Abstract
The contributions of hemispheric-specific electrophysiology (electroencephalogram or EEG) and independent executive functions (inhibitory control, working memory, cognitive flexibility) to episodic memory performance were examined using abstract paintings. Right hemisphere frontotemporal functional connectivity during encoding and retrieval, measured via EEG alpha coherence, statistically predicted performance on recency but not recognition judgments for the abstract paintings. Theta coherence, however, did not predict performance. Likewise, cognitive flexibility statistically predicted performance on recency judgments, but not recognition. These findings suggest that recognition and recency operate via separate electrophysiological and executive mechanisms.
Keywords: episodic memory, recency, recognition, executive functions, EEG alpha coherence, EEG theta coherence
1. Introduction
Explicit memory requires conscious awareness of information processing (Schachter, 1987; Schacter & Graf, 1986) and interacts with many other cognitive processes, such as executive functions, to facilitate encoding and retrieval (Mazoyer et al., 2001). Explicit memory is typically associated with activation within the medial temporal lobe and the prefrontal cortex (Dolan & Flectcher, 1997; Lepage, Ghaffer, Nyberg, & Tulving, 2000; Shallice et al., 1994). A general understanding of how these brain regions contribute to explicit memory performance is known. However, it is important to examine the functional connectivity (e.g., coherence) of frontal and temporal regions during various explicit memory tasks (e.g. episodic memory) in order to better understand the neural networks involved in explicit memory. Additionally, examining cognitive processes related to explicit memory may allow for a more comprehensive picture of how explicit memory operates. Research does not exist exploring both the electrophysiological and individual executive function contributors to explicit memory.
In the current study, we recorded electroencephalogram (EEG) measures of frontotemporal coherence during an episodic memory task, broken into the components of recency and recognition. We also collected measures of individual EF processes. In the following sections we describe what is known about episodic memory processes, the neural mechanisms of episodic memory, and the relations between EFs and episodic memory.
1.1. Recency and Recognition
Episodic memory is a form of explicit memory and allows for the conscious recollection of past events (Moscovitch, Nadel, Winocur, Gilboa, & Rosenbaum, 2006; Tulving, 2002). Episodic memory operates via encoding and retrieval processes, and can be measured using recognition tasks (Diana, Yonelinas, & Ranganath, 2007; Haist, Shimamura, & Squire, 1992; Yotsumoto, McLaughlin, Kahana, & Sekuler, 2008). Recognition is the ability to determine if a currently presented item had been encountered previously (Yonelinas, 2002). Two processes can drive recognition: familiarity and recollection. The type of information that is retrieved to support recognition differentiates these processes. Familiarity relies exclusively on the strength of the item being recognized without awareness of additional information. Recollection indicates retrieval of contextual information that was associated with an item during encoding (Yonelinas, 2002). Recognition tasks are more difficult when context information must be retrieved in addition to item information (Brown, Vousden, & McCormak, 2009; Milner, Corsi, & Leonard, 1991). Relative recency judgments are a form of recognition task in which retrieval of temporal order information, a context detail (Riggins, Miller, Bauer, Georgieff, & Nelson, 2008) may drive performance. Therefore we might expect that recency judgments rely more heavily on the recollection process than do recognition judgments.
1.2. Brain Processes
Prefrontal and temporal interactions have been supported by empirical evidence (Simons & Spiers, 2003). Specifically, studies have found co-activation of these brain areas during memory tasks (e.g. McIntosh, Nyberg, Bookstein, & Tulving, 1997). Furthermore, activation of prefrontal and temporal regions during encoding statistically predicts success during retrieval (e.g., Menon, Boyett-Anderson, & Reiss, 2005). EEG can be used to quantify coupled neural activity and, thus, provide a measure of functional connectivity between prefrontal and temporal areas during cognition. The particular measure that can be used for this purpose is EEG coherence, which is the frequency-dependent squared cross-correlation of electrical signals between two scalp electrode sites (Nunez, 1981; Thatcher, Krause, & Hrybyk, 1986). If the EEG activity at two electrodes is synchronized, then coherence values approach 1; and if there is no synchronization, then coherence values approach 0. Theoretically, lower and higher levels of coherence reflect differentiation and integration of function between two brain areas, respectively (Thatcher, North, & Biver, 2008). Unlike EEG power values, EEG coherence is not affected by arousal, opening or closing of eyes, or changes in state (Thatcher, 1994).
Frontotemporal hemispheric differences have been found in relation to memory encoding and retrieval. The left hemisphere is usually biased toward local features of stimuli, while the right hemisphere is biased toward more global features (Mevorach, Humphreys, & Shalev, 2005). Abstract images require global processing because local (smaller) forms are not obvious, suggesting that abstract images would elicit more activation from right when compared to the left hemisphere. Visuo-spatial memory is predominantly active within the right hemisphere of the medial temporal lobe, providing further support that the right hemisphere would be active during encoding and retrieval of abstract images (Smith & Milner, 1981). The difficulty of forming verbal labels to abstract images would also support this claim, given research supporting left hemisphere’s role in language (Vigeau et al., 2006; Smith, Jonides, & Koeppe, 1996).
Although correlated with memory recognition, we propose that the measure of recency is separate. Recency requires retrieval of contextual information making it more reliant on recollection processes rather than familiarity (Yonelinas, 2002). Brain activity may distinguish the processes such that recency involves higher activation in the PFC than recognition, which may be indicative of higher cognitive processing demands (Tendolker & Rugg, 1997). Furthermore, during fMRI studies, activation necessary for difficult temporal order decisions involve the bilateral middle lateral prefrontal areas, left inferior lateral prefrontal area and left anterior prefrontal area, as well as bilateral medial temporal areas (Konishi et al., 2002). This activation was above that of less taxing recognition judgments. We expected frontotemporal coherence differences between recollection and familiarity task performance.
1.3. Executive Functions
EFs are a set of higher order cognitive control processes typically divided into three components: updating (working memory), inhibitory control, and cognitive flexibility (attention shifting; Miyake & Friedman, 2013). Working memory is often defined as the active process of updating and manipulating information (Miyake & Shah, 1999); inhibitory control is the suppression of a prepotent response in favor of another less dominant response; and cognitive flexibility is the ability to switch attention between tasks or mental sets (Miyake & Friedman, 2013). The literature focusing on EFs has suggested that, although interactions occur between the processes, they are separate mechanisms (Miyake et al., 2000). Rehearsal within working memory is crucial for the encoding and later retrieval process (Gallo & Wheeler, 2012), whereas the ability to suppress interference during retrieval is often associated with inhibitory control processes (Levy & Anderson, 2002). Considering that performance on the Wisconsin Card Sort Task has been related to episodic memory in adults, one would deduce that cognitive flexibility (an EF typified by the card sort task) is also a contributor to memory flexibility and thus encoding and retrieval (McCabe et al., 2010).
Recollection performance appears to be more reliant on EFs than familiarity-based processes (Bugaiska et al., 2007). Bugaiska and colleagues found that aging populations displayed deficits in recollection, but not familiarity, performance. Furthermore, the recollection deficits were predicted by EF ability. This suggests that recollection performance is reliant on EF ability, whereas familiarity is not. These findings led us to conclude that pure recognition performance would be less reliant on EFs than recency, since recency should elicit recollection whereas recognition, without reliance on contextual binding, should elicit familiarity (Yonelinas, 2002). This conclusion was drawn because recency tends to be more reliant on recollection processes while pure recognition tends to be more reliant on familiarity.
1.4. Overview of Current Study
The association between episodic memory and EF has typically been examined with composite measures of EF. We examined the individual components of EF, in an attempt to tease apart specific EF contributions to recency and recognition performance. We also investigated electrophysiological contributions to recency and recognition memory, with particular focus on frontotemporal coherence. We used abstract paintings as stimuli and examined EEG coherence during encoding and retrieval. Coherence was examined within both theta (4–7) and alpha (8–13) bands, based on research implementing these bands in memory processes (Nyhus & Curran, 2010; Klimesch, 1999). Finally, we included the EF and EEG coherence contributors in the same regression analyses in order to examine the unique contributions of EFs and frontotemporal coherence to recency and recognition performance. Our study focused on three questions: (a) Will electrophysiological differences exist when comparing recency and recognition performance? We hypothesized that frontotemporal coherence within both theta and alpha bands would be greater during recency than recognition performance. (b) Will individual executive functions display different prediction patterns for recency and recognition performance? We hypothesized that EFs would be associated with recency but not recognition performance. (c) Will electrophysiological and executive functions display unique contributions to recency and recognition performance? We hypothesized that both EFs and electrophysiology would contribute unique variance to recency performance. The inclusion of EFs and electrophysiology (frontotemporal EEG coherence) is a unique approach that will allow for a more holistic understanding of the processes associated with recency and recognition. Answering these questions will broaden our understanding of the differences between recognition and recency aspects of episodic memory.
2. Method
2.1. Participants
Our sample included 108 women ranging in age from 18 to 49 years (M = 32.4, SD = 6.1). Two-thirds of the women participated in our laboratory in a small urban area, after being recruited through community agencies and advertisements (e.g., flyers distributed in schools and common areas in the community, university website and email announcements) for a study focused on mothers' parenting of young children. The other third of the sample was from a cohort of families from an ongoing longitudinal community study, who participated in a visit to our rural university laboratory.
The sample was representative of our Appalachian region, according to US Census data. The participants were predominantly Caucasian (79.2%; 10% African American, 5% Hispanic, 1.6% Asian, 4.2% other). See Deater-Deckard and colleagues (2012) for more details on the sample.
2.2. Procedure
Signed consent was provided upon arrival to the research lab. Each woman was seated at a small table and EEG electrodes were applied. A battery of cognitive tasks was completed while EEG was recorded. This report focuses on episodic memory and EF tasks. Participants received an honorarium, and the institutional review board approved study procedures.
2.3. Cognitive Tasks
2.3.1. Episodic Memory – Recency and Recognition
An adaptation of Corsi-Milner’s (Milner et al., 1991) temporal order memory task was employed. The original task was developed to examine the ability to make serial ordered judgments with frontal lobe impairments and utilized abstract paintings on index cards. For our task, participants were instructed to make both recency and recognition judgments. To begin, two simple drawings were presented on a computer screen, followed by the presentation of practice recency and recognition questions. All mothers correctly answered the two questions. Then 40 abstract paintings were presented; each painting remained on the screen for 3 seconds with no inter-trial interval. Occasionally, the sequence was interrupted with questions regarding either recency or recognition-based judgments of the paintings previously viewed. For the recency judgments, participants were shown two previously viewed paintings, one which was denoted as A and the other B, and asked to indicate which they saw last. They were given as much time as needed and responded by typing either A or B into the keyboard. For the recognition judgments, participants were again shown two paintings denoted as either A or B. However, recognition questions contained one picture that was not viewed previously. This required the participants to make a familiarity-based judgment on which image they had seen previously. The first recency question was asked after 10 paintings were viewed. This was followed by 4 sets of 5 paintings during which recognition and recency questions were alternated. Then there were 5 sets of 2 paintings during which recognition and recency questions were alternated (see Figure 1). There were a total of 5 recency questions and 5 recognition questions that were always presented in the same order. Each participant received the same task presentation. There were no restrictions in sampling for the questions (i.e., samples did not have to be part of the immediately preceding subset). The task was presented using SuperLab 4.5 (SuperLab Pro Edition).
Figure 1.
Examples of stimuli used for episodic memory assessment. Example (a) represents recency (“Which of these paintings did you last see?”) and example (b) represents recognition (“Which of these painting have you seen before?”).
2.3.2. Executive Function – Cognitive Flexibility
A computerized version of the Wisconsin Card Sort Task (WCST; Hearton & PAR staff, 2003) was administered as our cognitive flexibility task (Miyake et al, 2000). Participants were instructed to sort cards by matching the stimulus card, at the bottom of the screen, with one of four key cards at the top of the screen. No information on the required sorting strategy was given, but the computer provided feedback regarding the current strategy. The dimensions used to sort the cards changed throughout the task, so that participants had to alter their sorting strategy for the three dimensions of color, shape and quantity. We focused on the number of perseverative errors, which represents mistakes made by continuously using the same incorrect matching rule even after receiving feedback that the rule was no longer correct.
2.3.3. Executive Function - Working Memory
A backward digit span task was administered to assess working memory. Participants initially were presented with three digits and instructed to repeat the sequence in reverse. Two practice trials were given initially to ensure understanding and then the task began. Recall of the same digit span for two trials was required before lengthening the span by one digit. The digit span was lengthened until errors were produced on two consecutive trials of the same span. The length of highest error-free trial was the variable of interest. The span length for the participants in this study ranged from 4 to 10.
2.3.4. Executive Function - Inhibitory Control
A computerized Stroop task was used to present three conditions of the task. In the congruent condition, color words were presented in the same color ink and in the incongruent condition color words were presented in different colored ink. The mixed block had both congruent and incongruent trials. Performance was the number of accurate trials for the 20 words in the mixed block. This task was presented using SuperLab software.
2.4. EEG Acquisition, Processing, and Analysis
EEG recordings were made from 16 left and right scalp sites [frontal pole (Fp1, Fp2), frontal (F3, F4, F7, F8), central (C3, C4), temporal (T7, T8), parietal (P3, P4, P7, P8), and occipital (O1, O2)]. All electrodes were referenced to Cz during the recordings. The recordings were obtained using a stretch cap (Electro-Cap, Inc.; Eaton, OH; E1-series cap) with 16 electrodes in the 10/20 system pattern. After the cap was placed on the head, a small amount of abrasive gel was placed into each recording site and the scalp was gently rubbed. Conductive gel was then added to the recording sites. Electrode impedances were measured and accepted if they were below 20 kΩ. The electrical activity from each lead was amplified using separate James Long Company Bioamps (James Long Company; Caroga Lake, NY). During data collection, the high pass filter was a single pole RC filer with a 0.1 Hz cut-off (3 dB or half-power point) and 6 dB per octave roll-off. The low pass filter was a two-pole Butterworth type with a 100 Hz cutoff (3 dB or half-power point) and 12 dB octave roll-off.
Activity for each scalp lead was displayed on the monitor of the acquisition computer. The EEG was digitized on-line at 512 samples per second for each channel to eliminate the effects of aliasing. The acquisition software used was Snapshot-Snapstream (HEM Data Corp., Southfield, MI) and the raw data was stored for later analyses. Prior to the recording of each subject a 10 Hz, 50 uV peak-to-peak sine wave was input through each amplifier. This calibration signal was digitized for 30 seconds and stored for subsequent analysis.
Spectral analysis of the calibration signal and computation of power at 9–11 Hz was accomplished. These power figures were used to calibrate the power derived from the subsequent spectral analysis of the EEG. Then, EEG data were examined and analyzed using EEG Analysis software developed by the James Long Company. Data were re-referenced via software to an average reference configuration and then artifact scored for eye movements using a peak-to-peak criterion of 100µV or greater. Gross motor movements over 200µV peak to peak were also scored. These artifact scored epochs were eliminated from all subsequent analyses. No artifact correction procedures were used. The data were then analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1 second width and 50% overlap. Power values were computed at each site during encoding and during retrieval (recency and recognition trials). Power was expressed as mean square microvolts, and data were transformed using the natural log (ln) to normalize the distribution. Coherence between frontal (F3, F4) and all other electrode sites within the same hemisphere was computed for the 4–7 Hz (theta) and 8–13 Hz (alpha) frequency bands using an algorithm by Saltzberg, Burton, Burch, Fletcher, and Michaels (1986;
equation 9).
During the EEG recording, event marks were inserted into the record to indicate when the participant was viewing the series of paintings and when the participant responded to each recency or recognition question. We considered the EEG during the viewing of the abstract paintings to be "encoding" EEG and the EEG during the responses to the memory questions to be "retrieval" EEG. For our analyses we used the encoding EEG coherence as our “baseline” because we were interested in the unique effects of retrieval EEG coherence during recency and recognition judgments. We focused our attention on EEG coherence at left and right hemisphere frontal and temporal sites (F3/T7, F4/T8) in an attempt to capture frontotemporal functional connectivity during our episodic memory task.
2.5 Data Analyses
We examined all variables for outliers and missing data. We calculated exploratory Pearson correlations, and then focused our attention on our main analyses. We used MANOVA to test differences in frontotemporal EEG coherence during recency and recognition performance within both theta and alpha bands. Then we used correlations to test our hypothesis that EFs would be related to recency but not recognition performance. Finally, we used four hierarchical regressions to examine unique contributions of EEG coherence (alpha and theta separately), participant age, and the performance on the EF tasks of cognitive flexibility, inhibitory control, and working memory to recency and recognition performance, respectively. Age and frontotemporal EEG coherence during encoding were entered into the first step of the regression equation. EEG frontotemporal coherence during retrieval for recency and recognition trials, respectively, as well as scores for the cognitive flexibility, inhibitory control, and working memory tasks were entered at the second step.
3. Theory
Examination of the various mechanisms associated with episodic memory is necessary to provide a coherent understanding of the differences between recognition and recency, and to address the possible outcomes or associated influences. We examined electrophysiological activation during a pictorial episodic memory task that included both recency and recognition judgments in an attempt to tease apart these processes. The differentiation of such memory processes is important for our overall understanding of retention.
Our study focused on whether functional connectivity contributed to both recency and recognition judgments. We wanted to replicate existing studies demonstrating frontal and temporal activation during an episodic memory task. Our study is unique, however, because of the inclusion of the alpha band (8–13 Hz). Theta is consistently related to episodic memory (Nyhus & Curran, 2010); there are also strong links between alpha EEG power and explicit memory (Klimesch, 1999). We therefore focused on both theta and alpha in our analyses. Much of the research on EF and EEG has focused on alpha activity (e.g., Michels et al., 2010); because we examined contributions of EF to recency and recognition performance, we analyzed EEG coherence in the alpha band in addition to EEG coherence in the theta band.
In addition to these functional brain mechanisms, we were motivated by the literature connecting memory to executive processes associated with the prefrontal cortex in order to determine their potential impact on episodic processes (Gallo & Wheeler, 2012; Levy & Anderson, 2002). Developmental studies have suggested unique contributions of working memory and inhibitory control to episodic memory (Ruffman, Rustin, Garnham, & Parkin, 2001; Marsh, Beaman, Hughes, & Jones, 2012). Research with adults typically has focused on the amalgamated contributions of executive processes to episodic memory. Our study attempted to tease apart the contributions of the components of EF to provide a more detailed understanding of EF contributions to episodic memory performance.
4. Results
Tables 1 and 2 display the descriptive statistics as well as the correlations among all behavioral and electrophysiological variables in the theta and alpha bands, respectively. Recency and recognition performance were positively correlated. Performance on the recognition portion of the memory task was better than performance on the recency portion, t(107) = −4.35, p < .001.
Table 1.
Descriptive Statistics and Bivariate Correlations for Theta Coherence
| Task | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Recency Performance |
-- | |||||||||||
| 2. Recog Performance |
.47*** | -- | ||||||||||
| 3. EF Cognitive Flexibility |
−.34*** | −.16 | -- | |||||||||
| 4. EF Inhibitory Control |
−.06 | .05 | −.17 | -- | ||||||||
| 5. EF Working Memory |
.07 | .11 | −.14 | .29** | -- | |||||||
| 6. F3/T7 Recency Retrieval |
.02 | −.14 | −.23* | .10 | −.12 | -- | ||||||
| 7. F4/T8 Recency Retrieval |
.05 | .05 | −.13 | .07 | .07 | .49*** | -- | |||||
| 8. F3/T7 Recog Retrieval |
.02 | .02 | −.21* | .04 | −.09 | .32*** | .27** | -- | ||||
| 9. F4/T8 Recog Retrieval |
−.23* | .01 | −.03 | .03 | −.01 | .15 | .35*** | .43*** | -- | |||
| 10. F3/T7 Encoding |
−.04 | −.01 | .04 | .02 | −.03 | .12 | −.02 | .18 | .16 | -- | ||
| 11. F4/T8 Encoding |
−.12 | .01 | .02 | .00 | .11 | .04 | .23* | .02 | .35*** | .15 | -- | |
| 12. Age | −.07 | −.03 | .06 | .28** | .21* | −.02 | .04 | .13 | .11 | .15 | .17 | -- |
| N | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 |
| M | 3.58 | 4.03 | 6.63 | 18.12 | 5.23 | .26 | .26 | .30 | .30 | .14 | .15 | 32.36 |
| SD | 1.06 | 1.01 | 4.75 | 3.65 | 2.13 | .15 | .17 | .19 | .20 | .07 | .09 | 6.11 |
| Min | .00 | 1.00 | 2.00 | 3.00 | .00 | .04 | .01 | .02 | .04 | .02 | .01 | 18.00 |
| Max | 5.00 | 5.00 | 26.00 | 20.00 | 9.00 | .86 | .93 | .89 | .99 | .34 | .45 | 49.00 |
Note:
p < .05,
p < .01,
p < .001 (two-tailed)
Table 2.
Descriptive Statistics and Bivariate Correlations for Alpha Coherence
| Task | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.Recency Performance |
-- | |||||||||||
| 2. Recog Performance |
.47*** | -- | ||||||||||
| 3. EF Cognitive Flexibility |
−.24*** | −.16 | -- | |||||||||
| 4. EF Inhibitory Control |
−.06 | .05 | −.17 | -- | ||||||||
| 5. EF Working Memory |
.07 | .11 | −.14 | .29** | -- | |||||||
| 6. F3/T7 Recency Retrieval |
.07 | −.02 | −.13 | −.19 | −.17 | -- | ||||||
| 7. F4/T8 Recency Retrieval |
.18 | .01 | −.23* | .07 | −.16 | .29** | -- | |||||
| 8. F3/T7 Recog Retrieval |
−.08 | .02 | −.15 | −.09 | −.11 | .34*** | .32** | -- | ||||
| 9. F4/T8 Recog Retrieval |
−.09 | −.06 | −.17 | .06 | −.04 | .25** | .23** | .33*** | -- | |||
| 10. F3/T7 Encoding |
.00 | −.11 | −.04 | −.21* | −.05 | .46*** | .09 | .27** | .03 | -- | ||
| 11. F4/T8 Encoding |
−.05 | −.09 | −.11 | −.10 | −.02 | .22* | .43*** | .17 | .37*** | .40*** | -- | |
| 12. Age | −.07 | −.03 | .06 | .28** | .21* | −.18 | .08 | .08 | .03 | .03 | .01 | -- |
| N | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 | 108 |
| M | 3.58 | 4.03 | 6.63 | 18.12 | 5.23 | .24 | .26 | .24 | .26 | .15 | .17 | 32.36 |
| SD | 1.06 | 1.01 | 4.75 | 3.65 | 2.13 | .12 | .12 | .12 | .14 | .08 | .09 | 6.11 |
| Min | .00 | 1.00 | 2.00 | 3.00 | .00 | .01 | .02 | .03 | .03 | .02 | .01 | 18.00 |
| Max | 5.00 | 5.00 | 26.00 | 20.00 | 9.00 | .54 | .63 | .71 | .94 | .42 | .38 | 49.00 |
Note: Behavioral and descriptive correlations repeat.
p < .05,
p < .01,
p < .001 (two-tailed).
4.1 Frontotemporal EEG coherence during Recency and Recognition Performance
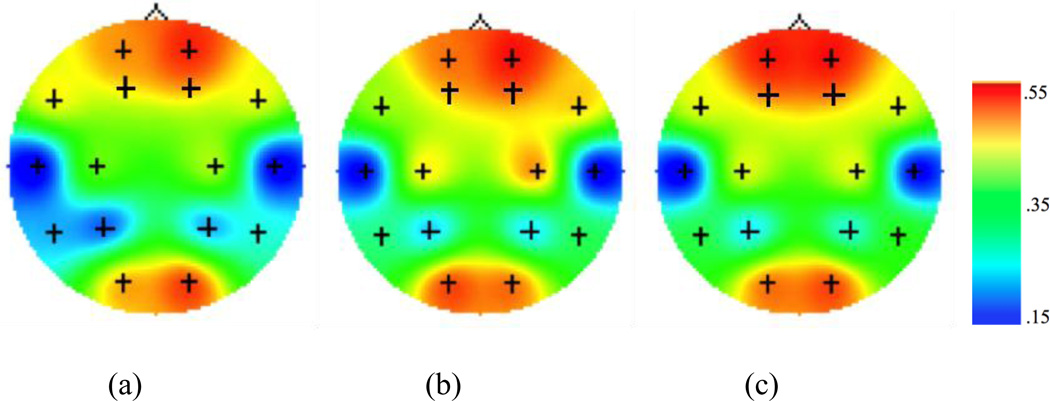
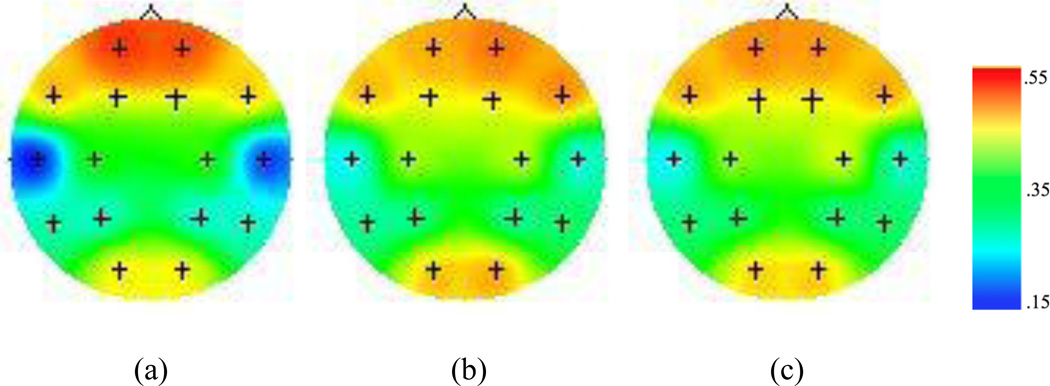
Figures 2 and 3 display the EEG coherence values for overall encoding, recency retrieval, and recognition retrieval within theta and alpha bands, respectively. Frontotemporal coherence during recognition was greater than frontotemporal coherence during recency within the theta band F (1, 107) = 6.14, p<.05. This is the opposite of what we predicted. There was no difference between frontotemporal coherence during recognition and recency retrieval within the alpha band, F (1,107) = .009, p = .93.
Figure 2.
Topographic maps for EEG coherence values within the theta band during (a) encoding, (b) recency, and (c) recognition conditions of the episodic memory task. Figures depict coherence between F3/F4 electrodes with all other channels within the same hemisphere. Higher values (i.e. warm colors) represent greater coherence.
Figure 3.
Topographic maps for EEG coherence values within the alpha band during (a) encoding, (b) recency, and (c) recognition conditions of the episodic memory task. Figures depict coherence between F3/F4 electrodes with all other channels within the same hemisphere. Higher values (i.e. warm colors) represent greater coherence.
4.2. Executive Function and Recency and Recognition Performance
Cognitive flexibility (i.e., WCST perseverative errors) was negatively correlated with recency performance. Working memory and inhibitory control were not correlated with recency performance. None of the executive functions were correlated with recognition performance (see Tables 1 and 2). These results partially support our second hypothesis that individual EFs would be associated with recency but not recognition performance.
4.3. Predicting Recency and Recognition Performance
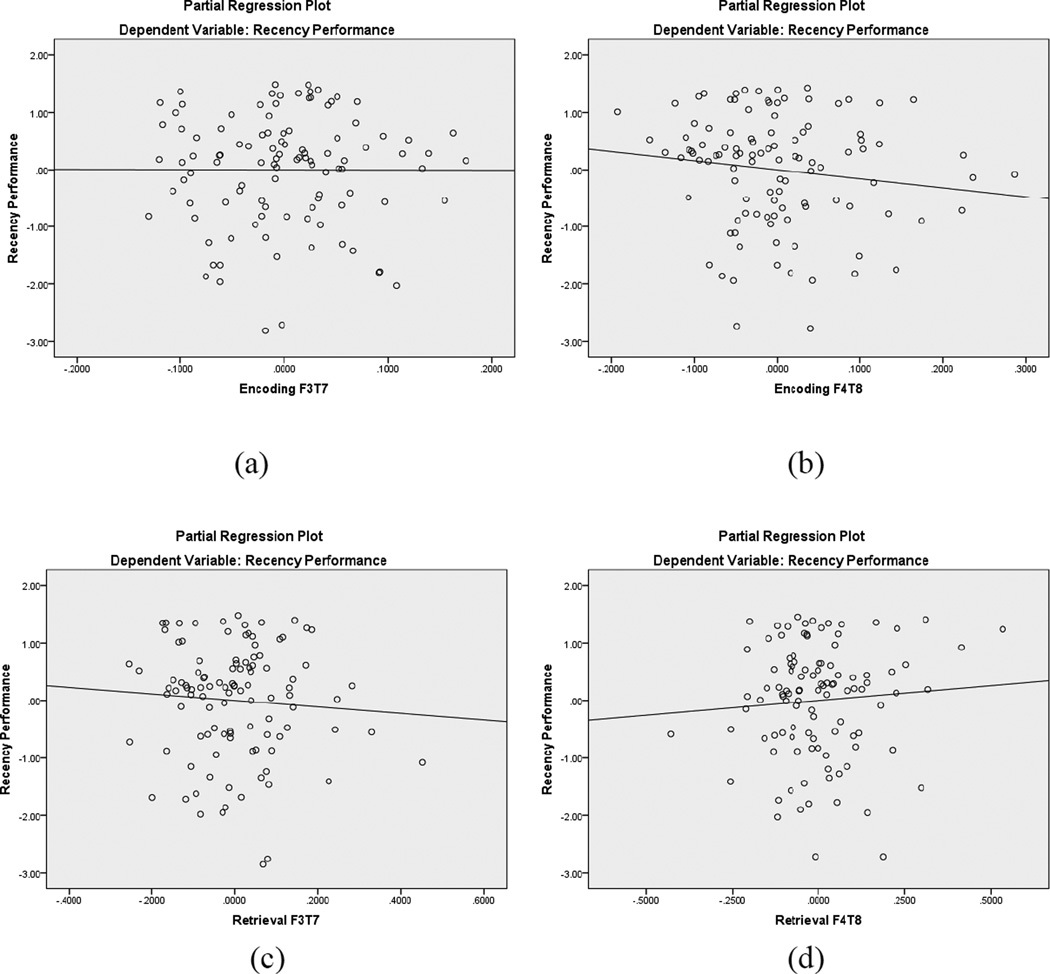
As shown in Tables 3 and 4, hierarchical regression equations were used to predict recency and recognition memory. Age and frontotemporal theta coherence during encoding were entered in the first steps and frontotemporal theta coherence during retrieval and performance on the EF tasks of inhibitory control, cognitive flexibility, and working memory were entered in the second steps of the equations. Cognitive flexibility (i.e., WCST preservative errors) was the only significant contributor within the equations, contributing 11% of the variance in recency performance. Theta EEG coherence did not contribute to either recency or recognition task performance. Figures 4 and 5 display the partial regression plots for the left and right hemisphere frontotemporal theta coherence predictors during the recency recognition task, highlighting the nonsignificant regression lines.
Table 3.
Results of Hierarchical Regression Statistically Predicting Theta Recency Performance
| R | R2 | R2 adj | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|---|
| Step 1: | .13 | .02 | −.01 | .57 | |||||
| Age | −.05 | −.50 | .00 | ||||||
| F3/T7 Coh Encoding | −.02 | −.17 | .00 | ||||||
| F4/T8 Coh Encoding | −.10 | −1.04 | .01 | ||||||
| Step 2: | .39 | .15 | .08 | .13 | 3.08* | 2.16* | |||
| Age | −.01 | −.10 | .00 | ||||||
| F3/T7 Coh Encoding | .01 | .08 | .00 | ||||||
| F4/T8 Coh Encoding | −.13 | −1.34 | .02 | ||||||
| F3/T7 Coh Recency Retrieval | −.08 | −.68 | .00 | ||||||
| F4/T8 Coh Recency Retrieval | .08 | .72 | .00 | ||||||
| EF Cognitive Flexibility (perseverative errors) | −.35 | −3.60** | * .11 | ||||||
| EF Working Memory | .06 | .60 | .00 | ||||||
| EF Inhibitory Control | −.12 | −1.25 | .01 | ||||||
Note: N = 108; df for Step 1 = (3, 104); df for Step 2 =(8, 99);
p ≤ .01;
p ≤ .05.
Table 4.
Results of Hierarchical Regression Statistically Predicting Theta Recognition Performance
| R | R2 | R2 adj | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|---|
| Step 1: | .03 | .00 | −.03 | .03 | |||||
| Age | −.03 | −.26 | .00 | ||||||
| F3/T7 Coh Encoding | −.01 | −.09 | .00 | ||||||
| F4/T8 Coh Encoding | .01 | .13 | .00 | ||||||
| Step 2: | .19 | .04 | −.04 | .03 | .70 | .45 | |||
| Age | −.04 | −.37 | .00 | ||||||
| F3/T7 Coh Encoding | .00 | .02 | .00 | ||||||
| F4/T8 Coh Encoding | .00 | .02 | .00 | ||||||
| F3/T7 Coh Recognition Retrieval | .00 | −.00 | .00 | ||||||
| F4/T8 Coh Recognition Retrieval | .01 | .10 | .00 | ||||||
| EF Cognitive Flexibility (perseverative errors) | −.15 | −1.40 | .02 | ||||||
| EF Working Memory | .09 | .85 | .01 | ||||||
| EF Inhibitory Control | .01 | .07 | .00 | ||||||
Note: N = 108; df for Step 1 = (3, 104); df for Step 2 =(8, 99);
p ≤ .01;
p ≤ .05.
Figure 4.
Partial regression plots for recency frontotemporal EEG coherence within the theta badn. The top plots are during encoding (a, b) and the bottom plots are during retrieval (c, d). In each pair of plots, the left image represents left hemisphere coherence and the right image represents right hemisphere coherence. None of the regression lines were nonsignificant.
Figure 5.
Partial regression plots for recognition frontotemporal EEG coherence within the theta band. The top plots are during encoding (a, b) and the bottom plots are during retrieval (c, d). In each pair of plots, the left image represents left hemisphere coherence and the right image represents right hemisphere coherence. None of the regression lines were significant.
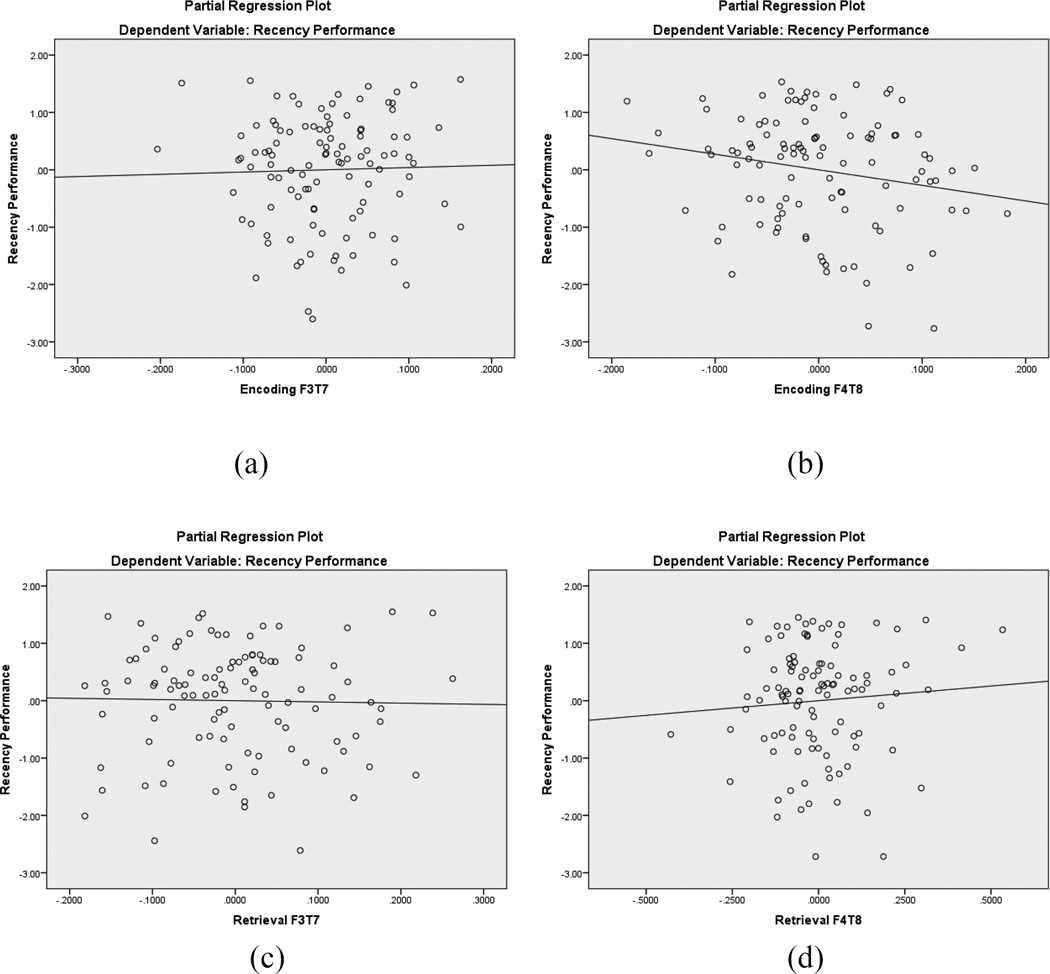
As shown in Table 5, the variables in Step 1 of the hierarchical regression (age, frontotemporal alpha coherence during encoding) accounted for a non-significant 1% of the variance in recency trials performance. The Step 2 variables (frontotemporal alpha coherence during recency retrieval, cognitive flexibility, working memory, inhibitory control) collectively accounted for an additional 17% of variance in recency task performance, with cognitive flexibility (i.e., WCST preservative errors) and right hemisphere frontotemporal alpha coherence during both encoding and retrieval contributing unique variance to recency performance. Figure 6 displays the partial regression plots for the left and right hemisphere frontotemporal alpha coherence predictors, highlighting the significant regression lines for the right hemisphere (Figure 6b and 6d). The beta weight for encoding was negative, whereas the beta weight for retrieval was positive. Thus, lower frontotemporal alpha coherence during encoding was associated with better recency performance, as was greater frontotemporal alpha coherence during retrieval. This regression analysis supports our hypothesis that both EFs and frontotemporal coherence would contribute unique variance to recency performance.
Table 5.
Results of Hierarchical Regression Statistically Predicting Alpha Recency Performance
| R | R2 | R2 adj | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|---|
| Step 1: | .09 | .01 | −.02 | .28 | |||||
| Age | −.07 | −.72 | .01 | ||||||
| F3/T7 Coh Encoding | .03 | .30 | .00 | ||||||
| F4/T8 Coh Encoding | −.06 | −.56 | .00 | ||||||
| Step 2: | .43 | .18 | .12 | .17 | 4.22** | 2.76** | |||
| Age | −.05 | −.55 | .00 | ||||||
| F3/T7 Coh Encoding | .03 | .28 | .00 | ||||||
| F4/T8 Coh Encoding | −.22 | −2.01* | .03 | ||||||
| F3/T7 Coh RecencyRetrieval | −.02 | −.21 | .00 | ||||||
| F4/T8 Coh RecencyRetrieval | .24 | 2.16* | .04 | ||||||
| 7EF CognitiveFlexibility (perseverative errors) | −.32 | −3.30*** | .09 | ||||||
| EFWorking Memory | .13 | 1.28 | .01 | ||||||
| EF InhibitoryControl | −.16 | −1.59 | .02 | ||||||
Note: 108; df for Step 1 = (3, 104); df for Step 2 = (8, 99);
p ≤ .001;
p ≤ .01;
p ≤ .05.
Figure 6.
Partial regression plots for recency frontotemporal EEG coherence within the alpha band. The top plots are during encoding (a, b) and the bottom plots are during retrieval (c, d). In each pair of plots, the left image represents left hemisphere coherence and the right image represents right hemisphere coherence. Right hemisphere coherence during encoding (b) displays a significant regression line, as does right hemisphere coherence during retrieval (d)
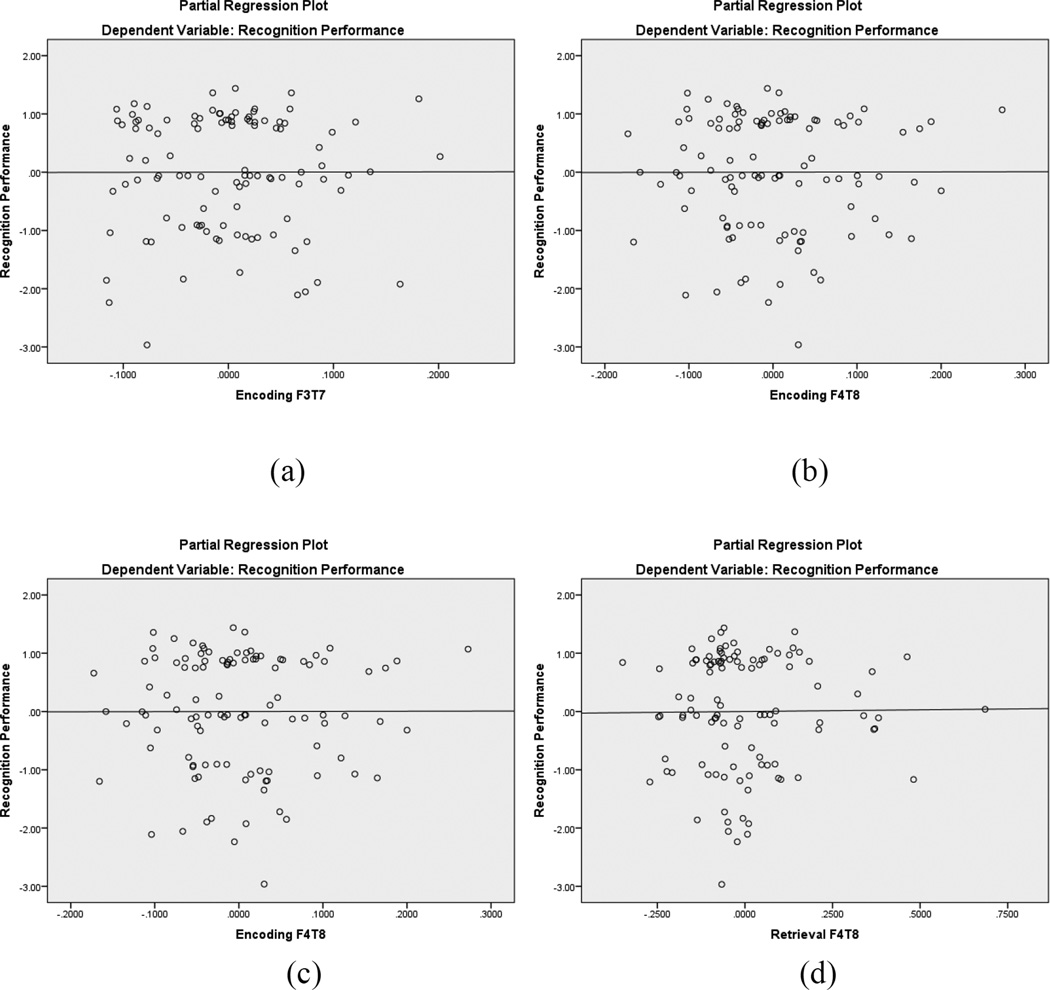
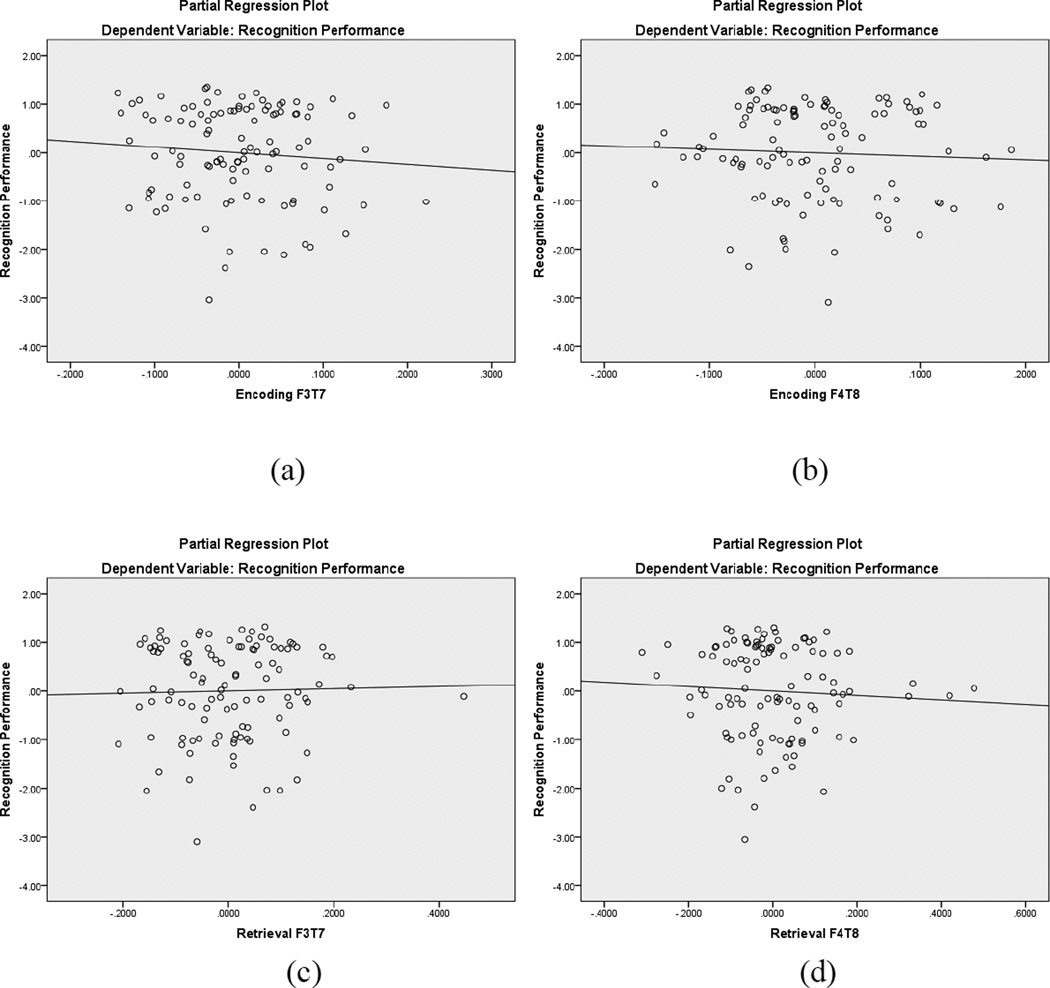
As shown in Table 6, a similar hierarchical regression equation was use to predict recognition memory, except that the EEG retrieval coherence was during recognition trials. Neither step was significant, meaning that collectively this group of independent variables did not predict recognition performance. Figure 7 displays the partial regression plots for the left and right hemisphere frontotemporal alpha coherence predictors, highlighting the nonsignificant regression lines.
Table 6.
Results of Hierarchical Regression Statistically Predicting Alpha Recognition Performance
| R | R2 | R2adj | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|---|
| Step 1: | .12 | .02 | −.01 | .527 | |||||
| Age | −.02 | −.23 | .00 | ||||||
| F3/T7Coh Encoding | −.09 | −.84 | .01 | ||||||
| F4/T8Coh Encoding | −.06 | −.53 | .00 | ||||||
| Step 2: | .24 | .06 | −.02 | .04 | .860 | .734 | |||
| Age | −.03 | −.26 | .00 | ||||||
| F3/T7Coh Encoding | −.09 | −.74 | .01 | ||||||
| F4/T8Coh Encoding | −.11 | −.93 | .01 | ||||||
| F3/T7Coh Recognition Retrieval | .02 | .15 | .00 | ||||||
| F4/T8Coh Recognition Retrieval | .05 | .41 | .00 | ||||||
| EF Cognitive Flexibility (perseverative errors) | −.16 | −1.51 | .02 | ||||||
| EF Working Memory | .11 | 1.01 | .01 | ||||||
| EF Inhibitory Control | −.03 | −.27 | .00 | ||||||
Note: N = 108; df for Step 1 = (3, 104); df for Step 2 =(8, 99);
p ≤ .01;
p ≤ .05.
Figure 7.
Partial regression plots for recognition frontotemporal EEG coherence within the alpha band. The top plots are during encoding (a, b) and the bottom plots are during retrieval (c, d). In each pair of plots, the left image represents left hemisphere coherence and the right image represents right hemisphere coherence. None of the regression lines were significant.
5. Discussion
The purpose of our study was to examine the contributions of EFs and frontotemporal coherence to episodic memory recency and recognition performance. Frontotemporal EEG alpha coherence within the right hemisphere during encoding and retrieval statistically predicted variance in recency memory performance, which replicates findings regarding frontotemporal coactivation (Simon & Spiers, 2003; Weiss & Rappelsberger, 2000) and right hemispheric dominance during non-verbal memory tasks (Kelley et al., 1998).
Our first hypothesis that there would be greater frontotemporal EEG coherence during the recency than recognition conditions was not supported by our analyses. Within the theta frequency band, recognition trials had greater coherence than recency trials across both hemispheres, which was not expected. No coherence differences were found between recency and recognition within the alpha band. The reasons for these unexpected findings are unclear. Our stimuli (i.e., abstract art) are not typical of those currently used during episodic memory studies but were very similar to those used in Milner’s early work on temporal ordering (Milner et al, 1991). However, the cognitive processes of recency and recognition should be the same regardless of the stimuli.
Regression analyses allowed for insight on how frontotemporal EEG coherence differentially contributes to recency and recognition performance and highlighted hemispheric differences when simultaneously considering the contributions of EF task performance. Frontotemporal EEG coherence failed to statistically predict variance in the less taxing recognition memory performance within both alpha and theta bands. However, right hemisphere frontotemporal EEG coherence had predictive power with respect to recency judgments within the alpha, but not theta band. Lower alpha coherence during encoding and greater alpha coherence during retrieval, both within the right hemisphere, predicted better recency performance. Thus, the association between brain and accuracy in outcome differed for the two types of episodic judgments within the same task. The associations were specific to the alpha frequency band.
The negative association between alpha coherence during encoding and recency performance was unexpected, as was the finding that theta coherence did not predict recency performance. We noted that our stimuli are not typical of contemporary episodic memory tasks. In addition, these unexpected results may be influenced by our unique sample. Rather than drawing from the typical undergraduate population, our sample consisted of women of wide ranging ages and education levels. These women are community mothers who participated in a larger study of cognitive and emotion processes associated with parenting (e.g., Deater-Deckard, Li, & Bell, 2016). It is possible that the typical theta associations with episodic memory may be a function of sample selection. Future studies should examine the neural correlates of recency in diverse samples to provide additional insight to episodic memory processes. Thus, our findings may be unique because we employed electrophysiological measures in a sample not typical of episodic memory studies to support the relation between coherence of right frontal and temporal regions and non-verbal episodic memory.
These findings are unique because they included executive processes along with recency and recognition differences. Evidence exists to suggest that EFs support episodic memory encoding and retrieval (Mantyla, Carelli, & Forman, 2007). However, research in episodic memory tends to explore the contributions of EFs as a composite score (McCabe et al., 2010). Perhaps a more informative method is to separate the processes and examine potential unique contributions in recency performance for each EF process. Our findings suggest that a relation exists between cognitive flexibility and recency memory performance. It was surprising that inhibitory control did not statistically predict recency performance because inhibitory control has been associated with memory performance in other studies (Alexander et. al, 2002; Marsh, Beaman, Hughes, & Jones, 2012). Of additional interest, is the lack of relation found between working memory and recency performance. This is contrary to previous research (Gallo & Wheeler, 2012; Marsh et al., 2012) and may be a result of the nature of the working memory task we used. Our working memory task required participants to repeat verbal numerical sequences. The results may have been somewhat different if pictorial items had been used instead. Because both working memory and inhibitory control were correlated with age, the lack of findings for these variables may also be a result of controlling for age. Future studies should examine EF contributions to recency and recognition in a narrower aged sample to test this association.
Additionally, our Corsi-Milner-type memory task may have required a greater level of cognitive flexibility processes than that required by other memory tasks. Participants were required to shift between the type of question asked (recency vs. recognition) and the encoding (viewing pictures) and retrieval (answering questions) process. The requirement of the participants to shift their attention to successfully complete the memory task could explain the relation found between cognitive flexibility and recency memory. However, inhibitory control has been proposed to aid in the retrieval of episodic memory through the inhibition of interference (Ruffman et al., 2001). It is possible that cognitive flexibility aids episodic memory in a similar fashion. However, limited research has explored the link between cognitive flexibility and episodic memory, so further investigation is necessary to draw such a conclusion.
Of particular interest is the difference in EF predictors between recency and recognition. Although cognitive flexibility contributed unique variance to the prediction of recency performance in our regression analysis, it did not contribute unique variance to the prediction of recognition performance; this may be attributed to the ease of the recognition task. Perhaps because the participants did not have to exert much effort to answer the recognition as compared to the recency questions, they were less likely to recruit the aid of higher order cognitive processes. To further test this explanation, we ran a post-hoc paired sample t-test comparing recency and recognition task performance. Participants performed better on the recognition portion of the memory task, demonstrating that the recognition task was easier than the recency. The ease of recognition questions (i.e. familiarity) is attributed to the fact that all studied material should elicit some form of familiarity, whereas the same is not true of recency (Yonelinas, Aly, Wang, & Koen, 2010). Furthermore, the discrepancy in ease may explain the lack of EF predictors found for recognition performance, as well as the lack of frontotemporal EEG coherence as a predictor.
Thus, the regression for the recency memory task showed significant statistical contributors but the recognition task did not. This suggests that recency and recognition tasks may rely on separate mechanisms; this result was expected considering that recency has been associated with increased use of recollection processes. However, research has implied that both recency and recognition require recollection to some extent, and that the same general areas of the brain (frontal and temporal) should be active for recency and recognition tasks (Casino et al., 2002). The findings of our study propose that recency and recognition do not elicit coherence from the same areas of the brain, at least for our episodic memory task. Perhaps recency, due to the temporal component, is more reliant on the coactivation of frontal and temporal regions when compared to recognition. In fact, it has been reported that recency-based memory requires more frontal activation when compared to recognition (Milner et. al, 1991). Still, additional information is needed to understand the neural network differences between recency and recognition.
Although the results of our study provide insights into the episodic memory process, there were some limitations to our work. First, only one measure was used to assess each individual EF. Multiple assessments of each construct yield a more reliable measure. The data we report here are from a larger study focused on cognition, emotion, and parenting. Because of the length of the protocol we were unable to administer multiple assessments for each EF task. Second, the cognitive flexibility task we administered (WCST) has been suggested to tap into other executive functions in addition to cognitive flexibility (Miyake et al., 2000). Third, while oscillatory communication may provide insight into scalp locations related to cognitive functioning, the spatial resolution of EEG is poor. Future studies should utilize imaging techniques that allow for localized spatial analyses. Finally, the generalizability may be restricted. Although research exists suggesting there are no differences between males and females on episodic memory tasks (Hyde, 2005), there has been evidence to suggest otherwise (Herlitz, Airaksinen, & Nordstrom, 1999).
6. Conclusions
We have provided evidence that the EF of cognitive flexibility contributes to recency, but not recognition, memory performance. Furthermore, we provided support for the role of frontotemporal functional connectivity during encoding and retrieval to recency, but not recognition, memory. Specifically, our results suggest that right hemisphere frontotemporal coherence contributes to episodic recency judgments during our memory task using abstract paintings. These findings indicate that recognition and recency operate via separate electrophysiological and executive mechanisms.
Highlights.
We examine EEG coherence and executive function contributions to episodic memory.
Frontal-Temporal coherence predicted recency performance, not recognition.
Set-Shifting predicted recency performance, not recognition.
Acknowledgments
This research was supported by grants HD057319 awarded to Martha Ann Bell and HD060110 awarded to Kirby Deater-Deckard from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our studies and to our research teams for their assistance with data collection and coding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav.Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Alexander KW, Goodman GS, Schaaf JM, Edelstein RS, Quas JA, Shaver PR. The role of attachment and cognitive inhibition in children’s memory and suggestibility for a stressful event. J. Exp. Child Psychol. 2002;83:262–290. doi: 10.1016/s0022-0965(02)00149-2. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nat. 2001;410:366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Levy BJ. Inhibitory processes and the control of memory retrieval. Trends Cognit. Sci. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Baumal K, Pastotter B, Hanslmayr S. Binding and inhibition in episodic memory-Cognitive, emotional, and neural processes. Neurosci. Biobehav. Rev. 2010;34:1047–1054. doi: 10.1016/j.neubiorev.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Brown GDA, Vousden JI, McCormack T. Memory retrieval as temporal discrimination. J. Mem. Lang. 2009;60:194–208. [Google Scholar]
- Bugaiska A, Clarys D, Jarry C, Taconnat L, Tapia G, Vanneste S, Isingrini M. The effect of aging in recollection experience: The processing speed and executive functioning hypothesis. Consciousness and Cognition. 2007;16:797–808. doi: 10.1016/j.concog.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Casino S, Maquet P, Dolan R, Rugg M. Brain activity underlying encoding and retrieval of source memory. Cereb. Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Kapur S, Craik FIM, McIntosh AR, Houle S, Tulving E. Functional neuroanatomy of recall and recognition: a PET study of episodic memory. J. Cognit. Neurosci. 1997;9:254–265. doi: 10.1162/jocn.1997.9.2.254. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum HE. Memory, amnesia, and the hippocampal system. Cambridge: MIT Press; 1993. [Google Scholar]
- Corkin S. What’s new with the amnesic patient H.M.? Nat. Rev. Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Li M, Bell MA. Multi-faceted emotion regulation, stress, and affect in mothers of young children. Cognition and Emotion. 2016;30:444–457. doi: 10.1080/02699931.2015.1013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Robertson LC, Efron R. Hemispheric specialization of memory for visual hierarchical stimuli. Neuropsychol. 1986;24:205–214. doi: 10.1016/0028-3932(86)90053-9. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging the medial temporal lobe: The roles of the hippocampus, parahippocampal cortex, and perirhinal cortex in recollection and familiarity. Trends Cog. Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Flectcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nat. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3rd. Circle Pines: American Guidance Service; 1997. [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Shallice CD, Frith CD, Frackowiak RS, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain. 1998;121:1249–1256. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Wheeler ME. Oxford handbook of cognitive psychology. New York: Oxford University Press; 2013. Episodic Memory; pp. 189–205. [Google Scholar]
- Güntekin B, Başar E. A new interpretation of P300 responses upon analysis of coherences. Cognit. Neurodyn. 2010;4:107–118. doi: 10.1007/s11571-010-9106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist F, Shimamura AP, Squire LR. On the relationship between recall and recognition memory. J. Exp. Psychol.: Learning, Mem. Cognit. 1992;18:691–702. doi: 10.1037//0278-7393.18.4.691. [DOI] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychol. 2008;22:491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitz A, Airksinen E, Nordstrom E. Sex differences in episodic memory: the impact of verbal and visuospatial ability. Neuropsychol. 1999;13:590–597. doi: 10.1037//0894-4105.13.4.590. [DOI] [PubMed] [Google Scholar]
- Hyde JS. The Gender Similarities Hypothesis. Am. Psychol. 2005;60:581–592. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Steven PE. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 1997;26:319–349. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci. Lett. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Konishi S, Uchida I, Okuaki T, Machida T, Shirouzu I, Miyashita Y. Neural correlates of recency judgment. J. Neurosci. 2002;22:9549–9555. doi: 10.1523/JNEUROSCI.22-21-09549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Natl. Acad. Sci. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and control of memory retrieval. Trends Cog. Sci. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Mantyla T, Carelli MG, Forman H. Time monitoring and executive functioning in children and adults. J. Exp. Child Psychol. 2007;96:1–19. doi: 10.1016/j.jecp.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Marsh JE, Beaman PC, Hughes RW, Jones DM. Inhibitory control in memory: evidence for negative priming in free recall. J. Exp. Psychol. 2012;38:1377–1388. doi: 10.1037/a0027849. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain research bulletin. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsychol. 2010;24:222–243. doi: 10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Nyberg L, Bookstein FL, Tulving E. Differential functional connectivity of prefrontal and medial temporal cortices during episodic memory retrieval. Human brain map. 1997;5:323–327. doi: 10.1002/(SICI)1097-0193(1997)5:4<323::AID-HBM20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Cognit. Brain Res. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mevorach C, Humphreys GW, Shalev L. Attending to local form while ignoring global aspects depends on handedness: evidence from TMS. Nat. Neurosci. 2005;8:276–277. doi: 10.1038/nn1400. [DOI] [PubMed] [Google Scholar]
- Michels L, Bucher IK, Luchinger R, Klaver P, Martin E, Jeanmonod D, Brandeis D. Simultaneous EEG-fMRI during a working memory task: Modulations in low and high frequency bands. PLoS ONE. 2010;5:e10298. doi: 10.1371/journal.pone.0010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contribution to recency judgements. Neuropsychol. 1991;29:601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: four general conclusions. Curr. Dir. Psychol. Sci. 2013;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cognit. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Shah P. Models of working memory: Mechanisms of active maintenance and executive control. New York: Cambridge University Press; 1999. Toward unified theories of working memory: Emerging general consensus, unresolved theoretical issues, and future research directions; pp. 442–481. [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr. Opin. Neurobiol. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Electric Fields of the Brain: The Neurophysics of EEG. New York: Oxford University Press; 1981. p. 484. [Google Scholar]
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci. Biobehav. Rev. 2010;34:1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. Binding items and contexts: the cognitive neuroscience of episodic memory. Curr. Dir. Psychol. Sci. 2010;19:131–137. [Google Scholar]
- Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Electrophysiological indices of memory for temporal order in early childhood: implications for the development of recollection. Dev. Sci. 2008;12:209–219. doi: 10.1111/j.1467-7687.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffman T, Rustin C, Garnham W, Parkin AJ. Source monitoring and false memories in children. Relation to certainty and executive functioning. J. Exp. Child Psychol. 2001;80:95–111. doi: 10.1006/jecp.2001.2632. [DOI] [PubMed] [Google Scholar]
- Saltzberg B, Burton WD, Jr, Burch NR, Fletcher J, Michaels R. Electrophysiological measures of regional neural interactive coupling. Linear and nonlinear dependence relationships among multiple channel electroencephalographic recordings. Int. J. Biomed. Comput. 1986;18:77–87. doi: 10.1016/0020-7101(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual attention is selectively associated with human alpha activity. Euro. J. Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Implicit memory: History and current status. J. Exp. Psychol.: Learning, Mem. Cognit. 1987;13:501–518. [Google Scholar]
- Schacter DL, Graf P. Effects of elaborative processing on implicit and explicit memory for new associations. J. Exp. Psychol.: Learning, Mem. Cognit. 1986;12:432. [Google Scholar]
- Shallice T, Flectcher P, Frith CD, Grasby P, Frackowiak RSJ, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nat. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Simon JA, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychol. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Neurosci. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Rugg MD. Electrophysiological dissociation of recency and recognition memory. Neuropsychol. 1998;36:477–490. doi: 10.1016/s0028-3932(97)00157-7. [DOI] [PubMed] [Google Scholar]
- Thatcher RW. Human behavior and the developing brain. New York: Guilford Press; 1994. Cyclic cortical reorganization: origins of human cognitive development; pp. 232–266. [Google Scholar]
- Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortisol associations and EEG coherence: a two-compartmental model. Electroencephalogr. Clin. Neurophysiol. 1986;64:123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, North DM, Biver CJ. Development of cortical connections as measured by EEG coherence and phase delays. Hum. Brain Mapp. 2008;29:1400–1415. doi: 10.1002/hbm.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Organization of memory. New York: Academic Press; 1972. Episodic and semantic memory; pp. 381–402. [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annu. Rev. Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiss S, Rappelsberger P. Long-range EEG synchronization during word encoding correlates with successful memory performance. Cognit. Brain Res. 2000;9:299–312. doi: 10.1016/s0926-6410(00)00011-2. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychol. 1998;37:103–118. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A Review of 30 years of research. J. Mem. Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, McLaughlin C, Kahana MJ, Sekuler R. Recognition and position information in working memory for visual textures. Mem. Cognit. 2008;36:282–294. doi: 10.3758/mc.36.2.282. [DOI] [PubMed] [Google Scholar]