Abstract
In the second part of this review article the management of medial elbow tendinopathy, distal biceps and distal triceps tendinopathy will be discussed. There is a scarcity of publications concerning any of these tendinopathies. This review will summarise the current best available evidence in their management. Medial elbow tendinopathy, also known as Golfer's elbow, is up to 6 times less common than lateral elbow tendinopathy. The tendinopathy occurs in the insertion of pronator teres and flexor carpi radialis. Diagnosis is usually apparent through a detailed history and examination but care must be made to exclude other conditions affecting the ulnar nerve or less commonly the ulnar collateral ligament complex. If doubt exists then MRI/US and electrophysiology can be used. Treatment follows a similar pattern to that of lateral elbow tendinopathy. Acute management is with activity modification and topical NSAIDs. Injection therapy and surgical excision are utilised for recalcitrant cases.
Distal biceps and triceps tendinopathies are very rare and there is limited evidence published. Sequelae of tendinopathy include tendon rupture and so it is vital to manage these tendinopathies appropriately in order to minimise this significant complication. Their management and that of partial tears will be considered.
Keywords: Golfer's elbow, tendinopathy, tendinosis, medial, elbow, biceps, triceps
INTRODUCTION
The tendinopathies to the common flexor origin, distal biceps and distal triceps occur much less commonly than lateral elbow tendinopathy. The second part of this two-part review article focuses on these tendinopathies and the current best evidence to manage them.
MEDIAL ELBOW TENDINOPATHY (GOLFER'S ELBOW)
The origins of the term ‘Golfer's Elbow’ are not as easily traceable as ‘Tennis Elbow’, most probably as a result of its reduced prevalence. The condition is similar to lateral elbow tendinopathy but affects the flexor-pronator origin on the medial epicondyle of the elbow and, similarly to ‘tennis elbow’, does not commonly trouble its namesake. It has sometimes been termed ‘Pitcher's elbow’, particularly in North America, as a result of baseball pitchers commonly being afflicted.
Epidemiology
The prevalence of medial elbow tendinopathy has been stated to be 0.4% [1] and is estimated to be between three- and six-fold less common than lateral elbow tendinopathy [1,2]. The prevalence in the working population is much higher and, in some cohorts, as high as 3.8% [3]. The peak incidence is in middle-aged patients in their fourth or fifth decade, commonly affecting women more than men. The condition, similar to lateral elbow tendinopathy, appears to be self-limiting, with resolution in approximately 80% within 1 years to 3 years [3,4].
Anatomy and pathogenesis
The flexor-pronator muscles attach to the medial epicondyle. These include the pronator teres, flexor carpi radialis, palmaris longus, flexor digitorum superficialis and flexor carpi ulnaris.
The pronator teres and flexor carpi radialis attach to the proximal margin on the anterior aspect of the medial epicondyle. These tendons are stretched as a result of valgus forces experienced in the throwing cycle and are therefore the most susceptible to microtrauma and the pathological changes seen in tendinopathy. Throwing athletes (e.g. baseball pitchers, javelin throwers and tennis players) typically have hypertrophy to the pronator teres and flexor carpi radialis [5].
Similar to lateral elbow tendinopathy, repetitive microtrauma to these tendons cause an inadequate healing response and the pathological changes of angiofibroblastic hyperplasia. The microtrauma, in the majority of cases, occurs because of a combination of repetitive valgus stresses to the elbow and resisted flexion of the flexor-pronator muscle group. This differs from lateral elbow tendinopathy where resisted wrist extension is the probable predisposing factor.
The biomechanics of elbow movement demonstrate that valgus stresses are maximal during the acceleration phase of throwing. This is most readily seen in baseball pitchers where, during this phase, the ball accelerates from a standstill to peak velocity at the point of release. When considering professional athletes, baseball pitchers regularly pitch at velocities of 90 miles per hour and tennis players regularly serve at over 100 miles per hour and so it is clear why these athletes are susceptible to medial elbow tendinopathy. In addition, Morris et al. demonstrated the highest activity in the pronator teres during tennis serves using electromyography [6]. The most activity in the acceleration phase of throwing is seen in the flexor carpi radialis and pronator teres [7]. Amateur golfers also have increased activity in pronator teres (compared to professional golfers), which explain the perceived predilection for ‘Golfer's elbow’ in this cohort [8].
The ulnar collateral ligament and the ulnar nerve must also be considered when assessing patients with medial elbow pain. The ulnar collateral ligament plays a fundamental role in resisting the valgus stresses through the elbow. The ulnar collateral ligament is composed of an anterior, posterior and transverse band [9]. It originates from the medial epicondyle and inserts distally at the base of the coronoid process. The anterior band is the primary restraint to valgus stress [10] and originates from the anteroinferior margin of the medial epicondyle inserting onto the sublime tubercle of the ulna. The ulnar nerve passes through the medial intermuscular septum, approximately 8 cm above the medial epicondyle, coursing along a groove in the medial head of triceps. It then travels posterior to the medial epicondyle in the cubital tunnel before passing between the two heads of the flexor carpi ulnaris.
Clinical features
The diagnosis of medial elbow tendinopathy is usually not as apparent as that of lateral elbow tendinopathy. This is a result of the possible differential diagnoses and the possibility of concomitant pathologies. For example, it is not uncommon for a patient to suffer with medial elbow tendinopathy and cubital tunnel syndrome. Symptoms are of activity-related medial elbow pain of gradual onset. Pain can be exacerbated during the acceleration phase, particularly of throwing.
Clinical examination commonly demonstrates pain just distal and anterior to the medial epicondyle over the insertion of the flexor-pronator muscles. The pain is worsened on resisted wrist flexion and pronation. The elbow should be flexed at 90° to isolate pronator teres. Flexion contractures may be present and are more commonly seen in overhead throwing athletes as a late sign. Grip strength is reduced and can be compared with the contralateral side either subjectively or objectively using a handheld dynamometer.
Assessment must be made to rule out ulnar nerve neuropathy. Tinel's sign should be elicited and a full neurological examination performed, including sensory and motor assessment. Ulnar collateral ligamentstability should also be assessed. This is a chieved with the elbow in 20° to 30° of flexion to unlock the olecranon from the olecranon fossa. Another method of assessment is the ‘milking test’ [11], where the elbow is flexed and supinated and the examiner pulls laterally on the patient's thumb at the same time as stabilizing the distal humerus against external rotation. A comparison of shoulder external rotation with the elbow in flexion and extension can also be used with good intra and interobserver reliability [12]. This method is effective because valgus instability at the elbow is only present with the elbow in flexion.
Investigations
Further investigations are not always necessary to diagnose medial elbow tendinopathy because the clinical history and an examination should be sufficient (Table 1). However, electromyography is useful if ulnar neuritis/neuropathy is suspected and plain radiography can rule out bony pathology. Radiographs may show calcification next to the medial epicondyle.
Table 1.
Differential diagnosis of medial elbow pain
| Ulnar neuropathy/neuritis |
| Medial collateral ligament injury |
| Osteoarthrosis |
| Osteochondritis dissecans |
| Occult fracture |
| Posteromedial elbow impingement |
| Synovial plica |
If further imaging is required, then magnetic resonance imaging (MRI) appears to be the investigation of choice as a result of the ease of assessing for intra-articular and alternative soft tissue pathology. A thickening of the common flexor tendon sheath with increased signal intensity on T1- and T2-weighted images is characteristic of medial elbow tendinopathy [13]. A high signal intensity on the T2-weighted images in the common flexor tendon and associated paratendinous oedema are the most specific findings (Fig. 1).
Fig. 1.
Magnetic resonance imaging showing a high signal intensity at the common flexor origin consistent with medial elbow tendinopathy.
Ultrasound can be used in the diagnosis with a sensitivity and specificity of 95.2% and 92%, respectively [14]. The most common abnormality seen is focal hypo-echoic areas within the common flexor tendon. Thickening, partial thickness and full thickness tears can also be demonstrated with this modality.
Treatment
As a result of the favourable natural history of medial elbow tendinopathy, conservative management is the basis of initial treatment. Poor prognostic factors include smoking, obesity, repetitive movements and forceful activities (work related activities), and so these should be sought for and addressed where possible [1]. Much of the evidence for treating medial elbow tendinopathy has been gained from studies on lateral elbow tendinopathy as a result of the common pathological process. There are very few studies specifically assessing the management of medial elbow tendinopathy, with only one randomized controlled trial found and no systematic reviews or meta-analyses. The lack of evidence most likely represents the reduced incidence compared to lateral elbow tendinopathy.
In the acute phase of medial elbow tendinopathy, treatment consists of stopping any activities that may be causing symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) can be used, preferably topically, to minimize side effects and the elbow can be iced. Injection therapy should be considered if these simple measures fail to control symptoms. Stahl and Kaufman performed a randomized controlled trial comparing corticosteroid injection with local anaesthetic in 60 elbows with a clinical diagnosis of medial epicondylitis [4]. There was significant less pain in the corticosteroid group at 6 weeks (p < 0.03), although the reduction of pain was the same by 3 months and at 1 year. Corticosteroid injection should be considered with caution given the high relapse rates seen with its use for lateral elbow tendinopathy at 6 months to 1 year. There is also the possibility of skin and fat atrophy following injection, which should not be underestimated [15]
There is very little evidence to support or refute other injection modalities in the treatment of medial elbow tendinopathy. However, one prospective study assessed the use of autologous blood injection and dry needling under ultrasound guidance [16]. It was found to be effective with respect to reducing pain and improving function in 17 out of 20 patients over a 10-month follow-up period, with improvement seen as early as 4 weeks.
Injections to the common flexor tendon are, however, not without risk as a result of the close proximity of the ulnar nerve [17] and the possibility of branches of the medial antebrachial cutaneous nerve crossing near the epicondyle [18]. It is important to examine the patient before injection to ensure the ulnar nerve is not subluxing. The safest method of injecting is with the elbow extended.
Extracorporeal shockwave therapy has also been assessed with unfavourable results. In a prospective, comparative study of 60 patients treated with extracorporeal shockwave therapy with a diagnosis of medial or lateral elbow tendinopathy, improvement was seen in only 27% and 60% of patients, respectively [19].
Ciccotti et al. described three phases of conservative management, particularly relevant for athletes [5]. Phase 1 consists of pain alleviation in the form of cessation of offending activities, NSAIDs, night splinting and a consideration of corticosteroid injection. Phase 2 starts once the pain has settled and focuses on physiotherapy and rehabilitation. The intention is to increase muscle strength to a level greater than that before injury. The concept being that pre-injury muscle strength was susceptible to injury. Phase 3 concentrates on modifications that the patient should consider in their sporting practice (or work practice) to minimize the risk of recurrence. This, for example, could involve a modification of the sporting equipment used.
Operative management
If a patient fails 6 months of conservative management, it is reasonable to consider surgery. The available evidence on the surgical options is limited, although some conclusions can be drawn. The principles of surgical management remain the same as surgery to the lateral side, with resection or release of the diseased tendon being fundamental to giving the best chances of improvement. Concomitant surgery to decompress the ulnar nerve or address ulnar collateral ligament pathology should also be considered if required.
Open surgery is the mainstay of treatment. Presently, there are no studies assessing the use of arthroscopy. One cadaveric study assessed the proximity of the ulnar nerve and the medial collateral ligament complex to the region of debridement, demonstrating a mean distance of 20.8 mm from the ulnar nerve and 8.3 mm from the anterior bundle of the medial collateral ligament [20]. Arthroscopic surgery is likely, at present, to remain experimental given the close proximity of these structures.
The operative technique involves a curvilinear incision centred on the medial epicondyle. The ulnar nerve should be identified and protected, as well should any branches from the medial antebrachial cutaneous nerve that are evident. Branches of the antebrachial cutaneous nerve have been shown to traverse the cubital tunnel in the subcutaneous tissue distal to the medial epicondyle in 100% of cases and proximal in 61% [21]. The flexor pronator origin is then identified. Two main surgical techniques for addressing the pathological tendon have been described similarly to those for lateral elbow tendinopathy. The flexor-pronator origin can be incised longitudinally with the pathological tissue being excised. Alternatively, the tendon can be incised transversely and released from its insertion. Some surgeons will decorticate or drill the medial epicondyle to increase vascularity.
The majority of studies on operative management have used a ‘resection and repair’ approach. Vangsness and Jobe reported the results obtained in 35 patients who were undergoing this procedure including drilling of the medial epicondyle with a mean follow-up of 85 months [22]. Using the grading system of Nirschl and Pettrone (Table 2), the results were excellent, good, and fair in 24, 10 and one patient, respectively [23]. Five patients required a concomitant ulnar nerve transposition. The results for these patients were excellent in four patients and fair in one.
Table 2.
Nirschl and Pettrone grading (1979)
| Excellent | Full return to all activity with no pain |
| Good | Full return to all activity with occasional mild pain |
| Fair | Normal activity with no pain, significant pain with heavy activity, and 75% or better overall improvement in pain |
| Failure | No relief of pre-operative symptoms |
Gabel and Morrey reported the results obtained in 30 surgically-treated cases of medial elbow tendinopathy with a mean follow-up of 7 years [24]. Their technique involved elevation of the flexor-pronator origin from the medial epicondyle, debridement, and epicondylar shaving with or without re-attachment. The results, using the same grading system, were excellent for 11 elbows, good for 15, fair for two and poor for two. It was noted that patients with an associated moderate to severe ulnar neuropathy comprised a poor prognostic sign, with only two out of five elbows achieving a good or excellent result compared to 24 out of 25 elbows without ulnar neuropathy.
Ollivierre et al. also reported on 50 surgically-treated cases using a resection and repair technique with drilling of the medial epicondyle [25]. Twelve had ulnar nerve symptoms, with eight undergoing cubital tunnel release and four undergoing subcutaneous transposition. All patients had partial or complete pain relief with increased grip dynamometer strength. However, 10 patients were unable to return to their premorbid sporting and occupational activities.
Cho et al. described a minimally invasive technique to perform resection and repair under local anaesthetic for both lateral and medial elbow tendinopathy [26]. In their study, there were 10 medial cases, although these results were not separated from those for the lateral side. The results using the Nirschl and Pettrone score were excellent in 23, good in 18 and fair in one. The ulnar nerve was not transposed in any of these cases.
There is only one published study assessing transverse release of the flexor-pronator origin [27]. This was performed in 40 consecutive elbows. Co-existent ulnar neuritis was evident in 24 elbows, with release being performed in 17 and transposition in two. Twenty-five elbows had a good outcome at final follow-up (mean of 44 months). The results were statistically significantly better in the patients with isolated medial elbow tendinopathy compared to those with co-existing ulnar neuritis. The results in the two patients undergoing ulnar nerve transposition were good, suggesting that transposition may lead to improved results in these patients.
Gong et al. provided further evidence to support ulnar nerve transposition in patients with co-existing pathology [28]. They performed musculofascial lengthening to the flexor-pronator origin using a technique originally described by Dellon and Coert [29] for submuscular ulnar nerve transposition. A retrospective review of 19 patients undergoing this procedure with a mean follow-up of 38 months was performed. All patients had clinical symptoms of ulnar neuritis/neuropathy with abnormal electrophysiology in six. Patients with severe neuropathy were excluded. The results obtained using the Nirschl and Pettrone grading system were excellent in six patients, good in 10 and fair in three (Table 3).
Table 3.
Summary of operative results
| Nirschl and Pettrone grading system | Vangsness and Jobe [22] (n = 35) | Gabel and Morrey [24] (n = 30) | Cho et al. [26] (n = 42) combined lateral and medial | Gong et al. [28] (n = 19) |
|---|---|---|---|---|
| Excellent | 24 (68.6%) | 11 (36.7%) | 23 (54.8%) | 6 (31.6%) |
| Good | 10 (28.6%) | 15 (50%) | 18 (42.9%) | 10 (52.3%) |
| Fair | 1 (2.9%) | 2 (6.7%) | 1 (2.4%) | 3 (15.8%) |
| Poor | 0 (0.0%) | 2 (6.7%) | 0 (0.0%) | 0 (0.0%) |
Postoperative management in all these studies vary slightly, although the principles are the same. Either rest or splintage is appropriate in the first week, followed by a return to normal daily activities. A progressive muscle strengthening programme should be started between 6 weeks and 12 weeks postoperatively, with sporting activities allowed at approximately 3 months.
Conclusions and preferred management
Medial elbow tendinopathy is much less common than lateral elbow tendinopathy. It is also far less common than cubital tunnel syndrome and so diligence in history taking and examination are important when identifying patients. Clearly, examination is vital when diagnosing co-existing pathology and ulnar neuritis can be present in up to 50% of cases. The incidence of co-existing medial collateral instability, however, appears relatively low, with only one reported case [25] in the publications discussed.
Once the diagnosis has been established, a period of conservative management is appropriate, particularly in the acute phase. This includes cessation of aggravating activities, simple analgesia and topical NSAIDs. Injection therapy would be the next logical step in patients with continuing symptoms. Given the evidence available, the injection of autologous blood and dry needling has a reasonable chance of improving symptoms. There are no studies assessing platelet-rich plasma or hyaluronic acid, both of which were effective for lateral elbow tendinopathy, and so, currently, their use cannot be recommended, although this may change in the future. Corticosteroid injection, however, should be avoided.
Should conservative measures fail, then open surgery using a resection and repair technique offers the best chance of success. If co-existing ulnar neuropathy is present, then patients should be counselled that prognosis is less favourable. Ulnar nerve transposition and not simple decompression should be performed for these patients.
DISTAL BICEPS TENDINOPATHY
A review of the literature on distal biceps tendinopathy demonstrates that the condition includes both tendinosis and partial tears. It is probable that complete tears of the distal biceps represent the terminal event of tendinopathy in the majority of cases, although the topic would be too vast to include these in the present review. This review therefore focuses on distal biceps tendinopathy including partial tears.
The current literature on distal biceps tendinopathy is sparse, with the majority consisting of case reports and retrospective case series. The first published case report was by Nielson, who described a partial tear to the distal biceps brachii [30]. The condition was also described by Bourne and Morrey a few years later, again as a partial tearing of the distal biceps tendon [31]. This amalgamation of distal biceps tendinosis and partial tears of the distal biceps has continued with very little differentiation made in the current literature. It is uncertain whether they represent the same pathological process because no histological studies have been performed, although there is certainly clinical evidence to support distal biceps tendinopathy and partial tears occurring in patients without a history of trauma [32]. Furthermore, MRI findings have shown abnormal intratendinous signal intensity consistent with tendinopathy in patients with partial tears [33].
Epidemiology
The incidence and prevalence of distal biceps tendinopathy has not been reported because of its rare occurrence. Complete distal biceps ruptures have a reported incidence of 1.2 per 100,000, with a 7.5-fold increased risk of rupture in patients who smoke [34]. There is, however, no conclusive link between complete ruptures and distal biceps tendinopathy and so it remains unclear whether smokers have a higher incidence of tendinopathy as well. Over 80% of cases are seen in males, with a peak incidence in the fifth and sixth decade of life. The natural history is unknown, with controversy persisting regarding the use and timing of surgical intervention.
Anatomy and pathogenesis
The biceps brachii contributes to flexion of the elbow with brachialis, although its most important role is as the principal supinator. The distal biceps tendon forms approximately 7 cm above the elbow joint at which point the biceps aponeurosis (lacertus fibrosus) arises, passing medially. The tendon initially is oval in cross-section before becoming broader and flatter at its insertion into the radial tuberosity. The fibres spiral in a predictable manner as described by Kulshreshthra et al. in an excellent cadaveric study on 74 elbows [35]. The fibres spiral clockwise and counter clockwise in the left and right elbows, respectively. This ensures the posterolateral fibres consistently insert superiorly, with the anteromedial fibres inserting inferiorly. The relevance of this anatomical arrangement is unclear.
There is very little evidence regarding the pathological process. It would be convenient to assume that distal biceps tendinopathy follows the same pathological process as other tendinopathies in the form of angiofibroplastic hyperplasia. This is most likely to be the case but, as yet, remains to be proven. Surgical and radiological findings demonstrate that the area of affected tendon is consistently just proximal to the osseotendinous junction. This correlates with an area of hypovascularity that may predispose the tendon to an impaired healing response and consequent tendinopathy or rupture [36].
Clinical features
A significant proportion of patients relate the onset of symptoms to an acute event. Frazier et al. reported the largest case series of partial distal biceps tendon ruptures with 17 patients [37]. They also demonstrated that 10 out of the 17 relate their symptoms to a specific event, with over 50% being caused by lifting or catching a heavy object.
Patients complain of pain in the antecubital region with palpable tenderness over the distal biceps tendon. The pain is exacerbated with resisted supination and, to a lesser degree, resisted flexion. Supination strength is reduced compared to the contralateral side. Limitation in movement secondary to pain inhibition is occasionally seen, with a loss of terminal extension being most common [38].
On clinical examination, it is important to differentiate a complete rupture from distal biceps tendinopathy or partial rupture. The hook test, described by O'Driscoll [39], places the elbow in 90° flexion and full supination. The examiner's finger is then brought in laterally to hook under the biceps tendon. An abnormal test, when the biceps tendon cannot be ‘hooked’, is indicative of a complete rupture. If the tendon is intact, then the examiner should pull on this, with pain being indicative of a partial tear or tendinopathy. Forty-five patients underwent surgical exploration of their distal biceps tendon that had pre-operatively been examined using this method. The examination was retrospectively assessed for accuracy. One hundred percent of abnormal hook tests had complete rupture and 100% of normal hook tests had a partial rupture, with 75% having pain on examination. The sensitivity and specificity of the hook test (100% for both) was higher than that of MRI (92% and 85%, respectively) in this cohort, although this has not yet been assessed in other studies.
Investigations
Plain radiography is unlikely to aid significantly in the diagnosis but may show some osseous reaction to the radial tuberosity [31]. MRI and ultrasonography remain the investigations of choice. MRI findings (Fig. 2) include an abnormal signal intensity on fluid sensitive images within the tendon at its insertion, bicipitoradial bursitis, and focal marrow oedema in the radial tuberosity [33]. Performing the MRI with the patient lying prone, the shoulder abducted, elbow flexed to 90° and fully supinated (‘FABS’ position) allows visualization of the whole distal biceps tendon on a single image [40]. The sensitivity and specificity for diagnosing partial tears of the distal biceps tendon using conventional MRI (i.e. not FABS position) is 59.1% and 100%, respectively [41]. No studies have assessed the sensitivity and specificity in the FABS position.
Fig. 2.
Magnetic resonance imaging showing a normal and tendinopathic distal biceps tendon.
Ultrasound can also be used and has the advantages of being dynamic and easy to compare to the contralateral side. However, it is sometimes difficult to image the whole of the distal biceps tendon, and the technique is user-dependent and thus less reproducible [42]. Findings can include reduced echogenicity and peritendinous fluid.
Treatment
The treatment of complete ruptures of the distal biceps tendon is now well established, with surgical anatomic repair being necessary to regain supination strength [43]. However, the optimum treatment for partial tears or tendinopathy remains contentious. Conservative management appears to be a logical place to start; however, there is very little evidence in the literature to support (or refute) this. Durr et al. reported on four MRI proven cases of partial tears where three were successfully treated with non-operative management [32]. This included splint immobilization for a period of 2 weeks, injection of local anaesthetic (one patient), NSAIDs and physiotherapy. A further case report highlighted the efficacy of conservative management in an elite athlete with full resolution of their symptoms [44].
It is not clear how long conservative treatment should be pursued before considering surgery. It is also unclear whether the natural history of partial tears or tendinopathy is complete rupture as is evident in other tendinopathies. Kelly et al. commented on two patients undergoing conservative management who subsequently developed complete rupture following minimal trauma and so this must remain a concern [45].
Operative treatment was first advocated by Bourne and Morrey [31]. Their case series of three patients included two patients who were undergoing a two-incision technique to release the remaining distal biceps, debridement and re-attachment. The other patient underwent re-attachment of the torn fibres alone without ‘release and repair’. The outcome for this patient was significantly worse, requiring further surgery to tenodese the distal biceps to brachialis. Subsequent to their experience, they advised a ‘release and repair’ approach to partial tears. It should be noted that this patient underwent a previous operation to debride the tendon before having the re-attachment and so conclusions regarding the outcome should be guarded.
There have been variations in surgical technique subsequent to this original description. The tendon has been approached using an anterior approach [32,38,46], a posterior approach [45] and a two-incision approach [31,47], with one case series including patients where a variety of these approaches were used [37]. The tendon has been re-attached without release or released and then repaired. The technique of re-attachment, however, has remained largely the same, with suture anchors being used in most cohorts. The largest case series of surgically treated partial tears used differing surgical techniques via differing approaches depending on the operating surgeon's preference [37]. Thirteen patients were treated with repair without release through an anterior approach, three with ‘release and repair’ via a posterior approach and one via a two-incision approach. Universally good results were reported regardless of the technique or approach. All patients stated that they would undergo the procedure again in a similar situation. Elbow flexion was equivalent or stronger compared to the contralateral side, with supination strength being marginally weaker. The results were not separated depending on technique. Complications included a case of asymptomatic heterotopic ossification, two transient sensory neurapraxias to the lateral antebrachial cutaneous nerve, and a late (4 years postoperatively) partial rerupture. This patient had an isolated suture repair originally with suture anchors used in the revision procedure.
Vardakas et al. performed a ‘release and repair’ via an anterior incision in seven patients [46]. Similar results were found, with all patients stating that they would undergo the same procedure again. The flexion and supination strength was stronger compared to the contralateral side and there were no complications. Dellaero and Mallon performed a similar technique on seven patients with again excellent results [38]. However, two patients had transient neurapraxia to the lateral antebrachial cutaneous nerve and six out of the seven patients had a mean loss of active elbow extension of 8.9°. Kelly et al. performed a ‘release and repair’ using a posterior incision in eight patients [45]. Six of these patients were completely satisfied and all would have the procedure again in a similar situation. There were no complications in this cohort. It would therefore appear that surgical intervention is efficacious regardless of approach or technique.
An endoscopic technique to assess and treat distal biceps pathology has been described by Bain et al. [48]. This utilizes a portal in the bicipitoradial bursa. The distal biceps can be adequately assessed and even debrided by this method. Clearly, it remains a technique appropriate only for a skilled elbow arthroscopist at present.
Complications
The major complication of conservative management is complete rupture of the distal biceps tendon. This, however, makes the decision-making process with respect to further management easier because anatomic repair should then be performed. Operative intervention has risks associated with it, although these remain rare in the published case series to date. A loss of extension appears to be the most likely loss to movement as seen in the case series of Dellaero and Mallon [38]. This did not affect function. The proximity of the lateral antebrachial cutaneous nerve renders this at risk of injury. The posterior interosseus nerve is also at risk, particularly if a repair is performed using an endobutton [49]. None of the case series have described this technique, although it is popular for complete tears and thus should be noted. One case of heterotopic ossification occurred but this was asymptomatic.
Conclusions and preferred management
Distal biceps tendinopathy is a rare condition and is unlikely to feature regularly in our orthopaedic practice. This is reflected in the paucity of cases available for review in the literature. Careful examination including the hook test should differentiate between partial tears/tendinopathy and complete tears. If any doubt remains, then MRI is the investigation of choice, ideally with the arm in a flexed, abducted and supinated position.
The literature would favour operative treatment for distal biceps tendinopathy, although this conclusion is susceptible to significant publication bias given that all publications are case series or reports. Therefore, a trial of conservative management would be appropriate. This would include a short period of splintage followed by physiotherapy and analgesia. Steroid injections are not advised as a result of the poor outcomes seen with other tendinopathies. Conservative management should persist for a minimum of 6 months before surgical intervention.
Surgical intervention should release the remaining distal biceps tendon and re-attach it using suture anchors. This technique, used in the majority of case reports, has low complication rates and minimizes the risk of late partial rupture. The surgical approach is dependent on the performing surgeon, with no perceived benefit between approaches being seen. In our practice, we would favour an anterior approach.
DISTAL TRICEPS TENDINOPATHY
Distal triceps tendinopathy is the rarest of the tendinopathies around the elbow and so, understandably, little evidence has been published in peer-reviewed journals. Nirschl described it as ‘posterior tennis elbow’, although there have been only sporadic descriptions of its existence [50]. Triceps rupture likely represents the terminal event of tendinopathy as is seen with distal biceps tendinopathy. Anzel et al. reported a case series of 1014 tendon ruptures of which only four (0.4%) were closed triceps ruptures [51]. The incidence of triceps tendinopathy is unknown.
The management of acute triceps rupture is relatively well established, with anatomic repair resulting in good functional results [52,53]. The management of tendinopathy and partial tears remains contentious and the present review aims to focus on this. Regrettably, there are less than 10 publications on partial ruptures, all of which are case reports/series, with no cases of tendinopathy. Understandably, this limits the robustness of the evidence.
Anatomy, pathogenesis and clinical features
The triceps brachii has the long, lateral and medial (deep) heads of origin. The long head arises from the infraglenoid tubercle of the scapula. The lateral head arises from posterolateral aspect of the proximal humerus and the medial arises from the humeral shaft inferior to the spiral groove. The lateral and long heads converge to produce the superficial portion of the triceps tendon. Medially, these insert straight into the medial aspect of the olecranon, whereas, laterally, the fibres insert at an angle and then continue to blend with the superficial fascia of anconeus. The precise anatomy of the deep portion of the tendon remains contested. Madsen et al. described the medial head of triceps as having a separate, deep insertion from the central tendon in a cadaveric study [54]. Keener et al., however, in another cadaveric study, described a thickening of the medial aspect of the tendon that was not distinct from the central tendon with fibres from the medial and long heads of triceps [55]. The insertion was not separate from the central tendon. The relevance of the deep anatomy is not fully understood but may help to explain the presence of partial ruptures.
Triceps rupture most commonly occurs at the osseotendinous junction although other sites have been reported. Tendon ruptures typically occur through areas of abnormal tendon [56] and so it can be assumed that tendinopathy most likely occurs at the triceps insertion. Imaging and/or operative findings of partial ruptures in the orthopaedic literature have shown the medial [57–60] portion of the tendon avulses with the lateral aspect remaining in continuity. This implies that the deep portion or medial head insertion may be the origin of tendinopathy of the distal triceps, although no studies have confirmed this. Interestingly, the radiological literature demonstrates partial ruptures of the lateral/superficial tendon with an intact medial head [61,62].
Predisposing factors for triceps rupture have been described in the literature and include chronic renal disease with secondary hyperparathyroidism [59,63], olecranon bursitis [62,64] and steroid injections [65]. Of the six case reports of partial ruptures in the literature, only one patient had renal failure [59] and one patient had a previous history of anabolic steroid use [66]. All these patients describe a traumatic incident, with half occurring following a fall onto an outstretched hand [57,59,67] and half occurring in body builders performing bench presses [54,58,60,66]. There are no pre-injury symptoms documented in any of these patients and so the presence of pre-existing tendinopathy is unknown.
Symptoms for distal triceps tendinopathy are consistent with other tendinopathies in that an activity-related pain is the predominant feature. Swelling and palpable tenderness may be present on examination. Resisted extension will exacerbate symptoms but strength is usually maintained. Partial ruptures present following an acute injury and, consequently, will have clinical features relating to this, including haematoma and swelling. There should be no palpable gap in the tendon because the partial rupture occurs on the deep portion [60]. However, strength will be diminished.
INVESTIGATIONS
Plain radiography has limited use but may show an avulsion fragment proximal to the olecranon representing the triceps insertion [66]. Both ultrasound and MRI can be used with the usual advantages and disadvantages applicable to each modality. The superficial location of the triceps tendon does allow easier evaluation using ultrasonography compared to imaging the distal biceps tendon. Ultrasound is performed with the elbow in flexion and shows reduced echogenicity and occasional calcification in tendinopathy. Partial tears of the superficial or deep insertion of the triceps can also be readily seen [61,62].
MRI has the advantage of assessing for other causes of symptoms within the elbow. The findings for tendinopathy demonstrate an abnormal signal intensity on fluid sensitive sequences consistent with all forms of tendinopathy (Fig. 3). The insertions of the superficial and deep portions of the tendon are also easily evaluated.
Fig. 3.
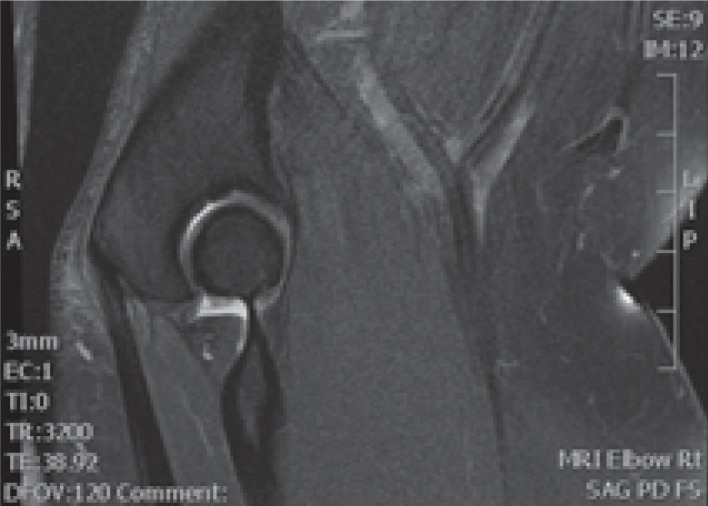
Magnetic resonance imaging showing a high signal in the distal triceps tendon.
Treatment
A detailed clinical assessment should determine whether a patient has suffered a complete triceps rupture or has symptoms from a partial rupture or tendinosis. The gold standard of management for complete ruptures is well established to comprise early anatomic repair [53,67,68]. The management of partial tears is less clear. Ultrasonography and MRI may help in this evaluation because this will allow an appraisal of the degree of tear and associated muscle retraction.
Presently, within the literature, there is no consensus whether conservative management or surgical repair is best for partial ruptures. Moreover, the decision-making to proceed to surgical repair is ill defined within these case reports. The available evidence is limited to one case series and six case reports. Mair et al. identified 21 partial or complete triceps ruptures in 19 National Football League players during a 6-year period [69]. Ten of these were considered partial ruptures following MRI (10% to 75% tears). The treatment for all these patients was conservative, with an average of 4.8 weeks (range 0 to 9) of the season being missed. One patient sustained a complete rupture within 6 weeks of his original injury requiring surgical repair. Six patients had successful conservative management with no residual loss of function or weakness. Three of these patients underwent follow-up MRI showing a healed tendon. The other three patients underwent delayed surgical repair at the end of the season for continuing weakness and pain. All players returned to their previous level of sport for a minimum of one further season.
The six case reports have three elbows treated conservatively [59,66] and five treated surgically [54,57,58], two of which had an arthroscopic repair [60]. Bos et al. reported on the successful conservative management of a partial rupture in a 36-year-old lady with chronic renal failure secondary to glomerulonephritis [59]. Harris et al. described the successful conservative management of a 39-year-old body builder with bilateral partial ruptures [66]. This patient was managed in broad arm slings and was noncompliant throughout his treatment, returning to weightlifting 4 weeks after injury.
Athwal et al. demonstrated, via a cadaveric study and case report on two patients, the feasibility of arthroscopic repair of the medial head of triceps insertion [60]. Repair was performed approximately 9 months post-injury having failed non-operative management. Madsen et al. performed an open repair using metal suture anchors on a 22-year-old who had injured his triceps weight training 4 months post-injury as a result of persistent weakness [54]. Foulk and Galloway performed an acute, transosseus anatomic repair on a 16-year-old whose mechanism of injury was a fall onto an outstretched hand [57]. MRI demonstrated a 65% tear presumably being the stimulus to proceed to early operative intervention. Khiami et al. also performed early transosseus surgical repair on a 28-year-old body builder with a 50% partial rupture diagnosed on MRI [58]. Again, the size of the tear and functional demands of the patient were the reason for early surgical repair.
Downey et al. reported on the ultrasound findings of partial thickness tears [62]. They identified 30 tears of which five underwent surgical intervention. The timing and reason for surgery in these patients is unclear because the study was targeted for the radiologists, although it helps gauge the conversion rate from conservative to surgical treatment for patients with partial thickness tears.
Although there are no studies available reporting on the management of distal triceps tendinopathy, the general impression is that it is a self-limiting condition that resolves with conservative management. As a result of a lack of evidence, management should follow the principles of treating other types of tendinopathies. Therefore, activity modification, including the cessation of weight training, NSAIDs and splinting, should be tried in the first instance. If this fails, then progression to more aggressive nonoperative treatments should be considered. This may include injection therapy with platelet-rich plasma or autologous blood (steroids should be avoided) or radiofrequency ablation. Surgical intervention should be reserved for refractory cases. Nirschl described surgical excision of affected tendon as a treatment for triceps tendinopathy [50], although there have been no case series of this treatment presented. The principles appear to follow the technique that Nirschl described for tennis elbow.
Conclusions and preferred management
Triceps rupture is uncommon with cases of partial ruptures and tendinopathy is even rarer. In the majority of cases, non-operative management of partial ruptures was utilized as an initial treatment of choice with only two patients undergoing early surgical repair. The reason for early surgical repair was that the tears were greater than 50% and, in accordance with the recommendations of Strauch [68], are therefore worthy of repair given the low surgical morbidity and reasonable success rate. However, in Mair's series of 10 partial ruptures, there were patients with tears of up to 75% who healed without loss of function through conservative means [69]. Indeed, the three patients who underwent delayed repair did not necessarily have the largest tears. Those undergoing delayed surgical repair also appeared to make a successful recovery and the surgery was not reported to be any more challenging than acute repair.
For these reasons, we would advocate an initial conservative approach for partial tears up to 75% with the use of a sling or brace. Regular follow-up is required to ensure the tear does not propagate to a complete tear, although this appears to be a rare occurrence. If symptoms persist after 6 months of conservative management, than delayed open anatomic repair using suture anchors or transosseus repair should be performed.
Triceps tendinopathy should also initially be managed conservatively with activity modification, NSAIDs and, occasionally, resting splints. If these simple measures fail to improve symptoms, then an injection of platelet-rich plasma can be administered. Surgical intervention should be considered after a minimum of 1 year of attempted conservative treatment with debridement of the affected tendon and early mobilization.
References
- 1. Shiri R, Viikari-Juntura E, Varonen H, Heliövaara M. Prevalence and determinants of lateral and medial epicondylitis: a population study. Am J Epidemiol 2006; 164: 1065–74. [DOI] [PubMed] [Google Scholar]
- 2. Hamilton P. The prevalence of humeral epicondylitis: a survey in general practice. J R Coll Gen Pract 1986; 36: 464–5. [PMC free article] [PubMed] [Google Scholar]
- 3. Descatha A, Leclerc A, Chastang JF, Roquelaure Y. Medial epicondylitis in occupational settings: prevalence, incidence and associated risk factors. J Occup Environ Med 2003; 45: 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stahl S, Kaufman T. The efficacy of an injection of steroids for medial epicondylitis. A prospective study of sixty elbows. J Bone Joint Surg Am 1997; 79: 1648–52. [DOI] [PubMed] [Google Scholar]
- 5. Ciccotti MC, Schwartz MA, Ciccotti MG. Diagnosis and treatment of medial epicondylitis of the elbow. Clin Sports Med 2004; 23: 693–705. [DOI] [PubMed] [Google Scholar]
- 6. Morris M, Jobe FW, Perry J, Pink M, Healy BS. Electromyographic analysis of elbow function in tennis players. Am J Sports Med 1989; 17: 241–7. [DOI] [PubMed] [Google Scholar]
- 7. Glousman RE, Barron J, Jobe FW, et al. An electromyographic analysis of the elbow in the normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med 1992; 20: 311–17. [DOI] [PubMed] [Google Scholar]
- 8. Farber AJ, Smith JS, Kvitne RS, Mohr KJ, Shin SS. Electromyographic analysis of forearm muscles in professional and amateur golfers. Am J Sports Med 2009; 37: 396–401. [DOI] [PubMed] [Google Scholar]
- 9. Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop 1985; 201: 84–90. [PubMed] [Google Scholar]
- 10. Nazarian LN, McShane JM, Ciccotti MG, O'Kane PL, Harwood MI. Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003; 227: 149–54. [DOI] [PubMed] [Google Scholar]
- 11. Safran MR, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy 2005; 21: 1381–95. [DOI] [PubMed] [Google Scholar]
- 12. Yasui K, Mihata T, Takeda A, Watanabe C, Kinoshita M. Anew method for assessing elbow valgus laxity. Sports Med Arthrosc Rehabil Ther Technol 2012; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kijowski R, De Smet AA. Magnetic resonance imaging findings in patients with medial epicondylitis. Skeletal Radiol 2005; 34: 196–202. [DOI] [PubMed] [Google Scholar]
- 14. Park GY, Lee SM, Lee MY. Diagnostic value of ultrasonography for clinical medial epicondylitis. Arch Phys Med Rehabil 2008; 89: 738–42. [DOI] [PubMed] [Google Scholar]
- 15. Beyzadeoglou T, Bekler H, Gokce A. Skin and subcutaneous fat atrophy after corticosteroid injection for medial epicondylitis. Orthopedics 2011; 34: 570. [DOI] [PubMed] [Google Scholar]
- 16. Suresh SPS, Ali KE, Jones H, Connell DA. Medial epicondylitis: is ultrasound guided autologous blood injection an effective treatment? Br J Sports Med 2006; 40: 935–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stahl S, Kaufman T. Ulnar nerve injury at the elbow after steroid injection for medial epicondylitis. J Hand Surg Br 1997; 22: 69–70. [DOI] [PubMed] [Google Scholar]
- 18. Richards RR, Regan WD. Medial epicondylitis caused by injury to the medial antebrachial cutaneous nerve: a case report. Can J Surg 1989; 32: 366–7. [PubMed] [Google Scholar]
- 19. Krischek O, Hopf C, Nafe B, Pompe JD. Shock-wave therapy for tennis and golfer's elbow – 1 year follow up. Arch Orthop Trauma Surg 1999; 119: 62–6. [DOI] [PubMed] [Google Scholar]
- 20. Zonno A, Manuel J, Merrell G, Ramos P, Akelman E, DaSilva MF. Arthroscopic technique for medial epicondylitis: technique and safety analysis. Arthroscopy 2010; 26: 610–16. [DOI] [PubMed] [Google Scholar]
- 21. Lowe JB, III, Maggi SP, Mackinnon SE. The position of crossing branches of the medial antebrachial cutaneous nerve during cubital tunnel surgery in humans. Plast Reconstr Surg 2004; 114: 692–6. [DOI] [PubMed] [Google Scholar]
- 22. Vangsness CT, Jr, Jobe FW. Surgical treatment of medial epicondylitis. Results in 35 elbows. J Bone Joint Surg Br 1991; 73: 409–11. [DOI] [PubMed] [Google Scholar]
- 23. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am 1979; 61: 832–9. [PubMed] [Google Scholar]
- 24. Gabel GT, Morrey BF. Operative treatment of medial epicondylitis. Influence of concomitant ulnar neuropathy at the elbow. J Bone Joint Surg Am 1995; 77: 1065–9. [DOI] [PubMed] [Google Scholar]
- 25. Ollivierre CO, Nirschl RP, Pettrone FA. Resection and repair for medial tennis elbow. A prospective analysis. Am J Sports Med 1995; 23: 214–21. [DOI] [PubMed] [Google Scholar]
- 26. Cho BK, Kim YM, Kim DS, etal. Mini-open muscle resection procedure under local anaesthesia for lateral and medial epicondylitis. Clin Orthop Surg 2009; 1: 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurvers H, Verhaar J. The results of operative treatment of medial epicondylitis. J Bone Joint Surg Am 1995; 77: 1374–9. [DOI] [PubMed] [Google Scholar]
- 28. Gong HS, Chung MS, Kang ES, Oh JH, Lee YH, Baek GH. Musculofascial lengthening for the treatment of patients with medial epicondylitis and coexisting ulnar neuropathy. J Bone Joint Surg Br 2010; 92: 823–7. [DOI] [PubMed] [Google Scholar]
- 29. Dellon AL, Coert JH. Results of the musculofascial lengthening technique for submuscular transposition of the ulnar nerve at the elbow. J Bone Joint Surg Am 2003; 85: 1314–20. [DOI] [PubMed] [Google Scholar]
- 30. Nielson K. Partial rupture of the distal biceps brachii tendon: a case report. Acta Orthop Scand 1987; 58: 287–8. [DOI] [PubMed] [Google Scholar]
- 31. Bourne MH, Morrey BF. Partial rupture of the distal biceps tendon. Clin Orthop Relat Res 1991; 271: 143–8. [PubMed] [Google Scholar]
- 32. Durr HR, Stabler A, Pfahler M, Matzko M, Fefior JJ. Partial rupture of the distal biceps tendon. Clin Orthop Relat Res 2000; 374: 195–200. [DOI] [PubMed] [Google Scholar]
- 33. Williams BD, Schweitzer ME, Weishaupt D, et al. Partial tears of the distal biceps tendon: MR appearance and associated clinical findings. Skeletal Radiol 2001; 30: 560–4. [DOI] [PubMed] [Google Scholar]
- 34. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics and the effect of smoking. Clin Orthop Relat Res 2002; 404: 275–83. [PubMed] [Google Scholar]
- 35. Kulshreshthra R, Singh R, Sinha J, Hall S. Anatomy of the distal biceps brachii tendon and its clinical relevance. Clin Orthop Relat Res 2006; 456: 117–20. [DOI] [PubMed] [Google Scholar]
- 36. Seiler JG, Parker LM, Chamberland PDC, Sherbourne GM, Carpenter WA. The distal biceps tendon. Two potential mechanisms involved in its rupture: arterial supply and mechanical impingement. J Shoulder Elbow Surg 1995; 4: 149–56. [DOI] [PubMed] [Google Scholar]
- 37. Frazier MS, Boardman MJ, Westland M, Imbriglia JE. Surgical treatment of partial distal biceps tendon ruptures. J Hand Surg Am 2010; 35: 1111–14. [DOI] [PubMed] [Google Scholar]
- 38. Dellaero DT, Mallon WJ. Surgical treatment of partial biceps tendon ruptures at the elbow. J Shoulder Elbow Surg 2006; 15: 215–17. [DOI] [PubMed] [Google Scholar]
- 39. O'Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med 2007; 35: 1865–9. [DOI] [PubMed] [Google Scholar]
- 40. Giuffrè BM, Moss MJ. Optimal positioning for MRI of the distal biceps brachii tendon: flexed abducted supinated view. Am J Roentgenol 2004; 182: 944–6. [DOI] [PubMed] [Google Scholar]
- 41. Festa A, Mulieri PJ, Newman JS, Spitz DJ, Leslie BM. Effectiveness of magnetic resonance imaging in detecting partial and complete distal biceps tendon rupture. J Hand Surg Am 2010; 35: 77–83. [DOI] [PubMed] [Google Scholar]
- 42. Chew ML, Giuffrè BM. Disorders of the distal biceps brachii tendon. Radiographics 2005; 25: 1227–37. [DOI] [PubMed] [Google Scholar]
- 43. Nesterenko S, Domire ZJ, Morrey BF, Sanchez-Sotelo J. Elbow strength and endurance in patients with a ruptured distal biceps tendon. J Shoulder Elbow Surg 2010; 19: 184–9. [DOI] [PubMed] [Google Scholar]
- 44. Giombini A, Innocenzi L, Di Cesare A, Di Salvo W, Fagnani F, Pigozzi F. Partial rupture of the distal biceps brachii tendon in elite water polo goalkeeper: a case report of conservative management. J Sports Med Phys Fitness 2007; 47: 79–83. [PubMed] [Google Scholar]
- 45. Kelly EW, Steinmann S, O'Driscoll SW. Surgical treatment of partial distal biceps tendon ruptures through a single posterior incision. J Shoulder Elbow Surg 2003; 12: 456–61. [DOI] [PubMed] [Google Scholar]
- 46. Vardakas DG, Musgrave DS, Varitimidis SE, Goebel F, Sotereanos DG. Partial rupture of the distal biceps tendon. J Shoulder Elbow Surg 2001; 10: 377–9. [DOI] [PubMed] [Google Scholar]
- 47. Rokito AS, McLaughlin JA, Gallagher MA, Zuckerman JD. Partial rupture of the distal biceps tendon. J Shoulder Elbow Surg 1996; 5: 73–5. [DOI] [PubMed] [Google Scholar]
- 48. Bain GI, Johnson LJ, Turner PC. Treatment of partial distal biceps tendon tears. Sports Med Arthrosc 2008; 16: 154–61. [DOI] [PubMed] [Google Scholar]
- 49. Lo EY, Li CS, Van den Bogaerde JM. The effect of drill trajectory on proximity to the posterior interosseus nerve during cortical button distal biceps repair. Arthroscopy 2011; 27: 1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nirschl RP. Prevention and treatment of elbow and shoulder injuries in the tennis player. Clin Sports Med 1988; 7: 289–308. [PubMed] [Google Scholar]
- 51. Anzel SH, Covey KW, Weiner AD. Disruption of muscles, tendons. An analysis of 1014 cases. Surgery 1959; 45: 406–14. [PubMed] [Google Scholar]
- 52. Levy M. Repair of triceps tendon avulsion or ruptures. J Bone Joint Surg Br 1987; 69: 115. [DOI] [PubMed] [Google Scholar]
- 53. Sierra RJ, Weiss NG, Shrader MW, Steinmann SP. Acute triceps ruptures: case report and retrospective chart review. J Shoulder Elbow Surg 2006; 15: 130–4. [DOI] [PubMed] [Google Scholar]
- 54. Madsen M, Marx RG, Millett PF, Rodeo SA, Sperling JW, Warren RF. Surgical anatomy of the triceps brachii tendon. Am J Sports Med 2006; 34: 1839–43. [DOI] [PubMed] [Google Scholar]
- 55. Keener J, Chafik D, Kim H, Galatz I, Yamaguchi K. Insertional anatomy of the triceps brachii tendon. J Shoulder Elbow Surg 2010; 19: 399–405. [DOI] [PubMed] [Google Scholar]
- 56. Kannus P, Jozsa L. Histopathologic changes preceding spontaneous tendon rupture. A controlled study of 891 patients. Suom Orthop Traumatol 1991; 14: 302–13 (in Finnish). [PubMed] [Google Scholar]
- 57. Foulk DM, Galloway MT. Partial triceps disruption: a case report. Sports Health 2011; 3: 175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khiami F, Tavassoli S, De Ridder Bauer L, Catonné Y, Sariali E. Distal partial ruptures of triceps brachii tendon in an athlete. Orthop Traumatol Surg Res 2012; 98: 242–6. [DOI] [PubMed] [Google Scholar]
- 59. Bos CF, Nelissen RG, Bloem JL. Incomplete rupture of the tendon of triceps brachii. A case report. Int Orthop 1994; 18: 273–5. [DOI] [PubMed] [Google Scholar]
- 60. Athwal GS, McGill RJ, Rispoli DM. Isolated avulsion of the medial head of the triceps tendon: an anatomic study and arthroscopic repair in 2 cases. Arthroscopy 2009; 25: 983–8. [DOI] [PubMed] [Google Scholar]
- 61. Tagliafico A, Gandolfo N, Michaud J, Perez MM, Palmieri F, Martinoli C. Ultrasound demonstration of distal triceps tendon tears. Eur J Radiol 2012; 81: 1207–10. [DOI] [PubMed] [Google Scholar]
- 62. Downey R, Jacobsen JA, Fessell DP, Tran N, Moraq Y, Kim SM. Sonography of partial thickness tears of the distal triceps brachii tendon. J Ultrasound Med 2011; 30: 1351–6. [DOI] [PubMed] [Google Scholar]
- 63. Malefijt MCD, Beeker TW. Avulsion of the triceps tendon in secondary hyperparathyroidism. Acta Orthop Scand 1987; 58: 434–5. [DOI] [PubMed] [Google Scholar]
- 64. Clayton ML, Thirupathi RG. Rupture of the triceps tendon with olecranon bursitis: a case report and a new method of repair. Clin Orthop 1984; 184: 183–5. [PubMed] [Google Scholar]
- 65. Stannard JP, Bucknell AL. Rupture of the triceps tendon associated with steroid injections. Am J Sports Med 1993; 21: 482–5. [DOI] [PubMed] [Google Scholar]
- 66. Harris PC, Atkinson D, Moorehead JD. Bilateral partial rupture of the triceps tendon: case report and quantitative assessment of recovery. Am J Sports Med 2004; 32: 787–92. [DOI] [PubMed] [Google Scholar]
- 67. Farrar EL, Lippert FG. Avulsion of the triceps tendon. Clin Orthop Relat Res 1981; 161: 242–6. [PubMed] [Google Scholar]
- 68. Strauch RJ. Biceps and triceps injuries of the elbow. Orthop Clin North Am 1999; 30: 95–107. [DOI] [PubMed] [Google Scholar]
- 69. Mair SD, Isbell WM, Gill TJ, Schlegel TF, Hawkins RJ. Triceps tendon ruptures in professional football players. Am J Sports Med 2004; 32: 431–4. [DOI] [PubMed] [Google Scholar]




