International Prostate Cancer Dream Team, and the following persons at our respective institutions who helped with this study: Hiep Nguyen, Mary-Claire King, Barbara Norquist, Celestia Higano, Lawrence True, and Robert Vessella (University of Washington); Claudia Bertan, Susana Miranda, Penny Flohr, Roberta Ferraldeschi, Zsofia Kote-Jarai, Bindu Raobaikady, Ajit Sarvadikar, Dionne Alleyne, Lucy Hamilton, Sheena Vadgama, and Ada Balasopoulou (Institute for Cancer Research); Jacob Musinsky, Josh Armenia, Diana Mandelker, Maria Arcila, and David Hyman (Memorial Sloan Kettering Cancer Center); Xuhong Cao, Yi-Mi Wu, and Felix Feng (University of Michigan); Elizabeth Heath (Wayne State University); and Tuo Zhang (Weill Cornell Medical College). We also thank the Exome Aggregation Consortium and the groups that provided exome variant data for comparison; a full list of groups contributing to the Exome Aggregation Consortium can be found at http://exac.broadinstitute.org/about.
APPENDIX
The authors’ full names and academic degrees are as follows: Colin C. Pritchard, M.D., Ph.D., Joaquin Mateo, M.D., Michael F. Walsh, M.D., Navonil De Sarkar, Ph.D., Wassim Abida, M.D., Ph.D., Himisha Beltran, M.D., Andrea Garofalo, B.Sc., Roman Gulati, M.Sc., Suzanne Carreira, Ph.D., Rosalind Eeles, M.D., Ph.D., Olivier Elemento, Ph.D., Mark A. Rubin, M.D., Dan Robinson, Ph.D., Robert Lonigro, Ph.D., Maha Hussain, M.D., Arul Chinnaiyan, M.D., Ph.D., Jake Vinson, B.Sc., Julie Filipenko, M.Sc., Levi Garraway, M.D., Ph.D., Mary–Ellen Taplin, M.D., Saud AlDubayan, M.D., G. Celine Han, Ph.D., Mallory Beightol, B.Sc., Colm Morrissey, Ph.D., Belinda Nghiem, B.Sc., Heather H. Cheng, M.D., Ph.D., Bruce Montgomery, M.D., Tom Walsh, Ph.D., Silvia Casadei, Ph.D., Michael Berger, Ph.D., Liying Zhang, M.D., Ph.D., Ahmet Zehir, Ph.D., Joseph Vijai, Ph.D., Howard I. Scher, M.D., Charles Sawyers, M.D., Nikolaus Schultz, Ph.D., Philip W. Kantoff, M.D., David Solit, M.D., Mark Robson, M.D., Eliezer M. Van Allen, M.D., Kenneth Offit, M.D., Johann de Bono, M.B., Ch.B., Ph.D., and Peter S. Nelson, M.D.
The authors’ affiliations are as follows: the University of Washington (C.C.P., M. Beightol, C.M., B.N., H.H.C., B.M., T.W., S. Casadei, P.S.N.) and Fred Hutchinson Cancer Research Center (N.D.S., R.G., P.S.N.) — both in Seattle; the Institute of Cancer Research and Royal Marsden Hospital, London (J.M., S. Carreira, R.E., J.B.); Memorial Sloan Kettering Cancer Center (M.F.W., W.A., M. Berger, L.Z., A.Z., J. Vijai, H.I.S., C.S., N.S., P.W.K., D.S., M.R., K.O.), Weill Cornell Medical College (H.B., O.E., M.A.R.), and the Prostate Cancer Clinical Trials Consortium (J. Vinson, J.F.) — all in New York; the University of Michigan, Ann Arbor (D.R., R.L., M.H., A.C.); Howard Hughes Medical Institute, Chevy Chase, MD (A.C., C.S.); and Dana–Farber Cancer Institute, Boston (A.G., L.G., M.-E.T., S.A., G.C.H., E.M.V.A.).
REFERENCES
- 1.Albertsen PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315:68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin Al Olama A, Kote-Jarai Z, Schumacher FR, et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum Mol Genet. 2013;22:408–415. doi: 10.1093/hmg/dds425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szulkin R, Karlsson R, Whitington T, et al. Genome-wide association study of prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev. 2015;24:1796–1800. doi: 10.1158/1055-9965.EPI-15-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helfand BT, Roehl KA, Cooper PR, et al. Associations of prostate cancer risk variants with disease aggressiveness: results of the NCI-SPORE Genetics Working Group analysis of 18,343 cases. Hum Genet. 2015;134:439–450. doi: 10.1007/s00439-015-1534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berndt SI, Wang Z, Yeager M, et al. Two susceptibility loci identified for prostate cancer aggressiveness. Nat Commun. 2015;6:6889. doi: 10.1038/ncomms7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haraldsdottir S, Hampel H, Wei L, et al. Prostate cancer incidence in males with Lynch syndrome. Genet Med. 2014;16:553–557. doi: 10.1038/gim.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to youngonset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105:1230–1234. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leongamornlert D, Mahmud N, Tymrakiewicz M, et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106:1697–1701. doi: 10.1038/bjc.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Lange EM, Lu L, et al. HOXB13 is a susceptibility gene for prostate cancer: results from the International Consortium for Prostate Cancer Genetics (ICPCG) Hum Genet. 2013;132:5–14. doi: 10.1007/s00439-012-1229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16:2115–2121. doi: 10.1158/1078-0432.CCR-09-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leongamornlert D, Saunders E, Dadaev T, et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br J Cancer. 2014;110:1663–1672. doi: 10.1038/bjc.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol. 2016;69:992–995. doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchard CC, Morrissey C, Kumar A, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988. doi: 10.1038/ncomms5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchard CC, Smith C, Salipante SJ, et al. ColoSeq provides comprehensive Lynch and polyposis syndrome mutational analysis using massively parallel sequencing. J Mol Diagn. 2012;14:357–366. doi: 10.1016/j.jmoldx.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16:56–67. doi: 10.1016/j.jmoldx.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beltran H, Eng K, Mosquera JM, et al. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol. 2015;1:466–474. doi: 10.1001/jamaoncol.2015.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrader KA, Cheng DT, Joseph V, et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016;2:104–111. doi: 10.1001/jamaoncol.2015.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirts BH, Casadei S, Jacobson AL, et al. Improving performance of multigene panels for genomic analysis of cancer predisposition. Genet Med. 2016 Feb 4; doi: 10.1038/gim.2015.212. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 27.Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci USA. 2010;107:12629–12633. doi: 10.1073/pnas.1007983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373:2336–2346. doi: 10.1056/NEJMoa1508054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tapia T, Smalley SV, Kohen P, et al. Promoter hypermethylation of BRCA1 correlates with absence of expression in hereditary breast cancer tumors. Epigenetics. 2008;3:157–163. doi: 10.4161/epi.3.3.6387. [DOI] [PubMed] [Google Scholar]
- 30.Meeks HD, Song H, Michailidou K, et al. BRCA2 polymorphic stop codon K3326X and the risk of breast, prostate, and ovarian cancers. J Natl Cancer Inst. 2016;108:108. doi: 10.1093/jnci/djv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 33.Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2012;62:745–752. doi: 10.1016/j.eururo.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 34.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 35.Jeggo PA, Pearl LH, Carr AM. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer. 2016;16:35–42. doi: 10.1038/nrc.2015.4. [DOI] [PubMed] [Google Scholar]
- 36.Isakoff SJ, Mayer EL, He L, et al. TB-CRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 39.Weitzel JN, Lagos VI, Cullinane CA, et al. Limited family structure and BRCA gene mutation status in single cases of breast cancer. JAMA. 2007;297:2587–2595. doi: 10.1001/jama.297.23.2587. [DOI] [PubMed] [Google Scholar]
- 40.National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian, version 2.2016. doi: 10.6004/jnccn.2016.0018. ( https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.