Abstract
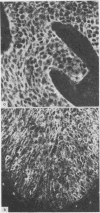
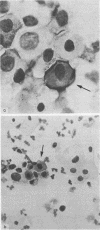
The distribution of an epitope of the transferrin receptor in the human uterine cervical epithelium has been investigated. Immunohistochemical staining, both immunofluorescent and immunoperoxidase, was performed on biopsy specimens and cytological samples from normal, dysplastic, and neoplastic cervical epithelia using the monoclonal OKT9 antibody. The results of staining 145 cervical biopsy specimens with OKT9 showed widespread staining in all malignant epithelia and most severely dysplastic epithelia. No such staining was seen in either normal epithelia or in mildly dysplastic epithelia apart from the staining of the basal cell layer in some normal epithelia. The incidence of staining in the 50 cervical cytocentrifuge preparations was not as high as that in the 145 tissue sections. The potential role of the OKT9 antibody in both the screening of cervical cytocentrifuge preparations and the prediction of malignancy is discussed. The antibody is considered to be of more value in the examination of biopsy material than of cytocentrifuge preparations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Enns C. A., Shindelman J. E., Tonik S. E., Sussman H. H. Radioimmunochemical measurement of the transferrin receptor in human trophoblast and reticulocyte membranes with a specific anti-receptor antibody. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4222–4225. doi: 10.1073/pnas.78.7.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Hsi B. L., Stevens P. J. Transferrin and transferrin receptors in carcinoma of the breast. Lancet. 1980 Aug 23;2(8191):390–392. doi: 10.1016/s0140-6736(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Faulk W. P. Immunological studies of transferrin and transferrin receptors of human placental trophoblast. Placenta. 1980 Jan-Mar;1(1):33–46. doi: 10.1016/s0143-4004(80)80014-2. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Faulk W. P. Transferrin binding by human lymphoblastoid cell lines and other transformed cells. Cell Immunol. 1980 Jan;49(1):215–222. doi: 10.1016/0008-8749(80)90072-6. [DOI] [PubMed] [Google Scholar]
- Gatter K. C., Brown G., Trowbridge I. S., Woolston R. E., Mason D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983 May;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines H. G., McCoy J. P., Hofheinz D. E., Ng A. B., Nordqvist S. R., Leif R. C. Cervical carcinoma antigens in the diagnosis of human squamous cell carcinoma of the cervix. J Natl Cancer Inst. 1981 Mar;66(3):465–474. [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Leif R. C., Easter H. N., Jr, Warters R. L., Thomas R. A., Dunlap L. A., Austin M. F. Centrifugal cytology. I. A quantitative technique for the preparation of glutaraldehyde-fixed cells for the light and scanning electron microscope. J Histochem Cytochem. 1971 Apr;19(4):203–215. doi: 10.1177/19.4.203. [DOI] [PubMed] [Google Scholar]
- Lloyd J. M., O'Dowd T., Driver M., Tee D. E. Immunohistochemical detection of Ca antigen in normal, dysplastic and neoplastic squamous epithelia of the human uterine cervix. J Clin Pathol. 1984 Jan;37(1):14–19. doi: 10.1136/jcp.37.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacsa A. S., Kummerländer L., Pejtsik B., Krommer K., Pali K. Herpes simplex virus-specific antigens in exfoliated cervical cells from women with and without cervical anaplasia. Cancer Res. 1976 Jul;36(7 Pt 1):2130–2132. [PubMed] [Google Scholar]
- Sincock A. M., Middleton J., Moncrieff D. Towards an automated procedure for the quantitative cytological screening of cervical neoplasms. J Clin Pathol. 1983 May;36(5):535–538. doi: 10.1136/jcp.36.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Domingo D. L. Anti-transferrin receptor monoclonal antibody and toxin-antibody conjugates affect growth of human tumour cells. Nature. 1981 Nov 12;294(5837):171–173. doi: 10.1038/294171a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]