Abstract
Background A wide range of methods have been used for estimating influenza‐associated deaths in temperate countries. Direct comparisons of estimates produced by using different models with US mortality data have not been published.
Objective Compare estimates of US influenza‐associated deaths made by using four models and summarize strengths and weaknesses of each model.
Methods US mortality data from the 1972–1973 through 2002–2003 respiratory seasons and World Health Organization influenza surveillance data were used to estimate influenza‐associated respiratory and circulatory deaths. Four models were used: (i) rate‐difference (using peri‐season or summer‐season baselines), (ii) Serfling least squares cyclical regression, (iii) Serfling–Poisson regression, (iv) and autoregressive integrated moving average models.
Results Annual estimates of influenza‐associated deaths made using each model were similar and positively correlated, except for estimates from the summer‐season rate‐difference model, which were consistently higher. From the 1976/1977 through the 2002/2003 seasons the, the Poisson regression models estimated that an annual average of 25 470 [95% confidence interval (CI) 19 781–31 159] influenza‐associated respiratory and circulatory deaths [9·9 deaths per 100 000 (95% CI 7·9–11·9)], while peri‐season rate‐difference models using a 15% threshold estimated an annual average of 22 454 (95% CI 16 189–28 719) deaths [8·6 deaths per 100 000 (95% CI 6·4–10·9)].
Conclusions Estimates of influenza‐associated mortality were of similar magnitude. Poisson regression models permit the estimation of deaths associated with influenza A and B, but require robust viral surveillance data. By contrast, simple peri‐season rate‐difference models may prove useful for estimating mortality in countries with sparse viral surveillance data or complex influenza seasonality.
Keywords: Excess mortality, human, Influenza, mortality
Introduction
For several decades, the Centers for Disease Control and Prevention (CDC) has made annual estimates of influenza‐associated deaths in the US. 1 , 2 , 3 , 4 We use the term influenza‐associated death herein to refer to a death for which influenza infection was likely a contributor to mortality, but not necessarily the sole reason for the acute illness that led to the death. Estimates of influenza‐associated deaths have been used to determine costs and benefits associated with influenza prevention and control strategies (including vaccination) and in preparing for both seasonal epidemics and future pandemics. 5 , 6 , 7
Influenza infections result in morbidity and mortality nearly every season in the US. 4 , 8 Mortality associated with influenza varies by age group, by chronic disease status, and by influenza virus type and subtype. 4 , 9 , 10 , 11 Introductions of a novel efficiently transmitted influenza A virus into the population can result in pandemics, which are often associated with more deaths than annual influenza epidemics. Primarily because the US population of those aged ≥65 years has increased substantially since the last pandemic in 1968–1969, current annual estimates of influenza‐associated deaths exceed the annual estimates of deaths associated with that pandemic. 4
Previous estimates for both pandemic and epidemic influenza‐associated deaths have varied, based on outcomes modeled and the specific statistical methods used. 4 , 9 , 12 , 13 Four classes of models have been used by CDC to estimate influenza‐associated deaths in the US: (i) rate‐difference models, 14 , 15 , 16 , 17 (ii) Serfling least squares cyclical regression models which do not incorporate influenza viral surveillance data, 1 , 2 , 18 (iii) Serfling–Poisson regression models which do incorporate influenza viral surveillance data, 4 , 19 and (iv) autoregressive integrated moving average (ARIMA) models which do not use influenza surveillance data. 13 , 20 , 21 In this study, we used these four classes of models to estimate underlying respiratory and circulatory deaths that were associated with influenza among persons aged <65 or ≥65 years. Our objectives were to compare estimates made by using each of the models, to assess similarities and differences among the estimates produced by using each model, and to suggest several strengths and weakness of each model. We believe these results will be of interest not only to researchers and health officials in countries that currently use these models, but also to those in countries that are considering methods to estimate the mortality burden of influenza.
Methods
Data and analyses
United States laboratory‐based surveillance for influenza viruses was conducted from October through mid‐May (calendar week 40 through week 20). During the 1976–1977 through 2002–2003 respiratory seasons, we obtained weekly influenza test results from 50 to 75 World Health Organization (WHO) collaborating virology laboratories in the US. The laboratories provided weekly numbers of total respiratory specimens tested for influenza and the number of positive influenza tests by virus type and subtype. 22
National mortality data were obtained from the National Center for Health Statistics. 23 Deaths were categorized using the International Classification of Diseases eighth revision (ICD‐8), ninth revision (ICD‐9) 24 or tenth revision (ICD‐10), as appropriate. We modeled underlying respiratory and circulatory deaths (ICD‐8 codes 390–519; ICD‐9 codes 390–519; ICD‐10 codes I00–I99, J00–J99). Underlying respiratory and circulatory deaths provide an estimate of death‐associated respiratory infections that is more sensitive than underlying pneumonia and influenza deaths and more specific than all‐cause deaths. 4
We used four types of models to estimate influenza‐associated deaths: (i)rate‐difference models, 16 (ii) Serfling least squares cyclical regression models, 18 (iii) Serfling–Poisson regression models, 4 and (iv) autoregressive integrated moving average models. 20 Human subject review was not required for this study as only aggregate national data without personal identifiers were used in analyses.
Peri‐ and summer‐season rate‐difference models
Incidence rate‐difference models have been used frequently to estimate influenza‐associated hospitalizations and deaths. 14 , 16 , 17 , 25 We defined five periods for each season: (i) a period when ≥10% of specimens tested were positive for influenza, (ii) a period when ≥15% of specimens were positive, (iii) a peri‐season baseline period when <10% of specimens were positive, (iv) a peri‐season baseline period when <15% of specimens were positive, and (v) a summer‐season baseline period. The summer‐season baseline period was defined as the weeks from July through September at the beginning of each season and May and June at the end of each season when there is little influenza activity.
The peri‐season excess mortality rates were defined as the difference in the average weekly mortality rates between an influenza period and a peri‐season period for a particular season. The summer‐season excess mortality rates were defined as the difference in the average weekly rates between an influenza period and a summer‐season period.
Weekly excess mortality rates were converted to annual excess numbers of deaths by using the number of weeks that were above an epidemic threshold, and available US census data: annual excess deaths = (excess weekly rate) × (number of epidemic weeks) × (population).
Serfling least squares cyclical regression model
A previously published Serfling least squares cyclical regression model was used to estimate annual numbers of influenza‐associated deaths. 18 In this model
where Y i represented the number of deaths in a particular week i, β0 represented the intercept, β1 represented a coefficient for the linear time trend, β2 represented a coefficient for the quadratic time trend, β3 and β4 represented coefficients associated with seasonal fluctuations in deaths, and ei represented the error term. Epidemic thresholds were defined for the first 5 years of data for each age‐group based primarily on visual inspection of the data. These thresholds were based on the 1978/1979 influenza season, when influenza A(H1N1) viruses predominated and other evidence suggested that few deaths were attributable to influenza. 18 , 26 For subsequent seasons, annual baselines were forecasted using the prior 5‐year non‐epidemic data.
Serfling–Poisson regression model
Poisson regression models which incorporated weekly influenza circulation data were used to estimate influenza‐associated deaths by age group. 4 , 27 , 28 The models included coefficients similar to those described for the least squares regression models as well as additional terms corresponding to the circulation of influenza A(H3N2), A(H1N1), and B viruses. 4 The three terms represented the percentages of specimens testing positive by subtype during a particular week. The age‐specific population size was used as an offset term. Weekly estimates of the US population by age group were obtained from the US Census Bureau. 29
In each Poisson regression model,
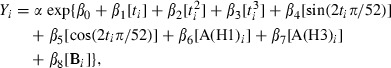 |
where, Y i represented the number of deaths at week i, α was the population offset, β0 represented the intercept, β1 through β3 represented coefficients associated with secular trends, β4 and β5 represented coefficients associated with seasonal changes in deaths, and β6–β8 represented coefficients associated with the percentages of specimens testing positive for each influenza virus type and sub‐type during a given week. We did not have data for respiratory syncytial virus (RSV) before 1990 so an RSV term was not included in the model. Previous estimates of influenza‐associated deaths have suggested that the total estimates of influenza‐associated deaths are not significantly influenced by the inclusion of a RSV term. 30 However, it is possible that if we made age‐specific estimates for young children that the inclusion of RSV in the model could lead to significant differences in death estimates.
Predicted values for the full model for a given week were estimated and then predicted values for models that excluded one viral term were subtracted to estimate influenza‐associated deaths associated with that viral type/subtype. The weekly influenza‐associated deaths were summed for each viral term across the influenza season.
Autoregressive integrated moving average (ARIMA) models
Previously published methods developed by Choi and Thacker 13 , 20 , 31 , 32 were used to estimate influenza‐associated deaths. For each age group, a Fourier equation was used to estimate baseline, non‐influenza deaths during the influenza epidemic weeks of 1972–1973 and 1973–1974; epidemic weeks were defined as two or more consecutive weeks when mortality was greater than two standard deviations (SD) above the mean. Influenza‐related excess deaths were defined as the difference between actual deaths and estimated non‐influenza deaths. During epidemic weeks, total deaths were replaced with estimates of non‐influenza deaths. Following Box‐Jenkins procedures, 33 we removed seasonal patterns from the data by taking the difference in weekly deaths one year apart (e.g., deaths week 40 in 1974–deaths week 40 in 1973). We used the actual deaths during non‐epidemic weeks and the Fourier‐estimated non‐influenza deaths to build the model to estimate deaths for the next 52 weeks. Influenza epidemic weeks were defined as two or more consecutive weeks when actual mortality was greater than the upper bound of a 95% confidence interval (CI) around the non‐influenza deaths. We replaced actual deaths for epidemic weeks with estimated non‐influenza deaths, and repeated the process for each subsequent season, re‐estimating the coefficients from the ARIMA equation. Goodness‐of‐fit was tested by using the Ljung modification of the Box‐Pierce Q statistic. 39
Comparisons of annual numbers of influenza‐associated deaths by age group
We compared the annual numbers of deaths for each model by age group using Wilcoxon signed‐rank tests with a Bonferroni adjustment for multiple comparisons; an adjusted P‐value of <0·05 was considered statistically significant.
Results
Estimates of influenza‐associated deaths using rate‐difference models with a 15% threshold
Among persons aged <65 years, the average annual excess death rate using the peri‐season baseline was 0·15 (95% CI 0·11–1·8) deaths per 100 000 person‐weeks and ranged from 0 to 0·42 deaths per 100 000 person‐weeks (Appendix S1). The annual average summer‐season excess rate was 0·27 deaths per 100 000 person‐weeks (95% CI 0·22–0·31). Among persons aged ≥65 years, excess mortality rates were substantially higher. Using the peri‐season baseline, there were 8·00 (95% CI 6·16–9·84) deaths per 100 000 person‐weeks with substantial variation by seasons (0–18·5 deaths per 100 000 person‐weeks). The average annual excess rate using the summer‐season baseline was 15·1 deaths per 100 000 person‐weeks (95% CI 12·9–17·3).
The annual average number of epidemic weeks (when >15% of specimens tested were positive for influenza) was 7·4 (range 0–15 weeks) (Table 1). Among persons aged <65 years, the estimated number of influenza‐associated deaths for the peri‐season model ranged from 0 to 6574 deaths with an annual average of 2507 deaths. Similarly, using the summer‐season model, the number of deaths ranged from 0 to 9264 deaths with an annual average of 4509 deaths. Among those aged ≥65 years, the estimated number of influenza‐associated deaths for the peri‐season model ranged from 0 to 51 122 deaths with an annual average of 19 954 deaths. Using the summer‐season model, the number of deaths ranged from 0 to 74 821 deaths with an annual average of 36 430 deaths. Eighty‐nine percent of all deaths occurred among persons aged 65 and older. Among all persons, the peri‐season model estimated an annual average of 22 454 (95% CI 16 189–28 179) influenza‐associated deaths.
Table 1.
Incidence rate‐difference model annual estimates for underlying respiratory and circulatory deaths using a 15% threshold*.
| Season | Epi weeks | Age < 65 years | Age >65 years | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual excess numbers | Annual excess rates | Annual excess numbers | Annual excess rates | Annual excess numbers | |||||||
| Peri** | Summer*** | Peri** | Summer*** | Peri** | Summer*** | Peri** | Summer*** | Peri | Summer | ||
| 1976 | 3 | 0 | 844 | 0·0 | 0·4 | 0 | 5749 | 0·0 | 24·2 | 0 | 6593 |
| 1977 | 5 | 4169 | 6107 | 2·1 | 3·1 | 18 658 | 29 272 | 77·3 | 121·2 | 22 827 | 35 379 |
| 1978 | 4 | 985 | 2228 | 0·5 | 1·1 | 3337 | 10 002 | 13·5 | 40·4 | 4322 | 12 230 |
| 1979 | 4 | 1275 | 2616 | 0·6 | 1·3 | 7896 | 17 461 | 31·2 | 68·9 | 9171 | 20 077 |
| 1980 | 3 | 2072 | 3170 | 1·0 | 1·6 | 14 334 | 21 753 | 55·4 | 84·1 | 16 406 | 24 923 |
| 1981 | 0 | 0 | 0 | 0·0 | 0·0 | 0 | 0 | 0·0 | 0·0 | 0 | 0 |
| 1982 | 6 | 1038 | 2714 | 0·5 | 1·3 | 7591 | 20 122 | 28·0 | 74·2 | 8629 | 22 836 |
| 1983 | 12 | 1080 | 4788 | 0·5 | 2·3 | 8197 | 33 704 | 29·6 | 121·6 | 9277 | 38 492 |
| 1984 | 9 | 3311 | 6360 | 1·6 | 3·1 | 29 960 | 50 028 | 106·0 | 176·9 | 33 271 | 56 388 |
| 1985 | 7 | 2022 | 4147 | 1·0 | 2·0 | 16 454 | 33 590 | 57·0 | 116·4 | 18 476 | 37 737 |
| 1986 | 8 | 2664 | 4789 | 1·3 | 2·3 | 17 107 | 32 709 | 58·3 | 111·4 | 19 771 | 37 498 |
| 1987 | 3 | 502 | 1488 | 0·2 | 0·7 | 5285 | 13 886 | 17·6 | 46·3 | 5787 | 15 374 |
| 1988 | 11 | 2511 | 5059 | 1·2 | 2·3 | 19 314 | 41 823 | 63·2 | 136·9 | 21 825 | 46 882 |
| 1989 | 7 | 1429 | 3897 | 0·7 | 1·8 | 17 743 | 36 461 | 57·1 | 117·3 | 19 172 | 40 358 |
| 1990 | 5 | 963 | 2361 | 0·4 | 1·1 | 5575 | 17 393 | 17·6 | 55·0 | 6538 | 19 754 |
| 1991 | 8 | 3269 | 5080 | 1·5 | 2·3 | 25 555 | 42 434 | 79·8 | 132·4 | 28 824 | 47 514 |
| 1992 | 8 | 2440 | 4767 | 1·1 | 2·1 | 20 442 | 40 631 | 62·7 | 124·7 | 22 882 | 45 398 |
| 1993 | 7 | 4193 | 5647 | 1·8 | 2·5 | 35 972 | 51 450 | 109·0 | 155·9 | 40 165 | 57 097 |
| 1994 | 7 | 1457 | 3319 | 0·6 | 1·4 | 14 795 | 31 670 | 44·2 | 94·6 | 16 252 | 34 989 |
| 1995 | 7 | 2631 | 4565 | 1·1 | 2·0 | 16 434 | 34 265 | 48·6 | 101·3 | 19 065 | 38 830 |
| 1996 | 10 | 3522 | 6201 | 1·5 | 2·6 | 35 806 | 61 385 | 104·7 | 179·5 | 39 328 | 67 586 |
| 1997 | 9 | 4498 | 6593 | 1·9 | 2·8 | 45 431 | 66 220 | 131·5 | 191·6 | 49 929 | 72 813 |
| 1998 | 11 | 3945 | 6777 | 1·6 | 2·8 | 43 398 | 70 799 | 124·4 | 203·0 | 47 343 | 77 576 |
| 1999 | 12 | 6574 | 9264 | 2·7 | 3·8 | 51 122 | 74 821 | 145·7 | 213·2 | 57 696 | 84 085 |
| 2000 | 10 | 3486 | 5924 | 1·4 | 2·4 | 21 171 | 43 358 | 59·9 | 122·7 | 24 657 | 49 282 |
| 2001 | 15 | 5050 | 7665 | 2·0 | 3·0 | 41 801 | 67 143 | 117·7 | 189·1 | 46 851 | 74 808 |
| 2002 | 10 | 2614 | 5384 | 1·0 | 2·1 | 15 374 | 35 482 | 43·2 | 99·6 | 17 988 | 40 866 |
| Average during the 1976/77 through the 2002/03 seasons | 7·4 | 2507 | 4509 | 1·2 | 2·1 | 19 954 | 36 430 | 64·7 | 119·3 | 22 461 | 40 939 |
*The 15% threshold represent weeks in which the number of positive influenza isolates exceeded 15% of the total specimen tested.
**The Peri‐season model estimates are calculated by multiplying the peri‐season rates in Appendix S1 times the number of epiweeks times the population divided by 100 000.
***The summer‐season model estimates are calculated by multiplying the summer‐season rates in Appendix S1 times the number of epiweeks times the population divided by 100 000.
Estimates of influenza‐associated deaths using rate‐difference models with a 10% threshold
As expected, estimates of numbers and rates of influenza‐associated deaths were higher with this model than the model using a 15% threshold (Appendices S2 and S3). The annual average number of epidemic weeks (when >10% of specimens tested positive for influenza) was 11·8 (range 2–20 weeks). For persons aged <65 years, the estimated number of influenza‐associated deaths for the peri‐season model ranged from 0 to 7084 deaths with an annual average of 3819 deaths. Using the summer‐season model, the number of deaths ranged from 436 to 10 069 deaths with an annual average of 6574 deaths. For persons aged ≥65 years, the estimated number of influenza‐associated deaths for the peri‐season model ranged from 0 to 57 844 deaths with an annual average of 29 971 deaths. Using the summer‐season model, the number of deaths ranged from 4072 to 93 789 deaths with an annual average of 52 795 deaths.
Estimates of influenza‐associated deaths using Serfling least squares regression models
Among persons aged <65 or ≥65 years, the average annual number of epidemic weeks estimated during the 1976–1977 through 2002–2003 seasons was 3·6 and 9·7, respectively (Table 2). Among persons aged ≥65 years during the 2000/2001 season, the model estimated 28 epidemic weeks, which represented an outlier. Among persons aged <65 or ≥65 years, the model estimated annual averages of 1475 (95% CI 855–2095) and 20 161 (95% CI 14 907–25 415) influenza‐associated deaths, respectively. The average annual rates of influenza‐associated deaths among those aged <65 or ≥65 years were 0·7 (range 0–2·7) and 65·0 (range 0–134·2) per 100 000 person‐weeks, respectively. The total number of influenza‐associated deaths annually was 21 636 (95% CI 15 914–27 358). More than 90% of influenza‐associated pneumonia and influenza deaths occurred among persons aged ≥65 years.
Table 2.
Linear regression model annual estimates using underlying respiratory and circulatory deaths*
| Season | Age < 65 years | Age ≥ 65 years | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epi weeks | Annual excess numbers | L 95% CI | U 95% CI | Annual excess rate** | Epi weeks | Annual excess numbers | L 95% CI | U 95% CI | Annual excess rate** | Annual excess numbers | L 95% CI | U 95% CI | |
| 1972 | 9 | 5206 | 2212 | 8200 | 2·7 | 6 | 12 174 | 5763 | 18 585 | 56·9 | 17 380 | 7975 | 26 785 |
| 1973 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| 1974 | 2 | 836 | 175 | 1497 | 0·4 | 7 | 12 140 | 4693 | 19 587 | 54·1 | 12 976 | 4868 | 21 084 |
| 1975 | 0 | 0 | 0 | 0 | 0·0 | 8 | 22 543 | 14 047 | 31 039 | 98·0 | 22 543 | 14 047 | 31 039 |
| 1976 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| 1977 | 7 | 5299 | 2904 | 7695 | 2·7 | 9 | 30 387 | 20 485 | 40 289 | 125·9 | 35 687 | 23 389 | 47 984 |
| 1978 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| 1979 | 0 | 0 | 0 | 0 | 0·0 | 7 | 14 494 | 7673 | 21 314 | 57·3 | 14 494 | 7673 | 21 314 |
| 1980 | 7 | 4035 | 2233 | 5837 | 2·0 | 11 | 34 726 | 25 292 | 44 159 | 134·2 | 38 761 | 27 525 | 49 996 |
| 1981 | 0 | 0 | 0 | 0 | 0·0 | 7 | 7758 | 1947 | 13 568 | 29·3 | 7758 | 1947 | 13 568 |
| 1982 | 0 | 0 | 0 | 0 | 0·0 | 6 | 8600 | 3373 | 13 827 | 31·8 | 8600 | 3373 | 13 827 |
| 1983 | 2 | 743 | 267 | 1220 | 0·4 | 2 | 3467 | 1707 | 5226 | 12·5 | 4210 | 1975 | 6446 |
| 1984 | 7 | 2595 | 1044 | 4147 | 1·2 | 9 | 21 908 | 13 843 | 29 972 | 77·6 | 24 503 | 14 887 | 34 119 |
| 1985 | 2 | 742 | 300 | 1185 | 0·4 | 12 | 20 426 | 8798 | 32 055 | 70·9 | 21 169 | 9097 | 33 240 |
| 1986 | 2 | 530 | 126 | 934 | 0·2 | 7 | 10 974 | 5117 | 16 831 | 37·4 | 11 504 | 5243 | 17 765 |
| 1987 | 0 | 0 | 0 | 0 | 0·0 | 16 | 26 857 | 15 518 | 38 197 | 89·8 | 26 857 | 15 518 | 38 197 |
| 1988 | 0 | 0 | 0 | 0 | 0·0 | 4 | 3446 | 848 | 6043 | 11·3 | 3446 | 848 | 6043 |
| 1989 | 5 | 2495 | 1503 | 3487 | 1·1 | 9 | 27 016 | 20 426 | 33 606 | 87·2 | 29 510 | 21 928 | 37 093 |
| 1990 | 2 | 642 | 254 | 1030 | 0·3 | 18 | 20 881 | 7632 | 34 130 | 66·3 | 21 523 | 7887 | 35 160 |
| 1991 | 5 | 2085 | 1101 | 3069 | 0·9 | 20 | 36 658 | 21 855 | 51 461 | 114·5 | 38 743 | 22 956 | 54 530 |
| 1992 | 3 | 885 | 283 | 1487 | 0·4 | 21 | 35 302 | 20 132 | 50 472 | 108·6 | 36 187 | 20 415 | 51 959 |
| 1993 | 4 | 2026 | 1266 | 2785 | 0·9 | 8 | 30 908 | 24 860 | 36 956 | 93·8 | 32 934 | 26 126 | 39 742 |
| 1994 | 0 | 0 | 0 | 0 | 0·0 | 8 | 10 569 | 4259 | 16 879 | 31·7 | 10 569 | 4259 | 16 879 |
| 1995 | 3 | 1449 | 872 | 2027 | 0·6 | 6 | 13 408 | 8805 | 18 011 | 39·7 | 14 857 | 9677 | 20 037 |
| 1996 | 4 | 1542 | 755 | 2329 | 0·7 | 9 | 26 841 | 19 702 | 33 980 | 78·5 | 28 383 | 20 457 | 36 309 |
| 1997 | 8 | 2604 | 1130 | 4077 | 1·1 | 11 | 33 901 | 26 058 | 41 744 | 98·2 | 36 504 | 27 188 | 45 821 |
| 1998 | 7 | 2290 | 983 | 3597 | 0·9 | 15 | 41 106 | 29 934 | 52 279 | 117·9 | 43 396 | 30 917 | 55 875 |
| 1999 | 8 | 4157 | 2698 | 5616 | 1·7 | 10 | 37 805 | 31 379 | 44 230 | 107·6 | 41 962 | 34 077 | 49 847 |
| 2000 | 15 | 4303 | 1475 | 7131 | 1·7 | 28 | 34 195 | 15 010 | 53 380 | 96·6 | 38 498 | 16 485 | 60 511 |
| 2001 | 5 | 1403 | 438 | 2368 | 0·6 | 8 | 12 712 | 6665 | 18 760 | 35·7 | 14 116 | 7103 | 21 128 |
| 2002 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| Average during the 1976/77 through the 2002/03 seasons | 3·6 | 1475 | 855 | 2095 | 0·7 | 9·7 | 20 161 | 14 907 | 25 415 | 65·0 | 21 636 | 15 914 | 27 358 |
*Model estimates are based on the linear regression model used in Simonsen et al. (1997).
**Deaths per 100 000 person years.
Estimates of influenza‐associated deaths using Serfling Poisson regression models
Among persons aged <65 years, the models estimated an annual average of 2680 (95% CI 2188–3171) deaths annually (Table 3). The annual rates of influenza‐associated deaths ranged from 0·29 to 2·06 deaths per 100 000 person years. Among persons aged ≥65 years, the models estimated an annual average of 22 790 (95% CI 17 565–28 033), and annual rates of influenza‐associated deaths ranged from 11·7 to 144·7 deaths per 100 000 person years. The average annual total number of influenza‐associated deaths estimated from this model was 25 470 (95% CI 19 781–31 159). Eighty‐nine percent of the estimated deaths occurred among persons aged ≥65 years.
Table 3.
Poisson regression model annual estimates using underlying respiratory and circulatory deaths*
| Season | Age < 65 years | Age ≥ 65 years | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual excess numbers | L 95% CI | U 95% CI | Annual excess rate** | Annual excess numbers | L 95% CI | U 95% CI | Annual excess rate** | Annual excess numbers | L 95% CI | U 95% CI | Annual excess rate** | |
| 1976 | 1549 | 1474 | 1628 | 0·79 | 10 889 | 10,686 | 11 095 | 45·9 | 12 438 | 12 221 | 12 659 | 5·7 |
| 1977 | 3618 | 3502 | 3738 | 1·84 | 21 370 | 21 085 | 21 658 | 88·5 | 24 988 | 24 680 | 25 300 | 11·3 |
| 1978 | 1504 | 1430 | 1582 | 0·76 | 3318 | 3207 | 3433 | 13·4 | 4822 | 4688 | 4960 | 2·2 |
| 1979 | 906 | 849 | 967 | 0·45 | 9107 | 8922 | 9296 | 35·9 | 10 013 | 9819 | 10 211 | 4·4 |
| 1980 | 2914 | 2810 | 3022 | 1·45 | 17 979 | 17 718 | 18 244 | 69·5 | 20 893 | 20 612 | 21 178 | 9·2 |
| 1981 | 584 | 539 | 633 | 0·29 | 4702 | 4570 | 4838 | 17·7 | 5286 | 5145 | 5430 | 2·3 |
| 1982 | 3375 | 3263 | 3491 | 1·64 | 23 881 | 23 580 | 24 186 | 88·0 | 27 256 | 26 934 | 27 582 | 11·7 |
| 1983 | 1822 | 1740 | 1908 | 0·88 | 11 706 | 11 496 | 11 920 | 42·2 | 13 528 | 13 302 | 13 758 | 5·8 |
| 1984 | 4294 | 4167 | 4424 | 2·06 | 33 448 | 33 091 | 33 808 | 118·3 | 37 742 | 37 363 | 38 125 | 15·9 |
| 1985 | 1638 | 1561 | 1719 | 0·78 | 16 139 | 15 892 | 16 390 | 55·9 | 17 777 | 17 518 | 18 040 | 7·4 |
| 1986 | 1303 | 1234 | 1376 | 0·61 | 3429 | 3316 | 3546 | 11·7 | 4732 | 4599 | 4869 | 2·0 |
| 1987 | 2328 | 2235 | 2425 | 1·08 | 19 598 | 19 326 | 19 874 | 65·3 | 21 926 | 21 638 | 22 218 | 9·0 |
| 1988 | 2049 | 1962 | 2140 | 0·95 | 15 272 | 15 032 | 15 516 | 50·0 | 17 321 | 17 065 | 17 581 | 7·0 |
| 1989 | 3299 | 3188 | 3414 | 1·51 | 29 005 | 28 673 | 29 341 | 93·3 | 32 304 | 31 954 | 32 658 | 12·9 |
| 1990 | 1232 | 1165 | 1303 | 0·56 | 13 888 | 13 659 | 14 121 | 43·9 | 15 120 | 14 881 | 15 363 | 6·0 |
| 1991 | 3632 | 3516 | 3752 | 1·63 | 31 073 | 30 729 | 31 420 | 97·0 | 34 705 | 34 342 | 35 072 | 13·6 |
| 1992 | 2110 | 2022 | 2202 | 0·93 | 23 022 | 22 727 | 23 321 | 70·6 | 25 132 | 24 823 | 25 445 | 9·7 |
| 1993 | 3297 | 3186 | 3411 | 1·45 | 31 452 | 31 106 | 31 802 | 95·3 | 34 749 | 34 386 | 35 116 | 13·3 |
| 1994 | 2481 | 2385 | 2581 | 1·07 | 25 000 | 24 692 | 25 312 | 74·7 | 27 481 | 27 158 | 27 808 | 10·4 |
| 1995 | 2531 | 2434 | 2632 | 1·09 | 20 564 | 20 285 | 20 847 | 60·8 | 23 095 | 22 799 | 23 395 | 8·6 |
| 1996 | 3948 | 3827 | 4073 | 1·67 | 41 220 | 40 824 | 41 620 | 120·5 | 45 168 | 44 753 | 45 586 | 16·7 |
| 1997 | 4429 | 4300 | 4561 | 1·85 | 43 824 | 43 416 | 44 236 | 126·8 | 48 253 | 47 824 | 48 685 | 17·6 |
| 1998 | 3763 | 3645 | 3885 | 1·55 | 38 884 | 38 499 | 39 272 | 111·5 | 42 647 | 42 244 | 43 054 | 15·4 |
| 1999 | 4678 | 4546 | 4814 | 1·91 | 44 818 | 44 405 | 45 235 | 127·7 | 49 496 | 49 062 | 49,934 | 17·7 |
| 2000 | 1608 | 1531 | 1689 | 0·65 | 12 013 | 11 800 | 12 230 | 34·0 | 13 621 | 13 394 | 13 852 | 4·8 |
| 2001 | 5187 | 5048 | 5330 | 2·06 | 51 390 | 50 948 | 51 836 | 144·7 | 56 577 | 56 113 | 57 045 | 19·7 |
| 2002 | 2269 | 2178 | 2364 | 0·89 | 18 351 | 18 087 | 18 618 | 51·5 | 20 620 | 20 340 | 20 903 | 7·1 |
| Average during 1976/77 through the 2002/03 seasons | 2680 | 2188 | 3171 | 1·20 | 22 790 | 17 565 | 28 016 | 72·4 | 25 470 | 19 781 | 31 159 | 9·90 |
*The Poisson regression model is based on the methods described in Thompson et al. (2003).
**Deaths per 100 000 person years.
Age‐specific annual estimates for the Poisson regression model were made by influenza virus type and subtype (Table 4). Among persons aged <65 years, the models estimated annual averages of 345 (range 0–1462), 2027 (range 0–4743), and 307 (range 0–825) influenza‐associated deaths for A(H1), A(H3) and B viruses, respectively. Among persons aged ≥65 years, the models estimated annual averages of 887 (range 0–3241), 17 797 (range 0–45 339), and 4107 (4–10 342) influenza‐associated deaths for A(H1), A(H3) and B viruses, respectively.
Table 4.
Poisson regression model annual estimates by virus type and subtype using underlying respiratory and circulatory deaths*
| Age group | Season | A(H1) viruses | A(H3) viruses | B viruses | All influenza | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual excess number | L 95% CI | U 95% CI | Annual excess number | L 95% CI | U 95% CI | Annual excess number | L 95% CI | U 95% CI | Annual excess number | L 95% CI | U 95% CI | ||
| <65 years | 1976 | 5 | 2 | 12 | 1103 | 1040 | 1170 | 441 | 402 | 484 | 1549 | 1474 | 1628 |
| 1977 | 290 | 258 | 325 | 3323 | 3212 | 3438 | 5 | 2 | 12 | 3618 | 3502 | 3738 | |
| 1978 | 1462 | 1389 | 1539 | 2 | 1 | 8 | 40 | 29 | 55 | 1504 | 1430 | 1582 | |
| 1979 | 25 | 17 | 37 | 56 | 43 | 73 | 825 | 771 | 883 | 906 | 849 | 967 | |
| 1980 | 382 | 346 | 422 | 2532 | 2435 | 2633 | 0 | 0 | 0 | 2914 | 2810 | 3022 | |
| 1981 | 199 | 173 | 229 | 0 | 0 | 0 | 385 | 348 | 425 | 584 | 539 | 633 | |
| 1982 | 250 | 221 | 283 | 2937 | 2833 | 3045 | 188 | 163 | 217 | 3375 | 3263 | 3491 | |
| 1983 | 924 | 866 | 986 | 228 | 200 | 260 | 670 | 621 | 723 | 1822 | 1740 | 1908 | |
| 1984 | 3 | 1 | 9 | 4237 | 4111 | 4367 | 54 | 41 | 71 | 4294 | 4167 | 4424 | |
| 1985 | 1 | 0 | 7 | 977 | 918 | 1040 | 660 | 612 | 712 | 1638 | 1561 | 1719 | |
| 1986 | 1283 | 1215 | 1355 | 14 | 8 | 24 | 6 | 3 | 13 | 1303 | 1234 | 1376 | |
| 1987 | 103 | 85 | 125 | 2058 | 1971 | 2149 | 167 | 143 | 194 | 2328 | 2235 | 2425 | |
| 1988 | 949 | 891 | 1011 | 435 | 396 | 478 | 665 | 616 | 718 | 2049 | 1962 | 2140 | |
| 1989 | 25 | 17 | 37 | 3268 | 3158 | 3382 | 6 | 3 | 13 | 3299 | 3188 | 3414 | |
| 1990 | 116 | 97 | 139 | 438 | 399 | 481 | 678 | 629 | 731 | 1232 | 1165 | 1303 | |
| 1991 | 369 | 333 | 409 | 3243 | 3133 | 3357 | 20 | 13 | 31 | 3632 | 3516 | 3752 | |
| 1992 | 65 | 51 | 83 | 1356 | 1286 | 1430 | 689 | 639 | 742 | 2110 | 2022 | 2202 | |
| 1993 | 9 | 5 | 17 | 3276 | 3166 | 3390 | 12 | 7 | 21 | 3297 | 3186 | 3411 | |
| 1994 | 28 | 19 | 41 | 2207 | 2117 | 2301 | 246 | 217 | 279 | 2481 | 2385 | 2581 | |
| 1995 | 760 | 708 | 816 | 1513 | 1439 | 1591 | 258 | 228 | 291 | 2531 | 2434 | 2632 | |
| 1996 | 0 | 0 | 0 | 3525 | 3411 | 3643 | 423 | 385 | 465 | 3948 | 3827 | 4073 | |
| 1997 | 4 | 2 | 11 | 4404 | 4276 | 4536 | 21 | 14 | 32 | 4429 | 4300 | 4561 | |
| 1998 | 16 | 10 | 26 | 3426 | 3313 | 3543 | 321 | 288 | 358 | 3763 | 3645 | 3885 | |
| 1999 | 153 | 131 | 179 | 4506 | 4376 | 4640 | 19 | 12 | 30 | 4678 | 4546 | 4814 | |
| 2000 | 985 | 925 | 1048 | 74 | 59 | 93 | 549 | 505 | 597 | 1608 | 1531 | 1689 | |
| 2001 | 45 | 34 | 60 | 4743 | 4610 | 4880 | 399 | 362 | 440 | 5187 | 5048 | 5330 | |
| 2002 | 873 | 817 | 933 | 858 | 802 | 917 | 538 | 494 | 585 | 2269 | 2178 | 2364 | |
| Average 1976/1977–2002/2003 | 345 | 169 | 521 | 2027 | 1388 | 2667 | 307 | 198 | 416 | 2680 | 2188 | 3171 | |
| ≥65 years | 1976 | 8 | 4 | 16 | 6609 | 6452 | 6770 | 4272 | 4146 | 4402 | 10 889 | 10 686 | 11 095 |
| 1977 | 570 | 525 | 619 | 20 759 | 20 479 | 21 043 | 41 | 30 | 56 | 21 370 | 21 085 | 21 658 | |
| 1978 | 2916 | 2812 | 3024 | 10 | 5 | 19 | 392 | 355 | 433 | 3318 | 3207 | 3433 | |
| 1978 | 51 | 39 | 67 | 377 | 341 | 417 | 8679 | 8498 | 8864 | 9107 | 8922 | 9296 | |
| 1980 | 824 | 770 | 882 | 17 151 | 16 896 | 17 410 | 4 | 2 | 11 | 17 979 | 17 718 | 18 244 | |
| 1981 | 435 | 396 | 478 | 0 | 0 | 0 | 4267 | 4141 | 4397 | 4702 | 4570 | 4838 | |
| 1982 | 569 | 524 | 618 | 21 196 | 20 913 | 21 483 | 2116 | 2028 | 2208 | 23 881 | 23 580 | 24 186 | |
| 1983 | 2173 | 2084 | 2266 | 1674 | 1596 | 1756 | 7859 | 7687 | 8035 | 11 706 | 11 496 | 11 920 | |
| 1984 | 6 | 3 | 13 | 32 775 | 32 422 | 33 132 | 667 | 618 | 720 | 33 448 | 33 091 | 33 808 | |
| 1985 | 4 | 2 | 11 | 7729 | 7559 | 7903 | 8406 | 8228 | 8588 | 16 139 | 15 892 | 16 390 | |
| 1986 | 3241 | 3131 | 3355 | 110 | 91 | 133 | 78 | 62 | 97 | 3429 | 3316 | 3546 | |
| 1987 | 273 | 242 | 307 | 17 105 | 16 851 | 17 363 | 2220 | 2130 | 2314 | 19 598 | 19 326 | 19 874 | |
| 1988 | 2591 | 2493 | 2693 | 3675 | 3558 | 3796 | 9006 | 8822 | 9194 | 15 272 | 15 032 | 15 516 | |
| 1989 | 66 | 52 | 84 | 28 821 | 28 490 | 29 156 | 118 | 99 | 141 | 29 005 | 28 673 | 29 341 | |
| 1990 | 324 | 291 | 361 | 3862 | 3742 | 3986 | 9702 | 9511 | 9897 | 13 888 | 13 659 | 14 121 | |
| 1991 | 1084 | 1021 | 1150 | 29 683 | 29 347 | 30 023 | 306 | 274 | 342 | 31 073 | 30 729 | 31 420 | |
| 1992 | 191 | 166 | 220 | 12 489 | 12 272 | 12 710 | 10 342 | 10 145 | 10 543 | 23 022 | 22 727 | 23 321 | |
| 1993 | 28 | 19 | 41 | 31 209 | 30 865 | 31 557 | 215 | 188 | 246 | 31 452 | 31 106 | 31 802 | |
| 1994 | 89 | 72 | 110 | 21 130 | 20 847 | 21 417 | 3781 | 3662 | 3903 | 25 000 | 24 692 | 25 312 | |
| 1995 | 2319 | 2227 | 2415 | 14 353 | 14 120 | 14 590 | 3892 | 3772 | 4016 | 20 564 | 20 285 | 20 847 | |
| 1996 | 0 | 0 | 0 | 34 692 | 34 329 | 35 059 | 6528 | 6372 | 6688 | 41 220 | 40 824 | 41 620 | |
| 1997 | 12 | 7 | 21 | 43 484 | 43 077 | 43 895 | 328 | 294 | 365 | 43 824 | 43 416 | 44 236 | |
| 1998 | 45 | 34 | 60 | 33 707 | 33 349 | 34 069 | 5132 | 4993 | 5274 | 38 884 | 38 499 | 39 272 | |
| 1999 | 462 | 422 | 506 | 44 039 | 43 630 | 44 452 | 317 | 284 | 354 | 44 818 | 44 405 | 45 235 | |
| 2000 | 2975 | 2870 | 3084 | 714 | 664 | 768 | 8324 | 8147 | 8505 | 12 013 | 11 800 | 12 230 | |
| 2001 | 134 | 113 | 159 | 45 339 | 44 924 | 45 758 | 5917 | 5768 | 6070 | 51 390 | 50 948 | 51 836 | |
| 2002 | 2559 | 2462 | 2660 | 7818 | 7647 | 7993 | 7974 | 7801 | 8151 | 18 351 | 18 087 | 18 618 | |
| Average 1976/1977–2002/2003 | 887 | 440 | 1334 | 17 797 | 11 833 | 23 760 | 4107 | 2660 | 5554 | 22 790 | 17 565 | 28 016 | |
*The Poisson regression model is based on the methods described in Thompson et al. (2003).
Estimates of influenza‐associated deaths using ARIMA models
Using a two‐SD threshold and data for persons aged <65 years, the average annual number of epidemic weeks from the 1976/1977 through 2002/2003 seasons was 1·6 (range 0–7 weeks), and the average annual number of influenza‐associated deaths was 809 (95% CI 292–1326) (Table 5). Among persons aged ≥65 years, the average annual number of epidemic weeks was 9·0 and the average annual number of influenza‐associated deaths were 24 856 (95% CI 19 576–30 136). Using these models, more than 96% of all influenza‐associated deaths occurred among persons aged 65 and older (See Appendix S4 for ARIMA model estimates using a one standard deviation threshold).
Table 5.
Autoregressive integrated moving average (ARIMA) model annual estimates using two SD threshold using underlying respiratory and circulatory deaths*
| Season | Age < 65 years | Age ≥ 65 years | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epi weeks | Annual excess numbers | L 95% CI | U 95% CI | Annual excess rate | Epi weeks | Annual excess numbers | L 95% CI | U 95% CI | Annual excess rate | Annual excess numbers | L 95% CI | U 95% CI | |
| 1972 | 4 | 690 | NA** | NA** | 0·4 | 4 | 3698 | NA** | NA** | 17·3 | 4388 | NA** | NA** |
| 1973 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| 1974 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| 1975 | 2 | 1043 | 113 | 1974 | 0·5 | 6 | 17 879 | 7744 | 28 015 | 77·7 | 18 922 | 7857 | 29 989 |
| 1976 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| 1977 | 7 | 4702 | 1887 | 7517 | 2·4 | 8 | 25 246 | 13 064 | 37 426 | 104·6 | 29 948 | 14 951 | 44 943 |
| 1978 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| 1979 | 0 | 0 | 0 | 0 | 0·0 | 7 | 18 582 | 8958 | 28 205 | 73·5 | 18 582 | 8958 | 28 205 |
| 1980 | 6 | 3715 | 1562 | 5869 | 1·8 | 11 | 37 319 | 22 498 | 52 139 | 144·2 | 41 034 | 24 060 | 58 008 |
| 1981 | 2 | 892 | 172 | 1611 | 0·4 | 4 | 6376 | 1184 | 11 566 | 24·1 | 7268 | 1356 | 13 177 |
| 1982 | 0 | 0 | 0 | 0 | 0·0 | 10 | 19 732 | 6892 | 32 573 | 72·9 | 19 732 | 6892 | 32 573 |
| 1983 | 0 | 0 | 0 | 0 | 0·0 | 9 | 16 424 | 5109 | 27 739 | 59·4 | 16 424 | 5109 | 27 739 |
| 1984 | 6 | 3109 | 932 | 5285 | 1·5 | 12 | 37 535 | 22 663 | 52 407 | 133·0 | 40 644 | 23 595 | 57 692 |
| 1985 | 0 | 0 | 0 | 0 | 0·0 | 12 | 27 305 | 12 768 | 41 841 | 94·8 | 27 305 | 12 768 | 41 841 |
| 1986 | 0 | 0 | 0 | 0 | 0·0 | 10 | 18 252 | 6350 | 30 155 | 62·2 | 18 252 | 6350 | 30 155 |
| 1987 | 0 | 0 | 0 | 0 | 0·0 | 14 | 37 528 | 21 097 | 53 960 | 125·4 | 37 528 | 21 097 | 53 960 |
| 1988 | 0 | 0 | 0 | 0 | 0·0 | 11 | 22 967 | 10 398 | 35 537 | 75·4 | 22 967 | 10 398 | 35 537 |
| 1989 | 4 | 1846 | 617 | 3076 | 0·8 | 11 | 35 694 | 23 200 | 48 187 | 115·1 | 37 540 | 23 817 | 51 263 |
| 1990 | 0 | 0 | 0 | 0 | 0·0 | 5 | 10 038 | 4431 | 15 645 | 31·9 | 10 038 | 4431 | 15 645 |
| 1991 | 0 | 0 | 0 | 0 | 0·0 | 9 | 20 883 | 10 877 | 30 889 | 65·2 | 20 883 | 10 877 | 30 889 |
| 1992 | 3 | 1191 | 285 | 2097 | 0·5 | 13 | 36 381 | 22 082 | 50 681 | 112·0 | 37 572 | 22 367 | 52 778 |
| 1993 | 4 | 2058 | 884 | 3232 | 0·9 | 10 | 39 624 | 28 657 | 50 592 | 120·3 | 41 682 | 29 541 | 53 824 |
| 1994 | 0 | 0 | 0 | 0 | 0·0 | 10 | 23 065 | 12 971 | 33 158 | 69·1 | 23 065 | 12 971 | 33 158 |
| 1995 | 2 | 802 | 231 | 1373 | 0·3 | 10 | 22 406 | 12 379 | 32 433 | 66·3 | 23 208 | 12 610 | 33 806 |
| 1996 | 2 | 645 | 80 | 1210 | 0·3 | 14 | 36 951 | 23 016 | 50 887 | 108·1 | 37 596 | 23 096 | 52 097 |
| 1997 | 0 | 0 | 0 | 0 | 0·0 | 12 | 42 928 | 31 084 | 54 772 | 124·3 | 42 928 | 31 084 | 54 772 |
| 1998 | 2 | 617 | 50 | 1185 | 0·3 | 15 | 49 097 | 34 518 | 63 677 | 140·9 | 49 714 | 34 568 | 64 862 |
| 1999 | 4 | 2268 | 1157 | 3378 | 0·9 | 9 | 39 915 | 31 257 | 48 573 | 113·6 | 42 183 | 32 414 | 51 951 |
| 2000 | 0 | 0 | 0 | 0 | 0·0 | 6 | 17 967 | 10 329 | 25 604 | 50·7 | 17 967 | 10 329 | 25 604 |
| 2001 | 0 | 0 | 0 | 0 | 0·0 | 8 | 22 256 | 14 569 | 29 944 | 62·5 | 22 256 | 14 569 | 29 944 |
| 2002 | 0 | 0 | 0 | 0 | 0·0 | 3 | 6646 | 3717 | 9574 | 18·6 | 6646 | 3717 | 9574 |
| Average 1976/1977–2002/2003 | 1·6 | 809 | 292 | 1326 | 0·4 | 9·0 | 24 856 | 19 576 | 30 136 | 80·3 | 25 665 | 20 148 | 31 182 |
*The ARIMA models are based on the methods described in Choi & Thacker (1982).
**Excess death confidence intervals could not be estimated.
Comparisons of annual estimates of influenza‐associated deaths by age and model type
Annual estimates of influenza‐associated deaths for each model by season are summarized in Table 6. Correlations between annual estimates by model type were all at least moderately correlated (r > 0·53) and statistically significant (Table 7). The lowest correlations were seen for comparisons with the Serfling linear regression model.
Table 6.
Summary of average annual numbers of influenza‐associated deaths by model type
| Season | Peri‐10% | Sum‐10% | Peri‐15% | Sum‐15% | Linear | Poisson | ARIMA 2 SD |
|---|---|---|---|---|---|---|---|
| 1972 | NA* | NA* | NA* | NA* | 17 380 | NA* | 4388 |
| 1973 | NA* | NA* | NA* | NA* | 0 | NA* | 0 |
| 1974 | NA* | NA* | NA* | NA* | 12 976 | NA* | 0 |
| 1975 | NA* | NA* | NA* | NA* | 22 543 | NA* | 18 922 |
| 1976 | 0 | 16 610 | 0 | 6593 | 0 | 12 438 | 0 |
| 1977 | 37 785 | 56 323 | 22 827 | 35 379 | 35 687 | 24 988 | 29 948 |
| 1978 | 8565 | 23 353 | 4322 | 12 230 | 0 | 4822 | 0 |
| 1979 | 25 971 | 45 929 | 9171 | 20 077 | 14 494 | 10 013 | 18 582 |
| 1980 | 47 663 | 64 942 | 16 406 | 24 923 | 38 761 | 20 893 | 41 034 |
| 1981 | 0 | 4508 | 0 | 0 | 7758 | 5286 | 7268 |
| 1982 | 22 027 | 47 513 | 8629 | 22 836 | 8600 | 27 256 | 19 732 |
| 1983 | 9552 | 48 383 | 9277 | 38 492 | 4210 | 13 528 | 16 424 |
| 1984 | 36 389 | 66 081 | 33 271 | 56 388 | 24 503 | 37 742 | 40 644 |
| 1985 | 23 274 | 49 339 | 18 476 | 37 737 | 21 169 | 17 777 | 27 305 |
| 1986 | 19 771 | 37 498 | 19 771 | 37 498 | 11 504 | 4732 | 18 252 |
| 1987 | 23 767 | 47 605 | 5787 | 15 374 | 26 857 | 21 926 | 37 528 |
| 1988 | 26 386 | 56 341 | 21 825 | 46 882 | 3446 | 17 321 | 22 967 |
| 1989 | 57 268 | 81 590 | 19 172 | 40 358 | 29 510 | 32 304 | 37 540 |
| 1990 | 20 090 | 46 889 | 6538 | 19 754 | 21 523 | 15 120 | 10 038 |
| 1991 | 36 345 | 59 542 | 28 824 | 47 514 | 38 743 | 34 705 | 20 883 |
| 1992 | 40 293 | 74 836 | 22 882 | 45 398 | 36 187 | 25 132 | 37 572 |
| 1993 | 50 242 | 71 382 | 40 165 | 57 097 | 32 934 | 34 749 | 41 682 |
| 1994 | 35 325 | 62 601 | 16 252 | 34 989 | 10 569 | 27 481 | 23 065 |
| 1995 | 32 455 | 68 758 | 19 065 | 38 830 | 14 857 | 23 095 | 23 208 |
| 1996 | 64 791 | 103 858 | 39 328 | 67 586 | 28 383 | 45 168 | 37 596 |
| 1997 | 61 335 | 87 824 | 49 929 | 72 813 | 36 504 | 48 253 | 42 928 |
| 1998 | 58 638 | 89 915 | 47 343 | 77 576 | 43 396 | 42 647 | 49 714 |
| 1999 | 63 111 | 91 602 | 57 696 | 84 085 | 41 962 | 49 496 | 42 183 |
| 2000 | 30 311 | 57 805 | 24 657 | 49 282 | 38 498 | 13 621 | 17 967 |
| 2001 | 55 023 | 87 343 | 46 851 | 74 808 | 14 116 | 56 577 | 22 256 |
| 2002 | 25 957 | 54 606 | 17 988 | 40 866 | 0 | 20 620 | 6646 |
| Average during the 1976/77 through the 2002/03 seasons | 33 790 | 59 369 | 22 461 | 40 939 | 21 636 | 25 470 | 25 665 |
| SD during the 1976/77 through the 2002/03 seasons | 18 778 | 23 279 | 15 839 | 22 054 | 14 462 | 14 377 | 13 943 |
ARIMA, autoregressive integrated moving average; SD, standard deviation.
*Excess death numbers and rates could not be estimated due to lack of viral surveillance data.
Table 7.
Correlations between annual estimates of influenza‐associated deaths*
| Peri‐10% | Sum‐10% | Peri‐15% | Sum‐15% | Linear | Poisson | ARIMA | |
|---|---|---|---|---|---|---|---|
| Peri‐10% | 1·00 | ||||||
| Sum‐10% | 0·95 | 1·00 | |||||
| Peri‐15% | 0·86 | 0·84 | 1·00 | ||||
| Sum‐15% | 0·83 | 0·88 | 0·97 | 1·00 | |||
| Linear | 0·72 | 0·63 | 0·62 | 0·54 | 1·00 | ||
| Poisson | 0·86 | 0·86 | 0·86 | 0·83 | 0·54 | 1·00 | |
| ARIMA 2SD | 0·82 | 0·78 | 0·69 | 0·65 | 0·79 | 0·68 | 1·00 |
ARIMA, autoregressive integrated moving average; SD, standard deviation.
*All correlations were statistically significant.
Estimates from each model were compared using the Wilcoxon signed‐rank tests with a Bonferroni adjustment for multiple comparisons. For models that used viral surveillance data, these comparisons were limited to the 1976–1977 through the 2002–2003 seasons when viral surveillance data were available. For persons aged <65 years, the summer‐season 10% rate‐difference estimates were significantly higher than all other estimates. (See Appendix S5a for annual estimates) Summer‐season 15% estimates were significantly lower than the summer‐season 10% estimates and higher than the linear, Poisson, and ARIMA estimates. The peri‐season 10% estimates were significantly higher than the linear and ARIMA estimates. The ARIMA model estimates were significantly lower than estimates from all other models, except for the linear regression estimates.
For persons aged ≥65 years, the summer‐season 10% rate‐difference estimates were significantly higher than all other model estimates with the exception of the summer‐season 15% model. (See Appendix S5b for annual estimates).
Discussion
Annual estimates of influenza‐associated deaths have been used to describe the relative severity of inter‐pandemic and pandemic influenza seasons. Numbers and rates of influenza‐associated deaths also have been used in economic analyses to assess the costs and benefits of public health interventions. Specifically, estimates of influenza‐associated deaths have been influential in analyses of the cost‐effectiveness of possible expansions of US influenza vaccination recommendations. 34 , 35 Thus, estimates of influenza‐associated deaths on a national level have been directly relevant to US influenza control policies.
The four excess death models used by CDC over the past four decades to make estimates of influenza‐associated deaths produced a similar picture of the burden of influenza‐associated mortality during our 31‐year study period. While there is no gold standard currently available for assessing the performance of the different models, with the exception of estimates made by using the summer‐season 10% rate‐difference model, the models produced mortality estimates that were similar in absolute magnitude and similar across 31 influenza seasons.
While most models yielded similar excess death estimates, each model has several strengths and weaknesses. Rate‐difference models have been used for many years because they are straightforward and can be used with less than five seasons of baseline data. Rate‐difference models may be used in countries with more than a single peak in influenza activity each season. These models are easy to implement, they do not require the manual definition of epidemic thresholds, and they allow other factors (e.g., the circulation of RSV) to be incorporated into models, if viral data for other pathogens are available. However the many advantages of rate‐difference models must be balanced against their weaknesses. While peri‐season rate‐difference models produce estimates of US influenza‐associated deaths that are comparable with those produced by using other methods, the summer‐season rate‐difference models consistently produce estimates of mortality that appear inflated when compared with those obtained from other models. Rate‐difference models usually cannot be used to estimate influenza type‐ and subtype‐specific mortality, because circulation of influenza types and subtypes overlap, and overlapping viral data is difficult to incorporate in these simple models. Finally, seasonal factors other than influenza circulation are difficult to control for and therefore the results could be biased by such factors temperature and humidity.
A strength of the Serfling least squares regression model is that it provides estimates of influenza‐associated deaths without the need for influenza virus surveillance data, at least when these models are used in temperate areas in which the seasonality of influenza has been documented. While this may be a strength for countries that are not collecting consistent influenza virus surveillance data, the lack of such data may mean that the model’s underlying assumption that essentially all excess winter mortality is associated with influenza circulation may be unreasonable. These models also are simple when compared with other regression models. Particular weaknesses of the least squares regression model are the requirement to visually examine data to define initial baseline periods and the use of arbitrary statistical thresholds (e.g., z‐score cut‐points) to define influenza‐associated deaths.
The Serfling–Poisson regression models produce estimate of numbers and rates of deaths by influenza type and subtype, an advantage for countries like the US that have many years of robust influenza virus surveillance data. Other strengths of Poisson models include the ability to account for changes in population size over time and the ability to incorporate other variables, such as the circulation of other pathogens (e.g., RSV) or climatic variables such as temperature. Disadvantages of Poisson models as used by CDC include requirements for consistent, robust weekly viral surveillance data and for at least 5 years of mortality data before stable estimates of the effects of all three currently circulating influenza types and subtypes can be made. Nonetheless, when the necessary data are available the ability of these models to provide weekly estimates of type‐ and subtype‐specific deaths represent a step forward in efforts to better understand the burden of influenza on mortality. Another disadvantage of this method is that it makes an assumption that a linear relationship exists between the percentage of specimens testing positive for influenza and the log of the mortality rate. This assumption is difficult to test. However, it is logical to assume that increasing intensity of influenza circulation does lead to increases in influenza‐associated deaths.
The ARIMA method is a dynamic forecasting method that uses the relationship between past data to forecast future values. A strength of this method for estimating influenza‐associated deaths is that virologic data and manually setting baselines are not required. Another advantage is that as more data are collected the model can be updated and re‐validated (i.e., the coefficients changed) to improve model fit and accuracy. Autoregressive integrated moving average methods have several disadvantages when compared with more commonly used models. They can be complicated to implement successfully, provide relatively few advantages over the more simple linear regression models, and suffer from some of the same weaknesses as these models, including defining influenza seasons solely by the use of statistical thresholds.
Centers for Disease Control and Prevention’s most recent published estimates of influenza‐associated deaths for the 1990–1991 through 1998–1999 seasons made use of Poisson regression models. The annual average number of underlying respiratory and circulatory deaths associated with influenza during those nine seasons was 36 155 deaths. 4 An annual estimate for a longer period (the 1976/1977 season through the 1998/1999 season) of 25 420 deaths was also made by using the Poisson regression model. 4 The mortality estimates made in this study for 1976–1977 to 2002–2003 were similar to these previous estimates. The annual average for the 1990–1991 through 1998–1999 seasons was 32 928. The average annual estimate for the 1993–1994 through the 2002–2003 seasons, the last decade of the study period, was 36 171 deaths.
While the estimates of numbers and rates of influenza‐associated deaths were similar and highly correlated across models, the estimates of the numbers of epidemic weeks were less highly correlated. The beginning and end of the epidemic periods (i.e., the tails) are typically associated with small differences between expected mortality and observed mortality. Therefore, differences in epidemic weeks lead to smaller differences than might be expected in the estimated annual number of influenza‐associated deaths. Understanding why differences in estimates of epidemic weeks are found using various models is an area for future research.
In summary, each of the four models we used to estimate annual influenza‐associated mortality produced similar estimates, with the exception of summer baseline rate‐difference and the ARIMA models. Several factors must be considered when seeking to make the most efficient and reliable estimates of influenza‐associated deaths. Depending on the availability of consistent and robust surveillance data, the length of the period for which mortality estimates are being made, and the general seasonality of influenza circulation in area of the world being studied, different models might be selected for primary use. We suggest that as countries or areas that have not previously made estimates of influenza‐associated mortality begin this process, that it is reasonable to compare estimates made by using several different methods to see how similar the results are, and how they vary over time. Poisson models seem well‐suited for use in countries with robust viral surveillance data. In countries where viral surveillance data are limited and where the seasonality of influenza is more complex, rate‐difference models represent a reasonable starting point for making estimates of influenza‐associated mortality. An important area for additional research is how to apply statistical models to estimate influenza‐associated mortality in those subtropical and tropical countries that include the majority of the world’s population.
Financial Support
The work presented in this manuscript was funded solely by the US Centers for Disease Control and Prevention. The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of interest
We declare that we have no conflict of interest.
Supporting information
Supporting info item
Acknowledgement
We wish to thank Ericka Sinclair, Erin Murray, and Alicia Budd for assistance in organizing the WHO influenza isolate data.
References
- 1. Serfling RE. Methods for current statistical analysis of excess pneumonia‐influenza deaths. Public Health Rep 1963; 78:494–505. [PMC free article] [PubMed] [Google Scholar]
- 2. Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health 1987; 77:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis 2000; 181:831–837. [DOI] [PubMed] [Google Scholar]
- 4. Thompson WW, Shay DK, Weintraub E et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 5. Meltzer MI, Cox NJ, Fukuda K. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg Infect Dis 1999; 5:659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nichol KL. The efficacy, effectiveness and cost‐effectiveness of inactivated influenza virus vaccines. Vaccine 2003; 21:1769–1775. [DOI] [PubMed] [Google Scholar]
- 7. Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2003; 52(RR‐8):1–34. [PubMed] [Google Scholar]
- 8. Thompson WW, Shay DK, Weintraub E et al. Influenza‐associated hospitalizations in the United States. JAMA 2004; 292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 9. Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis 1998; 178:53–60. [DOI] [PubMed] [Google Scholar]
- 10. Nichol KL, Baken L, Nelson A. Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung disease. Ann Intern Med 1999; 130:397–403. [DOI] [PubMed] [Google Scholar]
- 11. Nichol KL, Wuorenma J, Von Sternberg T. Benefits of influenza vaccination for low‐, intermediate‐, and high‐risk senior citizens. Arch Intern Med 1998; 158:1769–1776. [DOI] [PubMed] [Google Scholar]
- 12. Noble GR. Epidemiogical and clinical aspects of influenza; in Bears AS. (ed): Basic and Applied Influenza Research. Boca Raton, FL: CRC Press, 1982, 11–50. [Google Scholar]
- 13. Choi K, Thacker SB. Mortality during influenza epidemics in the United States, 1967–1978. Am J Public Health 1982; 72:1280–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol 1980; 112:798–811. [DOI] [PubMed] [Google Scholar]
- 15. Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med 1982; 142:85–89. [PubMed] [Google Scholar]
- 16. Izurieta HS, Thompson WW, Kramarz P et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–239. [DOI] [PubMed] [Google Scholar]
- 17. O’Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004; 113(3 Pt 1):585–593. [DOI] [PubMed] [Google Scholar]
- 18. Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health 1997; 87:1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neuzil KM, Maynard C, Griffin MR, Heagerty P. Winter respiratory viruses and health care use: a population‐based study in the northwest United States. Clin Infect Dis 2003; 37:201–207. [DOI] [PubMed] [Google Scholar]
- 20. Choi K, Thacker SB. An evaluation of influenza mortality surveillance, 1962–1979 I. Time series forecasts of expected pneumonia and influenza deaths. Am J Epidemiol 1981; 113:215–226. [DOI] [PubMed] [Google Scholar]
- 21. Stroup DF, Thacker SB, Herndon JL. Application of multiple time series analysis to the estimation of pneumonia and influenza mortality by age 1962–1983. Stat Med 1988; 7:1045–1059. [DOI] [PubMed] [Google Scholar]
- 22. Brammer TL, Izurieta HS, Fukuda K et al. Surveillance for influenza–United States, 1994–95, 1995–96, and 1996–97 seasons. Morb Mortal Wkly Rep CDC Surveill Summ 2000; 49:13–28. [PubMed] [Google Scholar]
- 23. Vital Statistics Mortality Data, Multiple Cause Detail, 1972–2004 . Public Use Data Tapes Contents and Documentation Package. Hyattsville, MD: National Center for Health Statistics, 2004. [Google Scholar]
- 24. World Health Organization . Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, based on recommendations of the Ninth Revision Conference, 1975. Geneva: World Health Organization, 1977. [Google Scholar]
- 25. Mullooly JP, Bridges CB, Thompson WW et al. Influenza‐ and RSV‐associated hospitalizations among adults. Vaccine 2007; 25:846–855. [DOI] [PubMed] [Google Scholar]
- 26. Simonsen L, Clarke MJ, Stroup DF, Williamson GD, Arden NH, Cox NJ. A method for timely assessment of influenza‐associated mortality in the United States. Epidemiology 1997; 8:390–395. [DOI] [PubMed] [Google Scholar]
- 27. Thompson WW, Weintraub E, Shay DK, Brammer L, Cox NJ, Fukuda K. Influenza‐attributable hospitalizations in the United States; in Osterhaus A, Cox NJ. (eds): Options for the Control of Influenza V. Hampson: Elsevier Science, 2003. [Google Scholar]
- 28. Thompson WW, Shay DK, Weintraub E, Brammer L, Cox NJ, Fukuda K. Influenza‐attributable deaths among the elderly in the United States; in Osterhaus A, Cox NJ. (eds): Options for the Control of Influenza V. Hampson: Elsevier Science, 2003. [Google Scholar]
- 29. US Bureau of the Census . Intercensal Estimates of the Population by Age, Sex, and Race: 1970‐2000. Washington DC: US Bureau of the Census, 2005. [Google Scholar]
- 30. Thompson WW, Shay DK, Weintraub E et al. In Reply to Letters. JAMA 2003; 289:2500–2502. 12759318 [Google Scholar]
- 31. Choi K, Thacker SB. From the centers for disease control. Improved accuracy and specificity of forecasting deaths attributed to pneumonia and influenza. J Infect Dis 1981; 144:606–608. [DOI] [PubMed] [Google Scholar]
- 32. Choi K, Thacker SB. An evaluation of influenza mortality surveillance, 1962–1979. II. Percentage of pneumonia and influenza deaths as an indicator of influenza activity. Am J Epidemiol 1981; 113:227–235. [DOI] [PubMed] [Google Scholar]
- 33. Box G, Jenkins GM. Time‐Series Analysis; Forecasting and Control. San Francisco: Holden‐Day, 1970. [Google Scholar]
- 34. Meltzer MI, Neuzil KM, Griffin MR, Fukuda K. An economic analysis of annual influenza vaccination of children. Vaccine 2005; 23:1004–1014. [DOI] [PubMed] [Google Scholar]
- 35. Prosser LA, Bridges CB, Uyeki TM et al. Health benefits, risks, and cost‐effectiveness of influenza vaccination of children. Emerg Infect Dis 2006; 12:1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


