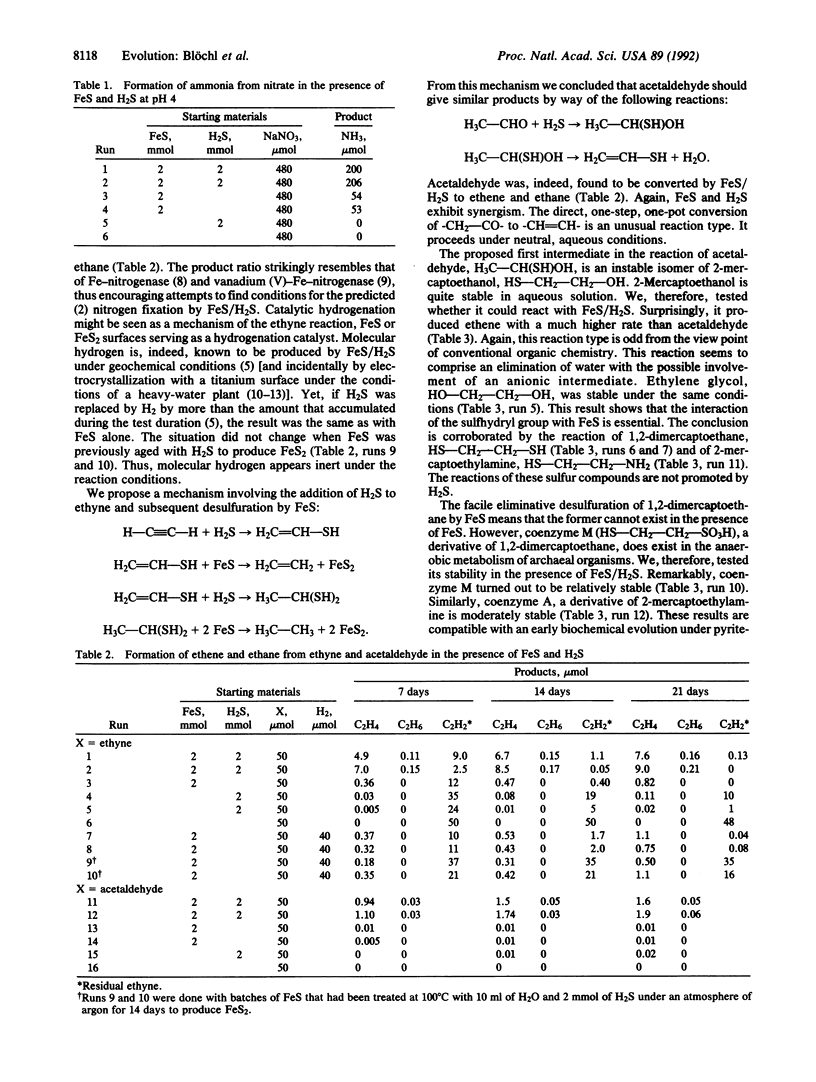
Abstract
Iron sulfide gives rise to unusual reducing reactions: some dependent on FeS/H2S synergism [NO-3 --> NH3; HC(three bonds)CH--> H2C=CH2, H3C-CH3; -CH2-CO- --> -CH=CH-, -CH2-CH2-; HS-CH2-COOH --> CH3-COOH; others dependent on FeS alone [HS-CH2-CH2-X --> CH2=CH2 (where X = OH, SH, or NH2)]. The experimental conditions are geochemically plausible: 100 degrees C, aqueous, nearly neutral, and fastidiously anaerobic. These reactions establish additional facts of soil chemistry, organic geochemistry, and the global nitrogen cycle. Further, they point to the common evolutionary denominator of geochemistry and biochemistry.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkowitz R. A., Abeles R. H. Mechanism of action of clostridial glycine reductase: isolation and characterization of a covalent acetyl enzyme intermediate. Biochemistry. 1991 Apr 23;30(16):4090–4097. doi: 10.1021/bi00230a039. [DOI] [PubMed] [Google Scholar]
- Beinert H., Kennedy M. C. 19th Sir Hans Krebs lecture. Engineering of protein bound iron-sulfur clusters. A tool for the study of protein and cluster chemistry and mechanism of iron-sulfur enzymes. Eur J Biochem. 1989 Dec 8;186(1-2):5–15. doi: 10.1111/j.1432-1033.1989.tb15170.x. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Kowal A. T., Morningstar J. E., Oliver M. E., Whittaker K., Gunsalus R. P., Ackrell B. A., Cecchini G. Subunit location of the iron-sulfur clusters in fumarate reductase from Escherichia coli. J Biol Chem. 1988 Oct 15;263(29):14732–14738. [PubMed] [Google Scholar]
- Pau R. N., Mitchenall L. A., Robson R. L. Genetic evidence for an Azotobacter vinelandii nitrogenase lacking molybdenum and vanadium. J Bacteriol. 1989 Jan;171(1):124–129. doi: 10.1128/jb.171.1.124-129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. Before enzymes and templates: theory of surface metabolism. Microbiol Rev. 1988 Dec;52(4):452–484. doi: 10.1128/mr.52.4.452-484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. Evolution of the first metabolic cycles. Proc Natl Acad Sci U S A. 1990 Jan;87(1):200–204. doi: 10.1073/pnas.87.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. Groundworks for an evolutionary biochemistry: the iron-sulphur world. Prog Biophys Mol Biol. 1992;58(2):85–201. doi: 10.1016/0079-6107(92)90022-x. [DOI] [PubMed] [Google Scholar]



