Abstract
Background
In the last 4 years, four novel oral anticoagulants have been developed as alternatives to warfarin and antiplatelet agents for stroke prevention in atrial fibrillation (AF) patients. The objective of this review was to estimate the comparative effectiveness of all antithrombotic treatments for AF patients.
Materials and methods
Data sources were Medline Ovid (1946 to October 2015), Embase Ovid (1980 to October 2015), and the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 9, 2015). Randomized controlled trials of AF patients were selected if they compared at least two of the following: placebo, aspirin, aspirin and clopidogrel combination therapy, adjusted-dose warfarin (target international normalized ratio 2.0–3.0), dabigatran, rivaroxaban, apixaban, and edoxaban. Bayesian network meta-analyses were conducted for outcomes of interest (all stroke, ischemic stroke, myocardial infarction, overall mortality, major bleeding, and intracranial hemorrhage).
Results
Based on 16 randomized controlled trials of 96,826 patients, all oral anticoagulants were more effective than antiplatelet agents at reducing the risk of ischemic stroke and all strokes. Compared to warfarin, dabigatran 150 mg (rate ratio 0.65, 95% credible interval 0.52–0.82) and apixaban (rate ratio 0.82, 95% credible interval 0.69–0.97) reduced the risk of all strokes. Dabigatran 150 mg was also more effective than warfarin at reducing ischemic stroke risk (rate ratio 0.76, 95% credible interval 0.59–0.99). Aspirin, apixaban, dabigatran 110 mg, and edoxaban were associated with less major bleeding than warfarin.
Conclusion
All oral anticoagulants reduce the risk of stroke in AF patients. Some novel oral anticoagulants are associated with a lower stroke and/or major bleeding risk than warfarin. In addition to the safety and effectiveness of drug therapy, as reported in this study, individual treatment recommendations should also consider the patient’s underlying stroke and bleeding risk profile.
Keywords: meta-analysis, cerebrovascular disorders/drug therapy, stroke prevention, platelet-aggregation inhibitors, atrial fibrillation/prevention and control
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, and affects between 0.5% and 2% of the population in Western countries. AF is also a growing health problem in developing countries, concordant with the increasing health burden of other chronic noncommunicable diseases.1
AF is associated with significant morbidity and a high risk of ischemic stroke. AF patients are five times more likely to experience an ischemic stroke than the general population, with 20% of patients dying within 1 year after stroke and 60% being left with a disability.2 Therefore, the majority of patients with AF must be on antithrombotic treatment for stroke prevention for the remainder of their lives. Patients are prescribed either antiplatelet drugs or oral anticoagulants (OACs) as antithrombotic therapy. As a result of the increased risk of bleeding associated with these agents, the benefits of treatment must be carefully weighed against the risks. Patients at low risk of stroke are typically prescribed antiplatelet drugs or in some cases no treatment. Similarly, patients at moderate-to-high risk of stroke are typically prescribed OACs, but may be prescribed antiplatelet drugs or even nothing.3–5
Rationale
For 50 years, warfarin was the only OAC indicated for antithrombotic therapy in AF patients. With the advent of the direct thrombin inhibitor dabigatran and the direct factor Xa inhibitors rivaroxaban, apixaban, and edoxaban (collectively called novel OACs [NOACs]), physicians and reimbursement decision makers are faced with a complex decision when selecting the optimal treatment for these patients. This decision is further complicated by the fact that new interventions are commonly compared with standardized therapies or placebo.6–9 Head-to-head trials are rarely conducted, because of the regulatory, budgetary, and time constraints faced by manufacturers.
Network meta-analyses (NMAs; also called mixed-treatment comparisons) allow for the comparison of all interventions, including those for which head-to-head comparisons have not been conducted.10,11 NMA is an extension of traditional meta-analysis, whereby multiple pairwise comparisons are conducted, involving three or more interventions.11 The advantages of NMAs are that they supplement direct estimates of relative efficacy with indirect estimates and provide indirect estimates where direct estimates are not available.
Objectives
The aim of this study was to compare the relative effectiveness and safety of aspirin (acetylsalicylic acid [ASA]), ASA and clopidogrel combination therapy (ASA + C), dose-adjusted warfarin, dabigatran 110 mg, dabigatran 150 mg, rivaroxaban, apixaban, edoxaban high dose (HD), edoxaban low dose (LD), and placebo in AF patients, using a Bayesian NMA approach.
Materials and methods
Information sources
Relevant studies were identified through a search of Medline Ovid (1946 to October 2015), Embase Ovid (1980 to October 2015), and the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 9, 2015) in October 2015. The search strategy was developed and conducted by an information specialist (JB), using validated randomized controlled trial (RCT) search filters in Medline and Embase, and by adapting the Medline filter for use in the Cochrane register.12,13 The search was conducted using the following terms and their derivatives: atrial fibrillation, warfarin, phenprocoumon, acenocoumarol, aspirin, apixaban, rivaroxaban, dabigatran, clopidogrel, edoxaban. The search was limited to English-language studies published from 1988 onward. The identified articles were scanned for further eligible studies, and experts in the field were consulted to identify unpublished studies. Trial briefing documents for the NOACs were also located online to provide data not included in the published RCTs. A protocol was developed and reviewed as part of thesis work, but not registered; the protocol is available from the authors upon request. The full Medline search strategy is available in Supplementary material.
Study selection and eligibility criteria
All titles and abstracts were initially screened by one investigator (AT) to identify potentially relevant studies for inclusion. Relevant studies were retrieved in full text, and were reassessed by two investigators (AT and PP) to determine eligibility for inclusion. For a study to be included in our analysis, it had to be a Phase III RCT of patients of any age with AF, comparing at least two of the antithrombotic treatments under investigation, or placebo. If a comparison included warfarin, it must have been administered at a target international normalized ratio (INR) of 2.0–3.0 to reflect current standard practice. Inconsistencies between the two investigators were resolved by discussion and review of the material.
Data collection and items
The same investigators (AT and PP) independently extracted data from each study on the following outcomes: number of strokes of any type (ischemic, hemorrhagic, or unspecified), ischemic strokes, myocardial infarctions (MIs), all-cause deaths, major bleeds, and intracranial hemorrhages (ICHs). Mean values of the following variables were also extracted: duration of follow-up, age, sex, time in therapeutic range (TTR), and CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke/transient ischemic attack) score. Also, the proportion of patients in each study with a history of stroke/transient ischemic attack, hypertension, MI, heart failure, and diabetes was extracted. Medians were used where means were not reported.
Risk of bias
Risk of bias in the included studies with respect to sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting was assessed using the Cochrane Collaboration’s risk-of-bias assessment tool.14 As recommended by the Cochrane Collaboration, all studies independent of their quality were included in the analysis.
Statistical analysis
Meta-analyses were used to synthesize RCT evidence for the direct comparisons, and NMAs were conducted to estimate relative effectiveness (as rate ratios) and credible intervals (CrIs) across all treatments for each outcome. Poisson models were used to adjust for possible differences in duration of follow-up between treatments and multiple outcomes per patient. A hierarchical Bayesian approach was followed, and models were fitted using Markov chain Monte Carlo simulations.15 Data were analyzed using fixed- and random-effect models, and prior distributions for each parameter of interest were assumed. For relative-effectiveness estimates, a vague normal prior probability (n0,1,000) was assumed. For the random-effect model, a γ-distribution (γ0.001) was used as a prior for the precision (inverse of the variance) parameter. The decision to use either a random-effect or fixed-effect model was based on clinical considerations, model convergence, and goodness of fit, as measured by the deviance information criterion. Studies with no events in any arm for a particular outcome were excluded from that outcome’s network of studies. All models were run until convergence was reached. Convergence was assessed through inspection of trace plots, consideration of potential scale-reduction factors, and inspection of multiple chains with different initial values.
Consistency, a key assumption underlying NMAs, requires that any differences between direct and indirect estimates are due to chance.11 Consistency was assessed through the inspection of coherence plots.16 Evidence of inconsistency was observed when CrIs around relative-effectiveness estimates did not cross 1. All analyses were performed using R version 2.14.1 statistical software (R Development Core Team, Vienna, Austria) and WinBUGS version 1.4 (MRC Biostatistics Unit, Cambridge, UK).
Interventions were compared based on the following outcomes: all strokes, ischemic stroke, MI, major bleeding, ICH, and overall mortality. For each outcome, treatment options were ranked at every iteration according to their effectiveness from best to worst. Ranking distributions, representing the proportions of iterations in which each treatment was ranked in position from first to tenth, were then estimated.
Differences between treatments were judged to be statistically significant when CrIs of risk ratios did not overlap 1. Differences were judged to be of borderline statistically significant when a boundary of the confidence interval equaled 1. Otherwise, differences were interpreted as nonsignificant.
Sensitivity analysis
Any RCTs investigating the effectiveness of antithrombotic treatments in AF patients deemed ineligible for warfarin were excluded in a sensitivity analysis. Criteria used to determine patient ineligibility for warfarin were highly variable across studies. The main criteria for ineligibility were patient or physician unwillingness due to fear of inadequate coagulation monitoring (or poor compliance), increased risk of hemorrhage, decreased risk of stroke due to the absence of other cardiovascular diseases, advanced age, and alcoholism. Given that patient and physician unwillingness to take or prescribe warfarin is a subjective eligibility criterion and that baseline characteristics of patients deemed ineligible for warfarin did not differ significantly from those of eligible patients, the distinction between these two patient groups is unclear.17 Therefore, we sought to determine whether their exclusion from the analysis would affect the results. We also examined the sensitivity of the results to assumptions related to the specification of prior distributions, by using vague uniform priors (U−10,10) for all log relative effectiveness estimates.
Results
Systematic review and network of evidence
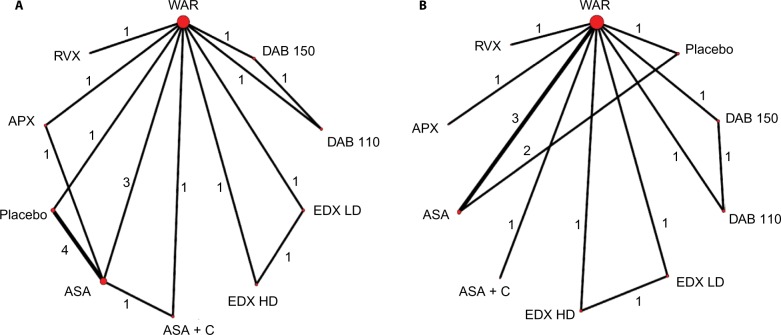
A total of 5,353 potentially relevant titles were identified through the systematic review. After our exclusion criteria were applied, 20 articles reporting on 16 Phase III RCTs in English were selected (Figure 1).6–9,18–33 Four of the RCTs were conducted on patients ineligible for warfarin,9,18,25,31 and were excluded in a sensitivity analysis. A diagram illustrating the networks of evidence in the base-case and sensitivity analysis can be found in Figure 2.
Figure 1.
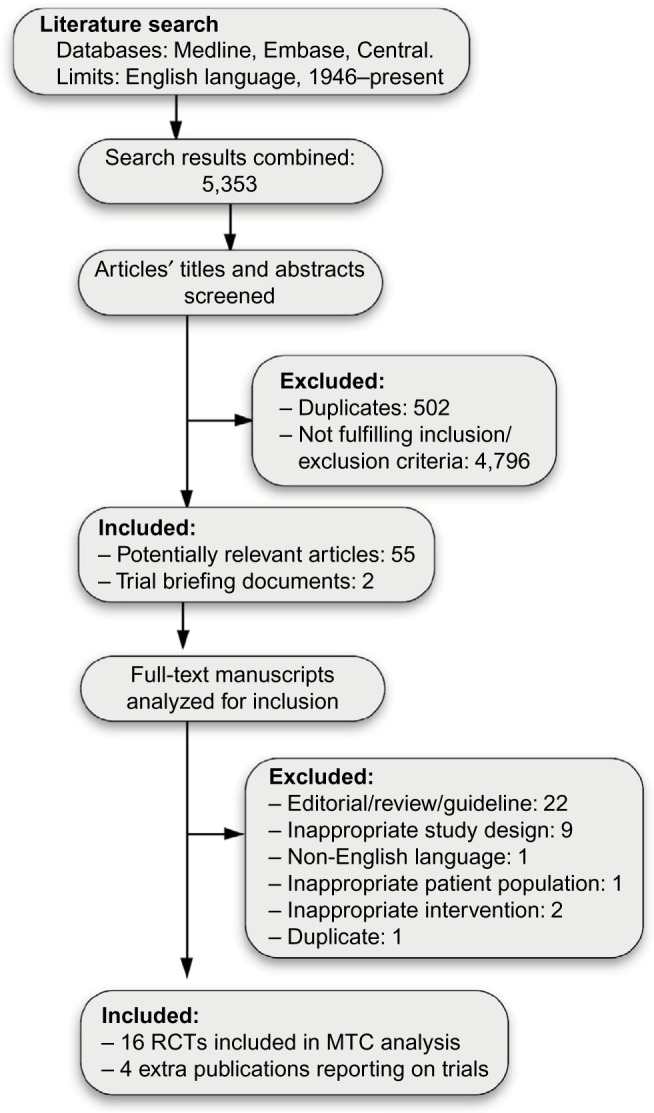
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study-selection process.
Abbreviations: RCTs, randomized controlled trials; MTC, multiple-treatment comparison.
Figure 2.
Network of evidence.
Notes: (A) Base-case multiple-treatment comparison analysis; (B) sensitivity analysis. The nodes represent the choice of stroke prophylactic treatment and the lines connecting the nodes represent direct comparisons from randomized controlled trials. The diameter of the nodes represents the number of patients receiving the intervention; the width of the lines and the numbers next to them indicate the number of direct comparisons.
Abbreviations: ASA, acetylsalicylic acid (aspirin); APX, apixaban; C, clopidogrel; DAB 110, dabigatran 110 mg; DAB 150, dabigatran 150 mg; EDX HD, high-dose edoxaban; EDX LD, low-dose EDX; RVX, rivaroxaban; WAR, warfarin.
Overall, eleven studies were assessed to be at low risk, four at unclear risk, and one at high risk of bias. All analyses were conducted on an intention-to-treat basis, except for two studies in which the method of analysis could not be determined. A more detailed description of bias assessment can be found in Table 1.
Table 1.
Risk-of-bias assessment
| Study | Sequence generation | Allocation concealment | Blinding of participants/personnel | Blinding of outcomes | Incomplete outcome data | Selective reporting | Intention-to-treat analysis | Overall |
|---|---|---|---|---|---|---|---|---|
| ACTIVE-A18 | Low | Low | Low | Unclear | Unclear | Low | Yes | Low |
| ACTIVE-W6 | Low | Low | High | Unclear | Unclear | Low | Yes | Unclear |
| AFASAK19 | Low | Low | Low | Unclear | Unclear | High* | Unclear | Unclear |
| AFASAK 221 | Low | Unclear | High | Low | Unclear | Low | Yes | Unclear |
| ARISTOTLE22 | Unclear | Unclear | Low | Low | Low | Low | Yes | Low |
| AVERROES9 | Low | Unclear | Low | Low | Low | Low | Yes | Low |
| BAFTA23 | Low | Low | High | Low | Low | Low | Yes | Low |
| CAFA24 | Unclear | Low | Low | Low | Low | Low | Yes | Low |
| EAFT25 | Low | Low | Low | Low | Unclear | Unclear | Yes | Low |
| ENGAGE-AF26 | Low | Unclear | Low | Low | Unclear | Low | Yes | Low |
| JAST27 | Unclear | Low | High | Low | Low | Low | Yes | Low |
| LASAF33 | Unclear | Unclear | High | High | Unclear | Unclear | Unclear | High |
| RE-LY7 | Low | Low | High | Low | Unclear | Low | Yes | Low |
| ROCKET-AF8 | Low | Low | Low | Low | Low | Low | Yes | Low |
| SPAF I31 | Low | Unclear | Low | Low | Low | Low | Yes | Low |
| WASPO32 | Low | Low | High | Unclear | High | Unclear | Yes | Unclear |
Note:
Authors did not report the number of events corresponding to a primary end point.
Study and patient characteristics
The selected RCTs included 96,826 patients followed for 184,370 patient-years. The average age of patients in the RCTs was 71.3 years. The studies in which mean CHADS2 scores were reported accounted for 94% of the total number of patients in the analysis, and all reported a mean score of 2 or more (ie, patients in the studies were on average at high risk for stroke). Patients in studies including warfarin as a comparator had a weighted average TTR of 62.5%. The main study characteristics are included in Table 2, and patient characteristics are included in Table 3.
Table 2.
Study characteristics
| Study | Treatment | Sample size (n) | Follow-up (years) |
Primary end points | All strokes (n) |
Ischemic stroke (n) |
Myocardial infarction (n) |
Overall mortality (n) |
Major bleeding (n) |
Intracranial hemorrhage (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| ACTIVE-A18 | ASA + C | 3,772 | 3.28 | S, SE, MI, VD | 296 | 235 | 90 | 825 | 251 | 54 |
| ASA | 3,782 | 3.24 | 408 | 343 | 115 | 841 | 162 | 29 | ||
| ACTIVE-W6 | ASA + C | 3,335 | 1.25 | S, SE, MI, VD | 100 | 90 | 36 | 159 | 101 | 21 |
| Warfarin | 3,371 | 1.25 | 59 | 42 | 23 | 158 | 93 | 11 | ||
| AFASAK19 | ASA | 336 | 1.08 | S, TIA, SE | 15 | NR | NR | NR | 2 | NR |
| Placebo | 336 | 1.13 | 16 | NR | NR | NR | 0 | 0 | ||
| AFASAK 220,21 | ASA | 169 | 2.16 | S, SE | 9 | 5 | 4 | 14 | 5 | 1 |
| Warfarin | 170 | 2.09 | 10 | 4 | 4 | 17 | 4 | 2 | ||
| ARISTOTLE22,* | Apixaban | 9,088 | 1.69 | S, SE, MB | 199 | 149 | 90 | 603 | 327 | 52 |
| Warfarin | 9,025 | 1.65 | 250 | 155 | 102 | 669 | 462 | 122 | ||
| AVERROES9 | Apixaban | 2,808 | 1.1 | S or SE | 49 | 35 | 24 | 111 | 44 | 11 |
| ASA | 2,791 | 1.1 | 105 | 93 | 28 | 140 | 39 | 13 | ||
| BAFTA23 | ASA | 485 | 2.7 | Disabling S, | 44 | 32 | 15 | 108 | 25 | 6 |
| Warfarin | 488 | 2.7 | ICH, AE | 21 | 10 | 15 | 107 | 25 | 8 | |
| CAFA24 | Placebo | 191 | 1.27 | Non-lacunar | 9 | 9 | NR | 8 | 2 | 0 |
| Warfarin | 187 | 1.27 | IS, SE, ICH, fatal bleeding | 7 | 6 | NR | 10 | 5 | 1 | |
| EAFT25 | ASA | 404 | 2.07 | VD, non-fatal | 88 | 64 | NR | 102 | 6 | 2 |
| Placebo | 378 | 1.89 | S, non-fatal MI, SE | 90 | 73 | NR | 99 | 4 | 1 | |
| ENGAGE-AF26,^ | Edoxaban HD | 7,035 | 2.19 | S, SE | 281 | 236 | 133 | 773 | 418 | 61 |
| Edoxaban LD | 7,034 | 2.24 | 360 | 233 | 169 | 737 | 254 | 41 | ||
| Warfarin | 7,036 | 2.21 | 317 | 235 | 141 | 839 | 425 | 132 | ||
| JAST27 | ASA | 426 | 2.1 | CVD, IS, TIA | 21 | 17 | NR | 10 | 7 | 4 |
| Placebo | 445 | 2.1 | 20 | 18 | NR | 9 | 2 | 2 | ||
| LASAF33 | ASA | 104 | 1.64 | CVD, OD | 4 | NR | NR | 7 | NR | NR |
| Placebo | 91 | 1.48 | 3 | NR | NR | 9 | NR | NR | ||
| RE-LY7,‡ | Dabigatran 110 | 6,015 | 2 | S, SE | 171 | 152 | 98 | 446 | 342 | 27 |
| Dabigatran 150 | 6,076 | 2 | 122 | 103 | 97 | 438 | 399 | 38 | ||
| Warfarin | 6,022 | 2 | 186 | 134 | 75 | 487 | 421 | 90 | ||
| ROCKET-AF8,§ | Rivaroxaban | 7,111 | 1.57 | S, SE, MB, | 184# | 149# | 101 | 582 | 395 | 55 |
| Warfarin | 7,125 | 1.58 | non-MB (clinically relevant) | 221# | 161# | 126 | 632 | 386 | 84 | |
| SPAF I31 | ASA | 552 | 1.3 | IS, SE | 24 | 23 | 7 | 39 | 10 | 2 |
| Placebo | 568 | 1.29 | 42 | 42 | 12 | 50 | 14 | 2 | ||
| WASPO32 | ASA | 39 | 1 | OD, S, SE, | 0 | 0 | NR | 2 | 3 | 0 |
| Warfarin | 36 | 1 | TIA, MB, withdrawal | 0 | 0 | NR | 1 | 0 | 0 |
Notes:
Sample sizes those used in efficacy analysis (sample sizes used in safety analysis 9,120 and 9,081 for apixaban and warfarin, respectively);
sample sizes those used in efficacy analysis (sample sizes used in safety analysis 7,012, 7,002, and 7,012 for edoxaban HD, edoxaban LD, and warfarin, respectively);
data updated to include new events reported in trial briefing document;
sample sizes those used in the analysis of all efficacy outcomes, except for overall mortality (7,081 and 7,090 for rivaroxaban and warfarin, respectively; sample sizes used in safety analysis 7,061 and 7,082 for rivaroxaban and warfarin, respectively);
data retrieved from supplementary appendix to trial.
Abbreviations: ASA, acetylsalicylic acid (aspirin); C, clopidogrel; LD, low dose; HD, high dose; S, stroke (including ischemic and hemorrhagic); SE, systemic embolism; MI, myocardial infarction; VD, vascular death; TIA, transient ischemic attack; MB, major bleeding; IS, ischemic stroke; ICH, intracranial hemorrhage; AE, arterial embolism; CVD, cardiovascular death; OD, overall death; NR, not reported.
Table 3.
Patient characteristics
| Study | Treatment | Age (years)* | Males (%) | CHADS2 score* | History of stroke/TIA (%) | History of hypertension (%) | Heart failure (%) | History of MI (%) | Diabetes (%) | TTR (%)* |
|---|---|---|---|---|---|---|---|---|---|---|
| ACTIVE-A18 | ASA + C | 70.9 | 58.6 | 2 | 13.2 | 85.3 | 32.9 | 13.9 | 19.5 | NA |
| ASA | 71.1 | 57.8 | 2 | 13 | 84.9 | 33.2 | 14.6 | 19.2 | ||
| ACTIVE-W6 | ASA + C | 70.2 | 67 | 2 | 15 | 83 | 30 | 17 | 21 | 63.8 |
| Warfarin | 70.2 | 66 | 2 | 15 | 82 | 31 | 18 | 21 | ||
| AFASAK19 | ASA | 75.1^ | 55 | NR | 5 | 33 | 54 | 7 | 8 | NA |
| Placebo | 74.6^ | 54 | NR | 6 | 31 | 51 | 8 | 10 | ||
| AFASAK 220,21 | ASA | 73.1 | 65 | NR | 8 | 43 | 70 | 7 | 10 | 73 |
| Warfarin | 73.2 | 57 | NR | 8 | 47 | 70 | 8 | 14 | ||
| ARISTOTLE22 | Apixaban | 70^ | 64.5 | 2.1 | 19.2‡ | 87.3 | 35.5§ | 14.5 | 25 | 62.2^ |
| Warfarin | 70^ | 65 | 2.1 | 19.7‡ | 87.6 | 35.4§ | 13.9 | 24.9 | ||
| AVERROES9 | Apixaban | 70 | 59 | 2 | 14 | 86 | 40 | NR | 19 | NA |
| ASA | 70 | 58 | 2.1 | 13 | 87 | 38 | NR | 20 | ||
| BAFTA23 | ASA | 81.5 | 54 | NR | 12 | 55 | 19 | 12 | 13 | 67 |
| Warfarin | 81.5 | 55 | NR | 13 | 53 | 20 | 10 | 14 | ||
| CAFA24 | Placebo | 67.4 | 73.3 | NR | 4.2 | 34 | 20.4 | 12 | 10 | 43.7^ |
| Warfarin | 68 | 75.9 | NR | 3.2 | 43.3 | 23.5 | 15 | 13.9 | ||
| EAFT25 | ASA | 73 | 59 | NR | 100 | 49 | 11 | 7 | 13 | NA |
| Placebo | 73 | 53 | NR | 100 | 47 | 12 | 9 | 13 | ||
| ENGAGE-AF26 | Edoxaban HD | 72^ | 62.1 | 2.8 | 28.1 | 93.7 | 58.2 | NR | 36.4 | 68.4^ |
| Edoxaban LD | 72^ | 61.2 | 2.8 | 28.5 | 93.5 | 56.6 | NR | 36.2 | ||
| Warfarin | 72^ | 62.5 | 2.8 | 28.3 | 93.6 | 57.5 | NR | 35.8 | ||
| JAST27 | ASA | 65.5 | 71.1 | NR | NR | 36.6 | 8.3 | NR | 12.7 | NA |
| Placebo | 64.8 | 69.7 | NR | NR | 40.4 | 10.1 | NR | 15.3 | ||
| LASAF33 | ASA | 66 | 55.5 | NR | NR | 53 | NR | NR | 5 | NA |
| Placebo | 67 | 49.5 | NR | NR | 49 | NR | NR | 9 | ||
| RE-LY7 | Dabigatran 110 | 71.4 | 64.3 | 2.1 | 19.9 | 78.8 | 32.2 | 16.8 | 23.4 | 64 |
| Dabigatran 150 | 71.5 | 63.2 | 2.2 | 20.3 | 78.9 | 31.8 | 16.9 | 23.1 | ||
| Warfarin | 71.6 | 63.3 | 2.1 | 19.8 | 78.9 | 31.9 | 16.1 | 23.4 | ||
| ROCKET-AF8 | Rivaroxaban | 73^ | 60.3 | 3.5 | 54.9 | 90.3 | 62.6 | 16.6 | 40.4 | 55^ |
| Warfarin | 73^ | 60.3 | 3.5 | 54.6 | 90.8 | 62.3 | 18 | 39.5 | ||
| SPAF I31 | ASA | 67 | 71 | NR | 6 | 53 | 20 | 7 | 16 | NA |
| Placebo | 67 | 70 | NR | 7 | 52 | 20 | 8 | 19 | ||
| WASPO32 | ASA | 82.6^ | 54 | NR | 0 | 46 | NR | NR | 5 | 69.2 |
| Warfarin | 83.5^ | 39 | NR | 0 | 49 | NR | NR | 3 |
Notes:
Data are mean values unless otherwise specified;
median values;
includes history of systemic embolism;
includes history of reduced left ventricular function.
Abbreviations: ASA, acetylsalicylic acid (aspirin); C, clopidogrel; LD, low dose; HD, high dose; TIA, transient ischemic attack; MI, myocardial infarction; TTR, time in therapeutic range; NA, not applicable; NR, not reported.
Relative effectiveness of treatments
Estimates from pairwise analyses and NMAs of relative effectiveness of all treatments are shown in Tables 4 and 5, respectively. Fixed-effect models were used for all outcomes, because they provided similar goodness of fit to the random-effect models, and the impact of the prior distributions on between-study variance in the random-effect models was too large.
Table 4.
Results of pairwise meta-analyses of direct evidence
| Reference | Comparator | All strokes | Ischemic stroke | Myocardial infarction | Overall mortality | Major bleeding | Intracranial hemorrhage |
|---|---|---|---|---|---|---|---|
| Warfarin | ASA + C | 1.7 | 2.15 | 1.57 | 1.01 | 1.09 | 1.92 |
| (1.23–2.35) | (1.49–3.1) | (0.93–2.65) | (0.81–1.26) | (0.82–1.45) | (0.92–3.98) | ||
| ASA | 1.95 | 2.33 | 1.06 | 1.01 | 1.29 | 0.54 | |
| (1.22–3.22) | (1.48–4.05) | (0.58–1.78) | (0.79–1.3) | (0.76–2.15) | (0.2–1.38) | ||
| Placebo | 1.26 | 1.47 | NA | 0.78 | 0.39 | 0.33* | |
| (0.47–3.38) | (0.52–4.13) | (0.31–1.98) | (0.08–2.02) | (0.01–8.01) | |||
| Apixaban | 0.79 | 0.96 | 0.88 | 0.9 | 0.69 | 0.42 | |
| (0.66–0.96) | (0.77–1.2) | (0.66–1.17) | (0.81–1) | (0.6–0.8) | (0.3–0.57) | ||
| Dabigatran 110 | 0.92 | 1.14 | 1.29 | 0.91 | 0.8 | 0.3 | |
| (0.75–1.13) | (0.9–1.43) | (0.96–1.75) | (0.8–1.03) | (0.7–0.93) | (0.19–0.45) | ||
| Dabigatran 150 | 0.64 | 0.76 | 1.27 | 0.88 | 0.93 | 0.41 | |
| (0.51–0.81) | (0.59–0.98) | (0.94–1.71) | (0.77–1) | (0.93–1.07) | (0.28–0.6) | ||
| Rivaroxaban | 0.85 | 0.94 | 0.82 | 0.94 | 1.03 | 0.67 | |
| (0.7–1.03) | (0.76–1.18) | (0.63–1.06) | (0.84–1.05) | (0.9–1.19) | (0.47–0.93) | ||
| Edoxaban HD | 0.88 | 1 | 0.94 | 0.92 | 0.80 | 0.47 | |
| (0.75–1.03) | (0.83–1.19) | (0.74–1.19) | (0.83–1.01) | (0.71–0.91) | (0.34–0.63) | ||
| Edoxaban LD | 1.13 | 1.41 | 1.19 | 0.87 | 0.47 | 0.3 | |
| (0.97–1.31) | (1.19–1.67) | (0.95–1.49) | (0.79–0.96) | (0.41–0.55) | (0.21–0.43) | ||
| ASA + C | ASA | 0.72 | 0.68 | 0.78 | 0.97 | 1.53 | 1.84 |
| (0.62–0.83) | (0.57–0.8) | (0.59–1.02) | (0.88–1.07) | (1.26–1.87) | (1.17–2.9) | ||
| ASA | Placebo | 1.25 | 1.42 | 0.59 | 1.19 | 0.75 | 0.46 |
| (1–1.55) | (1.1–1.84) | (0.23–1.5) | (0.96–1.48) | (0.42–1.31) | (0.14–1.31) | ||
| Dabigatran 110 | Dabigatran 150 | 0.7 | 0.67 | 0.98 | 0.97 | 1.16 | 1.39 |
| (0.56–0.89) | (0.52–0.86) | (0.74–1.3) | (0.85–1.11) | (1–1.34) | (0.85–2.28) | ||
| Edoxaban HD | Edoxaban LD | 1.26 | 1.38 | 1.25 | 0.94 | 0.60 | 0.66 |
| (1.08–1.47) | (1.17–1.63) | (0.99–1.57) | (0.85–1.04) | (0.51–0.7) | (0.44–0.98) |
Notes:
A correction of 0.5 was added to each count, due to zero events in the placebo arm. Results presented as rate ratios, with 95% confidence intervals in parentheses below. Significant results are in bold.
Abbreviations: ASA, acetylsalicylic acid (aspirin); C, clopidogrel; HD, high dose; LD, low dose; NA, not applicable.
Table 5.
Results of base-case mixed-treatment comparison analysis
| Reference | Comparator | All strokes | Ischemic stroke | Myocardial infarction | Overall mortality | Major bleeding | Intracranial hemorrhage |
|---|---|---|---|---|---|---|---|
| Warfarin | ASA + C | 1.44 | 1.87 | 1.05 | 1.02 | 1.16 | 1.32 |
| (1.15–1.8) | (1.45–2.42) | (0.75–1.47) | (0.88–1.18) | (0.92–1.45) | (0.81–2.19) | ||
| Aspirin | 1.93 | 2.68 | 1.22 | 1.05 | 0.77 | 0.64 | |
| (1.55–2.41) | (2.08–3.47) | (0.88–1.68) | (0.91–1.21) | (0.61–0.98) | (0.39–1.04) | ||
| Placebo | 2.39 | 3.75 | 1.96 | 1.22 | 0.57 | 0.22 | |
| (1.77–3.24) | (2.64–5.33) | (0.86–4.44) | (0.94–1.57) | (0.32–1) | (0.07–0.65) | ||
| Apixaban | 0.82 | 0.97 | 0.87 | 0.89 | 0.71 | 0.42 | |
| (0.69–0.97) | (0.79–1.19) | (0.67–1.13) | (0.8–0.98) | (0.62–0.81) | (0.31–0.57) | ||
| Dabigatran 110 | 0.92 | 1.14 | 1.23 | 0.92 | 0.82 | 0.3 | |
| (0.75–1.14) | (0.9–1.44) | (0.92–1.65) | (0.81–1.04) | (0.71–0.94) | (0.19–0.45) | ||
| Dabigatran 150 | 0.65 | 0.76 | 1.2 | 0.89 | 0.94 | 0.41 | |
| (0.52–0.82) | (0.59–0.99) | (0.9–1.61) | (0.79–1.01) | (0.82–1.08) | (0.28–0.6) | ||
| Rivaroxaban | 0.85 | 0.95 | 0.82 | 0.94 | 1.03 | 0.65 | |
| (0.7–1.03) | (0.76–1.18) | (0.64–1.07) | (0.84–1.05) | (0.9–1.19) | (0.46–0.91) | ||
| Edoxaban HD | 0.89 | 1.01 | 0.94 | 0.92 | 0.8 | 0.46 | |
| (0.76–1.04) | (0.84–1.21) | (0.74–1.18) | (0.84–1.02) | (0.71–0.91) | (0.34–0.62) | ||
| Edoxaban LD | 1.12 | 1.4 | 1.17 | 0.87 | 0.48 | 0.3 | |
| (0.96–1.3) | (1.18–1.65) | (0.94–1.46) | (0.78–0.96) | (0.41–0.56) | (0.21–0.43) | ||
| ASA + C | Aspirin | 1.34 | 1.44 | 1.16 | 1.03 | 0.67 | 0.48 |
| (1.17–1.55) | (1.23–1.68) | (0.91–1.48) | (0.94–1.13) | (0.56–0.8) | (0.32–0.72) | ||
| Placebo | 1.66 | 2.01 | 1.87 | 1.2 | 0.49 | 0.17 | |
| (1.29–2.15) | (1.5–2.69) | (0.84–4.07) | (0.95–1.51) | (0.28–0.85) | (0.05–0.47) | ||
| Apixaban | 0.57 | 0.52 | 0.83 | 0.87 | 0.61 | 0.32 | |
| (0.44–0.73) | (0.39–0.69) | (0.57–1.21) | (0.74–1.03) | (0.48–0.79) | (0.18–0.54) | ||
| Dabigatran 110 | 0.64 | 0.61 | 1.17 | 0.90 | 0.71 | 0.22 | |
| (0.47–0.87) | (0.43–0.86) | (0.76–1.82) | (0.74–1.1) | (0.54–0.93) | (0.11–0.43) | ||
| Dabigatran 150 | 0.45 | 0.41 | 1.15 | 0.88 | 0.82 | 0.31 | |
| (0.33–0.62) | (0.28–0.59) | (0.74–1.77) | (0.72–1.07) | (0.63–1.06) | (0.17–0.58) | ||
| Rivaroxaban | 0.59 | 0.51 | 0.78 | 0.92 | 0.9 | 0.49 | |
| (0.44–0.8) | (0.36–0.71) | (0.51–1.19) | (0.77–1.11) | (0.68–1.17) | (0.27–0.9) | ||
| Edoxaban HD | 0.62 | 0.54 | 0.89 | 0.91 | 0.7 | 0.35 | |
| (0.47–0.81) | (0.39–0.74) | (0.6–1.34) | (0.76–1.09) | (0.53–0.9) | (0.19–0.62) | ||
| Edoxaban LD | 0.78 | 0.75 | 1.12 | 0.85 | 0.41 | 0.23 | |
| (0.59–1.02) | (0.55–1.02) | (0.75–1.66) | (0.71–1.02) | (0.32–0.54) | (0.12–0.42) | ||
| ASA | Placebo | 1.24 | 1.4 | 1.62 | 1.16 | 0.74 | 0.35 |
| (1–1.54) | (1.09–1.8) | (0.75–3.36) | (0.94–1.44) | (0.42–1.25) | (0.11–0.91) | ||
| Apixaban | 0.42 | 0.36 | 0.72 | 0.85 | 0.92 | 0.66 | |
| (0.33–0.54) | (0.28–0.48) | (0.51–1.01) | (0.73–0.99) | (0.71–1.18) | (0.4–1.09) | ||
| Dabigatran 110 | 0.48 | 0.42 | 1.01 | 0.88 | 1.06 | 0.46 | |
| (0.35–0.64) | (0.3–0.6) | (0.66–1.55) | (0.72–1.06) | (0.8–1.4) | (0.24–0.88) | ||
| Dabigatran 150 | 0.34 | 0.28 | 0.99 | 0.85 | 1.22 | 0.65 | |
| (0.25–0.46) | (0.2–0.41) | (0.65–1.51) | (0.7–1.03) | (0.93–1.61) | (0.35–1.19) | ||
| Rivaroxaban | 0.44 | 0.35 | 0.68 | 0.9 | 1.34 | 1.03 | |
| (0.33–0.59) | (0.25–0.5) | (0.45–1.02) | (0.75–1.08) | (1.02–1.76) | (0.57–1.85) | ||
| Edoxaban HD | 0.46 | 0.38 | 0.77 | 0.88 | 1.04 | 0.72 | |
| (0.35–0.6) | (0.28–0.51) | (0.52–1.14) | (0.74–1.05) | (0.79–1.36) | (0.41–1.28) | ||
| Edoxaban LD | 0.58 | 0.52 | 0.96 | 0.83 | 0.62 | 0.48 | |
| (0.44–0.76) | (0.38–0.71) | (0.65–1.42) | (0.69–0.98) | (0.47–0.82) | (0.26–0.86) | ||
| Placebo | Apixaban | 0.34 | 0.26 | 0.44 | 0.73 | 1.24 | 1.9 |
| (0.25–0.47) | (0.18–0.37) | (0.2–1.03) | (0.56–0.95) | (0.7–2.26) | (0.64–6.49) | ||
| Dabigatran 110 | 0.39 | 0.3 | 0.62 | 0.75 | 1.43 | 1.33 | |
| (0.27–0.55) | (0.2–0.46) | (0.27–1.49) | (0.57–1) | (0.8–2.62) | (0.42–4.86) | ||
| Dabigatran 150 | 0.27 | 0.2 | 0.61 | 0.73 | 1.66 | 1.87 | |
| (0.19–0.4) | (0.13–0.31) | (0.26–1.47) | (0.55–0.97) | (0.93–3.02) | (0.59–6.69) | ||
| Rivaroxaban | 0.36 | 0.25 | 0.42 | 0.77 | 1.81 | 2.96 | |
| (0.25–0.51) | (0.17–0.38) | (0.18–1) | (0.58–1.02) | (1.02–3.32) | (0.95–10.4) | ||
| Edoxaban HD | 0.37 | 0.27 | 0.48 | 0.76 | 1.41 | 2.08 | |
| (0.26–0.52) | (0.18–0.4) | (0.21–1.12) | (0.58–1) | (0.79–2.57) | (0.68–7.3) | ||
| Edoxaban LD | 0.47 | 0.37 | 0.60 | 0.71 | 0.84 | 1.37 | |
| (0.33–0.66) | (0.25–0.55) | (0.26–1.4) | (0.54–0.93) | (0.47–1.54) | (0.44–4.86) | ||
| Apixaban | Dabigatran 110 | 1.13 | 1.17 | 1.41 | 1.03 | 1.16 | 0.7 |
| (0.86–1.47) | (0.86–1.6) | (0.96–2.07) | (0.88–1.22) | (0.95–1.41) | (0.41–1.18) | ||
| Dabigatran 150 | 0.8 | 0.78 | 1.38 | 1 | 1.33 | 0.98 | |
| (0.6–1.06) | (0.56–1.09) | (0.95–2.02) | (0.85–1.19) | (1.1–1.62) | (0.6–1.58) | ||
| Rivaroxaban | 1.04 | 0.97 | 0.95 | 1.06 | 1.46 | 1.55 | |
| (0.8–1.35) | (0.72–1.32) | (0.65–1.36) | (0.91–1.23) | (1.2–1.78) | (0.98–2.46) | ||
| Edoxaban HD | 1.09 | 1.04 | 1.08 | 1.04 | 1.13 | 1.1 | |
| (0.86–1.37) | (0.79–1.36) | (0.76–1.52) | (0.9–1.2) | (0.94–1.37) | (0.71–1.68) | ||
| Edoxaban LD | 1.37 | 1.44 | 1.35 | 0.97 | 0.68 | 0.72 | |
| (1.09–1.72) | (1.11–1.88) | (0.96–1.88) | (0.84–1.12) | (0.55–0.83) | (0.45–1.14) | ||
| Dabigatran 110 | Dabigatran 150 | 0.71 | 0.67 | 0.98 | 0.97 | 1.15 | 1.4 |
| (0.56–0.89) | (0.52–0.86) | (0.74–1.3) | (0.85–1.11) | (1–1.33) | (0.85–2.32) | ||
| Rivaroxaban | 0.92 | 0.83 | 0.67 | 1.02 | 1.27 | 2.21 | |
| (0.69–1.22) | (0.6–1.15) | (0.45–0.99) | (0.86–1.22) | (1.04–1.55) | (1.29–3.85) | ||
| Edoxaban HD | 0.97 | 0.89 | 0.76 | 1.01 | 0.98 | 1.56 | |
| (0.74–1.25) | (0.66–1.19) | (0.53–1.11) | (0.86–1.18) | (0.81–1.19) | (0.93–2.67) | ||
| Edoxaban LD | 1.21 | 1.23 | 0.96 | 0.94 | 0.59 | 1.02 | |
| (0.94–1.56) | (0.92–1.64) | (0.66–1.37) | (0.8–1.11) | (0.48–0.72) | (0.59–1.8) | ||
| Dabigatran 150 | Rivaroxaban | 1.31 | 1.24 | 0.68 | 1.05 | 1.1 | 1.58 |
| (0.97–1.77) | (0.88–1.75) | (0.46–1.01) | (0.89–1.25) | (0.9–1.33) | (0.96–2.64) | ||
| Edoxaban HD | 1.37 | 1.32 | 0.78 | 1.04 | 0.85 | 1.12 | |
| (1.04–1.81) | (0.96–1.82) | (0.54–1.13) | (0.88–1.22) | (0.71–1.02) | (0.69–1.83) | ||
| Edoxaban LD | 1.72 | 1.83 | 0.97 | 0.97 | 0.51 | 0.73 | |
| (1.31–2.26) | (1.35–2.5) | (0.68–1.4) | (0.82–1.14) | (0.41–0.62) | (0.44–1.23) | ||
| Rivaroxaban | Edoxaban HD | 1.05 | 1.07 | 1.14 | 0.98 | 0.78 | 0.71 |
| (0.81–1.35) | (0.8–1.42) | (0.8–1.62) | (0.85–1.14) | (0.64–0.94) | (0.45–1.11) | ||
| Edoxaban LD | 1.31 | 1.48 | 1.42 | 0.92 | 0.46 | 0.46 | |
| (1.03–1.68) | (1.12–1.95) | (1.01–2) | (0.79–1.07) | (0.38–0.57) | (0.29–0.75) | ||
| Edoxaban HD | Edoxaban LD | 1.26 | 1.38 | 1.25 | 0.94 | 0.60 | 0.66 |
| (1.08–1.47) | (1.17–1.64) | (0.99–1.57) | (0.85–1.03) | (0.51–0.7) | (0.44–0.97) |
Notes: Results presented as rate ratios, with 95% credible intervals in parentheses below. Significant results are in bold.
Abbreviations: ASA, acetylsalicylic acid (aspirin); C, clopidogrel; HD, high dose; LD, low dose.
All strokes
Dabigatran 150 mg and apixaban were the only NOACs that were superior to warfarin at reducing stroke of any type. All OACs were superior to ASA + C, ASA, and placebo, except for edoxaban LD, which was not significantly superior to ASA + C. ASA + C was superior to ASA alone and placebo, while ASA was only borderline superior to placebo.
Ischemic stroke
Dabigatran 150 mg was the only NOAC that was superior to warfarin at reducing ischemic stroke. Edoxaban LD was inferior to all other OACs, with the exception of dabigatran 110 mg. All OACs were superior to ASA + C, ASA, and placebo, except for edoxaban LD, which was not significantly superior to ASA + C. ASA and ASA + C were superior to placebo.
Myocardial infarction
Rivaroxaban was superior to dabigatran 110 mg at reducing MIs, borderline superior to placebo, and inferior to edoxaban LD.
Overall mortality
Apixaban and edoxaban LD offered mortality advantages over warfarin, ASA, and placebo. Also, dabigatran 150 mg was superior to placebo, while dabigatran 110 mg and edoxaban HD were only borderline superior to placebo.
Major bleeding
The following NOACs lowered the risk of major bleeding when compared to warfarin: apixaban, both doses of edoxaban, and dabigatran 110 mg. Warfarin was associated with a higher risk than ASA and a borderline higher risk than placebo. All treatments, except for warfarin, dabigatran 150 mg, and rivaroxaban, were associated with a lower risk than ASA + C. Edoxaban LD demonstrated a lower risk than apixaban, with both demonstrating a lower risk than dabigatran 150 mg. Finally, rivaroxaban was associated with a higher risk than all the other NOACs, with the exception of dabigatran 150 mg.
Intracranial hemorrhage
The risk of ICH on warfarin was higher than that of all other treatment options, with the exception of ASA and ASA + C. ASA + C was associated with a higher risk than all other treatment options. The risk of ICH on ASA was higher than on placebo. Finally, the risk of ICH on rivaroxaban was higher than that of dabigatran 110 mg and edoxaban LD.
Ranking and inconsistency
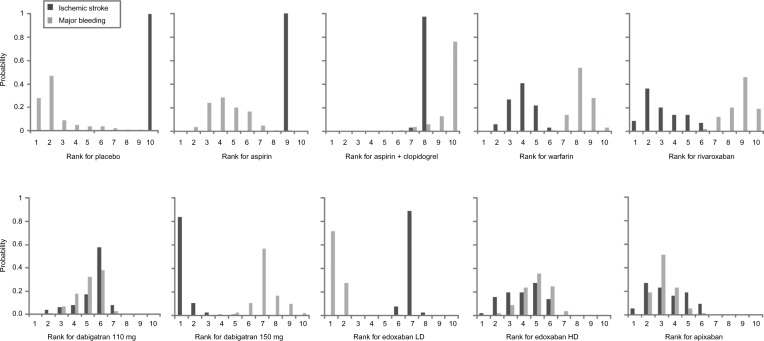
The ranking distributions in Figure 3 represent the proportions of simulations in which each treatment was ranked in each position (from best to worst) based on its effectiveness against ischemic stroke and major bleeding. For ischemic stroke, dabigatran 150 mg was shown to be the most effective option in 84% of simulations, followed by rivaroxaban (9%), apixaban (5%), and edoxaban HD (2%). None of the other treatments was the most effective option in any of the simulations. For major bleeding, edoxaban LD was the most effective option in 72% of simulations, followed by placebo (28%). None of the other treatments was the most effective option in any of the simulations. Although the risk of major bleeding on any OAC is higher than that on placebo, data on major bleeding while on placebo are scarce (ie, very few events occurring in smaller studies), resulting in weak evidence for this outcome on placebo. Through inspection of coherence plots, there was no evidence of inconsistency in any of the closed loops in the NMAs.
Figure 3.
Ranking distributions of each treatment for ischemic stroke (efficacy) and major bleeding (safety).
Notes: Distributions based on 100,000 Markov chain Monte Carlo simulations. Ranking indicates the probability that a treatment is ranked first (best) to tenth (worst) with respect to both outcomes.
Abbreviations: LD, low dose; HD, high dose.
Sensitivity analysis
Exclusion of the four studies in the sensitivity analysis decreased the total number of patients and patient-years to 81,771 and 150,589, respectively. The results of the NMAs using this smaller study set are presented in Table 6. There were some differences between the results of the base-case and sensitivity analyses. Notably, ASA + C combination therapy was no longer superior to ASA and placebo at reducing both ischemic and all strokes. Similarly, ASA was no longer superior to placebo at reducing ischemic and all strokes. In contrast, edoxaban LD was superior to ASA + C at reducing ischemic and all strokes, only borderline superior to ASA at reducing all strokes, and no longer superior to placebo at reducing ischemic strokes. Rivaroxaban was superior to dabigatran 150 mg at reducing MIs, and no longer borderline superior to placebo. Apixaban showed only a borderline reduction in overall mortality over warfarin, and no treatment showed any mortality advantage over ASA or placebo.
Table 6.
Results of sensitivity analysis*
| Reference | Comparator | All strokes | Ischemic strokes | Myocardial infarction | Overall mortality | Major bleeding | Intracranial hemorrhage |
|---|---|---|---|---|---|---|---|
| Warfarin | ASA + C | 1.72 | 2.16 | 1.37 | 1.01 | 1.10 | 1.47 |
| (1.25–2.39) | (1.54–3.04) | (0.84–2.25) | (0.82–1.27) | (0.83–1.46) | (0.75–3.02) | ||
| ASA | 1.8 | 2.27 | 1.03 | 0.99 | 1.24 | 0.52 | |
| (1.21–2.7) | (1.48–3.51) | (0.57–1.82) | (0.78–1.27) | (0.76–2.02) | (0.2–1.23) | ||
| Placebo | 1.9 | 2.9 | NA | 1.06 | 0.29 | 0.13 | |
| (1.1–3.32) | (1.54–5.17) | (0.59–1.86) | (0.08–0.85) | (0.02–0.64) | |||
| Apixaban | 0.80 | 0.95 | 0.85 | 0.9 | 0.69 | 0.41 | |
| (0.66–0.96) | (0.76–1.19) | (0.64–1.13) | (0.81–1) | (0.6–0.8) | (0.29–0.57) | ||
| Dabigatran 110 | 0.92 | 1.15 | 1.25 | 0.92 | 0.82 | 0.3 | |
| (0.75–1.14) | (0.91–1.44) | (0.93–1.69) | (0.81–1.04) | (0.71–0.94) | (0.19–0.45) | ||
| Dabigatran 150 | 0.65 | 0.77 | 1.23 | 0.89 | 0.94 | 0.41 | |
| (0.52–0.82) | (0.59–0.99) | (0.91–1.66) | (0.78–1.01) | (0.82–1.08) | (0.28–0.6) | ||
| Rivaroxaban | 0.85 | 0.97 | 0.82 | 0.94 | 1.03 | 0.65 | |
| (0.7–1.04) | (0.77–1.21) | (0.63–1.06) | (0.84–1.05) | (0.9–1.19) | (0.46–0.91) | ||
| Edoxaban HD | 0.89 | 1.03 | 0.94 | 0.92 | 0.8 | 0.46 | |
| (0.76–1.05) | (0.86–1.24) | (0.74–1.19) | (0.84–1.02) | (0.7–0.91) | (0.34–0.62) | ||
| Edoxaban LD | 1.12 | 1.43 | 1.17 | 0.87 | 0.48 | 0.3 | |
| (0.97–1.3) | (1.21–1.69) | (0.94–1.46) | (0.78–0.96) | (0.41–0.55) | (0.21–0.42) | ||
| ASA + C | ASA | 1.04 | 1.05 | 0.76 | 0.98 | 1.12 | 0.36 |
| (0.63–1.75) | (0.61–1.83) | (0.34–1.59) | (0.7–1.36) | (0.64–1.98) | (0.11–1.03) | ||
| Placebo | 1.1 | 1.35 | NA | 1.04 | 0.26 | 0.09 | |
| (0.58–2.09) | (0.65–2.62) | (0.56–1.89) | (0.07–0.79) | (0.01–0.47) | |||
| Apixaban | 0.46 | 0.44 | 0.62 | 0.89 | 0.63 | 0.28 | |
| (0.32–0.67) | (0.3–0.66) | (0.36–1.07) | (0.69–1.14) | (0.46–0.86) | (0.13–0.59) | ||
| Dabigatran 110 | 0.54 | 0.53 | 0.92 | 0.90 | 0.74 | 0.2 | |
| (0.36–0.78) | (0.35–0.8) | (0.52–1.61) | (0.7–1.17) | (0.54–1.02) | (0.09–0.44) | ||
| Dabigatran 150 | 0.38 | 0.36 | 0.90 | 0.88 | 0.86 | 0.28 | |
| (0.25–0.56) | (0.23–0.54) | (0.51–1.57) | (0.68–1.13) | (0.62–1.17) | (0.12–0.6) | ||
| Rivaroxaban | 0.49 | 0.45 | 0.6 | 0.93 | 0.94 | 0.44 | |
| (0.34–0.72) | (0.3–0.67) | (0.34–1.04) | (0.72–1.19) | (0.68–1.28) | (0.2–0.93) | ||
| Edoxaban HD | 0.52 | 0.48 | 0.69 | 0.91 | 0.73 | 0.31 | |
| (0.36–0.74) | (0.32–0.7) | (0.4–1.18) | (0.72–1.16) | (0.53–0.99) | (0.14–0.65) | ||
| Edoxaban LD | 0.65 | 0.66 | 0.86 | 0.85 | 0.43 | 0.21 | |
| (0.45–0.93) | (0.45–0.96) | (0.5–1.47) | (0.67–1.08) | (0.32–0.6) | (0.09–0.44) | ||
| ASA | Placebo | 1.05 | 1.28 | NA | 1.06 | 0.23 | 0.25 |
| (0.69–1.61) | (0.71–2.18) | (0.61–1.84) | (0.06–0.66) | (0.03–1.02) | |||
| Apixaban | 0.44 | 0.42 | 0.82 | 0.91 | 0.56 | 0.79 | |
| (0.28–0.69) | (0.26–0.68) | (0.44–1.61) | (0.69–1.18) | (0.34–0.93) | (0.31–2.13) | ||
| Dabigatran 110 | 0.51 | 0.5 | 1.22 | 0.92 | 0.66 | 0.56 | |
| (0.33–0.8) | (0.31–0.82) | (0.64–2.39) | (0.7–1.22) | (0.4–1.1) | (0.22–1.58) | ||
| Dabigatran 150 | 0.36 | 0.34 | 1.19 | 0.9 | 0.76 | 0.78 | |
| (0.23–0.57) | (0.2–0.55) | (0.63–2.33) | (0.68–1.19) | (0.46–1.26) | (0.31–2.16) | ||
| Rivaroxaban | 0.47 | 0.43 | 0.79 | 0.95 | 0.84 | 1.24 | |
| (0.3–0.74) | (0.26–0.69) | (0.43–1.53) | (0.72–1.24) | (0.5–1.39) | (0.5–3.39) | ||
| Edoxaban HD | 0.5 | 0.45 | 0.91 | 0.93 | 0.65 | 0.88 | |
| (0.32–0.76) | (0.28–0.72) | (0.49–1.73) | (0.72–1.21) | (0.39–1.07) | (0.35–2.37) | ||
| Edoxaban LD | 0.62 | 0.63 | 1.14 | 0.87 | 0.39 | 0.58 | |
| (0.4–0.95) | (0.39–1) | (0.62–2.15) | (0.67–1.13) | (0.23–0.64) | (0.23–1.58) | ||
| Placebo | Apixaban | 0.42 | 0.33 | NA | 0.85 | 2.41 | 3.26 |
| (0.23–0.75) | (0.18–0.64) | (0.48–1.53) | (0.81–8.93) | (0.62–27.71) | |||
| Dabigatran 110 | 0.49 | 0.39 | NA | 0.87 | 2.84 | 2.33 | |
| (0.27–0.87) | (0.21–0.77) | (0.49–1.57) | (0.97–10.67) | (0.43–19.75) | |||
| Dabigatran 150 | 0.34 | 0.26 | NA | 0.85 | 3.28 | 3.26 | |
| (0.19–0.62) | (0.14–0.52) | (0.47–1.53) | (1.11–12.2) | (0.61–27.92) | |||
| Rivaroxaban | 0.45 | 0.33 | NA | 0.89 | 3.59 | 5.14 | |
| (0.25–0.8) | (0.18–0.64) | (0.5–1.6) | (1.22–13.35) | (0.97–43.06) | |||
| Edoxaban HD | 0.47 | 0.35 | NA | 0.88 | 2.79 | 3.63 | |
| (0.26–0.83) | (0.19–0.68) | (0.49–1.57) | (0.95–10.32) | (0.7–30.74) | |||
| Edoxaban LD | 0.59 | 0.49 | NA | 0.82 | 1.66 | 2.39 | |
| (0.33–1.05) | (0.27–0.94) | (0.46–1.47) | (0.56–6.2) | (0.45–20.49) | |||
| Apixaban | Dabigatran 110 | 1.16 | 1.2 | 1.48 | 1.02 | 1.18 | 0.71 |
| (0.88–1.54) | (0.87–1.66) | (0.99–2.21) | (0.86–1.21) | (0.97–1.45) | (0.41–1.22) | ||
| Dabigatran 150 | 0.82 | 0.8 | 1.45 | 0.99 | 1.36 | 1 | |
| (0.61–1.1) | (0.57–1.13) | (0.97–2.17) | (0.84–1.17) | (1.12–1.66) | (0.6–1.64) | ||
| Rivaroxaban | 1.07 | 1.01 | 0.97 | 1.04 | 1.5 | 1.58 | |
| (0.82–1.41) | (0.74–1.39) | (0.66–1.42) | (0.89–1.22) | (1.23–1.83) | (0.99–2.53) | ||
| Edoxaban HD | 1.12 | 1.08 | 1.11 | 1.03 | 1.16 | 1.11 | |
| (0.88–1.44) | (0.81–1.44) | (0.77–1.6) | (0.89–1.19) | (0.96–1.41) | (0.72–1.75) | ||
| Edoxaban LD | 1.41 | 1.49 | 1.38 | 0.96 | 0.69 | 0.73 | |
| (1.11–1.79) | (1.13–1.97) | (0.97–1.97) | (0.83–1.11) | (0.56–0.85) | (0.45–1.18) | ||
| Dabigatran 110 | Dabigatran 150 | 0.71 | 0.67 | 0.98 | 0.97 | 1.16 | 1.4 |
| (0.56–0.89) | (0.52–0.86) | (0.74–1.3) | (0.85–1.11) | (1–1.34) | (0.85–2.32) | ||
| Rivaroxaban | 0.92 | 0.84 | 0.65 | 1.02 | 1.27 | 2.21 | |
| (0.69–1.23) | (0.62–1.16) | (0.44–0.97) | (0.86–1.22) | (1.04–1.55) | (1.28–3.88) | ||
| Edoxaban HD | 0.97 | 0.9 | 0.75 | 1.01 | 0.98 | 1.56 | |
| (0.74–1.26) | (0.67–1.2) | (0.51–1.09) | (0.86–1.18) | (0.81–1.19) | (0.93–2.67) | ||
| Edoxaban LD | 1.22 | 1.25 | 0.94 | 0.94 | 0.59 | 1.03 | |
| (0.94–1.57) | (0.94–1.65) | (0.65–1.35) | (0.8–1.11) | (0.48–0.72) | (0.59–1.79) | ||
| Dabigatran 150 | Rivaroxaban | 1.31 | 1.26 | 0.67 | 1.05 | 1.1 | 1.58 |
| (0.97–1.77) | (0.9–1.76) | (0.45–0.99) | (0.89–1.25) | (0.9–1.34) | (0.95–2.64) | ||
| Edoxaban HD | 1.37 | 1.34 | 0.77 | 1.04 | 0.85 | 1.11 | |
| (1.04–1.82) | (0.98–1.83) | (0.52–1.12) | (0.88–1.22) | (0.7–1.03) | (0.69–1.82) | ||
| Edoxaban LD | 1.72 | 1.86 | 0.96 | 0.97 | 0.51 | 0.73 | |
| (1.32–2.27) | (1.37–2.52) | (0.66–1.38) | (0.82–1.14) | (0.41–0.62) | (0.43–1.22) | ||
| Rivaroxaban | Edoxaban HD | 1.05 | 1.06 | 1.15 | 0.98 | 0.78 | 0.71 |
| (0.81–1.35) | (0.8–1.41) | (0.8–1.63) | (0.85–1.14) | (0.64–0.94) | (0.45–1.11) | ||
| Edoxaban LD | 1.32 | 1.47 | 1.43 | 0.92 | 0.46 | 0.47 | |
| (1.03–1.68) | (1.12–1.94) | (1.02–2.01) | (0.79–1.07) | (0.38–0.57) | (0.28–0.76) | ||
| Edoxaban HD | Edoxaban LD | 1.26 | 1.38 | 1.25 | 0.94 | 0.6 | 0.66 |
| (1.08–1.47) | (1.17–1.64) | (1–1.57) | (0.85–1.03) | (0.51–0.7) | (0.44–0.97) |
Notes:
Sensitivity analysis includes results for patients suitable for warfarin only. Results presented as rate ratios, with 95% credible intervals in parentheses below. Significant results are in bold.
Abbreviations: ASA, acetylsalicylic acid (aspirin); C, clopidogrel; HD, high dose; LD, low dose; NA, not applicable.
The risk of major bleeding on ASA was no longer lower than that on warfarin, ASA + C, or rivaroxaban, but was lower than that on apixaban. Also, the risk of major bleeding on dabigatran 150 mg was higher than that on placebo. The risk of major bleeding on placebo was lower than that on warfarin and ASA. The risk of ICH on placebo, dabigatran 110 mg, and edoxaban LD was no longer lower than the risk on ASA. When prior assumptions were varied, we found no significant deviations in the relative effectiveness estimates in any NMAs.
Discussion
In this analysis, we observed that most OACs were superior to antiplatelet agents and placebo in reducing ischemic and overall stroke risk, but results for risk of bleeding were mixed. Overall, we observed a reduction in ICHs with the NOACs when compared to warfarin. Although dabigatran 150 mg was shown to be superior to warfarin at reducing ischemic strokes, apixaban and edoxaban LD were the only treatments to demonstrate a mortality advantage over warfarin. Given that apixaban and edoxaban LD are associated with a lower major-bleeding risk than warfarin, these results may be an indication that overall mortality is driven more by major bleeding than ischemic stroke in AF patients.
One advantage of using a Bayesian NMA approach is the ability to rank treatments. This is in contrast to traditional meta-analysis, which must assume a class effect.34 Our ranking results have implications for clinical practice. For example, if a patient’s bleeding risk is higher than their risk of ischemic stroke, but their risk of ischemic stroke is high enough to require an OAC, apixaban may be a better option for them than dabigatran 150 mg. If a patient’s bleeding risk is very high, they might benefit from being on edoxaban LD or even no treatment, as edoxaban LD and placebo were ranked as the preferred options in reducing the risk of major bleeding.
When studies of patients ineligible for warfarin were excluded from the analysis, some important differences were observed. Since the excluded studies looked at ASA, apixaban, and placebo, there was less evidence for these treatments in the sensitivity analyses. This resulted in greater uncertainty around the relative-effectiveness estimates for these treatments. Additionally, ASA was shown to have a less favorable risk of major bleeding than that observed when all studies were included.
Although other NMAs have been conducted in this therapeutic area, we believe our study offers some unique insights, including reporting on a broader range of outcomes and inclusion of all existing treatments indicated for stroke prevention in AF patients. To the best of our knowledge, there exist four other NMAs comparing older treatments (placebo, antiplatelet drugs, warfarin) to some or all of the NOACs that address similar clinical questions in the same study population.35–38 Only one study included the Phase III trial of edoxaban.35 In this study, which had results similar to ours, ischemic stroke was not reported as a separate outcome.
One previous NMA37 did not include edoxaban as a comparator, and had several results inconsistent with our analysis. The most important difference was that the authors found dabigatran 150 mg and apixaban to be more effective at reducing stroke than warfarin, but did not achieve a level of statistical significance as in our study. Another NMA36 included Phase II trials of edoxaban, and found no significant differences between interventions in the risk of major bleeding or ICH, whereas our analysis found these differences to be significant. The authors of both published studies aimed to reflect current practice patterns by excluding trials or study arms where warfarin was administered at nonstandard doses. However, studies in which warfarin was administered outside of the standard target INR range of 2.0–3.0 were included in both. By including these studies in their analyses, warfarin’s effectiveness may be under or overestimated, since overcoagulation can lead to less bleeding but more ischemic strokes, and undercoagulation can lead to fewer ischemic strokes but more bleeding. This difference in included studies, coupled with differences in the choice of statistical models used, may explain inconsistencies between results.
There exist numerous published NMAs comparing the NOACs with each other.39–46 Overall, the results of these studies align well with ours, but these are only a subset of the treatments included in our analysis. Only one study included the Phase III trial results of edoxaban, but the authors looked at individual and pooled relative-effectiveness estimates of the NOACs compared to warfarin, and did not compare the NOACs with each other.46
This analysis has several limitations. As with any meta-analysis, NMAs are based on many simplifying assumptions. Although we assumed homogeneity of patient populations, the patients in the trials upon which we based our analysis were heterogeneous, particularly with respect to stroke and bleeding risk. For example, the average CHADS2 score of patients in the ROCKET-AF study was 3.5, which is significantly higher than that of most of the other studies, which was around 2. Generally, the trials were heterogeneous in terms of the definitions used for major bleeding, concomitant treatments allowed by protocols, discontinuation rates, and TTR for patients in the warfarin arms.
Other limitations related to patient characteristics may make using our findings for clinical decisions a challenge. For example, trials comparing ASA, placebo, and warfarin to each other are relatively old, and may not reflect the patients or outcomes seen today. These include high proportions of patients with valvular disease who are at much higher risk of stroke than those with nonvalvular disease.46 Additionally, as patient INRs may not be monitored as closely in practice as they are in clinical trials, the effectiveness of other treatments when compared to warfarin in clinical trials may be underestimated.47
A final limitation is the inclusion of only English-language studies, although there is currently a lack of evidence that excluding non-English studies from published systematic reviews of interventions in conventional medicine leads to biased estimates of an intervention’s effectiveness.47
In conclusion, in the absence of head-to-head RCTs of antithrombotic treatments in patients with AF, we can only estimate their relative effectiveness indirectly. This NMA supports the use of OACs over antiplatelet agents or no treatment for stroke prevention in AF patients, following careful consideration of their bleeding risk. Each treatment option compared in this analysis is associated with a unique safety and efficacy profile, but this alone does not suffice to make an individual treatment decision. Our results further confirm the difficulty in balancing the risk of ischemic stroke with that of bleeding associated with stroke prevention in AF patients. Decision-analytic models may assist in providing additional guidance for classes of patients with varying stroke and bleeding risk. This approach allows for a formal consideration of risk, benefit, and cost; factors that are all important in determining the optimal approach to managing this very challenging condition.
Acknowledgments
The primary author (AT) has received grants from the University of Toronto, the Toronto Health Economics and Technology Assessment Collaborative, and the government of Ontario in the form of the Ontario Graduate Scholarship to assist in the completion of her doctoral research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nguyen TN, Hilmer SN, Cumming RG. Review of epidemiology and management of atrial fibrillation in developing countries. Int J Cardiol. 2013;167:2412–2420. doi: 10.1016/j.ijcard.2013.01.184. [DOI] [PubMed] [Google Scholar]
- 2.Gladstone DJ, Bui E, Fang J, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anti-coagulated. Stroke. 2009;40:235–240. doi: 10.1161/STROKEAHA.108.516344. [DOI] [PubMed] [Google Scholar]
- 3.Skanes AC, Healey JS, Cairns JA, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28:125–136. doi: 10.1016/j.cjca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Lip GY, De Caterina R, et al. 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 5.Furie KL, Goldstein LB, Albers GW, et al. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:3442–3453. doi: 10.1161/STR.0b013e318266722a. [DOI] [PubMed] [Google Scholar]
- 6.ACTIVE Writing Group Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 7.Worthington JM, Gattellari M. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:2672–2675. [PubMed] [Google Scholar]
- 8.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 10.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 11.Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11:956–964. doi: 10.1111/j.1524-4733.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. London: Cochrane Collaboration; 2011. [Google Scholar]
- 13.Wong SL, Wilczynski N, Haynes R. Developing optimal search strategies for detecting clinically sound treatment studies in Embase. J Med Libr Assoc. 2006;94:41–47. [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper NJ, Sutton AJ, Lu G, Khunti K. Mixed comparison of stroke prevention treatments in individuals with nonrheumatic atrial fibrillation. Arch Intern Med. 2006;166:1269–1275. doi: 10.1001/archinte.166.12.1269. [DOI] [PubMed] [Google Scholar]
- 16.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Verheugt F. Who is ineligible for warfarin in atrial fibrillation? Lancet. 2009;374:510–511. doi: 10.1016/S0140-6736(09)61471-9. [DOI] [PubMed] [Google Scholar]
- 18.ACTIVE Investigators Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 19.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. Lancet. 1989;1:175–179. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 20.Gulløv AL, Koefoed BG, Petersen P, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation. Arch Intern Med. 1998;158:1513–1521. doi: 10.1001/archinte.158.14.1513. [DOI] [PubMed] [Google Scholar]
- 21.Gulløv AL, Koefoed BG, Petersen P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation: the AFASAK 2 study. Arch Intern Med. 1999;159:1322–1328. doi: 10.1001/archinte.159.12.1322. [DOI] [PubMed] [Google Scholar]
- 22.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 23.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 24.Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C. Canadian Atrial Fibrillation Anticoagulation (CAFA) study. J Am Coll Cardiol. 1991;18:349–355. doi: 10.1016/0735-1097(91)90585-w. [DOI] [PubMed] [Google Scholar]
- 25.Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255–1262. No authors listed. [PubMed] [Google Scholar]
- 26.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 27.Sato H, Ishikawa K, Kitabatake A, et al. Low-dose aspirin for prevention of stroke in low-risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke. 2006;37:447–451. doi: 10.1161/01.STR.0000198839.61112.ee. [DOI] [PubMed] [Google Scholar]
- 28.Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. New Engl J Med. 2010;363:1875–1876. doi: 10.1056/NEJMc1007378. [DOI] [PubMed] [Google Scholar]
- 29.Boehringer Ingelheim Advisory committee briefing document: dabigatran etexilate. 2010. [Accessed May 9, 2016]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM226009.pdf.
- 30.Johnson & Johnson Advisory committee briefing document: rivaroxaban for the prevention of stroke and non-central nervous system (CNS) systemic embolism in patients with atrial fibrillation. 2011. [Accessed May 9, 2016]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM270797.pdf.
- 31.Stroke Prevention in Atrial Fibrillation Study: final results. Circulation. 1991;84:527–539. doi: 10.1161/01.cir.84.2.527. No authors listed. [DOI] [PubMed] [Google Scholar]
- 32.Rash A, Downes T, Portner R, Yeo WW, Morgan N, Channer KS. A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO) Age Ageing. 2007;36:151–156. doi: 10.1093/ageing/afl129. [DOI] [PubMed] [Google Scholar]
- 33.Posada IS, Barriales V. Alternate-day dosing of aspirin in atrial fibrillation. Am Heart J. 1999;138:137–143. doi: 10.1016/s0002-8703(99)70259-0. [DOI] [PubMed] [Google Scholar]
- 34.Hicks T, Stewart F, Eisinga A. NOACs versus warfarin for stroke prevention in patients with AF: a systematic review and meta-analysis. Open Heart. 2016;3:e000279. doi: 10.1136/openhrt-2015-000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron C, Coyle D, Richter T, et al. Systematic review and network meta-analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open. 2014;4:e004301. doi: 10.1136/bmjopen-2013-004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assiri A, Al-Majzoub O, Kanaan AO, Donovan JL, Silva M. Mixed treatment comparison meta-analysis of aspirin, warfarin, and new anticoagulants for stroke prevention in patients with nonvalvular atrial fibrillation. Clin Ther. 2013;35:967–984. doi: 10.1016/j.clinthera.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Dogliotti A, Paolasso E, Giugliano RP. Current and new oral antithrombotics in non-valvular atrial fibrillation: a network meta-analysis of 79 808 patients. Heart. 2014;100:396–405. doi: 10.1136/heartjnl-2013-304347. [DOI] [PubMed] [Google Scholar]
- 38.Roskell NS, Lip GY, Noack H, Clemens A, Plumb JM. Treatments for stroke prevention in atrial fibrillation: a network meta-analysis and indirect comparisons versus dabigatran etexilate. Thromb Haemost. 2010;104:1106–1115. doi: 10.1160/TH10-10-0642. [DOI] [PubMed] [Google Scholar]
- 39.Harenberg J, Marx S, Diener HC, et al. Comparison of efficacy and safety of dabigatran, rivaroxaban and apixaban in patients with atrial fibrillation using network meta-analysis. Int Angiol. 2012;31:330–339. [PubMed] [Google Scholar]
- 40.Schneeweiss S, Gagne JJ, Patrick AR, Choudhry NK, Avorn J. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:480–486. doi: 10.1161/CIRCOUTCOMES.112.965988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lip GY, Larsen TB, Skjøth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60:738–746. doi: 10.1016/j.jacc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen LH, Larsen TB, Graungaard T, Skjøth F, Lip GY. Primary and secondary prevention with new oral anticoagulant drugs for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ. 2012;345:e7097. doi: 10.1136/bmj.e7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Testa L, Agnifili M, Latini RA, et al. Adjusted indirect comparison of new oral anticoagulants for stroke prevention in atrial fibrillation. QJM. 2012;105:949–957. doi: 10.1093/qjmed/hcs114. [DOI] [PubMed] [Google Scholar]
- 44.Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:711–719. doi: 10.1161/CIRCOUTCOMES.112.966572. [DOI] [PubMed] [Google Scholar]
- 45.Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth. 2013;5:40–54. [PMC free article] [PubMed] [Google Scholar]
- 46.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 47.Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–144. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]