Summary
Circuits distributed across cortico-limbic brain regions compose the networks that mediate emotional behavior. The prefrontal cortex (PFC) regulates ultraslow (<1Hz) dynamics across these networks, and PFC dysfunction is implicated in stress-related illnesses including major depressive disorder (MDD). To uncover the mechanism whereby stress-induced changes in PFC circuitry alter emotional networks to yield pathology, we used a multi-disciplinary approach including in vivo recordings in mice and chronic social-defeat stress. Our network model, inferred using machine learning, linked stress-induced behavioral pathology to the capacity of PFC to synchronize amygdala and VTA activity. Direct stimulation of PFC-amygdala circuitry with DREADDs normalized PFC-dependent limbic synchrony in stress-susceptible animals and restored normal behavior. In addition to providing insights into MDD mechanisms, our findings demonstrate an interdisciplinary approach that can be used to identify the large-scale network changes that underlie complex emotional pathologies and the specific network nodes that can be used to develop targeted interventions.
Keywords: Oscillations, Synchrony, Chronic Stress, major depressive disorder, neural networks
Introduction
MDD is a multifactorial mental disorder characterized by changes in mood, interests, sleep, and perception. While many genes and cells have been implicated in the onset and manifestation of depression, a concerted mechanism of causality has remained elusive, in part due to the heterogeneity of the disorder. Multiple human imaging studies have implicated altered functional activation of PFC in MDD (Mayberg et al., 1999). Hyper-connectivity (i.e. increased oscillatory synchrony) between PFC and the default mode network has been directly linked to the duration of depressive episodes (Greicius et al., 2007), and an increase in the functional connectivity between PFC and both cognitive and limbic affective networks has been described in depressed subjects as well (Sheline et al., 2010). Finally, direct stimulation of sub-regions of the PFC ameliorates MDD symptoms and related behaviors in select clinical populations (George et al., 2010; Mayberg et al., 2005) and in multiple preclinical models of the disorder (Covington et al., 2010; Kumar et al., 2013). These findings suggest that dysfunction within PFC-dependent networks may serve as a pathophysiological mechanism underlying MDD. Here, we employ a novel interdisciplinary approach to test this hypothesis. Our goal was to test whether functional changes in PFC-dependent networks directly contribute to (rather than simply reflect) the global changes in limbic oscillatory connectivity and the behavioral alterations that occur with the disorder.
In humans, MDD can be triggered, exacerbated, or re-occur in response to stress (Bartolomucci and Leopardi, 2009; Caspi et al., 2003; Kendler et al., 1999; Tennant, 2002). Similarly, in rodents, exposure to chronic social defeat stress induces a syndrome that recapitulates many phenotypes of MDD (Golden et al., 2011; Krishnan et al., 2007). In this paradigm animals are repeatedly exposed to larger aggressive animals for 10–15 consecutive days. At the end of this protocol, animals exhibit multiple depressive endophenotypes including hedonic dysfunction, circadian dysregulation, anxiety, and psychomotor retardation during the forced swim test (Krishnan et al., 2007; Warren et al., 2013). Furthermore, phenotypic responses to the paradigm are sensitive to multiple distinct treatments that ameliorate symptoms in major depressive disorder and bipolar depression in humans. These treatments include cortical brain stimulation (Covington et al., 2010; Kumar et al., 2013), tricyclic antidepressants (Berton et al., 2006), selective serotonin reuptake inhibitors (Berton et al., 2006), ketamine (Donahue et al., 2014), and anticonvulsants (Berton et al., 2006). Now, we have clarified the role of PFC-dependent circuitry in mediating MDD-related behavioral and network phenotypes using the rodent model.
To directly probe the relationship between PFC circuitry, limbic network oscillatory dysfunction, and the emergence of depression-related behavior, we used the following systematic approach. First, we implanted mice with microwire recording electrodes in PFC and three relevant limbic brain regions implicated in major depressive disorder: Nucleus Accumbens (NAc), amygdala (AMY), and VTA. We subjected the mice to chronic social defeat stress and recorded local field potential (LFP) oscillations and PFC unit activity. We identified all of the oscillatory frequencies at which the PFC influenced neural activity in the other limbic regions, and these oscillatory signals were selected as the input parameters for our neural network model of depression. Next, we used a translational depression-related assay, the forced interaction test (FIT) that involves exposure to an acute stressor. We measured the impact of this stressor on the oscillatory input parameters (Fig. 1, see also Supplemental Fig. S1), and then inferred a network model of depression that describes the relationship between the observed behavior and circuit physiology using supervised machine learning. Finally, we validated the ‘depression’ network by directly stimulating a key node at the intersection of PFC and AMY circuitry in chronically stressed susceptible mice. Importantly, rather than introducing artificial circuit activity, this network manipulation restored normal biological network oscillatory activity (i.e. mirroring the brain state of the unstressed and resilient animals) and behavioral function. The findings generated using this approach provided evidence that stress-induced dysregulation of PFC-AMY circuitry disrupts the PFC-dependent synchronization of ultraslow limbic activity. Furthermore, this dysregulation plays a key role in the manifestation of emotional behavior pathology in major depressive disorder.
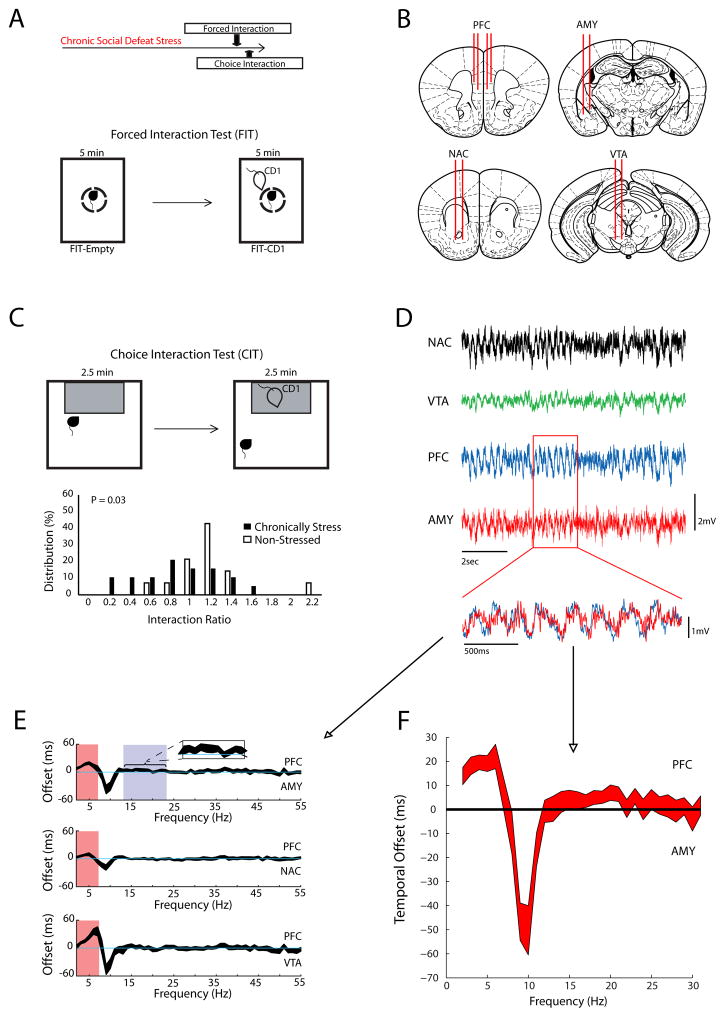
Figure 1. PFC directed oscillatory interactions.
A) Schematic of experimental timeline (top), and the forced interaction test (bottom). B) Electrophysiological recording sites. C) Schematic of classic choice social interaction test (top), and interaction ratio scores measured in stressed mice and non-stressed controls (bottom; p=0.03 using rank sum test). D) Representative LFP traces. In the overlaid traces below, note that PFC oscillations (blue) tended to precede AMY oscillations (red). E) Using LFP data obtained during the second half of the forced interaction, we quantified the extent to which PFC phase synchronized with activity in the limbic regions. We then introduced step-wise temporal shifts between PFC oscillations and the other LFPs. Finally, we re-calculated the phase synchrony between these two regions and extracted the directionality based on the optimal time offset at which each brain region synchronized with PFC for each frequency. Data is shown as the 95% confidence intervals (N=33 mice). PFC activity preceded all three of the other regions in 2–7Hz band (red highlight), and AMY activity in the 14–23Hz band. F) To confirm that PFC and AMY exhibited directionality in the beta frequency range (14–23Hz), we calculated LFP phase coherence at various temporal offsets in a second cohort of animals implanted with wires in PFC and AMY (N=46 mice; data shows 95% confidence interval).
Results
PFC Delta and Beta activity influences limbic oscillations
To identify neural circuit components underlying susceptibility, we implanted 33 animals with microwire electrodes (Fig. 1B); 19 of these mice were subjected to chronic social defeat stress, and 14 mice were used as non-stressed behavioral controls. The most established behavioral assay for measuring the impact of chronic social defeat stress in mice is the single chamber social interaction test, (hereafter referred to as the “choice interaction test”) (Golden et al., 2011). In this assay, the interaction ratio is defined as the total time an animal remains proximal to a CD1-strain mouse in a small chamber divided by the time spent proximal to that same chamber when it is empty. The interaction ratio has been validated as a behavioral measure of susceptibility or resilience to chronic social defeat, with susceptible mice exhibiting interaction ratios less than one (Krishnan et al., 2007). As expected, implanted mice subjected to chronic social defeat stress exhibited lower interaction ratios during the choice interaction test compared to control animals (p = 0.03 using rank-sum test; Fig. 1C). Furthermore, both susceptible and resilient animals were identified in the stressed group of implanted mice (Fig. 1C). Together, these results confirmed that mice implanted with recording electrodes continue to show normal behavioral responses to chronic stress. To discover the neural network state that accompanied stress susceptibility, we directly measured the impact of CD1 exposure on LFP activity in chronically stress mice using the FIT assay (Fig. 1A). Critically, this assay allows for the direct quantification of circuit responses to an aggressor mouse without the influence of the exploratory or escape behaviors exhibited during the choice interaction test (Kumar et al., 2014).
Given that dysregulation of PFC-dependent circuits has been implicated in contributing to major depressive disorder, we focused on PFC as the seed region to perform functional connectivity analysis. We initially sought to define which oscillatory frequency bands to investigate by determining those that were PFC-driven. We took advantage of prior studies that suggest that directional interactions across brain circuits can be extracted from phase relationships between concurrently recorded LFP signals (Dzirasa et al., 2013; Narayanan et al., 2011). We found that AMY, NAc, and VTA oscillatory activities were particularly influenced by PFC within the delta (2–7Hz) range (Fig. 1D–F) during exposure to the CD1 mouse. Beta (14–23Hz) activity in the AMY was sensitive to PFC input as well (Fig. 1E–F). Thus, PFC activity influenced limbic delta and beta oscillations, suggesting that PFC delta and beta oscillations could be used as the seed for our functional connectivity analysis.
PFC synchronizes slow evolving limbic beta dynamics
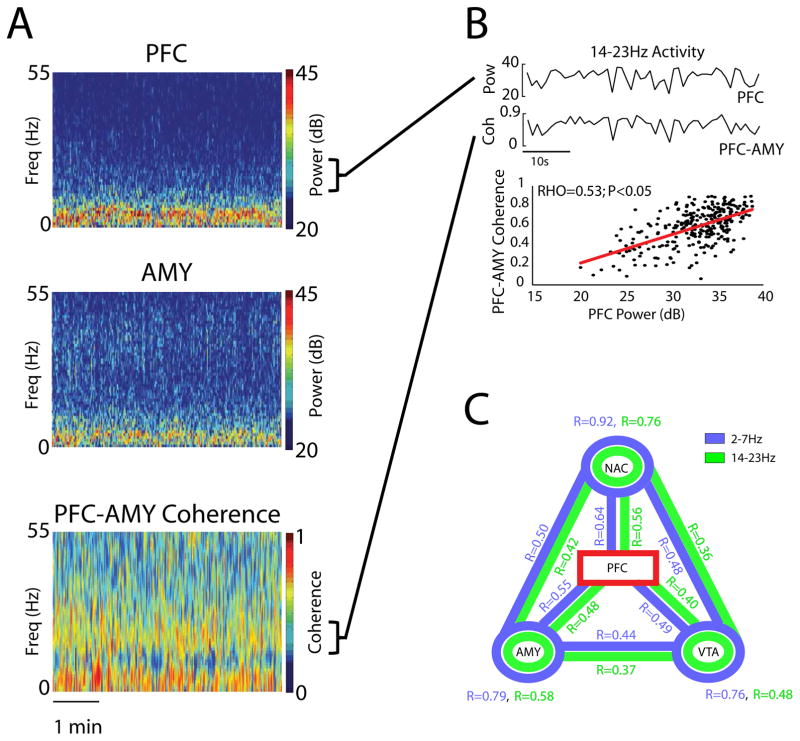
Next, we probed the relationship between PFC oscillations within these PFC-leading frequency bands (delta and beta) and LFP activity measured across the limbic regions. LFP power reflects global activity patterns within a given brain region, while LFP coherence quantifies the extent to which two distinct brain regions oscillate together across time. As such, LFP coherence reflects brain circuit activity (Igarashi et al., 2014; Jones and Wilson, 2005). To quantify activity across the cortico-limbic network, we calculated LFP power within each of the four brain regions and LFP coherence between the six pairs of brain regions we recorded. Since the dysfunctional networks previously uncovered in major depressive disorder using fMRI show slow evolving oscillatory periods that are more than 1 second (ultraslow; < 1Hz) (Greicius et al., 2007; Sheline et al., 2010), power and coherence were measured using 1 second temporal windows. This approach allowed us to identify the ultraslow patterns by which these measures change over extended periods of time (Fig. 2A–B). We then determined the extent to which PFC activity correlated with each measure of limbic brain activity (18 measures in this study: there were 3 power measures and 6 coherence measures for each of the two PFC-dependent frequency bands) over the recording period (Fig. 2B–C). Nearly all of the power and coherence measures we tested showed correlated activity with PFC (p<0.05 for 341/342 comparison of 18 correlations/animal using Spearman rank; N = 19 animals; p=0.06 for the remaining NAC-VTA coherence circuit measure from 1 animal; Fig. 2C), aligning with fMRI studies that show wide-spread functional connectivity between PFC and limbic regions (Sheline et al., 2010). After confirming that our approach quantified functional connectivity in mice using PFC as the seed region, we used the raw correlation measures obtained from each implanted animal as the parameters for a network analysis aimed identifying the neural architecture underlying the stress-susceptible state.
Figure 2. Limbic network dynamics exhibit functional connectivity with PFC.
A) Spectral plots showing PFC and AMY activity, and the coherence between the two regions (top). Sample time course trace of PFC power and PFC-AMY coherence in the 14–23Hz band (bottom). B) Correlation between PFC power and PFC-AMY coherence in a representative mouse. Each point corresponds to 1 second of LFP activity recorded during the 5 minute session (p<0.01, R=0.53 using Spearman rank correlation; top). C) Functional connectivity using PFC-seed analysis. RHO values were calculated using Spearman rank between PFC power and limbic power (circles) or coherence (links). Values shown on the plot were averaged across 33 mice. p<0.05 for all seed comparisons, with the exception of 1 measure (NAC-VTA; p=0.06).
Modeling depression network dysfunction using chronic social defeat stress
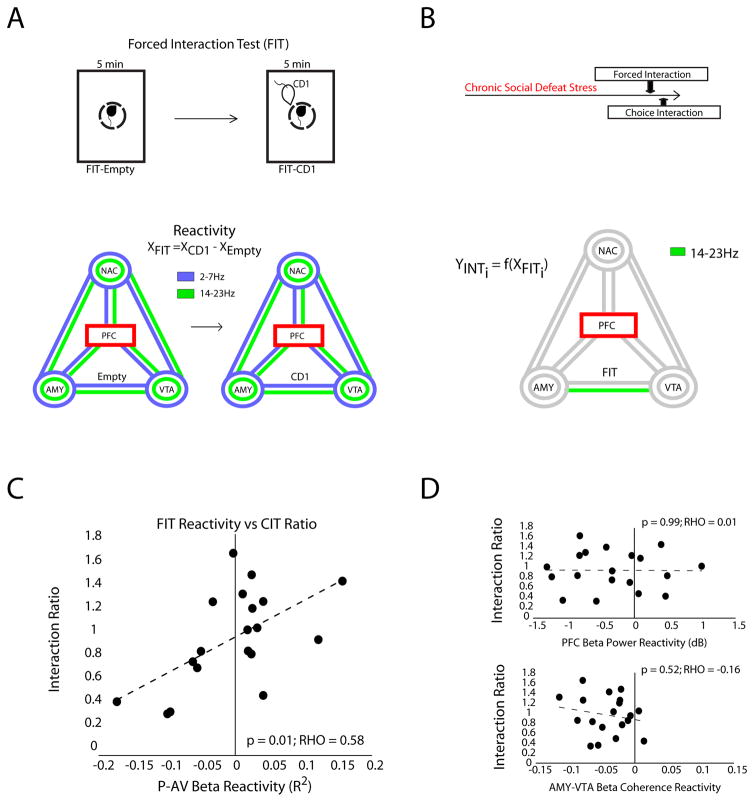
To infer a ‘depression network’ model that described the manifestation of the susceptible phenotype, we assayed coherence and power parameters during the forced interaction test. A forced interaction test reactivity score is defined as the difference in each measure between when the CD1 is and is not present (XForced Interaction Test = XCD1−XEmypty, Fig. 3A). We have shown previously that the PFC LFP-power reactivity score predicts susceptibility to social defeat stress in stress naive mice (Kumar et al., 2014). Here we used the forced interaction test assay to directly quantify the extent to which an acute exposure to a CD1 impacts neural circuit function within limbic regions and frequencies that we found to be PFC-directed (Fig. 3A).
Figure 3. PFC-centered network dynamics signal stress susceptibility.
A) The PFC-centered forced interaction test (FIT) reactivity was compared to the choice interaction ratio using the Elastic network. The PFC seed plot shows the 18 model parameters used for analysis (3 power measures shown as circles and 6 coherence measures shown as lines for each of the two PFC-dependent frequencies bands). B) Only the PFC beta (14–23 Hz) power vs. AMY-VTA beta coherence correlation was retained in the model. C) The correlation between these values is shown below (p=0.01 using Spearman rank correlation; bottom). D) The mean PFC beta power and mean AMY-VTA beta coherence were calculated for each segment of the FIT, and the reactivity of each measure was compared to the choice interaction test ratio using a Spearman rank regression. There was no significant relationship between behavioral responses and either brain measure.
We determined the contribution of the 18 neural circuit measures obtained for each animal to its individual behavioral responses using a machine learning approach, the Elastic Net (Zou and Hastie, 2005). This multivariate regularized regression method tests the relationship between the 18 circuit-reactivity values measured during the forced interaction test (i.e. change in R2 values; X1…n) and the behavioral interaction ratio measured during the choice interaction test (Y1…n). It infers a network model that explains the emergence of stress susceptibility. All circuit features retained in the network model contribute to the network, and because each of the circuit features is tested within a single multivariate model, there is no need for classical corrections for multiple comparisons. In our case, only one circuit feature was retained in the model, converging on the solution of a univariate regression.
The interaction ratio measured for each mouse’s behavior was directly and specifically related to the correlation between PFC power and AMY-VTA coherence in the beta oscillatory band during the forced interaction test (Fig. 3B; hereafter referred to as “P-AV” network). Exposure to the CD1 mouse decreased the correlation between PFC beta power and AMY-VTA beta coherence in the susceptible mice [(−)reactivity in the P-AV network], and increased this correlation in the resilient animals [(+)reactivity in the P-AV network; Fig. 3C; p<0.05; R = 0.58 using Pearson Correlation]. Notably, only the P-AV beta circuit reactivity measure was retained in the model, suggesting that reactivity in this network explained the susceptible phenotype independently of the other 17 neural measures we tested. Neither reactivity in PFC beta power nor AMY-VTA beta coherence alone correlated with the interaction ratio during social interaction testing (Fig. 3D). Thus, P-AV reactivity did not simply reflect a global increase or decrease in PFC power or AMY-VTA coherence induced by exposure to the CD1; rather, P-AV reactivity directly reflected changes in the dynamics that coordinated these two measures across time.
PFC neurons signal synchrony between amygdala and VTA
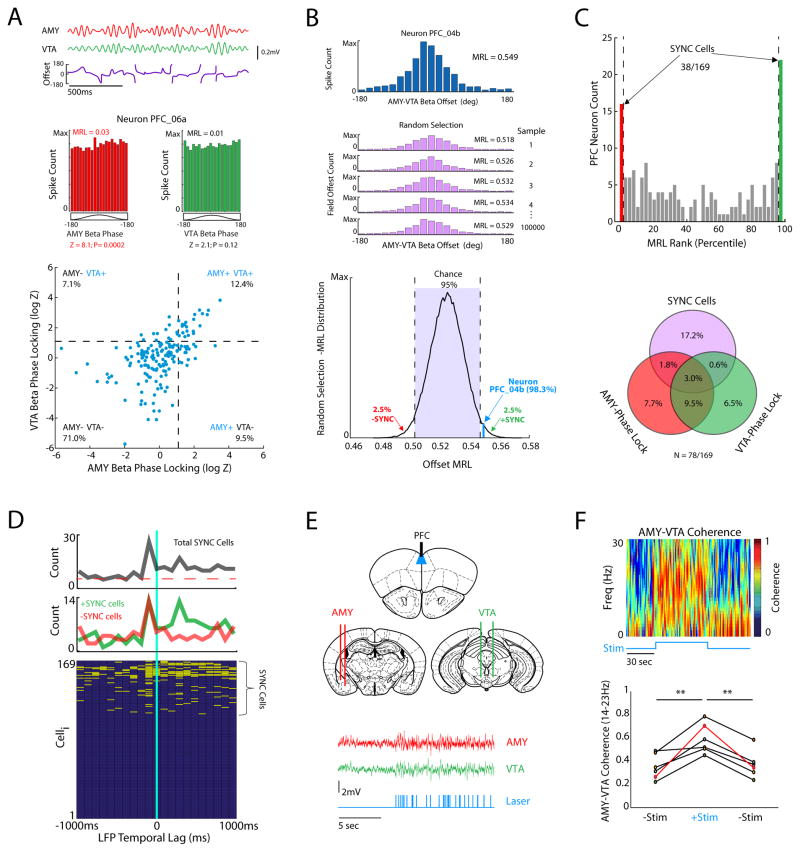
Since our depression network model suggested that PFC activity was causally linked to beta (14–23Hz) coherence between AMY and VTA, we set out to determine if PFC unit firing signaled the synchronization of beta oscillations in AMY and VTA. We compared the firing of 169 PFC neurons recorded during the forced interaction test to the instantaneous phase of AMY and VTA beta oscillations using phase-locking analysis (Fig. 4A, top). With this approach, we found that 37/169 PFC neurons phase-locked to AMY beta oscillations (Fig. 4A, bottom) and that 33/169 neurons phase-locked to VTA beta oscillations (Fig. 4A, bottom). Finally, we found that 21/169 PFC neurons phase-locked to beta oscillations in both AMY and VTA (Fig. 4A). Thus, the data suggest that the coupling between AMY and VTA beta oscillations can be signaled by the firing of PFC neurons.
Figure 4. PFC activity synchronizes AMY and VTA beta oscillations.
A) Traces shows beta filtered LFP activity recorded from AMY and VTA during forced interaction test, and their phase offset time series (top). Histograms depicts the instantaneous LFP beta oscillatory phases at which an example neuron fires (middle). This example PFC neuron phase-locked to AMY (left), but not VTA beta oscillations (right; p<0.05 using the Rayleigh test of circular uniformity, where Z = −log|p|). Rayleigh statistics (Z) for all neurons recorded during the FIT (N = 169 PFC neurons; bottom). The dashed lines correspond with the significance threshold for phase-locking to AMY and/or VTA beta oscillations. B) Histogram depicting the distribution of instantaneous phase offsets between AMY and VTA beta oscillations when an example neuron fires, and the corresponding mean resultant length (MRL; top). The AMY-VTA phase offset time series was randomly sampled the same number of times this neuron fired, yielding a chance MRL. Bootstrapping was repeated 105 times to create a distribution of chance MRL values for the neuron (bottom). C) Neurons that exhibited MRL values outside of the 95% confidence interval of their chance MRL distribution were classified as synchrony (SYNC)-cells. Venn diagram indicates the number of units that significantly phase-lock to AMY and VTA oscillations, and to the offset between them (SYNC cells). D) Phase-offset MRL values were calculated for PFC neurons at LFP temporal lags ranging from −1s to 1s in 100ms steps, and SYNC cells were detected based on a 99.7619% confidence interval (α = 0.05/21 bins). The majority of SYNC cells phase-offset locked to AMY-VTA synchrony 100ms in the past. SYNC cells that fired at periods of high synchrony (+SYNC cells, green) also coupled to AMY-VTA synchrony 300ms in the future. Plot below shows all PFC neurons at each of the temporal offsets we tested. Neurons that showed phase-offset locking at a given LFP temporal lag are shown in yellow. E) Experimental recording and stimulation sites for optogenetics experiment (top). LFP traces recorded from AMY and VTA during optogenetic stimulation of PFC (bottom). Light stimulation trace is shown below LFPs. F) PFC stimulation increased beta synchrony between AMY and VTA (**p<0.01 using Friedman’s test followed by sign-rank test). The animal in red corresponds with the coherence plot shown above.
PFC phase-locking to both AMY and VTA oscillations provides one putative mechanism whereby PFC could regulate AMY-VTA coherence. Nevertheless, there are other possible regulatory mechanisms. Specifically, PFC neurons could fire relative to the instantaneous relationship between oscillatory activity in AMY and VTA (rather than relative to the instantaneous phase of oscillations in each area; phase-locking). To probe for such a mechanism, we quantified the phase offset between AMY and VTA beta oscillations at the instant each PFC neuron fired.
Since the phase offset time series between AMY and VTA was not uniformly distributed (Fig. 4A), we expected that PFC neurons would also tend to fire at a non-uniform phase offset between AMY and VTA (i.e. mean resultant length [MRL] of the circular vector > 0; see Fig. 4B). To compare the actual firing of PFC neurons to the expectations based on purely random timing we: 1) randomly sampled the AMY-VTA phase offset time series an equivalent number of times that a PFC neuron fired, 2) grouped these randomly sampled phase offsets, and calculated their MRL, and 3) repeated this process 100,000 times, yielding a chance MRL distribution for that neuron. We then compared the phase offsets at which the PFC neurons actually fired to this random distribution. Neurons that exhibited MRL values outside of the 95% confidence interval of their chance MRL distribution were classified as synchrony (“SYNC”)-cells (Fig. 4B). SYNC cells thus represent neurons that fire according to specific AMY-VTA phase offsets (compared against a random population of offsets that occur by chance).
Nearly 23% (38/169) of PFC neurons fired relative to the phase offset between AMY and VTA (Phase-offset locking; Fig. 4B–C). The majority of SYNC cells (22/38) increased their firing when oscillatory activity in AMY and VTA was highly synchronized (+SYNC; neuronal firing increased at the dominant phase offset between AMY and VTA oscillations), while 16/38 of these SYNC cells increased their firing rate when AMY and VTA beta oscillations were desynchronized (−SYNC; neuronal firing increased at the non-dominant phase offset between AMY and VTA oscillations). Strikingly, the majority of SYNC cells did not phase lock to AMY or VTA beta oscillations, and the majority of PFC neurons that phase-locked to AMY and VTA oscillations were not SYNC cells (Fig. 4C, bottom).
Prior studies that probe spike-LFP relationships have shown that assessing the timing relationship between the two, using the introduction of temporal offsets between spikes and LFPs as part of the analysis, can clarify the mechanisms underlying spike-LFP interactions (Kumar et al., 2014; Siapas et al., 2005; Sigurdsson et al., 2010). Thus, we exploited this approach to probe the temporal mechanisms underlying the activity of SYNC cells (Fig. 4D). We introduced temporal offsets ranging from −1s to 1s in 100ms steps into our LFP analyses and recalculated the coupling between PFC activity and AMY-VTA synchrony in each of these shifted timeframes. The directionality of activity between PFC neuron firing and AMY-VTA was determined by the temporal offsets at which the coupling was observed. Strikingly, we found that the majority of SYNC cells fired relative to AMY-VTA synchrony 100ms in the past (Fig. 4D, top), indicating a strong relationship between SYNC cell firing and AMY-VTA synchrony in the immediate past. In contrast, few SYNC cells fired relative to the AMY-VTA synchrony measured at temporal offsets further in the past (6/38, 15%), demonstrating that phase offset locking reflected a neural mechanism that was coordinated on a sub-second time scale. Nearly half of the SYNC cells fired relative to the synchrony observed 300ms in the future (17/38, 45%). The majority of cells that fired relative to AMY-VTA synchrony in the future were +SYNC cells (i.e. the firing of these cells signaled an increase in synchrony). Together, our findings show that both types of SYNC cells detect AMY-VTA synchrony levels in the immediate past, and that +SYNC cells also predict increases in AMY-VTA synchrony in the immediate future. Notably, these findings also demonstrated that phase-locking and phase-offset locking were both distinct mechanisms whereby the activity of PFC neurons could signal AMY and VTA synchrony. Nearly 50% (78/169) of PFC neurons exhibited at least one of these mechanisms.
Direct PFC stimulation is sufficient to increase AMY-VTA synchrony
Our results so far confirm that PFC neurons signaled the synchronization of AMY-VTA oscillations. To test whether PFC activation was sufficient to cause coherence between AMY and VTA, we implanted transgenic mice engineered to express channelrhodopsin-2 in layer 5 pyramidal neurons of cortex with recording electrodes in AMY and VTA, and a stimulating fiber in PFC (Fig. 4E). We then recorded LFP activity during direct stimulation of PFC with a pre-recorded PFC spike pattern (mean stimulation rate of 4.02Hz for 60 seconds; see Fig. 4E). Direct stimulation of the subcortical projections in PFC significantly increased beta synchrony between AMY and VTA (Fig. 4F). Thus, PFC firing was indeed sufficient to increase AMY-VTA coherence. Together, these findings demonstrated a definitive physiological link between PFC activity and AMY-VTA coherence. The correlation between PFC beta power and AMY-VTA coherence in stressed animals is not simply a network epiphenomenon. Rather the correlation between these two neural measures reflected an emergent property of a PFC-regulated physiological network (P-AV network).
PFC-AMY circuit stimulation reverses network and behavioral phenotype induced by chronic stress
We next sought additional evidence of causality by manipulating the P-AV network in chronically stressed “susceptible” mice and measuring their behavioral response. Importantly, we sought to restore normal healthy function to the circuit. We targeted the PFC-AMY axis of the circuit because our LFP directionality analysis suggested that PFC exhibited a direct influence on AMY (but not VTA) activity in the beta frequency band, justifying the connection between PFC and AMY as a putative target for manipulating the P-AV network in a manner consistent with the endogenous network function that already exists.
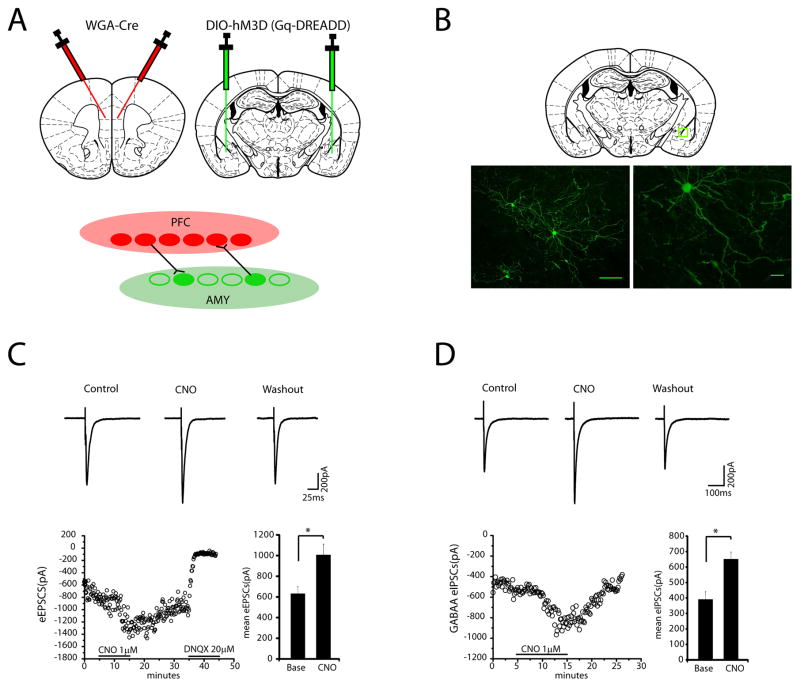
To modulate PFC-AMY circuit activity, we used a chemogenetic approach based on DREADDs. These DREADDs are variants of muscarinic receptors (−hM3D) that have been modified to be selectively activated by the pharmacologically inert compound, clozapine-N-oxide (CNO). When activated by CNO, hM3D increases the likelihood of neuronal firing through signaling by the G protein Gq (Alexander et al., 2009; Armbruster et al., 2007). To specifically target neurons within the PFC-AMY circuit, we used an intersectional approach described in the Methods. This strategy was designed to yield expression of the DREADDs in AMY neurons that form synaptic connections with neurons in PFC (i.e., both afferent and efferent connections; see Fig. 5A–B)(Gradinaru et al., 2010) (Supplemental Fig. S2). Next, we evaluated amygdalar cell types impacted by the DREADDs by measuring CNO-dependent evoked post synaptic currents in slices from these mice. Activation of the Gq-DREADDs by CNO reversibly enhanced both evoked excitatory post synaptic currents (eEPSCs) and GABAA evoked inhibitory post synaptic currents (eIPSCs) in AMY principal neurons (Fig. 5C–D; see also Supplemental Fig. S3), implying that the DREADDs were expressed in both excitatory pyramidal neurons and inhibitory GABAergic interneurons. The evoked IPSCs were completed blocked by bath application of 10 uM bicuculline methiodine, indicating that the IPSCs were mediated by GABAA receptors. Thus, our targeting strategy successfully rendered the cells in AMY that formed connections with PFC more likely to fire in response to excitatory input (PFC-AMY circuit stimulation).
Figure 5. In vitro confirmation of DREADD stimulation.
A) Schematic of viral infection strategy (top) designed to yield expression of Gq-DREADD in AMY neurons that form synaptic connections with PFC (bottom). Trans-synaptic wheat-germ agglutinin (WGA)-tagged Cre was injected into the PFC and floxed Gq-DREADD was injected into the AMY. B) Histology showing Gq-DREADD expression in AMY (green) using the viral targeting strategy described in A. C) Activation of Gq-DREADD by CNO reversibly enhances AMPA-excitatory post synaptic currents (EPSCs) in AMY principal neurons. Averaged traces of evoked EPSCs obtained during baseline, bath application of CNO (1uM), and washout periods are shown (top). Time course of amplitude of evoked EPSCs during baseline, bath application of CNO (1uM), washout and bath application of DNQX (20uM) (bottom left). CNO administration increased the peak mean evoked EPSCs (data were analyzed using two-tailed paired t-test; t3=8.09, *p=0.004, N=4 neurons; bottom right). D) Activation of Gq-DREADD by CNO reversibly augments GABAA-Inhibitory post synaptic currents (IPSCs) in AMY principal neurons. Averaged traces of evoked IPSC obtained during baseline, bath application of CNO (1uM), and washout are shown (top). Time course of amplitude of evoked IPSCs during control, bath application of CNO (1uM), and washout (bottom left). CNO administration increased the peak mean evoked IPSCs (data were analyzed using two-tailed paired t-test; t5=7.66, *p=0.0006, N=6 neurons; bottom right). These results demonstrated that the DREADDs were expressed in both excitatory pyramidal neurons and inhibitory GABAergic interneurons using this targeting strategy.
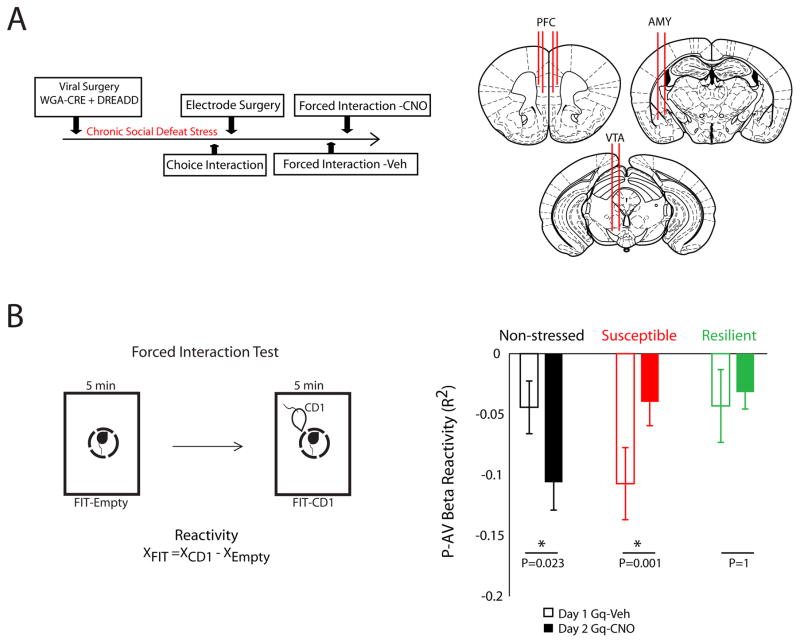
Next, we examined the network-level effects of DREADD stimulation in vivo. Mice were virally manipulated using our targeting strategy and either subjected to chronic social defeat stress or used as unstressed controls. Following chronic social defeat stress, stressed mice and the non-stressed controls were implanted with recording electrodes in AMY, PFC, and VTA. After surgical recovery, mice were treated with vehicle (i.p.) and neurophysiological data was collected during a forced interaction test. Twenty-four hours later, mice were treated with CNO (i.p.) to induce DREADD activation, and the forced interaction test was repeated (Fig. 6A).
Figure 6. PFC-AMY stimulation ameliorates stress induced network adaptations.
A) Schematic of experimental timeline (left) and electrode implantation sites (right). B) The correlation between PFC activity and AMY-VTA coherence (P-AV network) was quantified during each segment of the forced interaction test (FIT; left). DREAAD activation potentiated the FIT-reactivity in the P-AV network in non-stressed control mice, attenuated P-AV beta network reactivity in chronically-stressed susceptible mice, and had no effect in the resilient mice (*p<0.05 using sign-rank test; right).
During the initial forced interaction testing session, exposure to the CD1 mouse reduced connectivity in the P-AV network [e.g. a decrease in correlation between PFC power and AMY-VTA coherence; (−)reactivity] in all of the vehicle-treated stress-susceptible mice (N = 8; Fig. 6B). In stressed, susceptible animals, PFC-AMY circuit activation with CNO selectively attenuated P-AV network (−)reactivity, such that animals exhibited a smaller decrease in the correlation between PFC beta power and AMY-VTA beta coherence in response to the CD1 exposure. In non-stressed control animals, treatment with CNO potentiated P-AV network (−)reactivity. In the resilient mice, CNO had no impact (N = 6 mice; Fig. 6B). Critically, there was no difference in P-AV network (−)reactivity in uninfected susceptible mice subjected to repeat FIT testing (P = 0.84 using rank sum test; see Supplemental Figure (S4), demonstrating that activation of the PFC-AMY circuit (and not simply repeat FIT testing) suppressed the ‘susceptible-network’ phenotype we identified in chronically stressed mice. Control animals on CNO showed reactivity that resembled the susceptible animals on vehicle, and susceptible animals on CNO showed reactivity that resembled the resilient and unstressed animals on vehicle. Notably, stress-susceptible mice treated with vehicle tended to show (−)reactivity values that were more negative compared to the vehicle treated stress-resilient mice and the non-stressed controls (P = 0.08 and 0.05 using one-tailed rank-sum test; Fig. 6B, bottom), providing additional biological support for the neural network-based model we inferred using machine learning in which (−)reactivity signaled susceptibility.
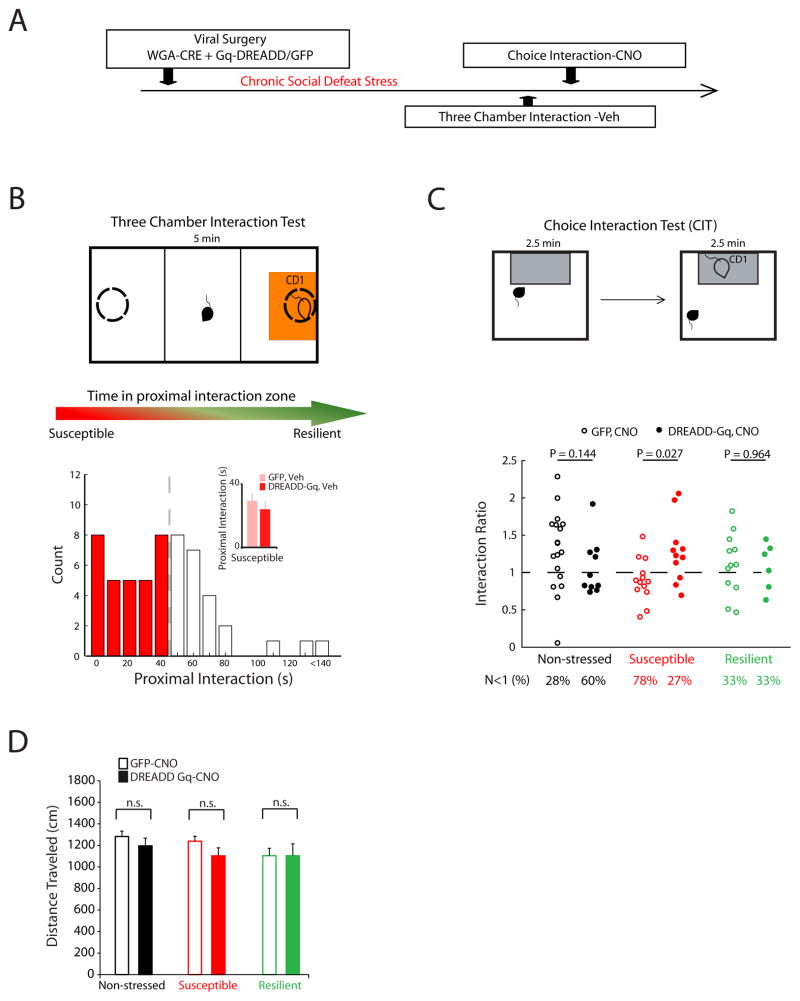
Since the DREADD strategy reversed the ‘susceptible-network’ neural phenotype in stressed mice (large P-AV reactivity), we hypothesized that activation of the PFC-AMY circuit might rescue their behavioral responses during the choice interaction test as well. We again used our viral infection strategy in mice with WGA-Cre in PFC and Gq-DREADDs or GFP control in AMY. After recovery, mice were subjected to chronic subordination stress or pair-housed as non-stressed controls (Fig. 7A). We initially identified susceptible mice by measuring social behavior using a three-chamber choice social interaction test (Fig. 7B). We used this modified three-chamber design in order to avoid habituating animals to the standard chamber and ultimately cofounding the measured interaction ratio during subsequent testing. Based on prior studies (Covington et al., 2010), the animals that exhibited the lowest 60% of social interaction scores in the three-chamber interaction test were treated as susceptible (Fig. 7B). Importantly, there was no difference in the distribution of interaction times between the DREADD and GFP infected groups of susceptible mice in the three-chamber test (p = 0.3088 using Kolmogorov-Smirnov test; Fig. 7B inset). Thus, simply expressing the DREADDs was not sufficient to alter the behavior of animals in the absence of CNO. Similarly, treatment with CNO did not impact social interaction behavior in mice that did not express the DREADD receptor (Supplemental Figure S5). After identifying the susceptible animals, all of the experimental mice were tested in the standard single chamber choice interaction test following CNO treatment to stimulate the DREADD (Fig. 7C, see Supplemental methods for further description). Importantly, DREADD stimulation had no effect on the locomotor profiles displayed by either of the groups (Fig. 7D). Furthermore, treatment with CNO did not impact social interaction behavior in mice that did not express the DREADD receptor (Supplemental Figure S5).
Figure 7. PFC-AMY stimulation reverses stress induced behavioral deficits.
A) Schematic of experimental timeline and B) Three chamber social interaction test (top). Distribution of proximal interaction scores during post-stress three-chamber social interaction test (top; N=55 mice). Similar interaction scores were observed between GFP and Gq-DREADD that displayed interaction scores within the lower 60% percentile (p = 0.366 using Wilcoxon rank-sum test; N=11 and 14 for Gq-DREADD-susceptible and GFP, respectively; bottom). C) PFC-AMY activation increased social interaction in the stress-susceptible mice in the social interaction test (p = 0.026 using Wilcoxon rank-sum for comparison between DREADD-Gq and GFP expressing mice), and tended to reduce social interaction in the non-stressed control mice (p = 0.14). PFC-AMY stimulation had no effect in the resilient mice. D) No significant effects of viral vector (GFP versus Gq-DREADD; F1,52 = 1.33, p = 0.25; N=10–18 mice per group) or viral vector × stress (F1,52 = 0.1, p = 0.76; N=10–18 mice per group) were observed on gross locomotor behavior in the susceptible mice using two way ANOVA.
GFP-expressing stressed mice (pooled susceptible and resilient animals) exhibited interaction ratios that were lower than the non-stressed controls (P = 0.024 using Kolmogorov-Smirnov test), demonstrating that the stressed animals exhibited the typical profile of behaviors that emerged following social defeat stress. Comparison of the interaction scores for the stressed DREADD-infected mice to their GFP-infected controls revealed that PFC-AMY stimulation increased social interaction in the stress-susceptible animals and had no effect in the stress-resilient mice (P = 0.027 and 0.96, respectively, using rank-sum test; Fig 7C). No differences in interaction ratios were observed between DREADD-expressing stressed mice (pooled susceptible and resilient animals) and their non-stressed GFP-controls (P = 0.43 using Kolmogorov-Smirnov test), demonstrating that social behavior was normalized in the stressed DREADD-expressing mice. Interestingly, activation of the PFC-AMY circuit tended to reduce the interaction ratio in the non-stressed controls as well (p=0.14 using Wilcoxon Rank-sum test; Fig. 7C). Thus, the behavioral responses induced by DREADD activation mirrored the neurophysiological responses we observed in each of the groups using in vivo recordings.
Finally, to ensure that the neurophysiological and behavioral responses observed in susceptible mice during PFC-AMY DREADD stimulation did not result simply from a DREADD-induced disruption of normal AMY activity, we repeated experiments in a new group of susceptible animals that expressed the Gq-DREADD in AMY in a non-circuit selective manner (Supplemental Fig. S6). Treatment with CNO in animals expressing Gq-DREADD under a CaMKII-driven proomter in the amygdala failed to restore normal social interaction behavior or P-AV reactivity in these mice. Taken together, these data affirm our hypothesis that stimulation of the PFC-AMY circuit reverses the expression of the “susceptible” network and susceptible behavioral phenotypes after chronic social defeat stress in mice.
Discussion
Animal studies have been particularly useful in dissecting the neural circuit mechanisms underlying sleep, habit formation, spatial processing, fear, motor and sensory processing, and motivation (Crawley, 2007). Nevertheless, there have been several barriers to using rodents to model the emotional behavior changes that characterize psychiatric disorders (Nestler and Hyman, 2010). For example, it has simply not been feasible to model dysfunction across many of the behavioral domains observed in major depressive disorder including guilt, mood, hallucinations, and suicidality. An alternative strategy for overcoming this hurdle is to create animal models that recapitulate the key disruptions in emotion networks that contribute to psychiatric disorders. Here we begin to identify and wield manipulations over such networks using a multidisciplinary approach including whole-circuit recordings, stress manipulations, behavioral analysis, machine learning, and direct brain stimulation using optogenetics, to demonstrate that changes in specific PFC-regulated neural networks signal the emergence of behavioral dysfunction in a widely validated rodent model of depression. Furthermore, we provide direct evidence using DREADDs that normal emotional behavior is restored by reversal of a naturally occurring network-level disruption brought on by chronic stress. To our knowledge, this is the first demonstration of electrophysiological parallels between a key neural network-level phenotype observed in patients with MDD and emotional deficits in a preclinical model of the disorder. The preclinical model now affords us detailed and more comprehensive understanding of the specific real time dynamics of network function that are not currently available in humans, thus uncovering novel targets for therapeutic development.
We began these studies with the well-recognized findings from both clinical and pre-clinical models that regulation of emotional behavior is highly dependent on the PFC. The PFC makes direct monosynaptic connections with multiple limbic brain regions including amygdala and VTA (Oh et al., 2014), and these PFC-dependent circuits have been shown to regulate anxiety (Likhtik et al., 2014), fear (Kumar et al., 2014), and psychomotor activity (Kim et al., 2015). Multiple studies have also implicated the dysregulation of PFC-dependent circuits as a central endophenotype of depression (Dzirasa et al., 2013; Greicius et al., 2007; Kumar et al., 2013; Mayberg et al., 1999; Salvadore et al., 2010; Sheline et al., 2010). Further, chronic antidepressant treatment reverses this neural circuit phenotype in the mouse model of depression (Dzirasa et al., 2013). We built on these findings in order to begin to dissect the distinct disruptions in emotion network critical for advancing understanding of psychiatric disorders. We now find that the emergence of behavioral pathology in a chronic stress-based model of major depressive disorder is associated with altered function across ultraslow PFC-dependent beta networks.
The studies presented here provide one of the first glimpses of large scale network analysis that will be critical to unraveling the complex encoding of emotional processing. We identified the P-AV network, whereby susceptible responses are reflected in failure of coordination between PFC power and AMY-VTA coherence, using a data driven strategy based in machine learning. The model solution generated by the Elastic Net is comprised by the non-zero coefficients that account best for the network recordings. Taken together, our behavioral, neurophysiological, and stimulation based experiments provide strong support for the network model generated using our data driven strategy.
On a cellular level, we show that nearly 50% of single units in the PFC neurons phase lock to oscillations in AMY and/or VTA, or to the phase offset between AMY and VTA oscillations (phase-offset locking; SYNC cells). To our knowledge, the identification of SYNC cells marks the first time that cortical activity has been shown to signal the phase offset between spatially distinct subcortical oscillations. Directionality studies of the phase-offset lock phenomenon indicated that the majority of SYNC cells phase-offset locked to limbic beta oscillatory activity 100ms in the immediate past (i.e. AMY-VTA phase synchrony state preceded PFC firing), and nearly half of the SYNC cells also phase-offset locked to activity nearly 900ms in the future (i.e. PFC firing preceded AMY-VTA phase synchrony state). Thus, SYNC cells may lie within a PFC-mediated feedback control circuit that detects and regulates the timing of sub-cortical oscillations to enable distinct network level computations. This is consistent with our optogenetic findings which showed that direct PFC activation was sufficient to increase the beta coherence between amygdala and VTA. Our findings also raise the hypothesis that phase-offset locking may serve as a general brain mechanism whereby the cortex coordinates spatially distributed brain regions to promote emotional behavior.
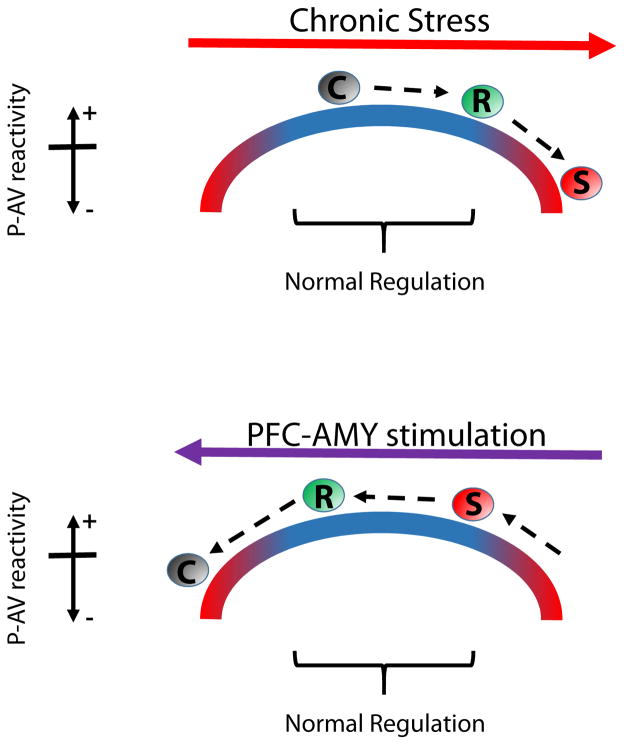
PFC activity has been shown to signal safety in threatening environments (Adhikari et al., 2010). In animals that exhibit behavioral dysfunction after stress (e.g. susceptible mice), acute CD1 exposure disrupts the regulation of AMY and VTA interactions by PFC. Furthermore, direct stimulation of PFC-AMY circuitry using DREADDs normalizes activity in the P-AV beta network and restores behavioral function in susceptible mice. Thus, the coherence between AMY and VTA may assign salience to threat cues in order to signal risk during various emotion experiences. When this coherence signal is regulated by PFC, an animal can maintain its normal function under threatening conditions. Thus, our findings suggest that the P-AV network contributes to the emotion experiences that guide behavior. Our observations suggest that the relationship between P-AV reactivity and emotional regulation may lie along a U-Shaped curve (Fig. 8). The state of a given emotional network along this curve is modulated by PFC inputs to AMY since this PFC-AMY circuit is altered by chronic stress and direct stimulation of this circuitry using DREADDs. Given that deficits in functional connectivity across PFC-dependent networks have been described in major depressive disorder (Greicius et al., 2007; Nugent et al., 2015), activity across the P-AV network may serve as a novel translational measure of face validity in preclinical models of major depressive disorder.
Figure 8. Model of stress effects on P-AV reactivity and threat regulation.
The relationship between P-AV reactivity and threat regulation can be modeled by the upside down U-shaped curve that is common to many biological systems. Chronic stress drives the network state to the right such that P-AV reactivity decreases. Susceptible mice show the largest P-AV reactivity change and the largest behavioral dysregulation, while little differences in either measure are observed in resilient mice (top). Stimulation of the PFC-AMY circuit moves the network state to the left such that P-AV reactivity increases in susceptible mice, restoring normal reactivity and behavioral regulation. In the control mice, this manipulation destabilizes the normal network state and decreases P-AV reactivity (bottom).
Using circuit manipulation, we were able to restore the P-AV network in stressed animals to the natural state that occurs in unstressed animals under standard physiological conditions. Our viral infection strategy resulted in DREADD expression in inhibitory and excitatory neurons. The PFC makes direct projections on glutamatergic neurons and feed-forward local inhibitory circuits in amygdala (Ehrlich et al., 2009). Given that local inhibitory-excitatory circuits are critical to generating oscillatory activity (Bartos et al., 2007), this feed-forward circuitry may play a central role in regulating local oscillatory timing, ultimately synchronizing AMY beta oscillations with efferent input from PFC. The susceptible brain state may then reflect the extent to which stress induces plasticity in these circuits. Our WGA-Cre viral targeting strategy also results in DREADD expression in AMY neurons that project to PFC. Many of these glutamatergic neurons also send axon collaterals to NAC (McDonald, 1991), providing a cellular mechanisms whereby decreasing the input resistance of AMY neurons could potentially serve to coordinate large scale subcortical limbic dynamics timed to PFC input.
Our DREADD manipulation directly targets the capacity of PFC to regulate AMY by increasingly the likelihood of AMY neuronal firing in response to efferent input (ultimately decreasing the input resistance of excitatory PFC efferents). In the absence of novel closed-loop optogenetic protocols which stimulate the brain timed to ongoing patterns of activity in PFC, even direct stimulation of PFC to AMY axon terminals would fail to achieve this type of regulation. For instance, optogenetic stimulation has been used to drive neurons based on their projection targets (Chaudhury et al., 2013; Stuber et al., 2011), and in this study we use a transgenic approach to selectively stimulate PFC efferents in layer V. Nevertheless, nearly all protocols for such open-loop optogenetic stimulation ‘override’ the ongoing oscillatory dynamics that coordinate PFC output under physiological conditions, thus failing to restore the regulation of AMY activity by PFC dynamics. To this point, we show that exposure to a CD1 decreases AMY-VTA coherence in both the susceptible and resilient mice (see Fig 3B Bottom). Thus, it is the regulation of AMY-VTA coherence by PFC, and not AMY-VTA coherence itself per se’ that underlies susceptibility. While multiple open-loop optogenetic stimulation protocols (including PFC layer V stimulation as shown here) may increase AMY-VTA coherence, they would be unlikely to achieve the type of regulation necessary to correct the P-AV network dysfunction that defines the susceptible brain state. Indeed there is a growing appreciation in the field that the timing, duration, as well as target and off-target effects from cellular manipulation are critical considerations for the interpretation of network and behavioral responses (Otchy et al., 2015).
Psychological stress is a major risk factor for the onset and exacerbation of major depressive disorder. Stress induces adaptations in cortical and limbic circuitry (Campioni et al., 2009; Dias-Ferreira et al., 2009; Ghosh et al., 2013; Kim et al., 1996; Yuen et al., 2012). For example, multiple distinct stress-induced cellular and molecular adaptations have been shown to regulate chronic social defeat syndrome in rodents. These adaptations include synaptic potentiation of thalamic inputs into Nucleus Accumbens (Christoffel et al., 2015), Ih current increases in ventral tegmental area (VTA) (Friedman et al., 2014), and gene expression changes in PFC (Covington et al., 2010; Vialou et al., 2014). Since manipulation of several distinct network nodes (circuits/brain areas) is sufficient to overcome these stress-induced cellular modifications to restore the normal emotional behavior (Bagot et al., 2015; Christoffel et al., 2015; Covington et al., 2010; Friedman et al., 2014), it is likely that these distinct molecular adaptations converge at the level of circuits or brain networks to yield the syndrome. Here we integrate the disparate neural sites involved in stress responses by showing how chronic stress alters the PFC-dependent coordination of long-range limbic networks to yield dysfunction in emotional behavior. Our findings suggest that a disruption in the capacity of PFC to coordinate neural interactions between AMY and VTA may be a central brain mechanism underlying major depressive disorder. Furthermore, therapeutics that directly target these network interactions may have the potential to rapidly ameliorate depression psychopathology.
Experimental Procedures
Animal Care and Use
Studies were conducted with approved protocols from the Duke University Institutional Animal Care and Use Committee and were in accordance with the NIH guidelines for the Care and Use of Laboratory Animals. See extended experimental procedures for additional details.
Electrode Implantation Surgery
At an age of six-seven weeks, C57 mice were anesthetized with isoflurane, placed in a stereotaxic device, and metal ground screws were secured to the cranium. A total of 32 tungsten microwires were arranged in array bundles and implanted in AMY (basolateral amygdala and central extended amygdala), NAC, PFC (prelimbic and infralimbic cortex), and VTA. See extended experimental procedures for additional details. Histological analysis of implantation sites was performed at the conclusion of experiments to confirm recording sites used for neurophysiological analysis (see Supplemental Figure S7).
Neurophysiological Data acquisition
Headstages were connected without anesthesia 30 minutes prior to recording sessions. All neurophysiological recordings were performed during the forced interaction test. Neuronal activity was sampled at 30kHz using the Cerebus acquisition system (Blackrock Microsystems Inc., UT). Local field potentials (LFPs) were bandpass filtered at 0.5–250Hz and stored at 1000Hz. Neurophysiological recordings were referenced to a ground wire connected to ground screws.
Determination of LFP oscillatory power and cross-area synchrony
Signals recorded from all of the implanted microwires were used for analysis. Using Matlab (The MathWorks, Inc., Natick, MA), a sliding Fourier transform with Hamming window was applied to the LFP signal using a 1 second window and a 1 second step. Frequencies were analyzed with a resolution of 1Hz. Additional analytical details are available in the extended experimental procedures.
Elastic net analysis
The spearman rank correlation coefficient was calculated between each limbic activity measure (three power measures and six coherence measures) and PFC power in the 2–7Hz and 14–23Hz range. Forced interaction test reactivity was then calculated for each of the 18 activity measures as RHO2(CD1) − RHO2(Empty). The 18 calculated reactivity measures were used as observation variables and the interaction scores measured during the choice interaction test were taken as the response at each observation.
The Elastic net is a regularized regression method that solves the problem:
where
with N the number of observations, yi the response at observation i. xi a vector of “feature” data (length p) at observation i, λ is a positive regularization parameter, β0 is a scalar “intercept” and β are coefficients on the different features (Zou and Hastie, 2005). The elastic net was solved using 10 fold cross validation, and optimized based on the global minimum of the mean squared error with alpha values ranging from 10^-6 to 0.9 incremented on a logarithmic scale. Data was analyzed using the Matlab Machine Learning toolbox (The MathWorks, Inc., Natick, MA).
Optogenetic stimulation
The optogenetic experiment reflects additional analyses of data described in a previous experiment (Kumar et al., 2013). Briefly, B6.Cg-Tg(Thy1-ChR2/EYFP)18Gfng/J were obtained from Jackson labs. Six 4–8 month old heterozygous mice were implanted with a stimulating fiber in PFC, and recording electrodes in PFC, AMY, NAC, and VTA. Following surgical recovery, neurophysiological recordings were obtained while mice were stimulated in PFC using a pre-recorded PrL neuron spike pattern with a mean rate (4.02Hz). Our physiology experiments were performed with a laser power of 2.5mW, and the light fiber was located directly above the superior sagittal sinus.
Viral Infusions
At an age of six-seven weeks, C57 mice were anesthetized with ketamine (100mg/kg) and xylazine (10mg/kg), placed in a stereotaxic device, and injected with AAV-EF1a-mCherry-IRES-WGA-Cre (WGA-Cre) based on stereotaxic coordinates measured from bregma at the skull (PFC: 1.96mm AP, ±0.0.62 mm ML, −2.28 mm DV at a 10° angle). Mice were also injected with AAV-hSyn-DIO-HAhM3D( Gq)-IRES-mCitrine (Gq-DREADD) or AAV-hSyn-DIO-EGFP (GFP) based on stereotaxic coordinates measured from bregma (AMY: −1.46mm AP, ±2.9 mm ML, −4.9 mm DV). A total of 0.5 μL of each virus was delivered bilaterally at each injection site over five minutes using a 5μL Hamilton syringe. All viruses were obtained from the UNC Gene Therapy Center (Chapel Hill, NC). Mice were singly housed for two weeks to allow for surgery recovery. Mice were then subjected to 15 days of chronic SDS. Social interaction behaviors were tested with either the three chamber social interaction test or the single chamber social interaction test (see Extended Experimental Procedures for more details).
For in vivo neurophysiology experiments in virally infused animals, mice were implanted with microwire recording electrodes after chronic social defeat stress as described above. It should be noted that these mice were anesthetized with isoflurane (1.5%); ketamine was not utilized in the post-chronic SDS surgery given its antidepressant-like effects on chronic stressed mice (Donahue et al., 2014). At the completion of all behavioral and neurophysiological studies, mice were perfused transcardially with 4% PFA (EM Sciences, Hatfield, PA) and brains were harvested, frozen, and sliced at 35μm using a cryostat (Cryocut 1800, Reichert-Jung, Depew, NY) and stained with an anti-GFP antibody at a dilution of 1:2000 (rabbit polyclonal, A11122, Invitrogen, Grand Island, NY) according to recommended Invitrogen protocol (see extended experimental procedures). Fluorescence microscopy was used to identify both mCherry expression co-expressed with the WGA-Cre in PFC as well as FITC-stained mCitrine in the AMY using a Nikon Eclipse 80i fluorescence microscope. Confocal images were taken using a Zeiss LSM 510 inverted confocal microscope.
Mouse brain slice preparation for single cell recordings
Mice infected with Gq-DREADDs were deeply anaesthetized with isoflurane. The brain was quickly removed from the skull after decapitation and immediately chilled in an ice cold, oxygenated artificial cerebrospinal fluid (aCSF) solution containing (in mM: NaCl 120, KCl 3.3, NaHCO3 25, NaH2PO4 H2O 1.23, CaCl2 1.8, MgSO4 1.2 and glucose 10). Coronal slices (300 μm thickness) containing the AMY were cut with a moving blade microtome and the slices were then kept in normal oxygenated aCSF at 35°C for 60 minutes. The slices were then kept at room temperature until used for recording.
A single slice was transferred to the recording chamber that was constantly perfused (~3 mL/min) with oxygenated aCSF at 35°C. The AMY principal neurons were viewed under a Zeiss upright microscope equipped with a 40× water immersion objective and an enhanced differential interference contrast (DIC) video microscope system. Recording electrodes with resistance of 4–8 MΩ were pulled from borosilicate glass capillaries (1.5 mm OD) using a P97 electrode puller. Access resistance and input capacitance were electronically compensated by approximately 60–70% and monitored throughout the experiment to confirm the stability of the recording. The internal pipette solution contained (in mM); CsCl 130, NaCL 4, EGTA 0.2, Mg-ATP 4, Tri-GTP 0.3, HEPES 10 and QX-314 6. The pH was adjusted to 7.4 with CsOH and osmolarity was 290 mOsm. Signals were filtered at 5 kHz and were digitized at 10 kHz through a Digidata1440 interface controlled by pClamp10 software (Molecular Devices).
In all instances, evoked excitatory postsynaptic currents (EPSCs) and inhibitory excitatory postsynaptic currents (IPSCs) were initially recorded for five minutes to establish stable baseline values. Without altering the perfusion rate and temperature, the perfusion medium was then switched to one containing clozapine-N-oxide (CNO, 1μM) for 10 min, followed by a drug washout. Synaptic responses were analyzed off-line using Clampfit 10 software (Molecular Devices, Sunnyvale, CA). A paired t-test was used to determine the statistical significance.
Statistics
All data are presented as mean ± s.e.m. unless otherwise specified.
Supplementary Material
Acknowledgments
We would like to thank A. Talbot, S. Lisberger, M. Halassa, and R. A. Adcock for helpful comments on this manuscript; G. Feng and B.L. Roth for providing reagents; Okechi Boms for technical support. This work was supported by funding from NIMH grants R37MH073853 and R01MH099192, and a DIBS Research Incubator award to KDz. A special thanks to Freeman Hrabowski, Robert and Jane Meyerhoff, and the Meyerhoff Scholarship Program.
Footnotes
Author Contributions
RH, QL, KDe, and KDz designed experiments; RH, SMa, BK, NM, LD, RC, SK, and KDz performed experiments; RH, SMa, QL, BK, NM, LL, JW, LD, CB, RC, LC, DD, SDM, and KDz analyzed the data; RH, SMa, QL, LC, DD, and KDz wrote the paper. Detailed author contributions are provided in the Supplemental Materials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, Topiwala MA, Gordon JA. Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolanos-Guzman CA, Cheer J, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Leopardi R. Stress and depression: preclinical research and clinical implications. PLoS One. 2009;4:e4265. doi: 10.1371/journal.pone.0004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, Dey A, Smith M, Rebusi N, Pfau M, et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci. 2015;18:962–964. doi: 10.1038/nn.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. What’s Wrong With My Mouse?: behavioral phenotyping of transgenic and knockout mice. 2. Hoboken, New Jersey: John Wiley & Sons, Inc; 2007. [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76:550–558. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K, Kumar S, Sachs BD, Caron MG, Nicolelis MA. Cortical-amygdalar circuit dysfunction in a genetic mouse model of serotonin deficiency. J Neurosci. 2013;33:4505–4513. doi: 10.1523/JNEUROSCI.4891-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Archives of general psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Laxmi TR, Chattarji S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J Neurosci. 2013;33:7234–7244. doi: 10.1523/JNEUROSCI.0638-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature protocols. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510:143–147. doi: 10.1038/nature13162. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS biology. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kim IH, Rossi MA, Aryal DK, Racz B, Kim N, Uezu A, Wang F, Wetsel WC, Weinberg RJ, Yin H, Soderling SH. Spine pruning drives antipsychotic-sensitive locomotion via circuit control of striatal dopamine. Nat Neurosci. 2015;18:883–891. doi: 10.1038/nn.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J, Katz BM, Feng G, Covington HE, 3rd, Dzirasa K. Cortical Control of Affective Networks. J Neurosci. 2013;33:1116–1129. doi: 10.1523/JNEUROSCI.0092-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Hultman R, Hughes D, Michel N, Katz BM, Dzirasa K. Prefrontal cortex reactivity underlies trait vulnerability to chronic social defeat stress. Nat Commun. 2014;5:4537. doi: 10.1038/ncomms5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McDonald A. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- Narayanan V, Heiming RS, Jansen F, Lesting J, Sachser N, Pape HC, Seidenbecher T. Social defeat: impact on fear extinction and amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS One. 2011;6:e22600. doi: 10.1371/journal.pone.0022600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Robinson SE, Coppola R, Furey ML, Zarate CA., Jr Group differences in MEG-ICA derived resting state networks: Application to major depressive disorder. NeuroImage. 2015;118:1–12. doi: 10.1016/j.neuroimage.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM, Olveczky BP. Acute off-target effects of neural circuit manipulations. Nature. 2015;528:358–363. doi: 10.1038/nature16442. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant C. Life events, stress and depression: a review of recent findings. Aust N Z J Psychiatry. 2002;36:173–182. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, et al. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T. Regularization and variable selection via the elastic net. J Roy Stat Soc B. 2005;67:301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.